Abstract
Background
We describe patterns of colorectal cancer screening uptake in a U.S. insured population as individuals become newly-eligible for screening at age 50 and assess temporal trends and patient characteristics with screening uptake.
Methods
We identified a cohort of 81,223 men and women who were members of Group Health and turned 50 years old from 1996 – 2010. We ascertained receipt of colorectal cancer screening within five years. Time to screening was estimated by year of cohort entry using cumulative incidence curves and Cox proportional hazards models estimated patient characteristics associated with screening uptake.
Results
Stool-based screening tests were the most common, 72% of first screening tests. The proportion of individuals initiating colorectal cancer screening via colonoscopy increased from 8% in 1996–98 to 33% in 2008–10. Patient factors associated with increased colorectal cancer screening were: turning 50 more recently (2008–10) (p-trend<0.0001) or Asian race (HR=1.14, 95% CI 1.10–1.19). Patient factors associated with decreased screening were: being a woman (HR=0.70, 95% CI 0.68–0.72), Native American (HR=0.68, 95% CI 0.60–0.78) or Pacific Islander race (HR=0.82, 95% CI 0.72–0.95), and having prevalent diabetes (HR=0.78, 95% CI 0.75–0.82) and higher body mass index (p-trend<0.0001).
Conclusions
Patient characteristics associated with initiation of colorectal cancer screening in a newly-eligible population are similar to characteristics associated with overall screening participation in all age-eligible adults. Our results identify patient populations to target in outreach programs.
Impact
Disparities in receipt of colorectal cancer screening are evident from onset of an age-eligible cohort, identifying key groups for future interventions for screening.
Keywords: colorectal cancer, screening, men and women, colonoscopy, fecal occult blood test
INTRODUCTION
Colorectal cancer screening is an effective way to reduce colorectal cancer mortality (1). Nearly two decades ago, the U. S. Preventive Services Task Force first recommended colorectal cancer (CRC) screening for average risk adults using flexible sigmoidoscopy and fecal occult blood testing (FOBT) beginning at age 50 years (2). Since the announcement of this recommendation, colorectal cancer screening in the U.S. has risen dramatically (3, 4), particularly with the availability of screening colonoscopy for average risk-individuals (3, 4).
However, colorectal cancer screening remains low. Only 63% of U.S. age-eligible adults report receiving colorectal cancer screening with FOBT in the prior 2 years or endoscopy in the prior 10 years. Reporting of recent screening differs by age; only 54% of adults aged 50–59 years report recent screening compared to 76% of older adults aged 70–75 year olds (4). Factors routinely associated with colorectal cancer screening include having health insurance, access to a usual source of care and a primary care doctor, and use of other preventive services (5–8). Men are also more likely to be screened for colorectal cancer than women (9). However, most of the evidence on factors associated with screening were conducted in all-age eligible populations. No studies have evaluated patient factors associated with screening initiation among adults that are newly-eligibly at age 50 for colorectal cancer screening, which is important in understanding which individuals might need additional outreach to improve colorectal cancer screening participation.
We describe patterns of colorectal cancer screening uptake in an insured population of men and women as they become newly-eligible for screening at age 50 years, including temporal trends of colorectal cancer screening initiation over a 15 year period and patient characteristics associated with screening uptake.
MATERIALS AND METHODS
Study population
We identified 83,777 men and women who were enrolled for at least one year in Group Health prior to their 50th birthday from 1996–2010. Group Health is a mixed model health insurance and care delivery system in Washington State. Our study is focused on average risk adults; hence we excluded participants who had prior diagnoses of colorectal cancer (n=380),(10) Crohn’s disease or colitis (n=760), a colectomy (n=258), or individuals with colonoscopy for any reason at age 49 (n=1,849). Our final sample included 81,223 eligible individuals during the study timeframe.
The study protocol received Institutional Review Board approval for a waiver of consent to enroll participants, link study data, and perform statistical analyses.
Colorectal cancer screening outreach
Colorectal cancer screening guidelines at Group Health follow the recommendations of the U. S. Preventive Services Task Force (11), and hence during this 15-year study period, providers could have recommended FOBT yearly, sigmoidoscopy every five years with or without interval FOBT, or colonoscopy every 10 years (12). From 1996–2006, patients learned about colorectal cancer screening from their providers during office visits or brochures in the clinics. In 2007, the Group Health Screening and Outreach Program began to send annual letters on individual’s birthdays as a reminder of upcoming clinical preventive services, including colorectal cancer screening. With the implementation of the Patient-Centered Medical Home in 2009, medical assistants or nurses sought to identify individuals not up-to-date for preventive services, and used electronic medical record alerts during patient visits (13). Finally, beginning in 2002, average-risk patients were able to self-refer to gastroenterology to receive a colonoscopy.
