Abstract
The association between Z α1-antitrypsin deficiency and juvenile cirrhosis is well-recognized, and there is now convincing evidence that the hepatic inclusions are the result of entangled polymers of mutant Z α1-antitrypsin. Four percent of the northern European Caucasian population are heterozygotes for the Z variant, but even more common is S α1-antitrypsin, which is found in up to 28% of southern Europeans. The S variant is known to have an increased susceptibility to polymerization, although this is marginal compared with the more conformationally unstable Z variant. There has been speculation that the two may interact to produce cirrhosis, but this has never been demonstrated experimentally. This hypothesis was raised again by the observation reported here of a mixed heterozygote for Z α1-antitrypsin and another conformationally unstable variant (I α1-antitrypsin; 39Arg→Cys) identified in a 34-year-old man with cirrhosis related to α1-antitrypsin deficiency. The conformational stability of the I variant has been characterized, and we have used fluorescence resonance energy transfer to demonstrate the formation of heteropolymers between S and Z α1-antitrypsin. Taken together, these results indicate that not only may mixed variants form heteropolymers, but that this can causally lead to the development of cirrhosis.
Introduction
Alpha1-antitrypsin (or α1-proteinase inhibitor) is the most abundant circulating proteinase inhibitor and acts to protect the tissues against indiscriminate proteolytic attack (1, 2). The clinical significance of this inhibitor is underscored by the association of severe genetic deficiency with early onset emphysema (3), bronchiectasis (4), asthma (5), and vasculitis (6, 7). Alpha1-antitrypsin is the archetypal member of the serine proteinase inhibitor or serpin superfamily, and like all members of this family it has a dominant A β-sheet that acts as a molecular scaffold to support a mobile reactive center loop (8, 9). This loop presents the key P1-P′1 methionine–serine bond as a pseudosubstrate for the cognate proteinase, neutrophil elastase (10). After docking, the reactive loop is cleaved and the proteinase is inactivated by the formation of a 1:1 enzyme/inhibitor complex .
Most individuals are homozygotes for the normal M α1-antitrypsin, but 4% of northern European caucasians carry the Z deficiency allele (342Glu→Lys). This mutation results in a block in α1-antitrypsin processing in the endoplasmic reticulum of the liver (11) and the formation of hepatic inclusions that, in the homozygote, are associated with neonatal hepatitis, juvenile cirrhosis, and hepatocellular carcinoma (12, 13). We have shown that Z α1-antitrypsin accumulates in the hepatocytes of affected individuals by loop-sheet polymerization in which the reactive center loop of one molecule is inserted into the A β-pleated sheet of a second (14, 15). The significance of this linkage was underscored by the finding that two other variants of α1-antitrypsin that are similarly associated with hepatic inclusions and plasma deficiency, Siiyama (53Ser→Phe; ref. 16) and Mmalton (52Phe deleted; ref. 17), similarly form loop-sheet polymers in vivo (18, 19). Polymerization also accounts for the mild deficiency of the common S variant (264Glu→Val) of α1-antitrypsin (20) and has been described in mutants of other members of the serpin family in association with angioedema (21, 22) and thrombosis (23, 24). Mutants that favor polymerization cluster in mobile structural domains of the serpins, particularly in the shutter domain that underlies the A β-pleated sheet (25). Shutter domain variants affect parting of strands 3 and 5 of the A β-sheet to make it more receptive to exogenous reactive loop peptides, thereby favoring polymer formation and disease.
There is a clear relationship between cirrhosis and homozygosity for the Z allele (13), but less is known about liver disease in mixed heterozygotes for the Z and other mutants of α1-antitrypsin (26, 27). We report here the molecular, biochemical, and structural basis of the I α1-antitrypsin allele identified in an IZ heterozygote who manifested cirrhosis secondary to α1-antitrypsin deficiency. In particular, we examine the interaction between the I and S shutter domain mutants and Z α1-antitrypsin, which are all conformationally labile, to determine whether heteropolymer formation may be a contributory factor to the development of cirrhosis.
Methods
Alpha1-antitrypsin phenotyping.
Isoelectric focusing was performed between pH 2.5 and 6.5, with the α1-antitrypsin bands being detected by immunoprecipitation and staining with Coomassie brilliant blue R250. Serum samples were diluted in water to give approximately 0.3 mg/ml α1-antitrypsin. Then, 5 μl of diluted serum or 5 μg of purified α1-antitrypsin was loaded in each lane.
Alpha1-antitrypsin genotyping.
