Abstract
Programmed cell death 1 ligand 1 (PD-L1, B7H1) is a cell-surface protein that suppresses the cytotoxic CD8+ T cell-mediated immune response. PD-L1 expression and its clinical relevance in sarcomas are not well understood. Therefore, we sought to measure RNA expression levels for PD-L1 in 38 clinically annotated osteosarcoma tumor samples, and aimed to determine if PD-L1 expression correlates with clinical features and tumor-infiltrating T-lymphocytes (TILs). Quantitative real-time RT-PCR for PD-L1 was optimized in 18 cell lines, of which 5 were osteosarcoma-derived. qRT-PCR results were validated via flow cytometry and immunohistochemistry (IHC) in select cell lines. Total RNA was isolated from 38 human osteosarcoma samples for qRT-PCR analysis. Clinical data were sorted and significance was determined by Student t-test. TILs were examined in patient samples by tissue microarray (TMA) hematoxylin-eosin (HE) staining. We confirmed the constitutive PD-L1 mRNA expression in cell lines by qRT-PCR, flow cytometry, and IHC. Across human osteosarcoma samples, PD-L1 mRNA gene expression ranged over four-log (>5000-fold difference). Relative expression levels were evaluated against clinical factors such as age/gender, metastasis, recurrence, chemotherapy, percent necrosis, and survival; no significant associations were identified. The presence of TILs was associated with high PD-L1 expression (R2=0.37, P=0.01). In summary, we developed an RNA-based assay to determine PD-L1 expression levels, and we show for the first time that high levels of PD-L1 are expressed in a subset of osteosarcoma, and PD-L1 expression is positively correlated with TILs. There are multiple agents targeting PD-1/PD-L1 in clinical development, and this may be a novel immunotherapeutic strategy for osteosarcoma clinical trials.
Keywords: PD-L1, osteosarcoma, RT-PCR, tumor-infiltrating lymphocytes
Introduction
Osteosarcoma is an aggressive malignant tumor of the bone thought to arise from mesenchymal stem cells (1). Though it is a rare tumor, osteosarcoma is the most common primary malignancy of the bone and the eighth most common form of childhood cancer (2, 3). With modern multimodality therapies, the five-year survival rate has increased to 70 percent (4). However, progress has slowed over the past thirty years and efforts to improve outcomes with intensifying regimens or adding novel agents have disappointing results (5-9). Moreover, for patients with metastatic osteosarcoma at diagnosis and for those with relapsed disease, outcomes are remarkably poor with 4-5 year overall survival at less than 20% (10, 11). Therefore development of novel therapeutic strategies is critical for this patient population.
There is growing interest in the oncology community in the immunoregulatory receptor PD-1 and the corresponding B7 family of ligands as a potential mechanism of tumor immune tolerance and escape. In normal physiologic state a T-cell response is a complex sequence of events involving clonal T-cell selection, activation, proliferation and trafficking to antigen sites to ultimately deliver immune effector functions (12). This is initiated through the T-cell receptor (TCR) followed by a series of highly regulated signals to either stimulate or inhibit the T-cell response. These inhibitory signals are critical to maintaining self-tolerance and protecting neighboring tissues during the immune response (12). PD-1 is a cell-surface receptor expressed on subsets of T and B lymphocytes, as well as other immune cells. In the inflammatory microenvironment, stimuli such as interferon-γ (13-15) may upregulate PD-L1 expression in peripheral tissues and immune cells to suppress the immune response.
Interestingly, many different malignancies can co-opt this checkpoint system by upregulating PD-L1 constitutively or in response to inflammation. Examples include melanoma, lung cancer, renal cell carcinoma, ovarian cancer, and colorectal cancers (14); however, the expression of PD-L1 in osteosarcoma remains unknown.
There are now multiple agents targeting the PD-1/PD-L1 system at different stages of clinical development (16-22). Recent early phase studies of inhibitory antibodies (aimed at PD-1 or PD-L1) have shown exciting clinical activity and durability across a range of malignancy subtypes, including non-small cell lung cancer (NSCLC), a histology not typically associated with immune responses. In these previously heavily treated patients the response rates ranged from 18-28%, depending on the tumor histology, and importantly the responses were durable.
At the moment there is no clear biomarker to predict anti-PD-1/PD-L1 tumor responsiveness. Recent data suggest that PD-L1 expression, determined by IHC, may be an important biomarker (14, 23-26) perhaps also when combined with T-cell activation signals (17, 27, 28). Despite these results, diagnostics for PD-L1 are limited by the lack of reliable protein-based assays and the literature remains controversial. Previous studies have shown a correlation between PD-L1 mRNA and protein expression (29, 30). To this end, we sought to develop an RNA-based PD-L1 assay to circumvent the technical issues constraining the immunohistochemistry-based strategies.
