Abstract
Background
Serum markers are used prior to pelvic imaging to improve specificity and positive predictive value (PPV) of ovarian cancer multimodal screening strategies.
Methods
We conducted a randomized controlled pilot trial to estimate surgical PPV of a “2 of 3 tests positive” screening rule, and to compare use of HE4 as a 1st-line (Arm 1) vs. a 2nd-line (Arm 2) screen, in women at high and elevated risk for EOC at five study sites. Semi-annual screening was offered to 208 women aged 25-80 with deleterious BRCA germ-line mutations, and to 834 women aged 35-80 with pedigrees suggesting inherited susceptibility. Annual screening was offered to 130 women aged 45-80 (Risk Group 3) with epidemiologic and serum marker risk factors. Rising marker levels were identified using the parametric empirical Bayes algorithm.
Results
Both strategies yielded surgical PPV above 25%. Protocol-indicated surgery was performed in six women, identifying two ovarian malignancies and yielding a surgical PPV in both arms combined of 33% (95% CI: 4%-78%), 25% in Arm 1 and 50% in Arm 2. Surgical consultation was recommended for 37 women (26 in Arm 1, 11 in Arm 2). Based on 12 women with at least 2 of 3 tests positive (CA125, HE4 or imaging), an intent-to-treat analysis yielded PPV of 14% in Arm 1 and 20% in Arm 2.
Conclusions
Positive screens were more frequent when HE4 was included in the primary screen. Impact. HE4 may be useful as a confirmatory screen when rising CA125 is used alone as a primary screen.
Keywords: Ovarian cancer, screening, CA125, HE4, high-risk population
Introduction
Epithelial ovarian cancer (EOC), including serous ovarian, fallopian tube and primary peritoneal carcinomas, is the most lethal gynecologic malignancy. Early detection could potentially reduce EOC mortality, but because efficacy has not been demonstrated, national guidelines [1] do not recommend EOC screening in the general population. For women with or at high risk for a germ-line BRCA mutation, semiannual screening with transvaginal ultrasound (TVU) and CA125 testing is recommended starting at age 35, or 5-10 years earlier than the earliest age of EOC diagnosis in the family. The U.K. familial study of EOC screening reported that frequent screening and prompt surgical intervention are needed to detect cancer at an early stage [2].
Only 0.8% of women in the general population are diagnosed with EOC by age 70; 39% and 22% of BRCA1 and BRCA2 mutation carriers respectively will experience EOC by age 70 [3] but hereditary EOC accounts for only 10-15% of cases. Epidemiologic risk factors [4, 5] and risk-associated circulating biomarkers [6] could be useful to expand the conventional hereditary high-risk population and identify a cohort of elevated risk women who may benefit from screening. CA125 is a predictive marker for EOC that becomes increasingly powerful with proximity to diagnosis [7, 8] and may signal the presence of precursor lesions such as adnexal dysplasia [9].
Interpreting CA125 trends using a longitudinal algorithm to select women for imaging potentially improves screening performance [8, 10]. This strategy is being tested in the three-arm U.K. Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) [11]. Results of the prevalence screen suggest that a multimodal approach using rising CA125 annually to select women for imaging is more sensitive as well as more specific than using TVU annually in all women, yielding positive predictive value (PPV) of 35% [11]. Data from a single-arm U.S. trial further support the potential efficacy of CA125 change over time as a means to triage women to TVU and surgery, demonstrating a PPV for early invasive EOC of 40% (95% CI= 12.2%-73.8%) and a specificity of 99.9% (95% CI= 99.7%-100%) [12].
The human epididymis 4 gene WFDC2 (HE4) [13-15] could potentially contribute to a screening strategy. Serum HE4 is more specific than CA125 in discriminating women with malignant tumors from those with benign tumors [15, 16] and was recently approved by the FDA for this use in combination with CA125 [17].
CA125 in combination with HE4 has not previously been prospectively examined in the screening setting. We performed a Phase I randomized controlled trial (RCT) using HE4 as either a primary (first-line) or a confirmatory (second-line) test to select women for TVU and clinical follow up. If 2 of the 3 tests (CA125, HE4 or imaging) are positive at the confirmatory screen, or if the confirmatory CA125 is sufficiently high, consultation with a gynecologic oncologist is recommended.
The parametric empirical Bayes (PEB) longitudinal algorithm [18, 19] is used to tailor marker thresholds to the individual woman based on her age and marker history. It provides a positive result at lower marker levels by accounting for marker history within each woman [18] without any sacrifice in specificity [19]. Cut-off levels assigned by the PEB will be lower for most women than a single threshold rule with comparable specificity [19], yielding longer lead times for screen-detected cancers, as demonstrated using serial CA125 data from the PLCO trial [10]. The PEB approach can be easily generalized to a panel that includes novel markers such as HE4. Requiring two tests such as CA125 and imaging [20], or CA125 and HE4 [21] both to be positive before recommending clinical follow up can improve specificity dramatically.
Materials and Methods
We conducted a RCT to estimate surgical PPV of the “2 of 3 tests positive” rule, and to compare use of HE4 as a 1st-line vs. a 2nd-line screen, in multimodal screening of women at high and elevated risk for EOC.
Study populations
Participants were recruited at five sites: Cedars-Sinai Medical Center, City of Hope, Stanford University, Swedish Cancer Institute/Fred Hutchinson Cancer Research Center, and Fox Chase Cancer Center. Sites recruited eligible women through their high-risk EOC screening programs (Cedars-Sinai, Fox Chase, Swedish), specimen donation programs (Cedars-Sinai, Fred Hutchinson), or local oncology and gynecology practices (Cedars-Sinai, City of Hope, Stanford, Swedish). The Fred Hutchinson Cancer Research Center served as the national Coordinating Center.
