Abstract
Background and Goals
Seroreactivity against the Saccharomyces cerevisiae (ASCA), Pseudomonas fluorescens-associated sequence (I2) and Bacteroides caccae TonB-linked outer membrane protein (OmpW) has been detected in celiac disease patients with small-bowel mucosal atrophy. Levels of these antibodies decrease during a gluten-free diet, but their functions and time of appearance in celiac disease is not known. We aimed to search for evidence of possible microbial targets of the immune responses in the early stage celiac disease patients who showed normal small-bowel mucosal architecture at the time of the first investigations, but later on a gluten-containing diet developed mucosal atrophy.
Methods
44 cases with proven early stage celiac disease and normal mucosal morphology were enrolled. Patients’ sera were tested for celiac disease antibodies against tissue transglutaminase (tTG-ab), endomysium (EmA) and for microbial antibodies against I2, OmpW and ASCA IgG and IgA isotypes in both at the time of diagnosis and while on a gluten-free diet.
Results
34 (77%) out of 44 patients with early stage celiac disease had elevated serum antibodies to one or more of the antibodies ASCA, I2 and OmpW. Furthermore, five out of the six cases negative for both tTG-ab and EmA showed positivity for the microbial markers. Seroreactivity to ASCA IgA, ASCA IgG and OmpW decreased significantly during gluten-free diet.
Conclusions
Seroreactivity to different microbial antigens is evident already in patients with early stage celiac disease. ASCA antibodies seem to be gluten-dependent. The results indicate that microbial targets might have a role in the early development of celiac disease.
Keywords: Celiac disease, Early-stage, Serology, Microbial markers
INTRODUCTION
The current diagnostic criteria of celiac disease require the demonstration of small-bowel mucosal villous atrophy and crypt hyperplasia.1-3 However, this structural damage caused by dietary gluten is only the final outcome of a gradually developing inflammatory process in previously normal mucosa.4-7 Furthermore, several recent studies have demonstrated that celiac patients may have gluten-dependent symptoms and serum autoantibodies against tissue transglutaminase (tTG-ab) and endomysium (EmA) already before the mucosal atrophy develops.8-15 The early positivity of tTG–ab and EmA is particularly important as they are strongly associated with celiac-type genetics and usually considered as the most specific diagnostic tools in screening of celiac disease.16-19
Interestingly, recent studies have proposed a role of enteric microbiota in the pathogenesis of celiac disease. It has been shown that seroreactivity against the Saccharomyces cerevisiae (ASCA), Pseudomonas fluorescens-associated sequence (I2) and Bacteroides caccae TonB-linked outer membrane protein (OmpW) can be detected in overt celiac disease (so-called Marsh III lesion).20-25 Positive seroreactivity towards ASCA has also been found in the first-degree relatives of celiac disease patients.26 Furthermore, the serum titers of these microbial seromarkers may decrease after initiation of gluten-free diet. 27-30 However, the natural history and the order of appearance of such serological responses remain obscure. Whether these antigens might have active role in the disease pathogenesis is also not clear. We aimed to investigate seroreactivity to these microbial antigens in the early stages of celiac disease when the small-bowel mucosa is still morphologically normal (Marsh I-II).
MATERIALS AND METHODS
Patients
The study was conducted at the Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital and in the Pediatric Research Centre, Tampere University and University Hospital. Patients having an early stage celiac disease with normal small-bowel mucosa (Marsh I-II) (n=44) were gathered from two separate subgroups. The first subgroup consisted of 16 subjects, all of whom showed normal small-bowel mucosal architecture at the time of the first investigations, but while continuing on a gluten-containing diet developed villous atrophy and crypt hyperplasia (Marsh III). The stored sera of these cases were available when they still had normal mucosal architecture and thus represented an early stage celiac disease. In addition, 14 serum samples from this subgroup were available when villous atrophy and crypt hyperplasia had been developed. The remaining 28 early stage celiac disease patients were collected from our previous series.12 All these cases were evaluated due to clinical suspicion of celiac disease but expressed normal small-bowel mucosal villous structure. Nevertheless, they had DQ2 or DQ8 HLA type and increased density of mucosal CD3+ and γδ+ intraepithelial lymphocytes characteristic for celiac disease.8,31-34 A subsequent beneficial response to a gluten-free diet confirmed their diagnosis. The serological samples of 33 out of the 44 early stage celiac disease patients were available for comparison while they had been on a gluten-free diet for one year.