In summary, we have developed an RNA-based assay to detect PD-L1 expression levels, which we used to demonstrate the expression of PD-L1 in 32 of 38 human osteosarcoma tumor samples. Given the clinical activity of anti-PD-1/PD-L1-based therapies across a range of tumor histologies, these data may lead to a promising target for novel immunotherapy strategies.
Materials and Methods
Institutional review board (IRB) approval was obtained to study all osteosarcoma samples from the Partners Human Research Office (2007P-002464). Written informed consent was obtained from all patients, whose specimens and clinical information were used for this research study.
Osteosarcoma patient surgical specimens
The study consisted of 38 fresh frozen tumor tissue samples of histologically confirmed osteosarcomas from 37 patients (15 female, 22 male) with an age range of 6 to 75 (median 29) years. Nine samples were from biopsy, 13 from primary tumor, 14 from metastases, and two from recurrent tumors. Two samples, P516 and P661 (clinical data are presented in Table 1), were metastases from the same patient and therefore P661 was excluded from statistical analyses. Fourteen out of 37 patients were treated with preoperative chemotherapy. Twenty-six patients died with a median survival of 36 months (range: 1 to 200 months).
Table 1.
Clinical data of RNA samples from osteosarcoma tissues
| Sample | age at diagnosis (median=29) |
gender (female=15; male=23) |
pre-op chemo |
percent necrosis |
metastasis, origin (lung=22; other=8) |
local relapse |
disease status |
follow up (months) (median=36) |
|---|---|---|---|---|---|---|---|---|
| P176 | 14 | male | yes | 95 | no | no | AWED | 231 |
| P180 | 15 | male | none | 20 | yes, lung | no | DOD | 29 |
| P188 | 75 | female | none | n/a | yes, other | yes | DOD | 110 |
| P208 | 17 | female | yes | 60 | yes, lung | no | DOD | 16 |
| P209 | 15 | male | yes | 40 | yes, lung | no | DOD | 14 |
| P214 | 28 | male | yes | 20 | yes, lung | no | DOD | 10 |
| P222 | 32 | male | yes | 50 | yes, lung | no | DOD | 28 |
| P237 | 31 | female | yes | 40 | yes, other | yes | DOD | 89 |
| P249 | 22 | male | none | n/a | yes, lung | yes | AWED | 36 |
| P263 | 45 | male | none | n/a | yes, lung | no | AWED | 234 |
| P286 | 15 | male | yes | 50 | yes, lung | no | DOD | 20 |
| P291* | 30 | female | none | n/a | no | no | NED | 233 |
| P322 | 42 | female | yes | 90 | yes, lung | no | AWED | 226 |
| P336* | 13 | male | yes | 25 | yes, other | no | DOD | 200 |
| P351 | 43 | male | yes | 95 | yes, lung | no | NED | 221 |
| P360 | 33 | male | none | n/a | yes, lung | no | DOD | 27 |
| P404 | 6 | female | yes | 50 | yes, lung | no | AWED | 27 |
| P466* | 15 | male | yes | 75 | no | no | NED | 156 |
| P483 | 18 | female | yes | 98 | yes, lung | no | DOD | 21 |
| P514* | 24 | female | yes | 90 | yes, lung | no | DOD | 39 |
| P516* | 20 | male | none | n/a | yes, other | no | DOD | 28 |
| P530 | 33 | male | yes | 50 | no | no | NED | 196 |
| P531* | 61 | female | none | n/a | yes, lung | no | DOD | 14 |
| P593 | 29 | male | yes | 30 | yes, other | no | DOD | 33 |
| P644 | 14 | female | yes | 20 | yes, other | no | NED | 193 |
| P661* | 20 | male | none | n/a | yes, other | no | DOD | 28 |
| P784 | 17 | male | yes | 50 | yes, lung | no | DOD | 22 |
| P793 | 63 | male | yes | 20 | yes, lung | yes | DOD | 138 |
| P794* | 37 | female | none | n/a | yes, lung | yes | DOD | 13 |
| P854 | 25 | female | yes | 25 | no | no | NED | 162 |
| P860 | 36 | male | none | n/a | no | no | AWED | 50 |
| P885 | 16 | male | yes | 20 | yes, lung | no | DOD | 37 |
| P945* | 34 | female | none | n/a | yes, lung | no | DOD | 5 |
| P969* | 73 | male | none | n/a | no | no | DOD | 1 |
| P974 | 38 | male | yes | 20 | yes, lung | yes | DOD | 36 |
| P983 | 26 | female | none | n/a | yes, lung | yes | DOD | 10 |
| P1028 | 70 | female | none | n/a | no | yes | DOD | 47 |
| P1037 | 37 | male | yes | n/a | yes, lung | yes | DOD | 16 |
no TIL data. Note that P516 and P661 are metastatic tumors from the same patient.
n/a = not available; NED = no evidence of disease; AWED = alive with evidence of disease; DOD = dead of disease.