Eligibility
Eligible participants were elevated-risk women aged 25 to 80, classified as belonging to the highest risk group for which they qualified. Risk Group 1 included women 25-80 years old carrying a deleterious BRCA1 or BRCA2 germ line mutation. Risk Group 2 included women 35-80 years old from a high-risk family, including women meeting NCCN V4.2013 Breast and Ovarian Cancer Genetic Assessment Guidelines for referral to a genetics professional, and women with a deleterious mutation in HNPCC or TP53 genes or a first or second degree relative positive for HNPCC. Risk Group 3 included women between 45-80 years old with epidemiologic risk factors [4, 5, 22, 23] or circulating proteins [6, 7] conferring EOC risk. The presence of either at least 3 of 6 risk factors (<1 year of oral contraceptive use, nulliparity, no breastfeeding, no tubal ligation, Ashkenazi Jewish, >1 year of menopausal hormone therapy [HT]), or a CA125, HE4, MMP7 or Mesothelin value exceeding the 95% percent population threshold, qualified a woman for Risk Group 3. Women were excluded if they had a personal history of EOC, no ovaries, abdominal surgery within the last 3 months, a current pregnancy, a medical condition precluding phlebotomy, untreated malignancy (other than non-melanoma skin cancer), or receipt of adjuvant chemotherapy or radiation therapy for cancer (tamoxifen, aromatase inhibitors, and/or GnRH agonist were allowed) within 3 months. Eligible women were enrolled if they provided informed consent, signed a medical records release form, and identified a care provider who agreed to receive screening results.
Randomization
At enrollment, participants were randomly allocated to screening that did or did not include HE4 in the primary screen by a computer algorithm generated by a statistician uninvolved in ascertainment of outcomes. Participants were randomized in blocks of 6 (3 to each arm) within study site and risk group.
Intervention
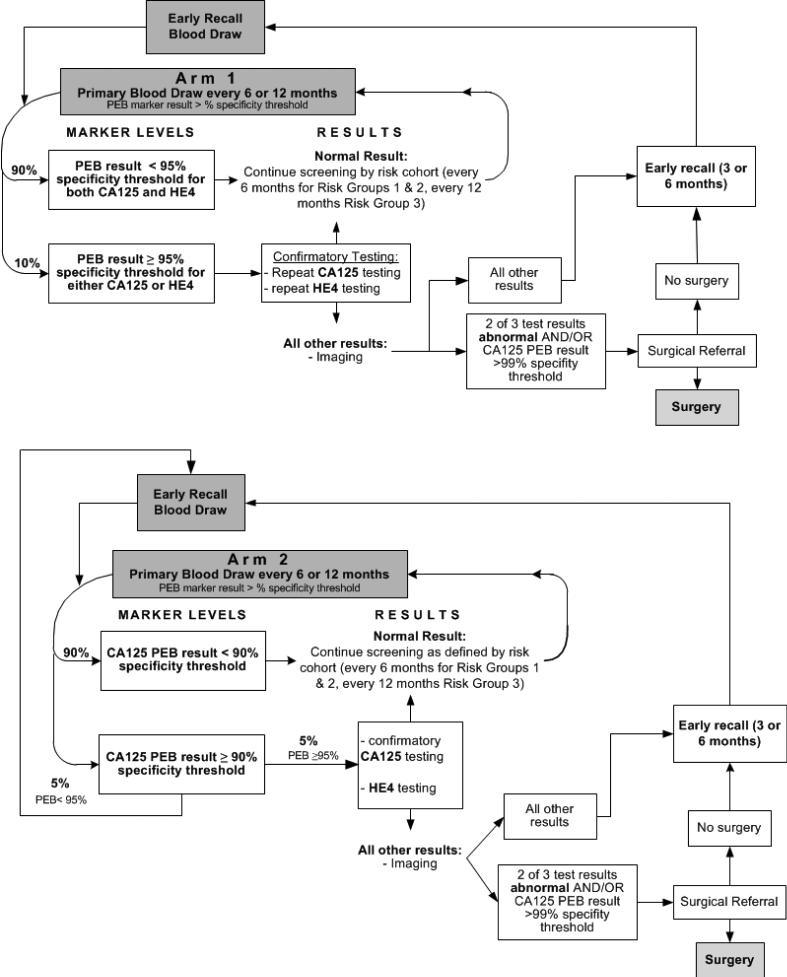
Two staged multimodal screening protocols were evaluated, as shown in Figure 1, differing only in the design of the primary screen which included both CA125 and HE4 in Arm 1 and only CA125 in Arm 2. In both arms, if the primary screen was positive, a confirmatory blood test that included both CA125 and HE4 was used to select women for follow-up imaging and potentially for clinical follow up. Screening was risk-based so that women in Risk Groups 1 and 2 were screened semi-annually with early recall at 3 months. Women in Risk Group 3 were screened annually with early recall at 6 months. The staged approach selects women for increasingly costly tests and procedures so that PPV improves with each stage of the protocol. CA125 and HE4 were interpreted using the previously developed PEB longitudinal algorithm (above a threshold corresponding to 90%, 95% or 99% specificity) to take advantage of rising trends in an individual woman's marker level as a signal of disease. The PEB determines the expected value of a marker for each individual woman based on her reference population and marker history. Age below or above 50 was used to define reference populations for the PEB, rather than pre- and post-menopause [24] which can be difficult to define.
Figure 1.
Overview of multimodal screening protocols by study arm
The PEB rule is designed to account for personal characteristics of women that affect levels of the markers, such as ethnicity for CA125 [25] and age and smoking for HE4 [24] which were tracked in this study, by looking at the deviation from expected level based on marker level history. Positivity thresholds for CA125 and HE4 were set at 95% specificity regardless of arm, so that 5% of participants would be positive on each marker at each screen. Because both markers were used only in Arm 1, 10% of women in Arm 1 and 5% of women in Arm 2 were expected to return for a confirmatory test. In Arm 2 only, women with CA125 above a 90% specificity threshold had early recall.
If either CA125 or HE4 was positive at the confirmatory screen, the woman was asked to return for imaging. Surgical consult was recommended if 2 of the 3 tests were positive or, to avoid missing any cancers, if confirmatory CA125 exceeded the PEB 99% specificity positivity threshold. The design assured that surgical consultation was recommended for relatively few women. Clinical follow-up was coordinated by the study oncologist(s).
Laboratory analyses
Both CA125 and HE4 were measured at FHCRC on the Abbott Architect™ automated platform in a Clinical Laboratory Improvement Amendments (CLIA)-approved laboratory using FDA-approved kits. CV's for CA125 and HE4 are 4% and 6% respectively.