Genomic DNA was prepared from the lymphocytes of the propositus by phenol/chloroform extraction and ethanol precipitation. Amplification of α1-antitrypsin DNA (∼1 μg) was performed by PCR in a 50-μl reaction volume with Taq polymerase (Promega Corp., Madison, Wisconsin, USA), 10× PCR buffer, 1.5 mM Mg2+, 200 μM dNTP, and 25 pmol primers: 5′-AGTCATCATGTGCCTTGACTC-3′ and 5′-ATTGCCAAGGAGAGTTCAAGA-3′ for exon II; 5′-CACTCTTCCAAACCTTCACT-3′ and 5′-TTATACAGAGTAGCAGTGACC-3′ for exon III; 5′-ACAAGAGGAATGCTGTGCCATG-3′ and 5′-CATCTTCAGGAGCTCAGCCA-3′ for exon IV; 5′-GATCAGCCTTACAACGTGTC-3′ and 5′-TACAGATCACATGCAGGCAG-3′ for exon V. The reactions were performed with an initial denaturing cycle of 5 min at 100°C followed by 40 cycles of 94°C for 20 s, 50°C for 20 s, and 74°C for 40 s. The PCR product was confirmed on a 1% wt/vol agarose gel; the primers and excess dNTP were digested with exonuclease I and shrimp alkaline phosphatase. The products were sequenced by cycle sequencing with Thermosequenase using the PCR primers and 33P-labeled terminators (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA).
Purification of plasma M, I, and Z α1-antitrypsin.
M and Z α1-antitrypsin were purified from plasma from M and Z homozygotes, and the mixed IZ α1-antitrypsin was purified from the propositus by 50% and 75% ammonium sulphate fractionation followed by glutathione and Q–Sepharose chromatography as detailed previously (15). The proteins migrated as a single band on SDS-PAGE, and the M and Z α1-antitrypsin controls had normal unfolding transitions on transverse urea gradient gel electrophoresis.
Characterization of the α1-antitrypsin variants.
SDS, nondenaturing and transverse urea gradient polyacrylamide gel electrophoresis, electron microscopy, the assessment of the rate of insertion of a synthetic 12-mer reactive center loop peptide (Ac-Ser-Glu-Ala-Ala-Ala-Ser-Thr-Ala-Val-Val-Ile-Ala) into the A β-sheet of α1-antitrypsin, and limited proteolysis of the reactive center loop of M and I α1-antitrypsin with papain were performed as detailed previously (28, 29). Staphylococcus aureus V8 proteinase digests were carried out in 50 mM ammonium acetate (pH 4.0), with the enzyme dissolved in an equal volume of 50 mM ammonium bicarbonate (pH 8.0). The proteolytic products were assessed by 7.5–15% wt/vol SDS-PAGE, and the NH2-terminal sequence of fragments was determined after separation on a PLRP-s column (30). The active-site values of M, Z, and I α1-antitrypsin, and kinetic parameters for the interaction of I α1-antitrypsin with bovine α-chymotrypsin and human neutrophil elastase, were assessed as described previously (15). The results for the kinetic parameters are the weighted mean of at least three independent experiments
Fluorescence measurement of polymer formation.
Fluorescence measurements were made using a Perkin-Elmer LS 50B spectrofluorometer (Norwalk, Connecticut, USA). Intrinsic tryptophan fluorescence of α1-antitrypsin was measured at 0.1 mg/ml and 45°C in 20 mM sodium phosphate, 100 mM NaCl, 0.1 mM EDTA, and 0.1% wt/vol polyethylene glycol (PEG) 8000 (pH 7.4) using an excitation wavelength of 295 nm and detecting photons emitted at 90° to the excitation beam. The slits controlling the intensity of the excitation light source were kept at the minimum machine-permissible limit of 2.5 nm, but emission slit widths were varied between 2.5 nm and 15 nm, depending on the experimental conditions, in order to give an optimal emission signal. The samples were incubated in a 0.5-ml cuvette with a path length of 1 cm on the excitation axis and 0.2 cm on the emission axis; throughout all experiments, the sample temperature was maintained at 45°C by a heated water jacket within the cuvette holder. The kinetic data were fitted to a single exponential function using Grafit (version 3.00; Erithracus Software Ltd.), and the rate of polymer formation (kapp) was determined from the equation F = A01(1–e–kappt) where F is signal fluorescence, A01 is the amplitude of the phase of rate kapp, and t is time.
Heteropolymer formation was assessed by resonance energy transfer (RET) after labeling of the free cysteines of M, Z, S, and I α1-antitrypsin with 5-iodoacetamidofluorescein (5-IAF) or tetramethylrhodamine-5-iodoacetamide (5-TMRIA) according to the instructions of the manufacturer (Molecular Probes Inc., Eugene, Oregon, USA).
Assessment of α1-antitrypsin secretion from the Xenopus oocyte.
The cDNA for M α1-antitrypsin was cloned into the sp64T expression vector (Promega Corp.) to prepare RNA for injection into Xenopus oocytes. I and Z α1-antitrypsin were prepared by site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, California, USA) and were confirmed by 33P thermal cycling using the primers detailed above. In vitro transcription and the assessment of secretion from Xenopus oocytes were performed as described by Sidhar et al. (31).
Results
Clinical features.