Cell lines, cell culture, and antibodies
The human osteoblast cell line HOB-c was purchased from PromoCell GmbH (Heidelberg, Germany), the human osteoblast cell line NHOS2 from American Type Culture Collection (ATCC) (Manassas, VA), and the human osteoblast cell line hFOB from Lonza Walkersville, Inc. (Walkersville, MD). The human stem cell line MCS was obtained from Invitrogen (Grand Island, NY). Two human muscle cell RNAs were purchased from Ambion (Grand Island, NY) and Stratagene (La Jolla, CA). The human osteosarcoma cell line SaOS was obtained from ATCC. The multidrug resistant human osteosarcoma cell line U-2OSTR was established in our lab from human osteosarcoma cell line U-2OS also purchased from ATCC. U-2OSTR was selected from U-2OS by stepwise increases in paclitaxel concentrations from 0.0001 μm to 0.1 μm. The human osteosarcoma cell lines KHOS and KHOSR2 were provided by Dr. Efstathios Gonos (Institute of Biological Research & Biotechnology, Athens, Greece). The human chondrosarcoma cell line Cs-1 was established in our lab from a surgically resected human high-grade chondrosarcoma removed from a 62 year-old male with metastatic chondrosarcoma. The human Ewing sarcoma TC-7 and the trabectedin-resistant TC-ET cell lines were donated by Dr. Katia Scotlandi (Rizzoli Orthopaedic Institute, Bologna, Italy). The human ovarian cancer cell line SKOV-3 was purchased from ATCC; the human ovarian cancer cell lines A2780 and OVCAR8 were obtained from Dr. Thomas Hamilton (Fox Chase Cancer Center, Philadelphia, PA) and from Dr. Patricia Donahue (Massachusetts General Hospital, Boston, MA), respectively. Cell lines were grown in DMEM (Invitrogen) supplemented with 10% FBS, 100U/ml penicillin and 100ug/ml streptomycin (Invitrogen). All cell lines were tested and validated to be mycoplasma-free. No other authentications were performed. U-2OS cells were treated with IFN-γ as previously described (31). Briefly, 3×105 cells were incubated at 37°C for 48h in 8ml medium supplemented with 100U/ml.
To detect PD-L1 by flow cytometry and immunohistochemistry, six antibodies were screened: four non-commercial mouse mAbs (CST1, CST2, XW, LC), a mouse mAb from eBioscience (clone MIH1), and a rabbit polyclonal from ProSci (cat # 4059).
Quantitative real-time RT-PCR
To determine the expression of PD-L1 mRNA in cell lines (n=18) and osteosarcoma surgical specimens (n=38), total RNA was extracted using TRIzol Reagent® (Life Technologies) according to the manufacturer’s protocol. RNA samples were quantified using the ultraviolet spectrophotometer at 260 nm (Beckman DU-640, Beckman Instruments, Fullerton, CA). Equal amounts of RNA were reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). cDNAs were then amplified by real-time PCR using TaqMan® gene expression assays (Applied Biosystems) for PD-L1 (Product ID: Hs01125301_m1; probe sequence: 5′-gtgatacacatttggaggagacgtaatccagcattggaacttctgatcttcaagcagggattctcaacctgtggtttaggggttcatcg-3′) and according to the manufacturer’s protocol. PCR was performed with TaqMan® gene expression master mix (Applied Biosystems) using 2 μL of cDNA in a 20-μL final reaction volume. The amplification cycles were performed by Applied Biosystems StepOnePlus™ System (Applied Biosystems) as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 seconds, and finally 60°C for 1 minute. The housekeeping gene β-actin (Product ID: Hs01060665_g1; probe sequence: 5′-ggcgtgatggtgggcatgggtcagaaggattcctatgtgggcgacgaggcccagagcaagaga-3′) expression level was used as an internal control to evaluate the integrity of each sample. The relative expression level of mRNA of PD-L1 was calculated as follows: 2^(threshold cycle of β-actin – threshold cycle of PD-L1). Relative expression was scored on a log10 scale (0-4).
Flow cytometry
Select cell lines were examined by flow cytometry to validate a correlation of PD-L1 mRNA and protein expression. MCF-7 and SKOV-3 cells were collected in 1mM EDTA/PBS and then washed with PBS. 2×105 cells of each were blocked in 2% BSA/PBS for 30 min at 4°C. Cells were then probed with 1vg of the primary antibody (eBioscience, XW, or ProSci) for 30 min at 4°C, washed, and then incubated at 1:100 with the secondary antibody (R-Phycoerythrin-AffiniPure F(ab’)2 Frag Goat Anti-Mouse IgG, Fcγ Frag Spec (min X Hu,Bov,Hrs Sr Prot), Jackson ImmunoResearch, West Grove, PA, or Alexa Fluor® 488 Goat Anti-Rabbit IgG (H+L) Antibody, Invitrogen). After washing and fixation in 2% PFA/PBS, cells were sorted using the BD FACSCalibur™ flow cytometer.