Imaging analyses
At each site protocol-indicated TVU tests were performed by certified ultrasonagraphers, interpreted by a radiologist and reported on standardized forms designed to capture data required by a morphology-based index [26]. Data were abstracted, entered into the research database by local study personnel, and reviewed centrally by the Coordinating Center research nurse. Women were considered post-menopausal if they had not had a menstrual period for at least one year. Screens in which the ovaries could not be visualized were considered negative. A scan was considered positive per study criteria if papillary nodules were present or if the volume of either ovary exceeded 20cc in pre-menopausal and 10cc in post-menopausal participants. Doppler studies were not performed regularly because their value has not been demonstrated consistently, the exam is operator dependent and poorly reproducible [27]. However, the form, generally including morphology index information and sometimes including blood flow to the ovaries, was provided to referring physicians along with any accompanying report.
Outcome ascertainment
The primary outcome was surgical PPV defined as malignant lesions identified among protocol-indicated surgical procedures performed; intermediate outcomes included surgical consultations recommended and performed. Pathology reports were obtained for all women reporting surgical removal of ovaries and were reviewed centrally. Lesions of interest included serous tubal intraepithelial carcinoma (STIC) lesions as well as invasive epithelial malignancy of the ovary, fallopian tube or peritoneum. Benign tumors, tumors of low malignant potential (LMP), and other types of malignancy were documented but were not the goal of screening.
Compliance with Screening
Attendance at screens by participants was tracked and a missed screen was defined as a failure to attend a screen before the next one was due. When 12 months elapsed without a screening examination, participants were considered to have dropped out.
Statistical analyses
Results were analyzed as of October 31, 2013; the trial is ongoing. Surgical PPV was calculated as the number of malignancies identified divided by the number of protocol-indicated surgeries performed; an intent-to-treat analysis was also performed based on recommendation for surgical consult in women with 2 of 3 tests positive. Participant characteristics were compared between study arms using Student's t-test for continuous variables, the chi-square test for categorical variables, and Poisson regression for counts of relatives with breast and ovarian cancer. Fisher's exact test was used to test for differences in the odds of having a positive primary or confirmatory screen between study arms, and for differences in the PPV based on the assigned treatment (intent-to-treat) between study arms. Statistical analyses were performed using the R statistical software [Version 2.14.0; The R Foundation for Statistical Computing, Seattle, WA]. All statistical tests were two-tailed.
Results
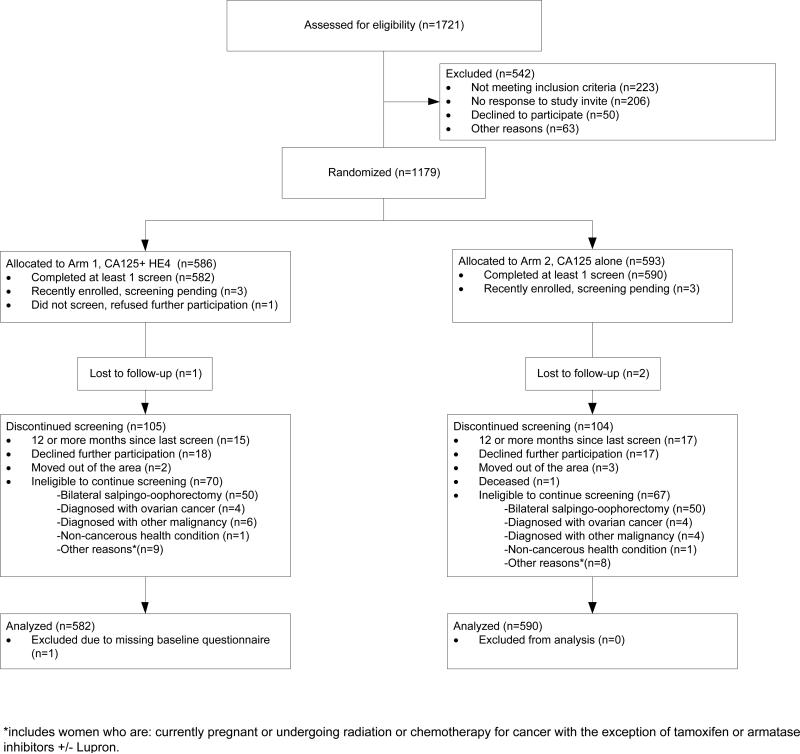
Between February 1, 2010 and October 31, 2013, a total of 1179 women were enrolled, of whom 1172 had at least one screen and were included in analyses, 582 in Arm 1 and 590 in Arm 2, as shown in Figure 2. Participants included 208, 834 and 130 women enrolled in Risk Groups 1, 2 and 3 respectively.
Figure 2.
Participation in the randomized controlled trial by study phase and study arm
The rate of missed primary screens in Arms 1 and 2 was 1.1% and 1.0%, respectively; it was 0.8%, 1.4%, and 0% in Risk Group 1, 2 and 3 participants respectively. Only 2.7% of study participants dropped out by being over 12 months late for a screening examination.
Clinical characteristics of women are reported in Table 1. There were no statistically significant differences between the study arms. Participants were predominantly between the ages of 45 and 65 (61%) and Caucasian (89%); about 19% were of Ashkenazi-Jewish descent. About 30% had a personal history of breast cancer, and 33% had a family history of EOC. About 41% had undergone genetic testing, of whom 44% had a deleterious mutation. Approximately 85% had ever-used OCP and 26% had ever-used HRT. About 12% had prior tubal ligation, about 38% had prior hysterectomy, and 39% were nulliparous. While not statistically significant, three times as many women qualified for Risk Group 3 on the basis of elevated HE4 in Arm 1 than in Arm 2.
Table 1.