The propositus was a 34-year-old man who had a 12-month history of lethargy and intermittent jaundice with pale stools and dark urine. There was no history of drug ingestion or excess alcohol consumption, and physical examination revealed hepatomegaly but no stigmata of chronic liver disease. Liver function tests showed a hepatitic pattern with an alanine transaminase of 729 U/l (normal range: 7–40), alkaline phosphatase of 180 U/l (normal range: 30–135), and bilirubin of 54 μmol/l (normal range: 2–17). Titers for hepatitis A, B, and C virus and autoantibodies were negative, and serum copper and ceruloplasmin levels were normal. Plasma α1-antitrypsin levels ranged from 0.5 to 0.8 mg/ml, and phenotyping by isoelectric focusing showed the Z deficiency variant and an unusual anodal band. Liver biopsy showed the features of a fully developed micronodular cirrhosis (Figure 1a) with florid bile duct proliferation and associated neutrophil infiltrates in the fibrous septa. There was no evidence of cholestasis, no increase in stainable iron, and the orcein stain for HepBsAg was negative. Immunostaining showed diffuse hepatocyte staining for α1-antitrypsin without globule formation (Figure 1b). There were no periodic acid-Schiff–positive inclusions. Alpha1-antitrypsin is not detectable in normal hepatocytes; thus, appearances support an etiological role for α1-antitrypsin retention in the propositus, as in cirrhosis, on the basis of the PiZZ phenotype. The propositus’ son was noted to have raised transaminase levels at the age of five, and phenotyping confirmed him to be a PiZ homozygote.
Figure 1.

IZ α1-antitrypsin accumulation in hepatocytes resulting in liver damage and cirrhosis. The liver biopsy from the propositus showed cirrhosis when stained with hematoxylin and eosin (a) and evidence of α1-antitrypsin retention when immunostained with polyclonal antibodies to human α1-antitrypsin (b).
Characterization of I α1-antitrypsin.
The Z allele of the propositus was confirmed as 342Glu→Lys by direct sequencing of PCR-amplified lymphocyte genomic DNA. The unusual α1-antitrypsin band seen on isoelectric focusing was identified as the 39Arg (CGC)→Cys (TGC), or I, mutation after complete sequencing of the α1-antitrypsin gene followed by sequencing of two PCR products containing the mutation on three separate occasions. IZ α1-antitrypsin was purified from plasma from the propositus by glutathione and anion exchange chromatography. Z and I α1-antitrypsin eluted from the anion exchange column at 0.09 and 0.15 M NaCl, respectively, and complete separation was confirmed by nondenaturing PAGE (Figure 2) and isoelectric focusing (data not shown). The difference in migration between Z α1-antitrypsin from the propositus (lane 4) and the control (lane 3) is due to NH2-terminal cleavage and the loss of five NH2-terminal amino acids (15). This gives a more cathodal migration profile on nondenaturing PAGE and characteristic bands that were clearly seen on isoelectric focusing.
Figure 2.
Elution of α1-antitrypsin from the anion exchange column allowed the separation of early eluting Z from late eluting I α1-antitrypsin. Nondenaturing PAGE (7.5–15% wt/vol). All lanes contain 4 μg protein. Lane 1, M α1-antitrypsin control; lane 2, 1:1 mixture of control M and Z α1-antitrypsin; lane 3, Z α1-antitrypsin control, lane 4, Z α1-antitrypsin from the plasma of the propositus; lane 5, a mixture of I and Z α1-antitrypsin isolated from the central fractions of the α1-antitrypsin peak from the anion exchange column; lane 6, I α1-antitrypsin from the plasma of the propositus; lane 7, M α1-antitrypsin control cleaved at the reactive center loop with Staphylococcus aureus V8 proteinase. The difference in migration between Z α1-antitrypsin from the propositus (lane 4) and the control (lane 3) is due to NH2-terminal cleavage.
I and Z α1-antitrypsin were intact as assessed by SDS-PAGE and were 55% and 61% active as inhibitors of bovine α-chymotrypsin, respectively. M α1-antitrypsin purified under identical conditions was typically 80% active. I α1-antitrypsin had a normal unfolding transition on a 0–8 M transverse urea gradient gel and became resistant to unfolding in 8 M urea after reactive loop cleavage with papain. The I mutation had little effect on the inhibitory kinetics with bovine α-chymotrypsin (3.0 ± 0.01 × 106 M-1s-1; Ki < 5 pM) with values similar to those reported previously for M and Z α1-antitrypsin (15). The association rate constant with the cognate proteinase human neutrophil elastase was 1.7 ± 0.04 × 107 M-1s-1 at 37°C, which is slower than M α1-antitrypsin (5.3 ± 0.06 × 107 M-1s-1; ref. 15), but comparable with the values obtained for Z α1-antitrypsin (1.2 ± 0.02 × 107 M-1s-1; ref. 15). I α1-antitrypsin formed stable complexes with both bovine α-chymotrypsin and human neutrophil elastase when assessed under physiological conditions (Ki < 5 pM) and with bovine α-chymotrypsin when assessed by SDS-PAGE (data not shown).