Tissue microarray construction
Representative areas of 100 paraffin-embedded osteosarcoma tumor specimens for each case were selected and prepared as previously described (32). Briefly, three areas of tumor parts per case were selected for assembling the recipient master block to ensure accurate representation of the selected cores. Each target area on the selected block was punched to form a 0.5 mm diameter tissue core and was placed consecutively on the recipient master block. The osteosarcoma TMA was constructed by the Tissue Microarray and Imaging Core at the Dana-Farber/Harvard Cancer Center (http://genepath.med.harvard.edu:8080/pathcore/).
PD-L1 detection by immunohistochemistry
TMA slides were stained using Cell and Tissue Staining Kit (R&D Systems) with slight modifications. Briefly, slides were dewaxed and rehydrated in xylene and graded ethanol solutions for antigen retrieval. Slides were then blocked with 3% H2O2, goat serum, avidin solution, and biotin solution. Primary antibody (eBioscience, CST1, CST2, LC) was added, and then probed with biotinylated goat anti-mouse secondary antibody (Vector Laboratories) and High-Sensitivity Streptavidin-HRP Conjugate. To visualize staining, slides were incubated in 3,3′-Diaminobenzidine in 0.1% H2O2 in Tris HCl Buffer, and subsequently counterstained with Hematoxylin QS (Vector Laboratories). PD-L1 positive samples were defined as those showing membrane and cytoplasmic staining pattern of tumor tissue. PD-L1 staining intensity was graded into four groups: no staining (0), weak staining (1+), moderate staining (2+), and intense staining (3+). The immunostained slide was evaluated under the microscope. The staining intensity of cells showing positive membrane and cytoplasmic staining for PD-L1 was calculated by reviewing the entire spot.
Detection of immune infiltrates
The TILs were examined on a HE stained osteosarcoma TMA slide. Tissue microarray was evaluated for the presence of tumor-infiltrating lymphocytes (TILs) at 400X magnification and scored semiquantitatively as follows: score 0 - no TILs; 1 - rare/few TILs; 2 - brisk/prominent TILs.
Statistical data analysis
Statistical analysis was done using GraphPad Prism 4 software. The 75th percentile was used as cutoff for high or low PD-L1 mRNA expression, as previous studies have suggested that high expression patients represented ~25% in other cancers (33, 34). For comparison between PD-L1 expression and clinical and pathologic variables, a two-sided Student’s t-test was used. The overall cancer-specific follow-up time was calculated in months from date of surgery to date of death due to osteosarcoma or the last follow-up date. Survival analysis was performed using the Kaplan-Meier method, and significance was determined by the log-rank test. For comparison between PD-L1 expression and immune infiltrates, a one-way ANOVA analysis was used. A P-value of <0.05 was considered as statistically significant.
Results
PD-L1 expression in cell lines
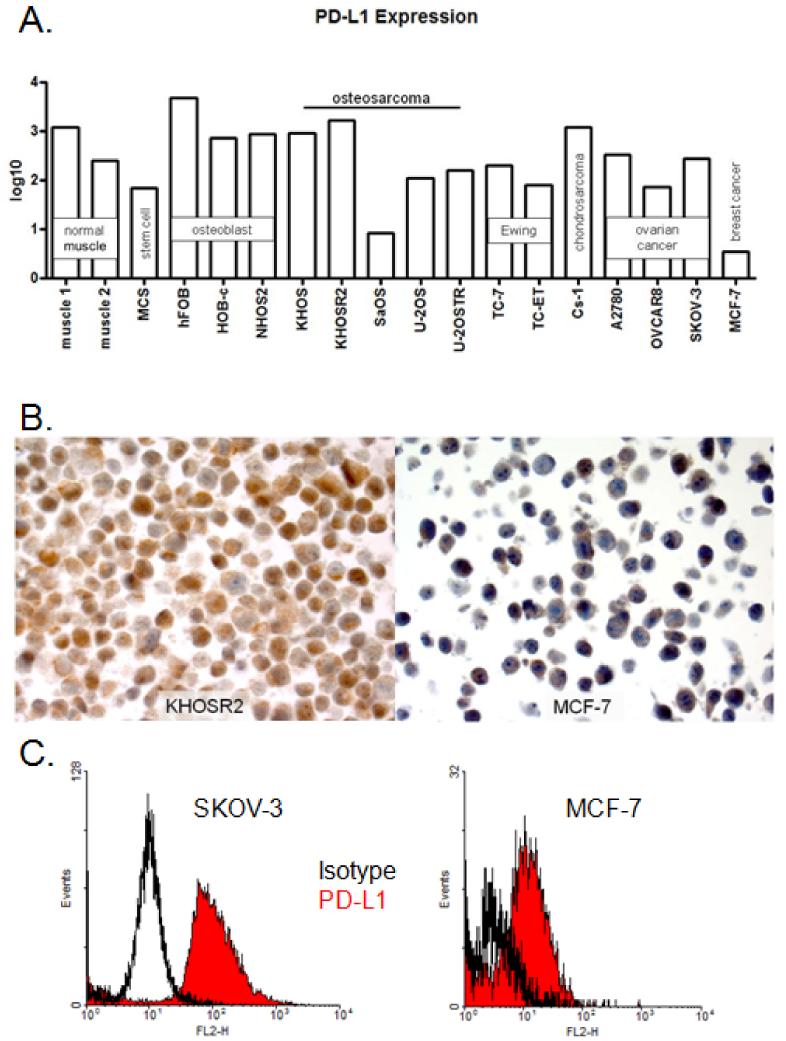