Small bowel mucosal morphology and immunohistochemistry
All 44 participants underwent upper gastrointestinal endoscopy and multiple biopsies were taken from the distal duodenum for routine morphological evaluation and immunohistochemistry. The biopsy specimens were embedded in optimal cutting temperature compound (Miles Labs, Elkhart, IN, USA) and stored at −70°C until testing. Cryostat sections were cut 5 μm thick and fixed in acetone for 10 min and thereafter in cloroform for 30 min at +4°C. After fixing, the specimens were washed three times in Tris buffer pH 7.4 and thereafter incubated in normal horse serum (dilution 1:60) for 20 min. They were then covered with diluted mAbs in tris/bovine serum for one hour. Primary antibodies were revealed using the Vectastain Elite ABC kit (Vectastain PK-6102, Vector Laboratories, Burlinghame, CA) according to manufacturers’ instructions. After removal of the buffer, the sections were covered with diluted MoAbs. Diaminobenzidine (DAB) was used as substrate.
The density of intraepithelial CD3+ and γδ+ lymphocytes was determined from frozen biopsy samples using monoclonal antibodies against CD3 (Leu-4; Becton Dickinson, San Jose, CA) and TCRγ (Endogen, Woburn, MA). Positively stained intraepithelial lymphocytes were counted per 100 epithelial cells. All histological evaluations were carried out without prior knowledge of the disease history or laboratory results of the patients.
Serum antibody tests
In each visit sera for the future antibody determinations were obtained and stored at −70°C until testing. Patients’ sera were tested for IgA class antibodies to tTG-ab (Celikey, Phadia, Freiburg, Germany) and EmA.17,35-37 Tests were considered positive in titers tTG ≥5.0 U and EmA 1: ≥5. Enzyme immunoassay kit Quanta Lite ASCA, INOVA Diagnostics Inc., San Diego, CA) was used according to manufacturer’s protocol for the determination of ASCA in both IgA and IgG isotypes. Results higher than 25 U for IgG and/or IgA ASCA were considered positive.
E. Coli XL-1 blue and E. Coli BL-21 (Stratagene, La Jolla, CA) strains were used for all cloning and recombinant expression experiments in our laboratory. I2 and OmpW were produced by using the antigen purification techniques as previously reported.38 Sera were analyzed with IgA enzyme-linked immunosorbent assays to I2 and OmpW. The cut-off level for positivity was set at 0.5 for I2 and 0.6 (children) and 1.0 (adults) for OmpW.39
Statistical analysis
Statistical analyses were carried out with PASW (Predictive Analytics SoftWare) version 18 (SPSS Inc., Chicago, IL). The differences between paired proportions were tested with McNemar's test and medians with Wilcoxon tests. P value <0.05 was considered as statistically significant.
Ethical considerations
The study protocol was approved by the Ethical Committee of Tampere University Hospital. All participants gave written informed consent.
RESULTS
The median age of the participants was 47 (range 13-72) years and 30 (68%) were females. A total of 38 (86%) out of the 44 patients expressed positive serum tTG-ab and/or EmA. At baseline 34 (77%) out of the 44 early stage celiac patients had positive seroreactivity to one or more of the studied microbial antigens (ASCA, I2, OmpW) (Figure 1). A significant decrease to the frequency of positive seroreactivity to tTG-ab and/or EmA and ASCA was found during a gluten-free diet (Table). Further, five out of six (83%) subjects negative for tTG-ab and EmA expressed positive serum I2 antibodies and/or OmpW antibodies, and three of them also had elevated levels of ASCA antibodies.