I α1-antitrypsin accepted a synthetic reactive loop peptide into the A β-sheet at a similar rate as control M α1-antitrypsin after incubation at 37°C for 24 hours, indicating that the mutation has little effect on the gross structure of the A β-sheet. Probing of the reactive loop with limited proteolysis by papain (which cleaves at P1-P′1 and P5-P6) gave similar results as M α1-antitrypsin. Limited proteolysis of M and I α1-antitrypsin with S. aureus V8 proteinase (which cleaves at P4-P5) showed similar rates of reactive loop cleavage, but I α1-antitrypsin produced a second minor cleavage product of approximately 46 kDa. Repeated high-performance liquid chromatography separation of cleavage products and NH2-terminal sequencing could not detect a novel NH2-terminal sequence to further characterize this minor band.
Assessment of rates of polymerization.
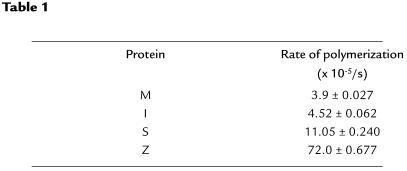
The I mutation lies in the shutter domain (32, 33) and rendered α1-antitrypsin more susceptible to heat-induced polymerization compared with M α1-antitrypsin after incubation at 37°C (Figure 3) and 41°C. The polymers that formed under physiological conditions showed characteristic high molecular mass bands on transverse urea gradient gels that were resistant to unfolding in 8 M urea (data not shown) and could be readily identified by electron microscopy (Figure 3). I α1-antitrypsin did not lose monomeric protein or form polymers as rapidly as a 1:1 mixture of IZ α1-antitrypsin purified from the plasma of the propositus (Figure 4a). Assessment of polymerization by change in intrinsic tryptophan fluorescence with respect to time showed that 45°C and 0.1 mg/ml were the optimum conditions to measure the rate of M α1-antitrypsin polymer formation. This assay confirmed that I α1-antitrypsin polymerized faster than M α1-antitrypsin but 14 and 2 times slower than Z and S α1-antitrypsin, respectively (Figure 4b and Table 1). The rate of polymerization of MZ α1-antitrypsin was compared with IZ α1-antitrypsin by mixing the variants in a 1:1 ratio at a final concentration of 0.1 mg/ml. The intrinsic fluorescence curves for IZ and MZ α1-antitrypsin could not be described with a single curve, and hence a single rate, but the rate of polymerization for individual components could be dissected out from the curves (Table 2). These show that I α1-antitrypsin polymerizes more rapidly in IZ α1-antitrypsin than does M α1-antitrypsin in a mixture of MZ α1-antitrypsin; the rate for Z α1-antitrypsin polymerization was similar in each mix. Taken together, the data show that a mix of IZ α1-antitrypsin polymerizes more rapidly than MZ α1-antitrypsin. The effect of the I mutation on α1-antitrypsin secretion in vivo was assessed by injecting RNA coding for the α1-antitrypsin variants into a Xenopus oocyte expression system. Both 39Arg→Cys (I) and 342Glu→Lys (Z) α1-antitrypsin resulted in reduced secretion of α1-antitrypsin, with more Z α1-antitrypsin being retained than I α1-antitrypsin (Figure 5).
Figure 3.
Nondenaturing PAGE (7.5–15% wt/vol) showing that I α1-antitrypsin polymerized more readily than M α1-antitrypsin after incubation at 37°C at 2 mg/ml in 50 mM Tris, 50 mM KCl (pH 7.4). All lanes contain 10 μg protein. (a) M α1-antitrypsin 0 days, 2 days, 3 days, 6 days, and 12 days at 37°C. (c) I α1-antitrypsin 0 days, 2 days, 3 days, 6 days, and 12 days at 37°C. The electron micrograph of M (b) and I (d) α1-antitrypsin incubated at 2 mg/ml and 37°C for 12 days confirmed that I α1-antitrypsin formed chains of polymers, but M α1-antitrypsin remained monomeric when incubated under identical conditions. The electron micrographs were stained with 1% wt/vol uranyl acetate. Scale bar: 100 nm.
Figure 4.
(a) Nondenaturing PAGE (7.5-15% wt/vol) showing that a mixture of IZ α1-antitrypsin loses protein from the monomeric band and forms high molecular mass polymers more readily than I α1-antitrypsin alone. The proteins were incubated at 2 mg/ml and 41°C in 50 mM Tris, 50 mM KCl (pH 7.4). All lanes contain 10 μg protein. Top: I α1-antitrypsin; bottom: IZ α1-antitrypsin. Lane 1, time 0; lane 2, 1 day; lane 3, 2 days; lane 4, 3 days; lane 5, 6 days; lane 6, 12 days. (b) Rate of polymerization of M, I, S, and Z α1-antitrypsin mutants at 0.1 mg/ml and 45°C determined from the measurement of intrinsic tryptophan fluorescence. The values for the rate of polymerization (Table 1) were obtained from fitting the data to Equation 1 and are the weighted mean and standard error of three (I, S, and Z α1-antitrypsin) or four (M α1-antitrypsin) experiments. The rate of polymer formation of mixtures of IZ and MZ α1-antitrypsin were calculated using a 1:1 mixture of the variants at 45°C. The concentration of each variant in the mixture was half that shown in b in order to keep the final protein concentration at 0.1 mg/ml. The curves for each component of the reaction were dissected from the final profile, and the rate of each component in the mixture was obtained by fitting the data to Equation 1. The results shown in Table 2 are the weighted mean and SE of two kinetic experiments.