Identification and indication of colorectal cancer screening tests
For each eligible individual, we identified the first colorectal cancer screening test within 5 years of their 50th birthday up to their 55th birthday. Data were available from administrative claims and electronic medical records. From clinical laboratory data, we identified the date of receipt of either guaiac FOBT or fecal immunochemical testing (FIT) with Current Procedural Terminology (CPT) codes (i.e., 82270, 82271, 82272, 82273, 82274) and Health Common Procedure Coding System (HCPCS) codes (i.e., G0107, G0328, G0394).
We identified colonoscopy based on CPT codes (45378–45386, 45391–45392), HCPCS (G01005, G0122), and International Classification of Diseases and Ninth Revision, Clinical Modification (ICD-9-CM) codes (45.23). We identified flexible sigmoidoscopy based on CPT (45300–45345), HCPCS (G0104), and ICD-9-CM codes (45.24, 48.21, 48.22, 48.23, 48.24, 48.36). We ascertained receipt of barium enema (CPT: 74270, 74280, HCPC G0106, G0120, G0122, ICD-9 87.64) and CT colonography (HCPCS 0066T and 0067T) through radiology imaging.
We assumed that all colorectal tests were conducted for the purpose of screening except for colonoscopy procedures. Colonoscopy is used for both screening and diagnostic evaluation of signs and symptoms, and the indication of the exam is not captured in administrative data. Therefore, we used an algorithm to identify screening colonoscopies using patient symptoms, prior procedures and patient demographics.(14) The algorithm was developed using Group Health administrative data available, including patient symptoms, prior procedures, and demographics, and performs better than those in the existing literature.(15) We dichotomized the predicted probabilities, categorizing colonoscopy exams with a probability of >0.261 as screening exams, which maximized the sensitivity and specificity at 88% and 90%, respectively.
Patient characteristics
Patient characteristics of interest were selected based on identified risk factors for colorectal cancer (16) and availability within our data systems (17). We identified patient characteristics through administrative patient files including sex (female/male), year of 50th birthday, and race/ethnicity (white, black, Hispanic, Asian, Pacific Islander, Native American, biracial, unknown), prior diagnosis of Type I or II diabetes mellitus (yes/no) (18), and a primary care visit in the year prior to their 50th birthday (yes/no). Additional patient characteristics were identified by the closest clinical encounter prior to their 50th birthday, including any family history of colorectal cancer (yes/no) (ICD9 code v16.0) and body mass index (kg/m2).
Statistical analysis
We developed an inception cohort of individuals newly-eligible for colorectal cancer screening at age 50 to evaluate patterns of screening tests and factors associated with initiation of screening. We described patient characteristics among men and women for the total population and by receipt of screening within 5 years of their 50th birthday.
To evaluate temporal trends among individuals who received colorectal cancer screening test, we calculated the distribution of colorectal cancer screening tests received, specifically stool-based, colonoscopy, flexible sigmoidoscopy, and other tests, stratified by year of 50th birthday (i.e., 1996–1998, 1999–2001, 2002–2004, 2005–2007, 2008–2010) in the entire age-eligible cohort. Barium enema and CT colonography are categorized as other screening tests because there were so few tests received (n=312 combined tests).
We also constructed cumulative incidence curves to demonstrate the time to receipt of the first screening test up to five years across all cohorts by year of 50th birthday (i.e., 1996–1998, 1999–2001, 2002–2004, 2005–2007, and 2008–2010).
Cox proportional hazards models were used to evaluate the association between time to first colorectal cancer screening after age 50 and patient characteristics including sex, year of 50th birthday, family history of colorectal cancer, race/ethnicity, prevalent diabetes, body mass index, and a primary care provider visit at age 49. Missing values were categorized as an unknown category and retained within the model. Person-time was calculated from the time of an individual’s 50th birthday to time of first colorectal cancer screening test, disenrollment from Group Health, a non-screening colonoscopy, or end of follow-up at five years or December 31, 2010, whichever came first. Fully adjusted models included all variables in the final model. Tests for trend were calculated by including the linear term of the categorical variable in the model. In sensitivity analyses, we evaluated the impact of missing data by rerunning the analysis on individuals with complete data. We also evaluated the impact of the family history variable on the results, and ran the model with this covariate excluded. All analyses were performed using SAS® Version 9.2 (SAS Institute, Cary, NC), and two-sided p<0.05 was considered statistically significant.