PD-L1 gene expression was quantitatively confirmed in all 18 cell lines. Absolute PD-L1 expression was normalized to that of the housekeeping gene β-actin. Breast cancer cell line MCF-7 has been shown previously to have relatively low PD-L1 expression (35). Drug-resistant osteosarcoma cell line KHOSR2 and virally-derived osteoblast cell line hFOB had high (3-log) PD-L1 gene expression, and osteosarcoma cell line SaOS and breast cancer cell line MCF-7 had low (< 1-log) expression. Other cell lines including stem cell (MCS), osteoblast (Obs, NHOS2), ovarian cancer (SKOV-3, OVCAR8, A2780), Ewing sarcoma (TC-7, TC-ET), chrondrosarcoma (Cs-1), and osteosarcoma (U-2OS, U-2OSTR, KHOS) ranged from intermediate (1-log) to high (2-log) expression of PD-L1 (Figure 1A). Each tumor cell type generally demonstrated varying PD-L1 expression. In non-malignant cell lines such as muscle, stem cells, and osteoblasts, PD-L1 gene expression ranged from moderate to high. Osteosarcoma cell lines ranged from low to high PD-L1 expression, with slightly higher expression from drug-resistant variants (KHOSR2 and U-2OSTR) than their parental cell lines (KHOS and U-2OS). Ewing sarcoma cell lines expressed moderate levels of PD-L1, and ovarian cancer cell lines demonstrated moderate PD-L1 expression as well. The one chondrosarcoma cell line showed high PD-L1 levels, and one breast cancer cell line exhibited low PD-L1 expression; however, conclusions on the expression of PD-L1 on these two tumor types cannot be drawn from one cell line. PD-L1 gene expression was induced 5-fold by IFN-γ treatment in osteosarcoma cell line U-2OS (data not shown).
Figure 1. PD-L1 expression in cancer and non-cancer cell lines.
A. Relative expression of PD-L1 from total RNA isolated from cell lines; B. Representative PD-L1 protein expression levels in corresponding high and low expressing cell lines by IHC; C. Representative PD-L1 protein expression levels in corresponding high and low expressing cell lines by flow cytometry.
To validate the RT-PCR data, immunohistochemistry and flow cytometry assays were performed on select cell lines. Consistent with the RT-PCR data there was low-level staining in the negative control MCF-7 cells and intense staining in the positive control KHOSR2 cells by IHC using mouse mAb LC (Figure 1B). This staining was both membranous and cytoplasmic. Flow cytometry of negative control MCF-7 and positive control SKOV-3 cells using the eBioscience clone MIH1 also showed consistent patterns of expression between RNA and protein (Figure 1C). Unfortunately, the ProSci rabbit polyclonal failed to detect PD-L1 on the positive control cell line SKOV-3, and the XW mouse mAb exhibited unspecific binding on the negative control cell line MCF-7 (Supplementary Figure S1). While the eBioscience mouse mAb demonstrated the expected results by flow cytometry, it produced weak staining by IHC TMA. The CST1, CST2, and LC antibodies also failed to show sufficient specificity for PD-L1 by IHC TMA.