Table 1.
Rates of polymerization of the M, I, S, and Z plasma variants of α1-antitrypsin
Table 2.
Rates of polymerization of M, Z, and I α1-antitrypsin in a 1:1 mix of MZ and IZ variants
Figure 5.
Secretion of M, Z, and I α1-antitrypsin by the Xenopus oocyte. The results are the average values (and SE) obtained by injection into batches of 20 oocytes on at least four separate occasions.
Assessment of heteropolymer formation in vitro.
If two species of α1-antitrypsin form heteropolymers, then the close proximity of fluorescent probes bound to 232cysteine should allow energy transfer from one to the other. This was tested by labeling aliquots of fully active plasma M α1-antitrypsin with the fluorophores 5-IAF and 5-TMRIA. The 5-IAF probe absorbed at 492 nm and emitted at 515 nm, while the 5-TMRIA probe absorbed at 543 nm and emitted at 567 nm. In both cases, the labeled protein was intact by SDS-PAGE, had 35% of its original activity as an inhibitor of bovine α-chymotrypsin, and formed a minor dimer band seen in lanes 2–4 in Figure 6a. The 5-IAF and 5-TMRIA M α1-antitrypsin were heated individually and mixed together in equal amounts at 41°C and 2 mg/ml for 14 days, and in all cases resulted in the formation of polymers as assessed by nondenaturing PAGE (Figure 6a). Excitation of the resulting mixed incubation at 492 nm resulted in a marked fluorescence peak at the emission wavelength of the 5-IAF probe (515 nm; data not shown) and a peak at 570 nm, which is within the emission wavelength of the 5-TMRIA α1-antitrypsin probe (Figure 6b). This was not seen in the preheated mixture or with M α1-antitrypsin labeled with either probe alone after polymer formation under identical conditions.
Figure 6.
(a) Polymerization of 5-IAF– and 5-TMRIA–labeled M α1-antitrypsin (2 mg/ml) at 41°C for 14 days. Nondenaturing PAGE (7.5–15% wt/vol), all lanes contain 5 μg protein. Lane 1, M α1-antitrypsin control; lane 2, 1:1 mix of M α1-antitrypsin labeled with 5-IAF and 5-TMRIA; lane 3, M α1-antitrypsin labeled with 5-IAF; lane 4, M α1-antitrypsin labeled with 5-TMRIA; lane 5, M α1-antitrypsin labeled with 5-IAF heated at 41°C for 14 days; lane 6, M α1-antitrypsin labeled with 5-TMRIA heated at 41°C for 14 days; lane 7, 1:1 mix of M α1-antitrypsin labeled with 5-IAF and 5-TMRIA heated at 41°C for 14 days. (b) A 1:1 mixture of 5-IAF– and 5-TMRIA–labeled M α1-antitrypsin incubated at 41°C for 14 days demonstrated RET (continuous line) when excited at 492 nm and fluorescence measured over a wavelength of 500–600 nm. The peak at approximately 570 nm was not seen in M α1-antitrypsin polymerized under identical conditions with either 5-IAF or 5-TMRIA alone (bold line). Labeled Z and S α1-antitrypsin both formed polymers when incubated alone and when mixed in a 1:1 ratio. The signal from the mixed Z and S α1-antitrypsin polymers excited at 492 nm shows RET (broken line), indicating the formation of heteropolymers. (c) Nondenaturing PAGE (7.5–15% wt/vol). All lanes contain 10 μg protein. Lane 1, I α1-antitrypsin labeled with 5-TMRIA heated at 41°C and 0.4 mg/ml for 12 days; lane 2, I α1-antitrypsin control heated at 41°C and 0.4 mg/ml for 12 days; lane 3, unlabeled I α1-antitrypsin control. 5-IAF, 5-iodoacetamidofluorescein; 5-TMRIA, tetramethylrhodamine-5-iodoacetamide; RET, resonance energy transfer.