RESULTS
Overall, the entire cohort contributed 285,250 person-years during follow-up. There were few differences in patient characteristics comparing individuals who initiated colorectal cancer screening after their 50th birthday with those who did not (Table 1). Patient characteristics which differed included the year of 50th birthday, family history of colorectal cancer, and receipt of a primary care visit at age 49 years. Person-time varied by year of 50th birthday and were calculated as 75,817 years for 1996–1998 cohort, 70,887 years for 1999–2001 cohort, 66,238 years for 2002–2004 cohort, 54,207 years for 2005–2007 cohort, and 18,301 years for 2008–2010 cohort.
TABLE 1.
Descriptive characteristics of Group Health members newly-eligible for colorectal cancer screening from 1996-2010 by receipt of screening and total population.
| Colorectal cancer screening |
||||||
|---|---|---|---|---|---|---|
| No | Yes | Total | ||||
| Characteristics | N | % | N | % | N | % |
| 47,950 | 33,275 | 81,225 | ||||
| Person-time | 147,400 | 138,050 | 285,450 | |||
| Sex | ||||||
| Female | 25,322 | 53% | 18,443 | 55% | 43,765 | 54% |
| Male | 22,628 | 47% | 14,832 | 45% | 37,460 | 46% |
| Year of 50th birthday | ||||||
| 1996-1998 | 11,796 | 24% | 6641 | 21% | 18,437 | 23% |
| 1999-2001 | 12,100 | 25% | 6767 | 21% | 18,867 | 24% |
| 2002-2004 | 8249 | 17% | 7242 | 23% | 15.671 | 19% |
| 2005-2007 | 7284 | 15% | 7371 | 23% | 14,655 | 18% |
| 2008-2010 | 9438 | 19% | 4155 | 13% | 13,593 | 17% |
| Race | ||||||
| White | 25,411 | 80% | 22,954 | 80% | 48,365 | 80% |
| Black | 1544 | 5% | 1237 | 4% | 2781 | 5% |
| Asian | 2174 | 7% | 2288 | 8% | 4462 | 7% |
| Hispanic | 1346 | 4% | 1232 | 4% | 2578 | 4% |
| Pacific Islander | 220 | 1% | 142 | <1% | 362 | 1% |
| Native American | 309 | 1% | 166 | 1% | 475 | 1% |
| Biracial | 594 | 2% | 529 | 2% | 1123 | 2% |
| Unknown | 16,352 | 34% | 4727 | 14% | 21,079 | 26% |
| Family history of colorectal cancer | ||||||
| No | 46,392 | 97% | 30,424 | 91% | 76,816 | 95% |
| Yes | 1558 | 3% | 2851 | 9% | 4409 | 5% |