Figure 1.
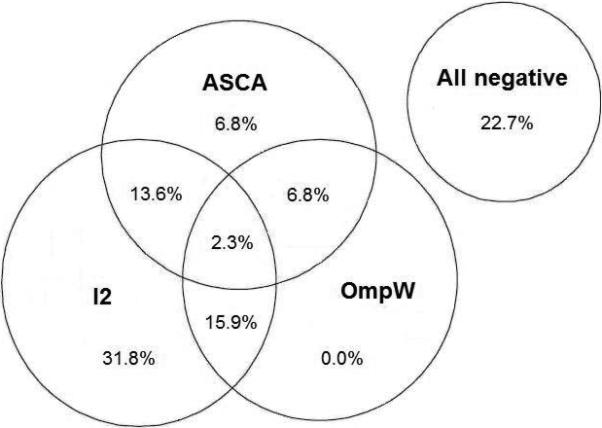
Concordance of the serum antibodies to microbial antigens against Saccharomyces cerevisiae (ASCA), Pseudomonas fluorescens-associated sequence (I2) and Bacteroides caccae TonB-linked outer membrane protein (OmpW) in the 44 patients with early stage celiac disease. The percentage of patients positive for each marker, any combination of two, or all markers is presented.
Table.
Frequency of Positive Seroreactivity in the Early Stage Celiac Disease at Baseline and During a Gluten-free Diet (n=33)
| Early stage celiac disease n (%) | Gluten-free diet n (%) | P value | |
|---|---|---|---|
| tTG/EmA* | 30 (83) | 4 (11) | <0.001 |
| ASCA | 8 (24) | 2 (6.1) | 0.031 |
| I2 | 23 (70) | 23 (70) | 1.00 |
| OmpW | 10 (30) | 7 (21) | 0.25 |
n=36
tTG/EmA indicate tissue transglutaminase/endomysium autoantibodies; ASCA, anti-Saccharomyces Cerevisiae antibodies; I2, anti-I2 antibodies to P. Fluorescence associated sequence I2; OmpW, a Bacteroides Caccae TonB-linked outer membrane protein
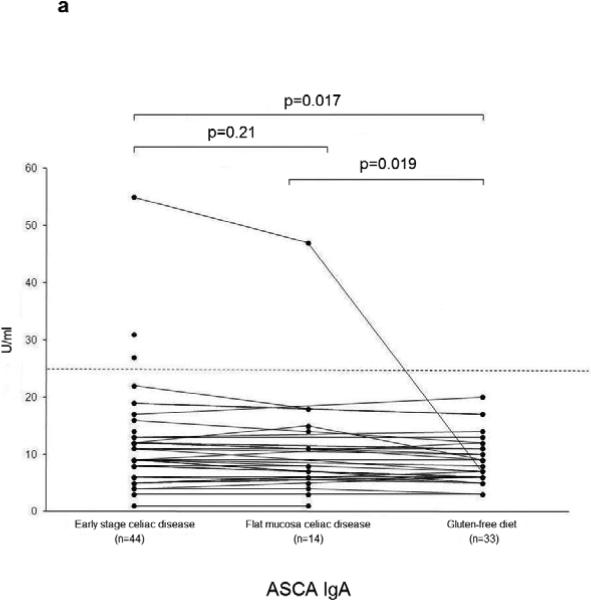
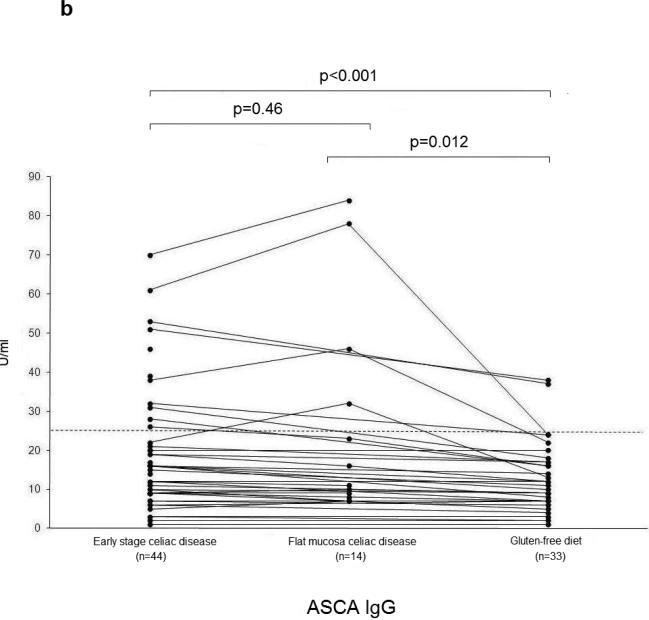