Summary of Participant Characteristics at Baseline by Study Arm
| Variable | Value | Arm1: CA125 + HE4 | Arm2: CA125 Alone | p-value |
|---|---|---|---|---|
| Study Arm | Sample Size | 582 (49.7%) | 590 (50.3%) | |
| Age at Enrollment | mean(sd) | 52(12) | 52(11) | 0.80 |
| Age at Enrollment (Categorical) | 35 ≤ age ≤ 44 | 146 (25.1%) | 151 (25.6%) | 0.97 |
| 45 ≤ age ≤ 54 | 185 (31.8%) | 189 (32.0%) | ||
| 55 ≤ age ≤ 64 | 171 (29.4%) | 175 (29.7%) | ||
| 65≤ age ≤ 85 | 80 (13.7%) | 75 (12.7%) | ||
| Self-Reported Race | White Caucasian | 520 (89.3%) | 527 (89.3%) | 1.00 |
| Non White | 51 (8.8%) | 62 (10.5%) | 0.32 | |
| Ethnicity | Ashkenazi Jewish | 119 (20.4%) | 105 (17.8%) | 0.27 |
| Hispanic | 29 (5.1%) | 25 (4.3%) | 0.58 | |
| Risk Group | RG1: BRCA Mutation Carrier | 106 (18.2%) | 102 (17.3%) | 0.98 |
| RG2: Significant Pedigree | 411 (70.6%) | 423 (71.7%) | ||
| RG3: Elevated Markers | 24 (4.1%) | 24 (4.1%) | ||
| RG3: Epidemiologic Risk Algorithm | 41 (7.0%) | 41 (6.9%) | ||
| Personal History of Breast Cancer | Yes | 167 (28.9%) | 180 (30.7%) | 0.52 |
| Number of First or Second Degree Relatives with Ovarian Cancer | 1 | 183 (31.4%) | 209 (35.4%) | 0.41 |
| 2 | 39 (6.7%) | 40 (6.8%) | ||
| 3+ | 7 (1.2%) | 10 (1.7%) | ||
| Prior Genetic Test | Tested | 229 (39.3%) | 250 (42.6%) | 0.28 |
| Genetic Test Results Among Participants with a Prior Genetic Test | Negative | 103 (45.4%) | 121 (51.1%) | 0.56 |
| Variant(s) of Unknown Significance | 10 (4.4%) | 8 (3.4%) | ||
| Confirmed Predisposition | 103 (45.4%) | 101 (42.6%) | ||
| Inconclusive | 11 (4.8%) | 7 (3.0%) | ||
| Use of Hormonal Contraceptives | Ever used | 487 (84.8%) | 493 (84.6%) | 0.94 |
| >1 year use | 466 (80.1%) | 472 (80.0%) | 1.00 | |
| Use of HRT (E alone, or E+P) | Ever used | 153 (26.3%) | 152 (25.8%) | 0.84 |
| One or more Years | 111 (19.1%) | 105 (17.8%) | 0.60 | |
| Prior Tubal ligation | Yes | 75 (13.0%) | 64 (10.9%) | 0.28 |
| Nulliparity | Yes | 220 (37.8%) | 232 (39.3%) | 0.63 |
| Prior Hysterectomy Number of First or Second Degree Relatives with Breast Cancer | Yes | 51 (8.9%) | 51 (8.8%) | 1.00 |
| 1 | 220 (37.8%) | 215 (36.4%) | 0.77 | |
| 2 | 105 (18.0%) | 120 (20.3%) | ||
| 3+ | 85 (14.6%) | 81 (13.7%) | ||
| First or Second Degree Relatives with BRCA1, BRCA2, or HNPCC | Yes | 93 (16.0%) | 96 (16.3%) | 0.95 |
| No | 269 (46.2%) | 267 (45.3%) | ||
| Unknown | 220 (37.8%) | 227 (38.5%) | ||
| Elevated Markers Among Elevated Marker Risk Group | HE4 | 3 (12.5%) | 9 (37.5%) | 0.09 |
| CA125 | 11 (45.8%) | 7 (29.2%) | 0.37 | |
| MMP7 | 7 (29.2%) | 8 (33.3%) | 1.00 | |
| Mesothelin | 5 (20.8%) | 5 (20.8%) | 1.00 | |
| Smoking (Past or Present) | Ever Smoker | 190 (32.6%) | 179 (30.3%) | 0.41 |
| Smoking Among Ever Smokers | Current Smoker' | 19 (10.0%) | 19 (10.6%) | 0.87 |
| Former Smoker | 171 (90.0%) | 160 (89.4%) |
Outcomes for the “2-of-3 tests positive” strategy (both arms combined) are summarized in Table 2. A total of 37 women received a recommendation for surgical consult; 28 had the consult, 6 had bilateral salpingooophorectomy (BSO) and 2 were diagnosed with EOC, yielding overall surgical PPV of 33% (95% CI: 4%-78%). Twelve women received surgical referral based on the criterion “at least 2 of 3 tests positive”; of these, all had the consult, 4 had BSO and 2 had EOC yielding surgical PPV of 50% and intent-to-treat PPV of 17%. Both CA125 and HE4 were positive on the 4 BSO cases and the 2 EOC were diagnosed in women who were positive on all three tests. One of the 2 participants who had BSO but no EOC was diagnosed with lung cancer based on her pre-operative chest x-ray. Of the 25 women who were positive on CA125 alone, 16 had the consult, 2 had BSO and none had EOC; two of these 25 were later found to have another malignancy (recurrent breast cancer and metastatic pancreatic cancer). Of the remaining nine, four and five women were in Risk Groups 1 and 2 respectively. Of these, three in Risk Group 1 were found to be pregnant and screening was suspended. Remaining women chose to attend an early recall examination; in all but one participant, CA125 returned to normal three months later.
Table 2.
Participants with a protocol derived recommendation for surgical consultation by confirmatory test result and follow up including surgical consult, surgical procedure, and diagnosis of EOC or other malignancy
| Participant's Test Results | Recommendation for surgical consultation | Consultation completed | BSO Performed | EOC Lesion identified | Other Cancer diagnosed |
|---|---|---|---|---|---|
| All 3 tests positive | 2 | 2 | 2 | 2 | 0 |
| CA125 and HE4 positive, TVS negative | 5 | 5 | 2 | 0 | 1 |
| CA125 and TVS positive, HE4 negative | 4 | 4 | 0 | 0 | 0 |
| HE4 and TVS positive, CA125 negative | 1 | 1 | 0 | 0 | 0 |
| CA125 positive,HE4 and TVS negative | 25 | 16 | 2 | 0 | 2 |
| Total | 37* | 28 | 6 | 2 | 3** |
37 women accounted for a total of 41 recommendations
recurrent breast cancer, metastatic pancreatic cancer (both were CA125 positive, HE4 and TVS negative), and suspected lung cancer (CA125 and HE4 positive, TVS negative). Of these 3 women only the participant with suspected lung cancer had BSO during the trial.