| Body mass index (kg/m2) | ||||||
| <18.5 | 194 | 1% | 184 | 1% | 378 | 1% |
| 18.5-24.9 | 6552 | 29% | 7262 | 34% | 13,814 | 32% |
| 25-29.9 | 7003 | 31% | 6798 | 32% | 13,801 | 32% |
| 30.0-34.9 | 4578 | 20% | 3782 | 18% | 8360 | 19% |
| ≥35 | 4367 | 19% | 3035 | 14% | 7402 | 17% |
| Unknown | 25,256 | 53% | 12,214 | 37% | 37,470 | 46% |
| Diagnosis of diabetes mellitus | ||||||
| No | 44,466 | 93% | 31,233 | 94% | 75,699 | 93% |
| Yes | 3484 | 7% | 2042 | 6% | 5526 | 7% |
| Geographic location | ||||||
| Urban | 45,237 | 94% | 32,377 | 97% | 77,614 | 96% |
| Rural | 1471 | 3% | 564 | 2% | 2035 | 3% |
| Suburban | 823 | 2% | 201 | 1% | 1024 | 1% |
| Unknown | 419 | 1% | 133 | <1% | 552 | 1% |
| Primary care visit at age 49 years | ||||||
| No | 18,092 | 38% | 7237 | 22% | 25,329 | 31% |
| Yes | 29,858 | 62% | 26,038 | 78% | 55,896 | 69% |
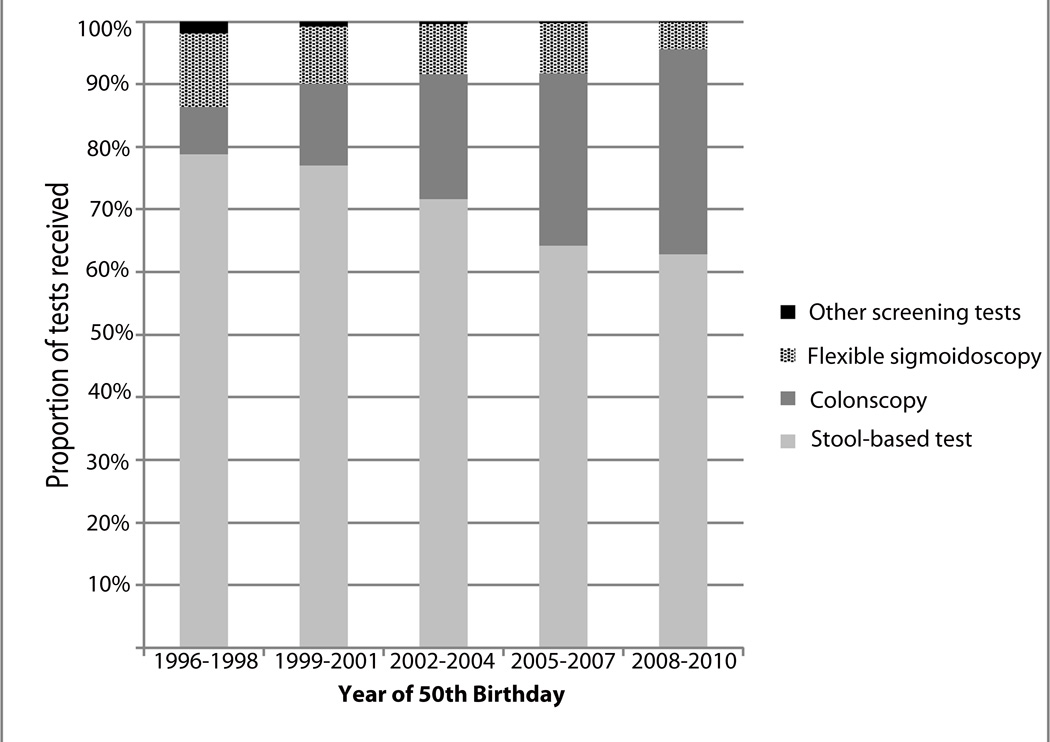
Stool-based tests were the most common initial screening test in this population, representing 72% of screening tests among 50 year olds who receive colorectal cancer screening (Figure 1). However, over time, the proportion of individuals receiving stool-based tests has dropped to about 63% of all tests in 2008–2010 cohort (Figure 1), and colonoscopy represents a larger proportion of screening tests among 50 year olds. The proportion of individuals initiating colorectal cancer screening via colonoscopy increased from 8% in 1996–1998 to 33% in 2008–2010
FIGURE 1.
The distribution of colorectal cancer screening test received during follow-up by year of 50th birthday.
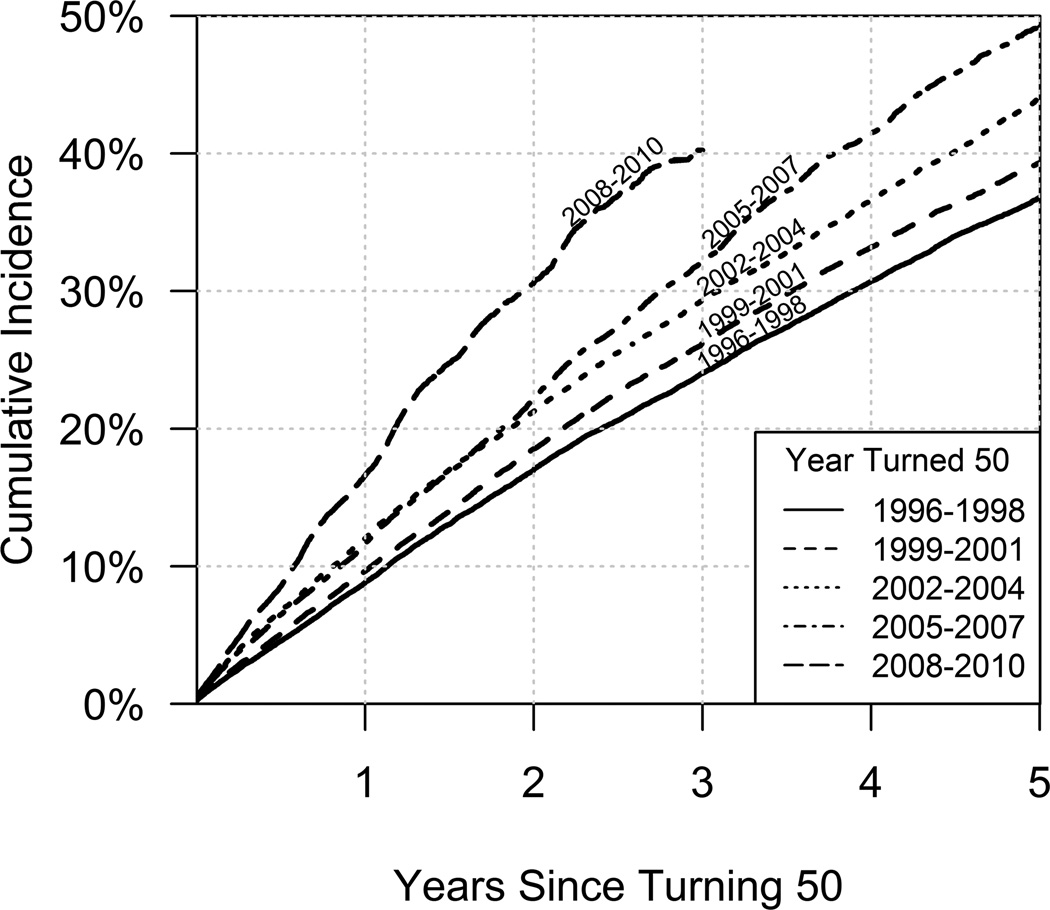
Cumulative incidence curves demonstrate a substantial increase in colorectal cancer screening rates over time (Figure 2). At two years since 50th birthday, approximately 17% of the 1996–1998 cohort had received colorectal cancer screening compared to about 30% of the individuals in the 2008–2010 cohort. By five years, approximately 36% of the 1996–1998 cohort had received colorectal cancer screening compared to 49% of the individuals in the 2005–2007 cohort.