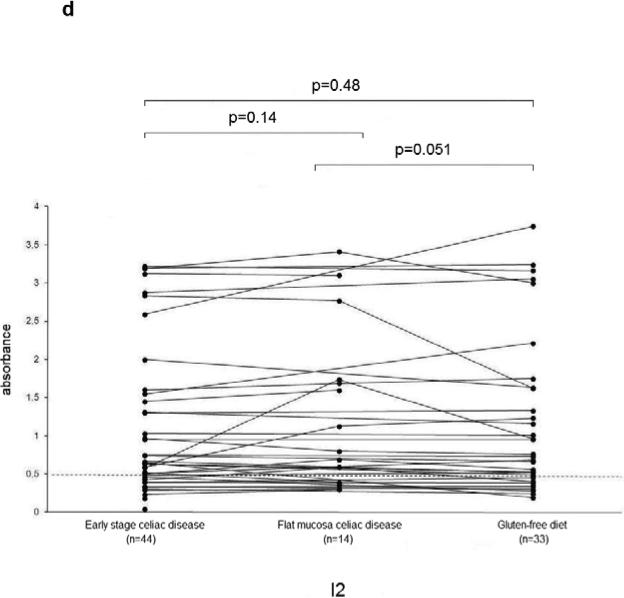
The serum ASCA IgA, IgG and OmpW titers decreased significantly during a gluten-free diet (Figure 2 a-c). Microbial serum titers were also measured in 14 serum samples from the subgroup of 16 celiac disease patients at the time they had developed flat mucosa while continuing on a gluten-containing diet. Significant decrease was seen in ASCA (IgA P=0.02, IgG P=0.01) (Figure 2 a-b) and I2 (P=0.05) (Figure 2d) titers during one year follow-up.
Figure 2.
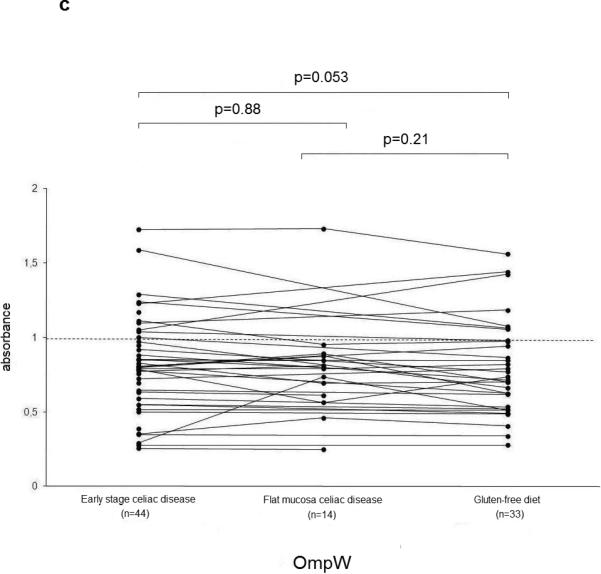
Serological responses to anti-Saccharomyces cerevisiae antibodies (ASCA) in IgA (2a) and IgG (2b) classes, to Bacteroides caccae TonB-linked outer membrane protein (OmpW) (2c) and to Pseudomonas fluorescens-associated sequence (I2) (2d) in the 44 patients with early stage celiac disease. The results are shown at the beginning of the study (Early stage celiac disease), at the time of small-bowel mucosal villous atrophy (Villous atrophy celiac disease, n=14) and while on treatment (Gluten-free diet, n=33). The dashed horizontal lines denote the cut-off value for seropositivity of each antibody in question.