Positive predictive value (PPV) by study arm is reported in Table 3 by level of screen; reason for surgical recommendation is reported in terms of combinations of tests that were positive. Based on surgeries performed for all 37 women for whom surgical consult was recommended, PPV was 33% for both arms combined, 25% in Arm 1 and 50% in Arm 2. Because surgery was seldom performed, we included an intent-to-treat analysis estimating PPV based on the 12 women with “at least 2 of 3 tests positive”, yielding PPV estimates of 14% in Arm 1 and 20% in Arm 2, respectively. Among the same 12 women, based on surgeries actually performed, PPV was 33% in Arm 1 and 100% in Arm 2.
Table 3.
Positive Predictive Value (PPV) by Arm, Level of Screen, and Reason for Recommendation for Gynecologic Oncology Consult
| Study Arm | CA125 + HE4 at Primary Screen | CA125 Alone at Primary Screen | Both Arms Combined |
|---|---|---|---|
| Cancers Diagnosed | 4 | 4 | 8 |
| Primary Screens | 2269 | 2269 | 4538 |
| Positive (% [95% CI]) | 233 (10.3% [9.0%, 11.6%]) | 69 (3.0% [2.4%, 3.8%]) | 302 (6.7% [5.9%, 7.4%]) |
| Cancers Diagnosed Following Positive Screen (PPV [95% CI]) | 2 (0.9% [0.1%, 3.1%]) | 1 (1.4% [0.0%, 7.8%]) | 3 (1.0% [0.2%, 2.9%]) |
| Confirmatory Screens | 217 | 64 | 281 |
| Positive (% [95% CI]) | 79 (36.4% [30.0%, 43.2%]) | 33 (51.6% [38.7%, 64.2%]) | 112 (39.9% [34.1%, 45.8%]) |
| Cancers Diagnosed Following Positive Screen (PPV [95% CI]) | 1 (1.3% [0.0%, 6.9%]) | 1 (3.0% [0.1%, 15.8%]) | 2 (1.8% [0.2%, 6.3%]) |
| Ultrasound Assessments | 79 | 31 | 110 |
| Positive (% [95% CI]) | 4 (5.1% [1.4%, 12.5%]) | 3 (9.7% [2.0%, 25.8%]) | 7 (6.4% [2.6%, 12.7%]) |
| Cancers Diagnosed Following Positive Screen (PPV [95% CI]) | 1 (25.0% [0.6%, 80.6%]) | 1 (33.3% [0.8%, 90.6%]) | 2 (28.6% [3.7%, 71.0%]) |
| Referrals for Consultation with Gynecologic Oncologist | 29 | 12 | 41 |
| Cancers Diagnosed (PPV [95% CI]) | 1 (3.4% [0.1%, 17.8%]) | 1 (8.3% [0.2%, 38.5%]) | 2 (4.9% [0.6%, 16.5%]) |
| Surgical procedures following a referral for consult | 4 | 2 | 6 |
| Cancers Diagnosed (PPV [95% CI]) | 1 (25.0% [0.6%, 80.6%]) | 1 (50.0% [1.3%, 98.7%]) | 2 (33.3% [4.3%, 77.7%]) |
| Referrals for Consultation with Gynecologic Oncologist (with 2 of 3 modalities positive) | 7 | 5 | 12 |
| Cancers Diagnosed (PPV [95% CI]) | 1 (14.3% [0.4%, 57.9%]) | 1 (20.0% [0.5%, 71.6%]) | 2 (16.7% [2.1%, 48.4%]) |
| Surgical procedures following a referral for consult (with 2 of 3 modalities positive) | 3 | 1 | 4 |
| Cancers Diagnosed (PPV [95% CI]) | 1 (33.3% [0.8%, 90.6%]) | 1 (100.0% [2.5%, 100.0%]) | 2 (50.0% [6.8%, 93.2%]) |
As reported in Table 3, three cancers were diagnosed in women with positive primary screens: twice as many cancers were identified in thrice as many positive screens in Arm 1 (2 cancers out of 233 positive screens) relative to Arm 2 (1 cancer out of 69 positive screens); primary screen PPV was 0.9% and 1.5% in Arms 1 and 2 respectively. The difference in PPV between arms was not statistically significant but there were significantly more positive primary screens in Arm 1 than in Arm 2: in each arm, 2269 primary screens were performed, of which 233 and 69 were positive in Arms 1 and 2, respectively (OR=3.5 [2.6,4.7], p<0.0001, Fisher's Exact test).
At the confirmatory screen, one cancer was identified in each arm in twice as many confirmatory screens in Arm 1 (1 out of 79 positive confirmatory screens) than in Arm 2 (1 out of 33 positive confirmatory screens); confirmatory screen PPV was 1.3% and 3.0% in Arms 1 and 2 respectively, not statistically significantly different. However, there were more positive confirmatory screens in Arm 1 than in Arm 2 leading to 29 and 12 recommendations for surgical consult (OR=2.4 [1.2,5.2], p=0.01, Fisher's Exact test) in Arms 1 and 2, respectively.
Positive confirmatory marker screens resulted in imaging of 79 women in Arm 1 and 31 women in Arm 2, leading to 4 and 3 positive imaging exams in Arms 1 and 2 respectively. Surgical consultation was recommended (following 29 and 12 screens in Arms 1 and 2 respectively) if 2 of the 3 confirmatory tests were positive, or if CA125 exceeded the 99% PEB threshold for positivity. However, decisions regarding surgery depended on joint decision making by the woman, her primary care physician, and a surgeon if one was consulted. As shown in Table 2, 28 participants consulted with a surgeon but most did not undergo surgery, instead returning to screening with early recall. Only 6 women had BSO following a recommendation for surgical consult, 4 in Arm 1 and 2 in Arm 2, yielding a diagnosis of EOC in one woman in each arm. No EOC has been identified in the remaining women for whom surgical consult was recommended; all but 7 continued screening.
Described in Table 4 are all eight EOC lesions identified in 108 BSOs performed in this high-risk study cohort, including the three signaled by screening. Six BSOs were protocol-indicated, as described above. Of the remaining 102, 62 were RRSOs performed in mutation carriers, 19 were in women with significant pedigrees whose only surgical indication was prevention, and 21 occurred in women with neither a documented mutation nor a significant pedigree for reasons other than or in addition to prophylaxis.
Table 4.