FIGURE 2.
Cumulative incidence curves for time to first colorectal cancer screening test by year individuals turned 50 years old.
In multivariable adjusted models (Table 2), there was a statistically significant increasing trend in use of colorectal cancer screening among men and women who turned 50 more recently compared to 1996–1998 (p<0.0001), with a two-fold increased receipt of colorectal cancer screening among 2008–2010 cohort compared to the 1996–1998 cohort. Other patient factors associated with an increase in uptake of colorectal cancer screening included having a family history of colorectal cancer (HR=1.78, 95% CI 1.71–1.84) and a primary care visit at age 49 years (HR=1.42, 95% CI 1.38–1.45). There were also racial/ethnic differences in uptake of colorectal cancer screening. Asian men and women were 14% more likely to screen for colorectal cancer compared to Caucasians; however, men and women who were black, Pacific Islander, or Native American were less likely to screen for colorectal cancer. Individuals with diabetes were 21% less likely to screen for colorectal cancer compared to individuals without diabetes. There is also a significant inverse trend in the relationship between increasing body mass index and use of colorectal cancer screening (p<0.0001). Individuals with a BMI >35 were 28% less likely to screen for colorectal cancer compared to normal weight individuals.
TABLE 2.
Patient characteristics associated with initiation of colorectal cancer screening in members of Group Health, 1996-2010.
| Patient characteristics | HR* | 95% CI |
|---|---|---|
| Year of 50th birthday | ||
| 1996-1998 | Referent | |
| 1999-2001 | 1.12 | (1.09-1.15) |
| 2002-2004 | 1.32 | (1.28-1.36) |
| 2005-2007 | 1.42 | (1.42-1.52) |
| 2008-2010 | 2.04 | (1.95-2.12) |
| p-trend | <0.0001 | |
| Gender | ||
| Men | Referent | |
| Women | 0.71 | (0.70-0.73) |
| Race | ||
| White | Referent | |
| Black | 0.96 | (0.91-1.01) |
| Asian | 1.14 | (1.10-1.19) |
| Hispanic | 1.01 | (0.96-1.07) |
| Pacific Islander | 0.80 | (0.69-0.92) |
| Native American | 0.70 | (0.61-0.80) |
| Multiracial | 0.98 | (0.90-1.05) |
| Unknown | 0.66 | (0.64-0.68) |
| Family history of colorectal cancer | ||
| No | Referent | |
| Yes | 1.78 | (1.71-1.84) |
| Diagnosis of diabetes mellitus | ||
| No | Referent | |
| Yes | 0.79 | (0.76-0.82) |
| Body Mass Index (kg/m2) | ||
| <18.5 | 0.98 | (0.85-1.12) |
| 18.5-24.9 | Referent | |
| 25-29.9 | 0.90 | (0.87-0.93) |
| 30-34.9 | 0.81 | (0.78-0.84) |
| >35 | 0.72 | (0.69-0.75) |
| Unknown | 0.56 | (0.54-0.58) |
| p-trend | <0.0001 | |
| Primary care visit at age 49 years | ||
| No | Referent | |
| Yes | 1.42 | (1.38-1.45) |
Analyses are adjusted for all variables presented in the table.
From our sensitivity analyses, there were no differences in the magnitude or direction of results in a population with complete data or when family history was dropped in the model.
DISCUSSION
Our results suggest that characteristics of newly-eligible individuals who initiate colorectal cancer screening are similar to the characteristics of all U. S. adults who receive colorectal cancer screening tests. That is, the disparity in receipt of colorectal cancer screening that occurs in all age-eligible adults is present within the first years of eligibility for colorectal cancer screening. Our study population is unique in that all study participants had health insurance, which offset patient costs for screening tests and subsequent diagnostic evaluations, removing some economic barriers. Even so, we still observed differences in the use of colorectal cancer screening across patient characteristics.