This technique was then applied to the naturally occurring I and Z variants of α1-antitrypsin to assess if they could also form heteropolymers in vitro. The I α1-antitrypsin mutation created a second cysteine residue at position 39, and labeling this protein with 5-TMRIA completely abolished polymerization when incubated at 41°C for 12 days (Figure 6c). The experiment was therefore performed with S α1-antitrypsin, which mediates its effect via the adjacent residue 38Tyr (20), has only one cysteine, has also been associated with cirrhosis in association with the Z allele (34, 35), and favors spontaneous polymer formation at a rate similar to I α1-antitrypsin. Incubation of Z and S α1-antitrypsin labeled with 5-IAF and 5-TMRIA, respectively, at 0.4 mg/ml and 41°C resulted in the formation of a similar quantity of loop-sheet polymers as unlabeled Z and S α1-antitrypsin controls when assessed by nondenaturing PAGE. Thus, the label did not interfere with the polymerization of these variants. Z and S α1-antitrypsin were then incubated together in a 1:1 ratio at 0.4 mg/ml and 41°C for 14 days, and the formation of polymers was confirmed by nondenaturing PAGE. Excitation of the mixture at 492 nm resulted in a peak at 570 nm, in keeping with RET and indicative of heteropolymer formation (Figure 6b).
Discussion
Alpha1-antitrypsin deficiency is characterized by the formation of intracellular inclusions that may be associated with neonatal hepatitis, cirrhosis, and hepatocellular carcinoma (12, 13). This is well recognized in Z α1-antitrypsin homozygotes, and there is now convincing evidence that a key factor in the accumulation of protein and hepatocellular damage are the entangled polymers of mutant Z α1-antitrypsin (14). The Z α1-antitrypsin allele is found in 4% of the northern European Caucasian population, but even more common is S α1-antitrypsin, with as many as 28% of southern Europeans carrying the S allele (36). The S variant is known to have an increased susceptibility to polymerization (20), although this increase is marginal compared with the more conformationally unstable Z variant (Figure 4). There has been speculation that the two may interact to produce cirrhosis in SZ heterozygotes (34, 35), but this has never been demonstrated experimentally. The formation of heteropolymers was investigated following our finding a 34-year-old man with cirrhosis related to α1–antitrypsin deficiency, who was a mixed heterozygote for Z α1-antitrypsin and another conformationally unstable variant, 39Arg→Cys. This, like S α1–antitrypsin (20), is in the shutter domain that underlies the A β-sheet (Figure 7a). The 39Arg→Cys mutation has been reported previously as the I phenotype (37) in association with mild plasma deficiency and minor impairment of lung function (38). It has not been reported with hepatitis or cirrhosis, but the finding of the allele in a patient with α1-antitrypsin deficiency–related liver disease suggests that the I allele may contribute to the hepatic damage.
Figure 7.
(a) The crystal structures of α1-antitrypsin (32, 33) demonstrated the availability of 232Cys and the position of the I (39Arg→Cys) mutation in helix A at the back of the molecule. The reactive center loop is shown in red; the A β-sheet, which must open to allow polymer formation, is illustrated in green. S α1-antitrypsin mediates its effect by breaking a hydrogen bond with 38Tyr in the shutter domain (20), which controls A β-sheet mobility. (b) Reactive loop/A-sheet polymerization with an open helical conformation (32) places the cysteine residues over 60 Å apart (right), but a closed helical conformation predictably brings the cysteine residues of different α1-antitrypsin molecules close to each other and therefore available for resonance energy transfer (left). In this model, the α1-antitrypsin molecules are ordered blue, green, and red (from bottom to top), with RET predictably occurring between the labeled cysteines of molecules of the same color.
The significance of I α1-antitrypsin in the IZ α1-antitrypsin propositus was investigated after its purification from the Z variant. The I mutation favored the formation of long-chain polymers when incubated under physiological conditions in vitro. These polymers formed more readily than those of M α1-antitrypsin (Figure 3). The rate of polymerization of I α1-antitrypsin was greatly accelerated by incubation at 41°C, which supports our previous suggestion that inclusion formation and liver disease are likely to be exacerbated by the inflammatory response (14). A 1:1 I/Z α1-antitrypsin mixture polymerized at a faster rate than I alone at 37°C and 41°C (Figure 4a), demonstrating that Z α1-antitrypsin was more polymerogenic than I α1-antitrypsin. The relative susceptibility of these variants to form polymers was examined in assays of intrinsic tryptophan fluorescence at 45°C in vitro (Figure 4) and by secretion from Xenopus oocytes in vivo (Figure 5). The results confirm that the ability of α1-antitrypsin variants to form polymers may be graded Z>S>I>M and demonstrate that a mixture of IZ α1-antitrypsin polymerizes more readily than MZ α1-antitrypsin. These data are consistent with less retention of α1-antitrypsin within hepatocytes and milder plasma deficiency in MZ heterozygotes (plasma level 0.9 mg/ml) when compared with the IZ heterozygote (0.5–0.8 mg/ml) and Z α1-antitrypsin homozygotes (< 0.3 mg/ml). Thus, I α1-antitrypsin undergoes the same conformational transition to form loop-sheet polymers as Z (14), Siiyama (18), Mmalton (19), and S (20) α1-antitrypsin at a rate that puts patients at risk for α1-antitrypsin deficiency–related liver disease when it is inherited in association with a severe polymerogenic allele such as Z α1-antitrypsin.