DISCUSSION
We and some other groups have recently shown positive seroreactivity against different microbial antigens in celiac disease patients having established small-bowel mucosal damage with villous atrophy and crypt hyperplasia .20-25 In our former study, 90% of celiac disease patients had positive serological responses towards one or more of these microbial markers.20 The present study showed for a first time that similar serological responses can be detected already in the early stages of celiac disease when the mucosal villi are still morphologically normal. In this study, 77% of the early stage celiac disease patients showed seropositivity to one or more of the studied microbial markers, suggesting possible over-representation of seropositivity to these markers already at the early stage of the disease. Although the intestinal villi were still morphologically normal, most of the patients here had an increased density of CD3+ and γδ+ intraepithelial lymphocytes.12 Previously, we have reported that this can predict forthcoming celiac disease in autoantibody positive subjects with normal small bowel mucosa.32-33 Moreover, the densities of intraepithelial lymphocytes decrease during gluten-free diet.8,34 These findings indicate that the microbial seroreactivity may actually correlate more with mucosal inflammation than with structural damage.
Previously, seroreactivity to different microbial components has been shown to be associated with disease severity and progression in patients with Crohn's disease.40-43 In fact, in such inflammatory bowel disease patients those with the highest levels of serum reactivity towards microbiota have been reported to show also the greatest frequency of complications. 40-43 In addition to Crohn's disease and overt celiac disease, our present findings suggest that immune responses to commensal enteric bacteria are also present in early stage celiac disease and thus may have some unidentified role in the early pathogenesis of celiac disease and the development of mucosal damage.
Besides being present in the sera of untreated patients with early stage celiac disease, a significant decrease was seen in the serum ASCA and OmpW titers on a gluten-free diet. Furthermore, while on diet the ASCA and I2 titers decreased in the 14 subjects who eventually developed villous atrophy. These findings are in accordance with our previous study in celiac patients with overt villous atrophy.30 However, in that study a significant decline was seen in all three microbial markers during gluten-free diet when compared to the time of diagnosis, whereas in the present study there was no significant change in the serum OmpW titers. This disparity may be at least partly explained by the different study designs and smaller number of celiac disease patients in the present trial. Furthermore, there might be differences in microbial species composition in the gut depending on the stage of the disease. For example, it has been reported that Bacteroides population in the duodenum of celiac children and controls differ in diversity and species composition which may contribute to features of the celiac disease.44-46
Interestingly, five out of the six cases who had an early stage celiac disease but negative tTG-ab and EmA were positive to either I2 or OmpW antibodies, and three of them also had elevated ASCA antibody levels. This could be important, since sometimes tTG-ab and EmA are present only as a form of local IgA deposits in the small-bowel mucosa of untreated celiac patients.18,47-48 Consequently, although the microbial seromarkers are not disease-specific, they might be helpful in identifying the autoantibody-negative celiac disease patients. Furthermore, due to the apparent gluten-dependency of the microbial antibodies, they might be utilized in the follow-up of dietary treatment in celiac patients seronegative for other markers.