Bilateral-salpingo-oophorectomies (BSO) and surgical pathologic findings
| Arm 1 | Arm 2 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cancer | no cancer | total | cancer | no cancer | total | cancer | no cancer | total | |
| BSOs performed: | 4 | 50 | 54 | 4 | 50 | 54 | 8 | 100 | 108 |
| Surgical Indication: | |||||||||
| protocol-indicated | 1 | 31 | 4 | 1 | 12 | 2 | 2 | 4 | 6 |
| RRSO (BRCA mutation) | 1 | 29 | 30 | 3 | 29 | 32 | 4 | 58 | 62 |
| BSO (significant pedigree) | 0 | 9 | 9 | 0 | 10 | 10 | 0 | 19 | 19 |
| BSO (other) | 23 | 94 | 11 | 0 | 105 | 10 | 2 | 19 | 21 |
| >Lesion Detail | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | mutation status | Cancer | Stage | Histology | Arm | CA125 | HE4 | PEB results | Surgical Indication |
| 1 | 66 | no testing reported | EOC | 3c6 | Serous | 2 | 256.1 | 59.9 | CA125 > 99%, HE4 > 95% | protocol |
| 2 | 61 | no testing reported | EOC | 2c | Serous | 1 | 104.9 | 63.1 | CA125 > 99%, HE4 > 95% | protocol |
| 3 | 55 | BRCA2 mutation | FTC | 1a | Serous | 1 | 37.8 | 25.2 | CA125>99% | prophylactic |
| 4 | 57 | BRCA2 mutation | FTC | 1a | Serous | 2 | 7.3 | 34.0 | normal | prophylactic |
| 5 | 47 | BRCA1 mutation | FTC | 1a | Serous | 2 | 3.9 | 30.4 | normal | prophylactic |
| 6 | 69 | not tested | FTC | 0 | Serous | 1 | 10.7 | 35.3 | normal | prophylactic |
| 7 | 36 | BRCA1 mutation | FTC | 0 | Serous | 2 | 10.7 | 27.9 | normal | prophylactic |
| Other Lesions Found: | ||||||||||
| 8 | 47 | negative | LMP | 1c | Mucinous | 1 | 8 | 33.3 | normal | symptomatic |
One participant had BSO at the same time as an indicated laparotomy to correct a complication caused by prior gastric bypass surgery.
suspected lung cancer based on imaging at time of surgery
participants presented with symptoms at time of surgery
2 participants were scheduled for BSO as part of treatment for breast cancer
2 participants diagnosed with endometrial cancer
participant had a STIC lesion present in her right fallopian tube
Two EOC lesions (#1 and #2) were classified as screen-detected (true-positive screens) because they were diagnosed following recommendation for surgical consultation: one stage IIC serous EOC diagnosed following the third screen in Arm 1, and one stage IIIC serous EOC diagnosed at the prevalence screen in Arm 2. Both had low-volume disease and were optimally cytoreduced with minimally invasive surgeries, but the one diagnosed at the prevalence screen recurred two years later. In both cases, CA125, HE4 and imaging were all positive at the confirmatory screen.
A mutation carrier diagnosed with Stage 1A invasive fallopian tube cancer (#3) at RRSO tested positive for CA125 at a primary screen but a confirmatory screen was not performed. This lesion was counted as a screen-detected cancer (true positive) for the primary screen only, and ignored for estimation of the primary outcome surgical PPV and also the intent-to-treat PPV, as reflected in Table 3.
Two lesions (#4 and #5) were classified as missed invasive cancers (false negative screens), both in mutation carriers diagnosed with Stage 1A invasive fallopian tube cancer found at previously planned RRSO following a normal primary screen.
Of the three remaining EOC lesions, two (#6 and #7) were non-invasive STIC lesions, one in a mutation carrier and one in an untested women with a significant pedigree. Both were found at a previously planned RRSO following a normal primary screen.
Only one woman (#8) was symptomatic; she had a low malignant potential (LMP) mucinous tumor that was not considered significant due to the generally indolent clinical course of these lesions.
Discussion
We performed a pilot RCT to learn how best to use HE4 in a staged multimodal screening protocol, to inform the design of future screening protocols. To assess safety, we chose trial size to yield primary outcome estimation (for both arms combined) of a 95% confidence interval for surgical PPV; we had 80% power to rule out inclusion of PPV of 10% or lower. We observed fewer surgeries and malignancies than expected, and observed an overall surgical PPV of 33%, with 95% CI of 4%-78%. The trial was not large enough to test the difference in PPV between arms, and no statistically significant difference was seen. Both screening protocols were found to be feasible and acceptable to women and physicians; neither resulted in excessive imaging, surgical consults or surgical procedures.
Positive screens were more frequent when HE4 was included in the primary screen. Consistent with retrospective analysis of PLCO data [21], these data suggest that HE4 may be useful as a confirmatory test in a multimodal strategy using CA125 alone in the primary screen. Women were more likely to complete a recommended surgical consultation and a surgical procedure when rising CA125 was confirmed by rising HE4. Of seven women who had positive confirmatory test results on both CA125 and HE4, four had surgery and two had ovarian malignancy, but women seldom had surgery if CA125 alone was positive.
Two EOC cases were preceded by positivity of all three tests. Both had low volume stage 3 disease that was optimally treated; one identified at the prevalence screen recurred with a progression free interval of 24 months. No advanced or symptomatic EOC cases were missed by screening, but no early-stage cancers were identified by screening. As shown in Table 4, five stage 0 and 1a tubal lesions were identified at RRSO; only one was preceded by elevation in CA125. These results are consistent with the literature suggesting that performance of the primary screen is critical and that better serum markers may be needed to reduce EOC mortality cost-effectively. Using a previously developed microsimulation model [28], we have recently reported that use of rising CA125 to select average-risk post-menopausal women for imaging as in the UKCTOCS is likely to reduce mortality by about 13% for a cost per year of life saved of about $89,000 [29]. Selection of women at elevated risk for screening combined with use of HE4 in a confirmatory screen would improve cost-effectiveness and might improve efficacy somewhat, but better markers will likely be needed to achieve mortality reduction of 30%.