The finding of cirrhosis in a patient who was an IZ α1-antitrypsin heterozygote prompted an examination of the interaction of the Z variant with I α1-antitrypsin and with the common S α1-antitrypsin. The fluorescence phenomenon of resonance energy transfer (RET) was used to assess whether these unstable mutants would form heteropolymers. This technique relies on labeling two populations of protein with different fluorescent probes via a free cysteine on the protein surface. The fluorescent groups are specifically chosen to have spectral characteristics that allow energy to pass between them when they are in close proximity to one another. The striking demonstration of RET between two aliquots of plasma M α1-antitrypsin labeled with different fluorescent probes afforded the first demonstration of a technique that could be used to examine α1-antitrypsin heteropolymer formation in vitro (Figure 6b). The fluorescent probes were then used to monitor the polymerization of I, S, and Z α1-antitrypsin. The I mutation generated a second cysteine, and labeling with the bulky rhodamine probe completely blocked the polymerization of I α1-antitrypsin in vitro (Figure 6c). This demonstrated that part of the dysfunction of I α1-antitrypsin resulted from the Arg→Cys mutation reducing the size of the residue at position 39 in the shutter domain that controls opening of the A β-sheet, reactive loop annealing, and polymer formation (20, 25). Labeling this residue with a large fluorescent moiety mimicked the original M α1-antitrypsin sequence at that position (arginine) and reversed the effect of the mutation. S α1-antitrypsin mediates its effects through the tyrosine residue at position 38 and may also be considered a shutter domain mutant (20). This variant also favors spontaneous polymer formation at a rate similar to I α1-antitrypsin but has only one cysteine residue and is therefore not affected by labeling with fluorescent probes. After a 14-day incubation, the SZ α1-antitrypsin polymers demonstrated RET in keeping with heteropolymer formation. This observation suggests that hepatic inclusions in patients with the Z α1-antitrypsin allele and a second polymerizing mutant (S or I) are likely to result from both homopolymers and mixed Z/mutant heteropolymers. RET will only occur if the cysteine residues labeled with 5-IAF and 5-TMRIA are less than 50 Å, and ideally less than 35 Å, apart (39). Molecular models of open helical A β-sheet polymers (32) place the cysteine residues over 60 Å apart. The requirements for RET can be best satisfied if the loop-A β-sheet heteropolymers form a closed helical structure, with RET occurring between molecules 1 and 4, 2 and 5, 3 and 6, and so on (Figure 7b). An alternative explanation is linkage of the reactive center loop to the C β-sheet (40), but recent studies suggest that this only occurs in α1-antitrypsin polymers formed by heating monomeric α1-antitrypsin in the presence of stabilizing concentrations of sodium citrate (28, 41).
In summary, these data demonstrate that loop-sheet polymerization underlies the plasma deficiency of the shutter domain variant I α1-antitrypsin and provide the first description of Z α1-antitrypsin forming heteropolymers with S α1-antitrypsin. The clinical relevance of this observation is underscored by our demonstration with the IZ α1-antitrypsin propositus, and the finding of others with the SZ phenotype (34, 35), that a mixed heterozygote for conformationally unstable variants can develop cirrhosis associated with α1-antitrypsin deficiency.
Acknowledgments
We are grateful to Alexander Gimson and Graeme Alexander (Department of Hepatology, Addenbrooke’s NHS Trust) for referring the propositus, John Finch (Laboratory of Molecular Biology, Medical Research Council Centre, University of Cambridge) for his help with the electron micrographs, and Robin Carrell (Department of Haematology, University of Cambridge) for critical reading of the manuscript. This work was supported by the Medical Research Council (UK), the Wellcome Trust, the Cystic Fibrosis Trust (UK), the Papworth Hospital Research Fund, the Sir Halley Stewart Trust, and the Wessex Medical Trust. R. Mahadeva is an Anglia and Oxford Research Fellow.