We demonstrated that individuals turning 50 more recently (i.e., 2008–2010) were more likely to receive colorectal cancer screening within five years of their 50th birthday compared to individuals who turned 50 in 1996–1998. Improvements in the initiation of colorectal cancer screening such as protocols for screening referral, tracking of patient outcomes, and addressing patient barriers, have demonstrated to increase colorectal cancer screening by 18% among adults <64 years (19). These types of initiatives could also have impacted our populations. A recent study at Group Health used mailings and additional telephone support to improve colorectal cancer screening (20). Further, Group Health’s implementation of the Patient Centered Medical Home in 2009 also may have led to improved colorectal cancer screening among younger adults in the most recent timeframe (13). Finally, national efforts to meet quality standards established by the National Committee for Quality Assurance (NCQA) have increased the need of all stakeholders to meet HEDIS performance measures (21). The increased initiation in cancer screening within our study population mimic similar trends in increased adherence to cancer screening in all age-eligible individuals (3) and changes in choice of colorectal cancer screening test nationally (4).
Women and some racial minority groups were less likely to initiate colorectal cancer screening within 5 years of their 50th birthdays. Women participate in screening for breast and cervical cancer at higher rates than colorectal cancer (22). Further, prior studies have demonstrated that younger men (41.0%) report higher rates of receipt of any recommended colorectal cancer screening compared to women (31.4%), which aligns with our study results (9). In assessing women’s perspectives for colorectal cancer screening, women report being more afraid or fearful of colorectal cancer screening and the unpleasant preparation compared to men (23). Several studies have documented that racial/ethnic minorities are less likely to receive colorectal cancer screening, even in insured populations. Recent analysis of Behavioral Health Risk Factor Survey data demonstrates that Hispanics, Asians, and American Indians/Alaska Natives have prevalent screening rates 11–15% below Whites and Blacks (24). In our analysis, Asian men and women were most likely to receive screening within 5 years compared to Whites. Reasons for differences by racial/ethnic groups are not clear in our study population and should be further investigated.
Despite having health insurance, only 69% of patients in our study population had seen a primary care provider at age 49 years. Contact with the health care system is an important first step to receiving cancer screening. A recent study of Group Health members evaluated receipt of FOBT among men and women aged 50–54 and found that up to 4.5% of women and 10.1% of men remain unscreened for colorectal cancer due to infrequent primary care visits (defined as <1 visit in 2 years) (25). Currently preventive well-care visits are recommended for men and women every 2 years at age 50, and attendance at well-care visits could influence the initiation of colorectal cancer screening (26).
Obesity was associated with reduced colorectal cancer screening. The majority of research suggests that being overweight and obese is associated with reduced participation in colorectal cancer screening compared with normal weight individuals (27, 28), particularly in women (29, 30). In the Reducing Barriers to Colorectal Cancer Screening study, Messina et al. (31) determined that women who were overweight or obese were 40% less likely to have had recent screening compared to normal weight women, while for men, there were no differences in recent screening by BMI category. A recent systematic review and meta-analysis demonstrated similar findings associating decreasing rates of colorectal cancer screening with increasing obesity class.(30) When evaluating perceptions about colorectal cancer and screening, obese women were less likely to report that obesity was a risk factor for colorectal cancer and to express worry about colorectal cancer. There were no significant differences in perceptions about colorectal cancer for overweight or obese men (31).
Prior studies have demonstrated that individuals with a family history of colorectal cancer are strongly motivated to receive screening, and in our analysis, a prior family history was associated with increased use of screening. Carney et al. recently documented that both men and women with a positive family history are significantly more likely to be up-to-date for colorectal cancer screening compared with individuals with a negative family history of cancer (32). The American Cancer Society recommends that individuals with a first degree family history screen at an earlier age prior to 50 (33). In our analysis, we only evaluated screening at age 50 and older and did not evaluate screening among individuals in their 40s.