In conclusion, the present study showed that seropositivity to microbial antigens can be present already in the early stages of celiac disease before structural damage of intestinal mucosa develops. The results also indicate that intestinal bacterial flora may play a role in the pathogenesis of celiac disease. Furthermore, due to the early appearance and gluten-dependency of the microbial seromarkers, they might provide new tools for the diagnosis and follow-up of autoantibody-negative celiac disease. In the future it would be interesting to investigate possible changes in microbiota for example in the siblings of known celiac patients and in celiac antibody-positive subjects who initially have completely normal small-bowel mucosa (Marsh 0) but later proceed to develop villous atrophy and crypt hyperplasia.49
ACKNOWLEDGEMENTS
The authors would like to acknowledge Ms Leena Ripsaluoma for assistance and Ms Marja-Leena Koskinen and Ms Eini Eskola for technical support.
This study is supported by the Foundation for Pediatric Research, the Finnish Medical Foundation, the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital, and US National Institutes of Health; Grant Number: DK 46763.
Footnotes
Conflicts of interest and financial disclosures: None.
REFERENCES
- 1.Walker-Smith J, Guandalini S, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäki M, Holm K, Koskimies S, et al. Normal small bowel biopsy followed by celiac disease. Arch Dis Child. 1990;65:1137–1141. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”). Gastroenterol. 1992;102:330–354. [PubMed] [Google Scholar]
- 4.Corazza G, Andreani M, Biagi F, et al. Clinical, pathological and antibody pattern of latent celiac disease: report of three adult cases. Am J Gastroenterol. 1996;91:2203–2207. [PubMed] [Google Scholar]
- 5.Kaukinen K, Mäki M, Partanen J, et al. Celiac disease without villous atrophy. Revision of criteria called for. Dig Dis Sci. 2001;46:879–887. doi: 10.1023/a:1010729207320. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Brandt L, Montgomery SM. Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 2009;9:57. doi: 10.1186/1471-230X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaukinen K, Lindfors K, Collin P, et al. Review: Coeliac disease – a diagnostic and therapeutic challenge. Clin Chem Lab Med. 2010;48:1205–1216. doi: 10.1515/CCLM.2010.241. [DOI] [PubMed] [Google Scholar]
- 8.Iltanen S, Holm K, Ashorn M, et al. Changing jejunal gammadelta+ T cell receptor (TCR) -bearing intraepithelial lymphocyte density in coeliac disease. Clin Exp Immunol. 1999;117:51–55. doi: 10.1046/j.1365-2249.1999.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paparo F, Petrone E, Tosco A, et al. Clinical, HLA, and small-bowel immunohistochemical features of children with positive serum antiendomysium antibodies and architecturally normal small-bowel intestinal mucosa. Am J Gastroenterol. 2005;100:2294–2298. doi: 10.1111/j.1572-0241.2005.41134.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaukinen K, Peräaho M, Collin P, et al. Small bowel mucosal transglutaminase-2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized study. Scand J Gastroenterol. 2005;40:564–572. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- 11.Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–823. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Kurppa K, Ashorn M, Iltanen S, et al. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157:373–380. doi: 10.1016/j.jpeds.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 13.Salmi TT, Collin P, Maki M, et al. Immunoglobulin a autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming celiac disease. Aliment Pharmacol Ther. 2006;24:541–552. doi: 10.1111/j.1365-2036.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurppa K, Rasanen T, Collin P, et al. Endomysial antibodies predict celiac disease irrespective of the titers or clinical presentation. World J Gastroenterol. 2012;18:2511–2516. doi: 10.3748/wjg.v18.i20.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurppa K, Lindfors K, Collin P, et al. Antibodies against deaminated gliadin peptides in early-stage celiac disease. J Clin Gastroenterol. 2011;45:673–678. doi: 10.1097/MCG.0b013e3181fbdfa6. [DOI] [PubMed] [Google Scholar]