PD-L1 expression in osteosarcoma tumor tissue
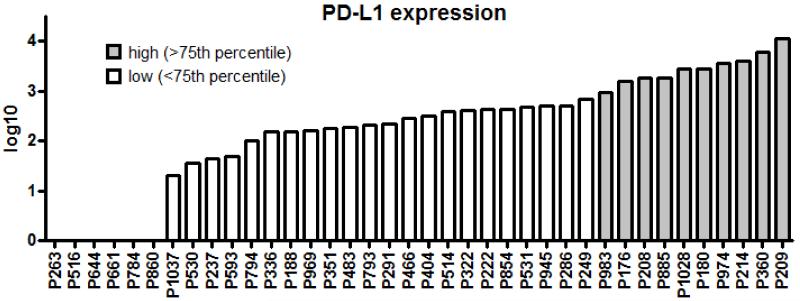
The PD-L1 RT-PCR assay was performed on total RNA isolated from 38 osteosarcoma human tumor samples (Figure 2). Absolute expression was normalized to that of housekeeping gene β-actin and categorized by log-transcript detection: low = 1-log, intermediate = 2-log, and high = 3- and 4-log. We found 9 samples with high-level expression (24%). There were 19 (50.0%) with intermediate expression, 4 (10.5%) with low-level expression, and 6 (15.8%) were negative.
Figure 2. Relative expression of PD-L1 in 38 osteosarcoma specimens.
PD-L1 expression was evaluated from total RNA by quantitative real-time RT-PCR and showed that the expression levels ranging over 4-log (5000-fold).
Prognostic value of PD-L1 expression
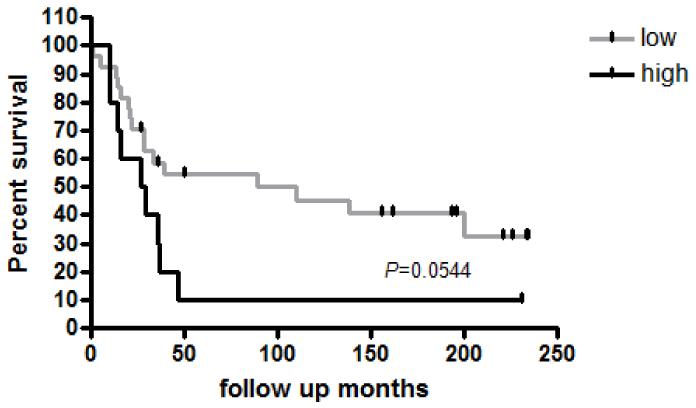
We evaluated the clinicopathologic features of the human tumor samples and found no significant relationship between PD-L1 gene expression and age at surgery, gender, neoadjuvant chemotherapy treatment, percent necrosis, metastatic status, relapse status, or survival. The median overall survival for PD-L1-low patients was 89 months compared with 28 months for PD-L1-high patients, exhibiting a borderline trend, but not achieving statistical significance (P=0.054) (Figure 3).
Figure 3. Overall survival of 37 patients with osteosarcoma in relation to PD-L1 gene expression.
The median overall survival for PD-L1-low patients was 89 months compared with 28 months for PD-L1-high patients showed a trend but was not statistically significant (P=0.0544).
Correlation between PD-L1 and tumor-infiltrating T lymphocytes
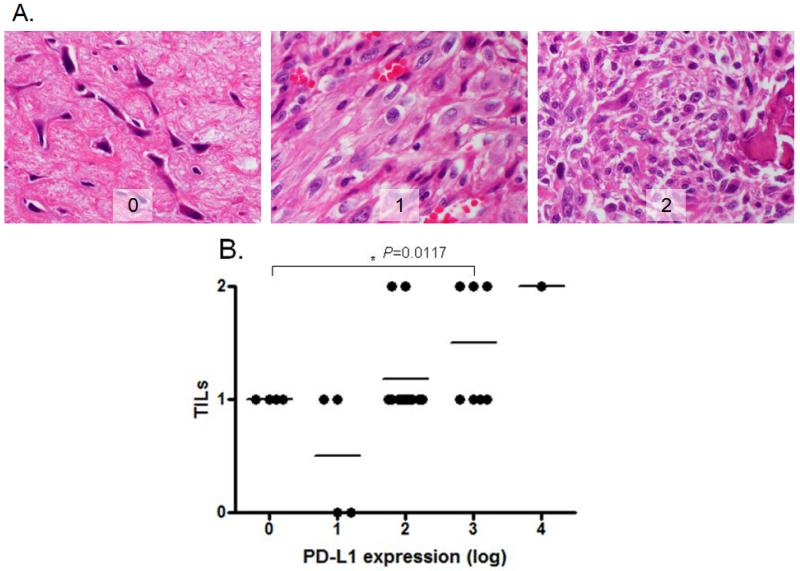
Immunohistochemistry identified TILs in 28 of 38 tumor samples on the tissue microarray. The TIL patterns were diffused within the 0.5mm TMA punches of the osteosarcoma tissues. Semi-quantitative scoring of the degree of TILs was determined by a pathologist, who was blinded to the PD-L1 results. There was a positive correlation between PD-L1 mRNA expression and TILs (R2 = 0.37, P=0.01) (Figure 4).
Figure 4. Correlation between PD-L1 gene expression and TILs by IHC.