Emerging evidence suggests that salpingectomy offers a complementary means to reduce EOC mortality [30]. In women with a mutation in BRCA1 or BRCA2, occult malignancy of serous histology is frequently found in the fimbrial end of the fallopian tube at the time of RRSO [31-34]. About half of such lesions are invasive, and long-term follow up confirms their aggressiveness. Of 15 women with invasive lesions, 7 recurred and 3 died in 88 months of follow up; of 17 women with non-invasive lesions, 1 recurred and none died in 80 months of follow up [35]. Serous EOC may originate in native serous epithelium of the fallopian tube in most mutation carriers and in many sporadic cases, suggesting that bilateral salpingectomy with ovarian retention (BSOR) may reduce cancer risk. We have previously noted different CA125 levels between women with tubal dysplasia and normal FTs at RRSO [36]. Only one woman in this study elected BSOR; no significant lesions were identified. Her CA125 levels were 35, 88, 58, and 57 U/ml prior to BSOR, and 7, 6, 6, 9 and 8 U/ml following BSOR, suggesting the need for more research. Markers may contribute to a reliable risk classification tool to inform a woman's decision-making regarding elective BSO or BSOR at the time of hysterectomy [37, 38]. The addition of BSOR to hysterectomy in women who do not carry BRCA1/2 mutations was recently reported to show no negative effects on ovarian function or perioperative complications [39]; efficacy of this approach remains to be demonstrated [40]. Results from a recent online survey conducted by a national patient advocacy group showed that one-third of BRCA mutation carriers indicated definite interest in prophylactic salpingectomy with delayed oophorectomy as a risk-reducing surgery [41].
Limitations
This report represents a snapshot in time of an evolving EOC screening trial. Age-specific elevation thresholds for CA125 and HE4 were not used in the original design, but it was learned from trial data that due to a strong effect of age on HE4 in healthy participants, thresholds for HE4 should be defined for women of specific ages. We estimated age-based positivity thresholds from healthy participants, reporting positivity thresholds yielding 90%, 95%, 98% and 99% specificity for age-defined populations of healthy women for HE4 and CA125 [24]. We considered STIC lesions to be outcomes of interest, but did not include other putative precursor lesions such as small benign paratubal cysts [42] reported on the right ovary of a woman with rising HE4 who had BSO off-protocol. Costly abdominal and pelvic imaging such as CT or MRI was not performed in women with positive tests; performance of those technologies in the context of screening this high-risk population might be of interest. Marker levels were not measured post-BSO; CA125 and HE4 both fall with EOC surgery and rise with recurrence [43].
CA125 level may not elevate in patients with stage 1 ovarian cancer [44], suggesting the need for HE4 (or another promising biomarker) in the primary screen to catch early disease. However, results suggest that a higher specificity should be required if HE4 is used in the primary screen, or that HE4 should be reserved for use in the 2nd-line screen, to reduce call-backs for confirmatory screens. Both protocols were safe with PPV comparing favorably to results reported by efficacy trials to date. Data from this pilot trial may be useful for designing future risk assessment and prevention studies and for estimating the costs of conducting a full-scale efficacy screening trial should such a study be undertaken in the future.
Acknowledgements
We gratefully acknowledge helpful comments provided during review of manuscript from Beth Schodin of Abbott Diagnostics. Support from the Canary Foundation, Marsha Rivkin Center for Ovarian Cancer Research, NCI P50 CA083636 (to NU), NCI U01 CA152637 (to CL), and NIH/NCATS Grant# UL1TR000124, American Cancer Society Clinical Research Professorship (SIOP-06-258-01-COUN) (to BYK), and a grant of no-charge study materials from Abbott Laboratories is gratefully acknowledged. Administrative and technical assistance from Kathy O'Briant, Hannah Purkey and Christine Poulin are much appreciated.
Financial Support: NCI P50 CA083636 (to NU), NCI U01 CA152637 (to CL), NIH/NCATS Grant# UL1TR000124 and SIOP-06-258-01-COUN (to BYK)
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT01121640
Conflict of Interest: None of the authors listed above (Beth Y. Karlan, Jason Thorpe, Kate Watabayashi, Charles W. Drescher, Melanie Palomares, Mary B. Daly, Pam Paley, Paula Hillard, M Robyn Andersen, Garnet Anderson, Ronny Drapkin, Nicole Urban) has declared any conflict of interest with the above manuscript.
References
- 1.Genetic/Familial High Risk Assessment Breast and Ovarian. 2006 [cited 2006]. v.1.2006:[Available from: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- 2.Rosenthal AN, Fraser L, Manchanda R, Badman P, Philpott S, Mozersky J, et al. Results of annual screening in phase I of the United kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol. 2013;31:49–57. doi: 10.1200/JCO.2011.39.7638. PMCID: PMC3530690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–71. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitonis AF, Titus-Ernstoff L, Cramer DW. Assessing ovarian cancer risk when considering elective oophorectomy at the time of hysterectomy. Obstet Gynecol. 2011;117:1042–50. doi: 10.1097/AOG.0b013e318212fcb7. PMCID: PMC3781934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morch LS, Lokkegaard E, Andreasen AH, Kjaer SK, Lidegaard O. Hormone therapy and different ovarian cancers: a national cohort study. Am J Epidemiol. 2012;175:1234–42. doi: 10.1093/aje/kwr446. [DOI] [PubMed] [Google Scholar]
- 6.Urban N, Drescher C. Current and future developments in screening for ovarian cancer. Women's Health. 2006;2:733–42. doi: 10.2217/17455057.2.5.733. [DOI] [PubMed] [Google Scholar]
- 7.Bjorge T, Lie A, Hovig E, Gislefoss R, Hansen S, Jellum E, et al. BRCA1 mutations in ovarian cancer and borderline tumours in Norway: a nested case-control study. Br J Cancer. 2004;91:1829–34. doi: 10.1038/sj.bjc.6602199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, et al. Screening for ovarian cancer: a pilot randomized controlled trial. Lancet. 1999;353:1207–10. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen BBJ, von Mensdorff-Pouilly S, Berkhof J, van Diest PJ, Gille JJP, Menko FH, et al. Serum CA-125 in Relation to Adnexal Dysplasia and Cancer in Women at Hereditary High Risk of Ovarian Cancer. J Clin Oncol. 2007;25:1383–9. doi: 10.1200/JCO.2006.06.7884. [DOI] [PubMed] [Google Scholar]