- 16.Mäki M, Holm K, Lipsanen V, et al. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet. 1991;338:1350–1353. doi: 10.1016/0140-6736(91)92234-s. [DOI] [PubMed] [Google Scholar]
- 17.Collin P, Kaukinen K, Vogelsang H, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91. doi: 10.1097/00042737-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Koskinen O, Collin P, Lindfors K, et al. Usefulness of small-bowel mucosal transglutaminase-2 specific autoantibody deposits in the diagnosis and follow-up of celiac disease. J Clin Gastroenterol. 2009;44:483–488. doi: 10.1097/MCG.0b013e3181b64557. [DOI] [PubMed] [Google Scholar]
- 19.Vives-Pi M, Takasawa S, Pujol-Autonell I, et al. Biomarkers for diagnosis and monitoring of celiac disease. J Clin Gastroenterol. 2013;47:308–313. doi: 10.1097/MCG.0b013e31827874e3. [DOI] [PubMed] [Google Scholar]
- 20.Ashorn S, Raukola H, Välineva T, et al. Elevated serum anti-Saccharomyces cerevisiae, anti-I2 and anti-OmpW antibody levels in patients with suspicion of celiac disease. J Clin Immunol. 2008;28:486–494. doi: 10.1007/s10875-008-9200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damoiseaux JG, Bouten B, Linders AM, et al. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies for inflammatory bowel disease: high prevalence in patients with celiac disease. J Clin Immunol. 2002;22:281–288. doi: 10.1023/a:1019926121972. [DOI] [PubMed] [Google Scholar]
- 22.Barta Z, Csipo I, Szabo GG, et al. Seroreactivity against Saccharomyces cerevisiae in patients with Crohn's disease and celiac disease. World J Gastroenterol. 2003;9:2308–2312. doi: 10.3748/wjg.v9.i10.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candelli M, Nista EC, Carloni E, et al. Anti-Saccharomyces cerevisiae antibodies and celiac disease. Scand J Gastroenterol. 2003;38:1191–1192. doi: 10.1080/00365520310005523. [DOI] [PubMed] [Google Scholar]
- 24.Kotze LM, Nisihara RM, Utiyama SR, et al. Antibodies anti-Saccharomyces cerevisiae (ASCA) do not differentiate Crohn's disease from celiac disease. Arq Gastroenterol. 2010;47:242–245. doi: 10.1590/s0004-28032010000300006. [DOI] [PubMed] [Google Scholar]
- 25.Biet F, Gendt L, Anton E, et al. Serum antibodies to Mycobacterium avium subspecies paratuberculosis combined with anti-Saccharomyces cerevisiae antibodies in Crohn's disease patients: Prevalence and diagnostic role. Dig Dis Sci. 2011;56:1794–1800. doi: 10.1007/s10620-010-1523-8. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva Kotze LM, Nisihara RM, Nass FR, et al. ASCA antibodies in first-degree relatives of celiac disease patients. J Clin Gastroenterol. 2010;44:308. doi: 10.1097/MCG.0b013e3181bea0d4. [DOI] [PubMed] [Google Scholar]
- 27.Granito A, Zauli D, Muratori P, et al. Anti-Saccharomyces cerevisiae and perinuclear antineutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment Pharmacol Ther. 2005;21:881–887. doi: 10.1111/j.1365-2036.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- 28.Mallant-Hent R, Mary B, von Blomberg E, et al. Disappearance of anti-Saccharomyces cerevisiae antibodies in coeliac disease during gluten-free diet. Eur J Gastroenterol Hepatol. 2006;18:75–78. doi: 10.1097/00042737-200601000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Toumi D, Mankaï A, Belhadj R, et al. Anti-Saccharomyces cerevisiae antibodies in celiac disease. Scand J Gastroenterol. 2007;42:821–826. doi: 10.1080/00365520601154996. [DOI] [PubMed] [Google Scholar]
- 30.Ashorn S, Välineva T, Kaukinen K, et al. Serological responses to microbial antigens in celiac disease patients during a gluten-free diet. J Clin Immunol. 2009;29:190–195. doi: 10.1007/s10875-008-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes PL, Asquith P, Holmes GK, et al. Histocompatibility antigens associated with adult celiac disease. Lancet. 1972;2:162–164. doi: 10.1016/s0140-6736(72)91330-x. [DOI] [PubMed] [Google Scholar]