We found decreased use of colorectal cancer screening among individuals with diagnosis of diabetes. Individuals with a diabetes diagnosis are similarly up-to-date for colorectal cancer screening compared to national averages, near 60% (34). However, women with diabetes are less likely to be up-to-date compared to men with diabetes. Further, women with diabetes tend to participate less in clinical preventive services (i.e., mammography screening) compared to women without diabetes (35). We did not specifically evaluate interactions between patient characteristics, but important subgroups, such as overweight women with diabetes could be potential target populations for screening outreach. Our study has several strengths including a large cohort to evaluate temporal trends by test type with ascertainment of all screening tests through our administrative data. Although indication for colonoscopy is routinely missing from administrative data, we were able to assign indication for colonoscopy using a new, accurate algorithm based on administrative data (14). While this is the first study to describe colorectal cancer screening uptake in a large cohort of newly-eligible 50 year old adults, there are several limitations to our analysis. First, we ascertained patient characteristics as close to age 50 as possible; however due to the limitations of administrative data and irregular timing of contact with the health care system, we were not always able to document patient characteristics which resulted in missing data, particularly for race and body mass index. In our analyses, we included “missing” as a category in multivariable models. When we restricted our analysis to members with complete data, we observed similar patterns of association. Second, our estimates of family history of colorectal cancer might be biased because the information was primarily obtained through patient visits. Documentation of family history is difficult because the variable will be documented affirmatively due to a positive family, and when there is no mention of a family history, we assumed this represented a negative family history. However, excluding the variable from our analysis did not widely vary our results. Finally, we ascertained receipt of colonoscopy in the year prior to individual’s 50th birthdays. The trends in use of colonoscopy prior to age 50 are not well-known, so it is not possible to determine what proportion of individuals might have had testing prior to this period. However, given that colorectal cancer screening is recommended in average risk adults beginning at age 50, we would expect few individuals to receive colonoscopy for screening prior to age 50 years.
Our results indicate that within 5 years of their 50th birthday almost 50% of men and women have received colorectal cancer screening. Physicians, medical teams, and support staff could focus on reducing disparities access to colorectal cancer screening among 50 year olds by targeting individuals who are most likely to remain non-adherent (e.g., overweight women, individuals with diabetes, racial/ethnic minorities) to screening with outreach and inreach and interventions to improve participation.
Acknowledgments
Acknowledgement of funding: All authors received research support in this publication by the National Cancer Institute of the National Institute of Health under Award Number U54 CA163261 and K. J. Wernli additionally by the Agency for Healthcare Research and Quality under Award Number K12 HS019482.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, National Institutes of Health or Agency for Healthcare Research and Quality.
The collection of cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Rebecca A. Hubbard, Email: Hubbard.r@ghc.org.
Eric Johnson, Email: Johnson.e@ghc.org.
Jessica Chubak, Email: Chubak.j@ghc.org.
Aruna Kamineni, Email: Kamineni.a@ghc.org.
Beverly B. Green, Email: green.b@ghc.org.
Carolyn M. Rutter, Email: rutter.c@ghc.org.
REFERENCES
- 1.Richardson LC, Tai E, Rim SH, Joseph D, Plescia M. Vital Signs: Colorectal cancer screening, incidence, and mortality - United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–889. [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore, MD, USA: 1996. [Google Scholar]
- 3.Sinicrope PS, Goode EL, Limburg PJ, Vernon SW, Wick JB, Patten CA, et al. A population-based study of prevalence and adherence trends in average risk colorectal cancer screening, 1997 to 2008. Cancer Epidemiol Biomarkers Prev. 2012;21:347–350. doi: 10.1158/1055-9965.EPI-11-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening among adults aged 50–75 years - United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–812. [PubMed] [Google Scholar]
- 5.Carlos RC, Fendrick AM, Patterson SK, Bernstein SJ. Associations in breast and colon cancer screening behavior in women. Acad Radiol. 2005;12:451–458. doi: 10.1016/j.acra.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Carlos RC, Underwood W, 3rd, Fendrick AM, Bernstein SJ. Behavioral associations between prostate and colon cancer screening. J Am Coll Surg. 2005;200:216–223. doi: 10.1016/j.jamcollsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Carlos RC, Fendrick AM, Abrahamse PH, Dong Q, Patterson SK, Bernstein SJ. Colorectal cancer screening behavior in women attending screening mammography: longitudinal trends and predictors. Womens Health Issues. 2005;15:249–257. doi: 10.1016/j.whi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez P, Castaneda SF, Mills PJ, Talavera GA, Elder JP, Gallo LC. Determinants of breast, cervical and colorectal cancer screening adherence in Mexican-American women. J Community Health. 2012;37:421–433. doi: 10.1007/s10900-011-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Surveillance System. [cited 9/25/2013];Cancer Surveillance System: Surveillance, Epidemiology, and End Results (SEER) Program. Available from: http://www.fhcrc.org/en/labs/phs/projects/cancer-surveillance-system.html.