A. Representative TILs in osteosarcoma tissues (400×); score 0 - no TILs; 1 - rare/few TILs; 2 - brisk/prominent TILs; B. There was significant positive correlation between PD-L1 gene expression and TILs in patients with osteosarcoma (P=0.0117).
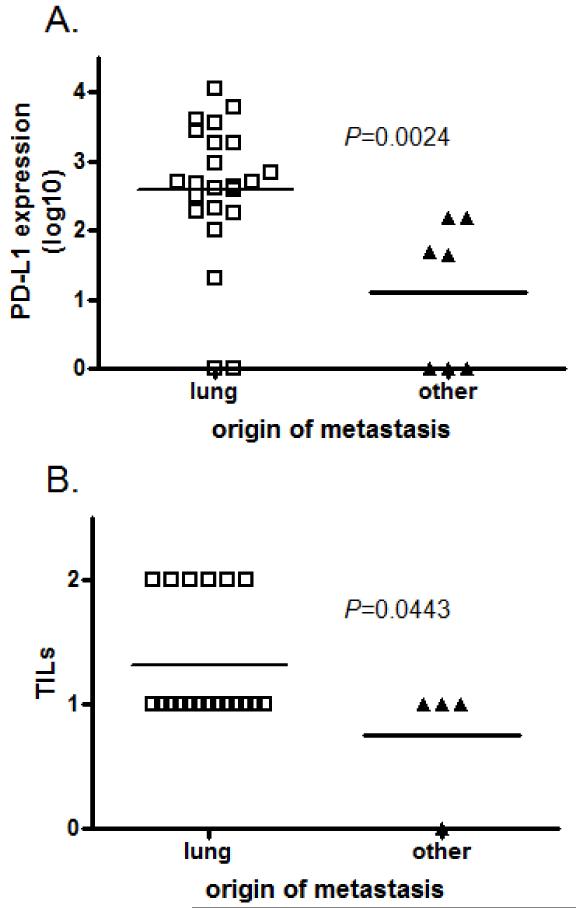
Characterization of origin of metastases
Thirty osteosarcoma tissue samples were derived from metastatic lesions (23/30 were pulmonary and 7/30 were non-pulmonary). Non-pulmonary metastases included the pelvis, humerus, ilium, flank, and anterior mediastinum. The mean PD-L1 expression for pulmonary metastases (2.6±0.21 −log) was significantly higher than the mean PD-L1 expression for non-pulmonary metastases (1.1±0.40 −log) (P=0.0024) (Figure 5A). Furthermore, the origin of the metastasis (pulmonary v. non-pulmonary) correlated with TILs (P=0.044) (Figure 5B).
Figure 5. Characterization of the origins of metastases.
A. PD-L1 expression is significantly higher in metastatic osteosarcoma tumors that originate from lung than from other locations (P=0.0024); B. TILs also exhibit a positive correlation with pulmonary osteosarcoma metastasis compared with non-pulmonary metastasis (P=0.0443).
Discussion
The field of immunotherapeutics is rapidly evolving with the recent successful early clinical studies targeting the PD-1/PD-L1 axis. There is much interest to see if these agents could be applied to other tumor subtypes. It has been suggested that tumor PD-L1 expression may be an adequate biomarker to predict responsiveness to these therapies. One limiting factor, however, has been a lack of reliable, and widely available, anti-PD-L1 antibodies for this important diagnostic step. In the first part of this study our objective was to develop an RNA-based assay to circumvent this issue.
With our quantitative RNA assay we were able to show a wide range of expression in cell lines including low-levels in MCF-7 cells, which is consistent with the literature. Moreover, we could demonstrate induction of PD-L1 transcript expression with interferon-γ, which is an important control for immune activation and regulation. The protein-based assays described in Figure 2 were used to validate our RNA findings as previous studies have demonstrated a correlation between PD-L1 mRNA and protein expression (29, 30). As other investigators have experienced, these were challenging experiments and six antibodies were used for these studies. However, despite the difficulties we were able to reliably and consistently show a correlation between PD-L1 RNA and protein expression in these cell lines.
In the second part of this study, we applied our quantitative PD-L1 RNA assay to human osteosarcoma samples. These tumor samples were selected intentionally quite heterogeneous to represent a wide range of age and clinical scenarios (e.g. localized/metastatic, pre-treatment/on-treatment, new diagnoses/relapsed disease, etc.). We show for the first time that PD-L1 is expressed in 32 of 38 (84.2%) osteosarcoma samples. One challenge to analyzing these data is determining how to normalize the expression levels. We used absolute values normalized to beta-actin as there is no specimen or comparable “normal” tissue to establish a baseline level of PD-L1 expression. With this approach we found 24% of patients had quite high levels of PD-L1 transcript (3-log or more). This perhaps suggests that there is a subset of patients for whom PD-L1-directed therapies could be relevant.