- 32.Iltanen S, Holm K, Partanen J, et al. Increased density of jejunal gammadelta+ T cells in patients having normal mucosa - marker of operative autoimmune mechanisms? Autoimmunity. 1999;29:179–187. doi: 10.3109/08916939908998533. [DOI] [PubMed] [Google Scholar]
- 33.Kaukinen K, Collin P, Holm K, et al. Small-bowel mucosal inflammation in reticulin or gliadin antibody-positive patients without villous atrophy. Scand J Gastroenterol. 1998;33:944–949. doi: 10.1080/003655298750026967. [DOI] [PubMed] [Google Scholar]
- 34.Jarvinen TT, Kaukinen K, Laurila K, et al. Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol. 2003;98:1332–1337. doi: 10.1111/j.1572-0241.2003.07456.x. [DOI] [PubMed] [Google Scholar]
- 35.Salmi T, Collin P, Reunala T, et al. Diagnostic methods beyond conventional histology in coeliac disease diagnosis. Dig Liver Dis. 2010;42:28–32. doi: 10.1016/j.dld.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Sulkanen S, Collin P, Laurila K, et al. IgA and IgG-class antihuman umbilical cord antibody tests in adult coeliac disease. Scand J Gastroenterol. 1998;33:251–254. doi: 10.1080/00365529850170810. [DOI] [PubMed] [Google Scholar]
- 37.Ladinser B, Rossipal E, Pittschieler K. Endomysium antibodies in coeliac disease: an improved method. Gut. 1994;35:776–778. doi: 10.1136/gut.35.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn's disease. Gastroenterol. 2000;119:23–31. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 39.Wei B, Dalwadi H, Gordon LK, et al. Molecular cloning of Bacteroides caccae TonB-linked outer membrane protein identified by an inflammatory bowel disease marker antibody. Infect Immun. 2001;69:6044–6054. doi: 10.1128/IAI.69.10.6044-6054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papp M, Altorjay I, Norman GL, et al. Seroreactivity to microbial components in Crohn's disease is associated with ileal involvement, noninflammatory disease behavior and NOD2/CARD15 genotype, but not with risk for surgery in a Hungarian cohort of IBD patients. Inflamm Bowel Dis. 2007;13:984–992. doi: 10.1002/ibd.20146. [DOI] [PubMed] [Google Scholar]
- 41.Arnott ID, Landers CJ, Nimmo EJ, et al. Seroreactivity to microbial components in Crohn's disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–2384. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 42.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Papp M, Altorjay I, Dotan N, et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103:665–681. doi: 10.1111/j.1572-0241.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez E, Donat E, Ribes-Koninckx C, et al. Intestinal Bacteroides species associated with celiac disease. J Clin Pathol. 2010;63:1105–1111. doi: 10.1136/jcp.2010.076950. [DOI] [PubMed] [Google Scholar]
- 45.Collado MC, Donat E, Ribes-Koninckx C, et al. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009;62:264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 46.Nadal I, Donat E, Ribes-Koninckx, et al. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 47.Koskinen O, Collin P, Korponay-Szabo I, et al. Gluten-dependent small bowel mucosal transglutaminase 2 -spesific IgA deposits in overt and mild enteropathy coeliac disease. J Pediatr Gastroenterol Nutr. 2008;47:436–442. doi: 10.1097/MPG.0b013e31817b6dec. [DOI] [PubMed] [Google Scholar]
- 48.Not T, Ziberna F, Vatta S, et al. Cryptic genetic gluten intolerance revealed by intestinal antitransglutaminase antibodies and response to gluten-free diet. Gut. 2011;60:1487–1493. doi: 10.1136/gut.2010.232900. [DOI] [PubMed] [Google Scholar]
- 49.Borrelli M, Salvati V, Maglio M, et al. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. Am J Gastroenterol. doi: 10.1038/ajg.2013.303. Doi: 10.1038/ajg.2013.303. [DOI] [PubMed] [Google Scholar]