- 10.Drescher CW, Shah C, Thorpe J, O'Briant K, Anderson GL, Berg CD, et al. Longitudinal screening algorithm that incorporates change over time in CA125 levels identifies ovarian cancer earlier than a single-threshold rule. J Clin Oncol. 2013;31:387–92. doi: 10.1200/JCO.2012.43.6691. PMCID: PMC3732015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005;23:7919–26. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 12.Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, et al. A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013;119:3454–61. doi: 10.1002/cncr.28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schummer M, Ng W, Bumgarner R, Nelson P, Schummer B, Bednarski D, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999;238:375–85. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- 14.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, IM DD, Rosenshein NB, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- 15.Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter J, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–700. [PubMed] [Google Scholar]
- 16.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011;118:280–8. doi: 10.1097/AOG.0b013e318224fce2. PMCID: PMC3594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh M, Urban N. A parametric empirical Bayes method for cancer screening using longitudinal observations of a biomarker. Biostatistics. 2003;4:27–40. doi: 10.1093/biostatistics/4.1.27. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh M, Urban N, Karlan B. Generating Longitudinal Screening Algorithms Using Novel Biomarkers for Disease. Cancer Epidemiol Biomarkers Prev. 2002;11:159–66. [PubMed] [Google Scholar]
- 20.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10:327–40. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 21.Urban N, Thorpe JD, Bergan LA, Forrest RM, Kampani AV, Scholler N, et al. Potential role of HE4 in multimodal screening for epithelial ovarian cancer. J Natl Cancer Inst. 2011;103:1630–4. doi: 10.1093/jnci/djr359. PMCID: PMC3206037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koskela-Niska V, Lyytinen H, Riska A, Pukkala E, Ylikorkala O. Ovarian cancer risk in postmenopausal women using estradiol-progestin therapy - a nationwide study. Climacteric : the journal of the International Menopause Society. 2013;16:48–53. doi: 10.3109/13697137.2012.663818. [DOI] [PubMed] [Google Scholar]
- 23.Koskela-Niska V, Pukkala E, Lyytinen H, Ylikorkala O, Dyba T. Effect of various forms of postmenopausal hormone therapy on the risk of ovarian cancer--a population-based case control study from Finland. Int J Cancer. 2013;133:1680–8. doi: 10.1002/ijc.28167. [DOI] [PubMed] [Google Scholar]
- 24.Urban N, Thorpe JD, Karlan BY, McIntosh MW, Palomares MR, Daly MB, et al. Interpretation of single and serial measures of HE4 and CA125 in asymptomatic women at high risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2087–94. doi: 10.1158/1055-9965.EPI-12-0616. PMCID: PMC3493821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauler D, Menon U, McIntosh M, Symecko H, Skates S, Jacobs I. Factors Influencing Serum CA125II Levels in Healthy Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2001;10:489–93. [PubMed] [Google Scholar]
- 26.DePriest PD, Shenson D, Fried A, Hunter JE, Andrews SJ, Gallion HH, et al. A morphology index based on sonographic findings in ovarian cancer. Gynecol Oncol. 1993;51:7–11. doi: 10.1006/gyno.1993.1238. [DOI] [PubMed] [Google Scholar]
- 27.Lutz AM, Willmann JK, Drescher CW, Ray P, Cochran FV, Urban N, et al. Early Diagnosis of Ovarian Carcinoma: Is a Solution in Sight? . Radiology. 2011;259:329–45. doi: 10.1148/radiol.11090563. [DOI] [PubMed] [Google Scholar]
- 28.Urban N, Drescher C, Etzioni R, Colby C. Use of a stochastic simulation model to identify an efficient protocol for ovarian cancer screening. Controlled Clinical Trials. 1997;18:251–70. doi: 10.1016/s0197-2456(96)00233-4. [DOI] [PubMed] [Google Scholar]
- 29.Drescher CW, Hawley S, Thorpe JD, Marticke S, McIntosh MW, Gambhir SS, et al. Impact of screening test performance and cost on mortality reduction and cost-effectiveness of multimodal ovarian cancer screening. Cancer Prev Res (Phila Pa) 2012;5:1015–24. doi: 10.1158/1940-6207.CAPR-11-0468. PMCID: PMC3729263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. 2013 Nov; [2013 Apr 1]. Available from: https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/
- 31.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 32.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 35.Powell CB, Swisher EM, Cass I, McLennan J, Norquist B, Garcia RL, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol. 2013;129:364–71. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlan BY, McIntosh M. The quest for ovarian cancer's Holy Grail: can CA-125 still be the chalice of early detection? J Clin Oncol. 2007;25:1303–4. doi: 10.1200/JCO.2006.09.7543. [DOI] [PubMed] [Google Scholar]
- 37.Tone AA, Salvador S, Finlayson SJ, Tinker AV, Kwon JS, Lee CH, et al. The role of the fallopian tube in ovarian cancer. Clinical advances in hematology & oncology : H&O. 2012;10:296–306. [PubMed] [Google Scholar]
- 38.Anderson GL, McIntosh MW, Wu L, Barnett M, Goodman G, Thorpe JD, et al. Assessing Lead Time of Selected Ovarian Cancer Biomarkers: A Nested Case–Control Study. J Natl Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. PMCID: PMC2802285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morelli M, Venturella R, Mocciaro R, Di Cello A, Rania E, Lico D, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: Primum non nocere. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.03.023. pii: S0090-8258:00178-9. [DOI] [PubMed] [Google Scholar]
- 40.Gilks CB, Miller D. Opportunistic salpingectomy for women at low risk for development of ovarian carcinoma: The time has come. Gynecol Oncol. 2013;129:443–4. doi: 10.1016/j.ygyno.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Holman LL, Friedman S, Daniels MS, Sun CC, Lu KH. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–7. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schummer M, Drescher C, Forrest R, Gough S, Thorpe J, Hellstrom I, et al. Evaluation of ovarian cancer remission markers HE4, MMP7 and Mesothelin by comparison to the established marker CA125. Gynecol Oncol. 2012;125:65–9. doi: 10.1016/j.ygyno.2011.11.050. PMCID: PMC3303992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paramasivam S, Tripcony L, Crandon A, Quinn M, Hammond I, Marsden D, et al. Prognostic importance of preoperative CA-125 in International Federation of Gynecology and Obstetrics stage I epithelial ovarian cancer: an Australian multicenter study. J Clin Oncol. 2005;23:5938–42. doi: 10.1200/JCO.2005.08.151. [DOI] [PubMed] [Google Scholar]