In our analyses, there was no correlation of PD-L1 expression with clinicopathologic features, such as age at surgery, gender, neoadjuvant chemotherapy treatment, percent necrosis, metastatic status, relapse status, or disease status at the time of biopsy. There was a slight trend for poorer overall survival for osteosarcoma patients with high PD-L1 expression. These data are likely confounded by the small, heterogeneous sample size, and larger studies with similar osteosarcoma patient samples are required. Several previous studies have demonstrated conflicting results varying from positive correlation of tumor aggressiveness in renal cell carcinoma (33, 36), to observing no correlation with clinicopathologic variables (24), to an inverse relationship in which high PD-L1 expression is associated with increased survival in colorectal cancer, melanoma, and NSCLC (15, 30, 37). Additionally, the role that TILs play in the mechanisms of PD-L1 expression and survival for osteosarcoma patients is not yet known.
In anti-PD-L1 clinical trial samples there are emerging data that PD-L1 expression plus a T-cell activation gene signature, including CD8 and IFN-γ, may be associated with treatment response (17). We found a correlation between TILs and PD-L1 expression in our tumor samples. In future studies we will examine the subtypes of lymphocyte present in biopsies, including B- and T-cells (CD20 and CD3), CD4/CD8 T-cell response, and macrophages (CD163 or CD68), as well as evidence for T-cell activation.
We found higher levels of expression of PD-L1 and TILs in metastases that originated in the lung versus other sites, including the pelvis, humerus, ilium, flank, and anterior mediastinum. The significance of this remains unclear but could be related to the lung microenvironment or perhaps the prior treatments (e.g. lung metastases are typically an early event in osteosarcoma). Future studies with larger patient samples will be needed to explore this further.
In summary, we have developed a sensitive quantitative RNA-based assay to detect PD-L1 expression. We showed PD-L1 expression in over 80% of osteosarcoma patient samples, with high levels in 24%, and that PD-L1 expression correlated with the presence of TILs. This study sets an important framework to design anti-PD-1 or anti-PD-L1 immune checkpoint therapies in osteosarcoma patients.
Supplementary Material
Acknowledgements
We thank Chungdak Namgyal in the Tissue Microarray and Imaging Core at the Dana-Farber/Harvard Cancer Center for help with the construction of osteosarcoma TMA.
We also thank Dr. Mari Mino-Kenudson in the Department of Pathology at Massachusetts General Hospital for help with the cell line IHC.
Financial support: This work was supported in part by grants from the Gattegno and Wechsler funds. JKS is supported by the Jennifer Hunter Yates Foundation and the Kenneth Stanton Osteosarcoma Research Fund. ZD is supported, in part, through a grant from Sarcoma Foundation of America (SFA), a developmental research award from Sarcoma SPORE (NCI), a grant from NCI/National Institutes of Health (NIH), UO1, CA151452-01, and a grant from an Academic Enrichment Fund of MGH Orthopedic Surgery.
Footnotes
Conflict of interest
EC is a compensated consultant for Amgen and Pfizer. The other authors cite no conflict of interest.
References
- 1.Fletcher CDM, World Health Organization. International Agency for Research on Cancer . WHO classification of tumours of soft tissue and bone. 4th ed. IARC Press; Lyon: p. 468. [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 5.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–28. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–89. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 7.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 8.O’Day K, Gorlick R. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther. 2009;9:511–23. doi: 10.1586/era.09.7. [DOI] [PubMed] [Google Scholar]
- 9.Whelan J, Seddon B, Perisoglou M. Management of osteosarcoma. Curr Treat Options Oncol. 2006;7:444–55. doi: 10.1007/s11864-006-0020-y. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 11.Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27:557–65. doi: 10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powderly JD, Koeppen H, Hodi FS, Sosman JA, Gettinger SN, Desai R, et al. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. ASCO Meeting Abstracts. 2013;31(15_suppl):3001. [Google Scholar]
- 18.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria J-C, Hamid O, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Meeting Abstracts. 2013;31(15_suppl):3000. [Google Scholar]
- 21.Spigel DR, Gettinger SN, Horn L, Herbst RS, Gandhi L, Gordon MS, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2013;31(15_suppl):8008. [Google Scholar]
- 22.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 24.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 25.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–5. [PubMed] [Google Scholar]
- 26.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- 27.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 29.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 30.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haile ST, Bosch JJ, Agu NI, Zeender AM, Somasundaram P, Srivastava MK, et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011;186:6822–9. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Guo S, Schwab JH, Nielsen GP, Choy E, Ye S, et al. Tissue microarray immunohistochemical detection of brachyury is not a prognostic indicator in chordoma. PloS One. 2013;8:e75851. doi: 10.1371/journal.pone.0075851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 34.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 35.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–42. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.