- 11.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 12.Tiro J, Kamineni A, Levin TR, Zheng Y, Schottinger JS, Rutter CM, et al. The colorectal cancer (CRC) screening process in community settings: A conceptual model for the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) Consortium. Cancer Epidemiol Biomarkers Prev Under review. doi: 10.1158/1055-9965.EPI-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158:301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams KF, Johnson EA, Chubak J, Kamineni A, Doubeni CA, Buist DSM, et al. Development of an algorithm to classify colonoscopy indication from health plan data. Med Care Under review. doi: 10.13063/2327-9214.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher DA, Grubber JM, Castor JM, Coffman CJ. Ascertainment of colonoscopy indication using administrative data. Dig Dis Sci. 2010;55:1721–1725. doi: 10.1007/s10620-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 16.Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 17.Hornbrook MC, Hart G, Ellis JL, Bachman DJ, Ansell G, Greene SM, et al. Building a virtual cancer research organization. J Natl Cancer Inst Monogr. 2005:12–25. doi: 10.1093/jncimonographs/lgi033. [DOI] [PubMed] [Google Scholar]
- 18.Chubak J, Anderson ML, Saunders KW, Hubbard RA, Tuzzio L, Liss DT, et al. Predictors of 1-year change in patient activation in older adults with diabetes mellitus and heart disease. J Am Geriatr Soc. 2012;60:1316–1321. doi: 10.1111/j.1532-5415.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 19.Ling BS, Schoen RE, Trauth JM, Wahed AS, Eury T, Simak DM, et al. Physicians encouraging colorectal screening: a randomized controlled trial of enhanced office and patient management on compliance with colorectal cancer screening. Arch Intern Med. 2009;169:47–55. doi: 10.1001/archinternmed.2008.519. [DOI] [PubMed] [Google Scholar]
- 20.Reid RJ, Coleman K, Johnson EA, Fishman PA, Hsu C, Soman MP, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood) 2010;29:835–843. doi: 10.1377/hlthaff.2010.0158. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Quality Assurance. HEDIS 2008 Volume 2 Technical Specifications. Washington, DC: 2007. [Google Scholar]
- 22.Henley SJ, King JB, German RR, Richardson LC, Plescia M. Surveillance of screening-detected cancers (colon and rectum, breast, and cervix) - United States, 2004–2006. MMWR Surveill Summ. 2010;59:1–25. [PubMed] [Google Scholar]
- 23.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38:508–516. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph DA, King JB, Miller JW, Richardson LC Centers for Disease Control and Prevention (CDC) Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):51–56. [PubMed] [Google Scholar]
- 25.Fenton JJ, Reid RJ, Baldwin LM, Elmore JG, Buist DS, Franks P. Influence of primary care use on population delivery of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18:640–645. doi: 10.1158/1055-9965.EPI-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group Health Cooperative. [cited 03/07/2014];Adult wellness visits, screenings and immunizations. Available from: http://www.ghc.org/healthAndWellness/?item=/common/healthAndWellness/tests/recommendedTests/adultTests.html.
- 27.Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med. 2004;19:332–338. doi: 10.1111/j.1525-1497.2004.30339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer R, Collet TH, Zeller A, Zimmerli L, Gaspoz JM, Giraudon K, et al. Obesity and overweight associated with lower rates of colorectal cancer screening in Switzerland. Eur J Cancer Prev. 2013;22:425–430. doi: 10.1097/CEJ.0b013e32835f3b87. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, Bradshaw P, et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer. 2008;112:1892–1904. doi: 10.1002/cncr.23408. [DOI] [PubMed] [Google Scholar]
- 30.Maruthur NM, Bolen S, Gudzune K, Brancati FL, Clark JM. Body mass index and colon cancer screening: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:737–746. doi: 10.1158/1055-9965.EPI-11-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messina CR, Lane DS, Anderson JC. Body mass index and screening for colorectal cancer: gender and attitudinal factors. Cancer Epidemiol. 2012;36:400–408. doi: 10.1016/j.canep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney PA, O'Malley JP, Gough A, Buckley DI, Wallace J, Fagnan LJ, et al. Association between documented family history of cancer and screening for breast and colorectal cancer. Prev Med. 2013;57:679–684. doi: 10.1016/j.ypmed.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Cancer Society. [cited 2013 Oct. 4];American Cancer Society recommendations for colorectal cancer early detection. Available from: http://www.cancer.org/cancer/colonandrectumcancer/moreinformation/colonandrectumcancerearlydetection/colorectal-cancer-early-detection-acs-recommendations.
- 34.Adjaye-Gbewonyo K, Sabatino SA, White MC. Exploring opportunities for colorectal cancer screening and prevention in the context of diabetes self-management: an analysis of the 2010 National Health Interview Survey. Transl Behav Med. 2013;3:72–81. doi: 10.1007/s13142-012-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan W, Yun L, Austin PC, Jaakkimainen RL, Booth GL, Hux J, et al. Impact of socio-economic status on breast cancer screening in women with diabetes: a population-based study. Diabet Med. 2014 doi: 10.1111/dme.12422. [DOI] [PubMed] [Google Scholar]