References
- 1.Carrell RW, et al. Structure and variation of human α1-antitrypsin. Nature. 1982;298:329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- 2.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(Suppl. 6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson S. Studies in α1-antitrypsin deficiency. Acta Med Scand Suppl. 1965;432:1–85. [PubMed] [Google Scholar]
- 4.King MA, et al. α1-antitrypsin deficiency: evaluation of bronchiectasis with CT. Radiology. 1996;199:137–141. doi: 10.1148/radiology.199.1.8633137. [DOI] [PubMed] [Google Scholar]
- 5.Colp C, Pappas J, Moran D, Lieberman J. Variants of α1-antitrypsin in Puerto Rican children with asthma. Chest. 1993;103:812–815. doi: 10.1378/chest.103.3.812. [DOI] [PubMed] [Google Scholar]
- 6.Esnault VLM, et al. Alpha1-antitrypsin genetic polymorphism in ANCA-positive systemic vasculitis. Kidney Int. 1993;43:1329–1332. doi: 10.1038/ki.1993.186. [DOI] [PubMed] [Google Scholar]
- 7.Segelmark M, Elzouki A-N, Wieslander J, Eriksson S. The PiZ gene of α1-antitrypsin as a determinant of outcome in PR3-ANCA-positive vasculitis. Kidney Int. 1995;48:844–850. doi: 10.1038/ki.1995.360. [DOI] [PubMed] [Google Scholar]
- 8.Huber R, Carrell RW. Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- 9.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 10.Johnson D, Travis J. Structural evidence for methionine at the reactive site of human α-1-proteinase inhibitor. J Biol Chem. 1978;253:7142–7144. [PubMed] [Google Scholar]
- 11.Le A, Ferrell GA, Dishon DS, Quyen-Quyen AL, Sifers RN. Soluble aggregates of the human PiZ α1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992;267:1072–1080. [PubMed] [Google Scholar]
- 12.Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- 14.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 15.Lomas DA, Evans DL, Stone SR, Chang W-SW, Carrell RW. Effect of the Z mutation on the physical and inhibitory properties of α1-antitrypsin. Biochemistry. 1993;32:500–508. doi: 10.1021/bi00053a014. [DOI] [PubMed] [Google Scholar]
- 16.Seyama K, et al. Siiyama (serine 53 (TCC) to phenylalanine 53 (TTC)). A new α1-antitrypsin-deficient variant with mutation on a predicted conserved residue of the serpin backbone. J Biol Chem. 1991;266:12627–12632. [PubMed] [Google Scholar]
- 17.Roberts EA, Cox DW, Medline A, Wanless IR. Occurrence of alpha-1-antitrypsin deficiency in 155 patients with alcoholic liver disease. Am J Clin Pathol. 1984;82:424–427. doi: 10.1093/ajcp/82.4.424. [DOI] [PubMed] [Google Scholar]
- 18.Lomas DA, Finch JT, Seyama K, Nukiwa T, Carrell RW. α1-antitrypsin Siiyama (Ser53→Phe): further evidence for intracellular loop-sheet polymerization. J Biol Chem. 1993;268:15333–15335. [PubMed] [Google Scholar]
- 19.Lomas DA, et al. Alpha1-antitrypsin Mmalton (52Phe deleted) forms loop-sheet polymers in vivo: evidence for the C sheet mechanism of polymerization. J Biol Chem. 1995;270:16864–16870. doi: 10.1074/jbc.270.28.16864. [DOI] [PubMed] [Google Scholar]
- 20.Elliott PR, Stein PE, Bilton D, Carrell RW, Lomas DA. Structural explanation for the dysfunction of S α1-antitrypsin. Nat Struct Biol. 1996;3:910–911. doi: 10.1038/nsb1196-910. [DOI] [PubMed] [Google Scholar]
- 21.Aulak KS, et al. A hinge region mutation in C1-inhibitor (Ala436→Thr) results in nonsubstrate-like behavior and in polymerization of the molecule. J Biol Chem. 1993;268:18088–18094. [PubMed] [Google Scholar]
- 22.Eldering E, Verpy E, Roem D, Meo T, Tosi M. COOH-terminal substitutions in the serpin C1 inhibitor that cause loop overinsertion and subsequent multimerization. J Biol Chem. 1995;270:2579–2587. doi: 10.1074/jbc.270.6.2579. [DOI] [PubMed] [Google Scholar]
- 23.Bruce D, Perry DJ, Borg J-Y, Carrell RW, Wardell MR. Thromboembolic disease due to thermolabile conformational changes of antithrombin Rouen VI (187 Asn→Asp) J Clin Invest. 1994;94:2265–2274. doi: 10.1172/JCI117589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindo VS, et al. Antithrombin-TRI (Ala382 to Thr) causing severe thromboembolic tendency undergoes the S-to-R transition and is associated with a plasma-inactive high-molecular-weight complex of aggregated antithrombin. Br J Haematol. 1995;89:589–601. doi: 10.1111/j.1365-2141.1995.tb08368.x. [DOI] [PubMed] [Google Scholar]
- 25.Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995;2:96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- 26.Morin T, et al. Heterozygous alpha1-antitrypsin deficiency and cirrhosis in adults, a fortuitous association. Lancet. 1975;1:250–251. doi: 10.1016/s0140-6736(75)91143-5. [DOI] [PubMed] [Google Scholar]
- 27.Hodges JR, Millward-Sadler GH, Barbatis C, Wright R. Heterozygous MZ alpha1-antitrypsin deficiency in adults with chronic active hepatitis and cryptogenic cirrhosis. N Engl J Med. 1981;304:557–560. doi: 10.1056/NEJM198103053041001. [DOI] [PubMed] [Google Scholar]
- 28.Lomas DA, Elliott PR, Chang W-SW, Wardell MR, Carrell RW. Preparation and characterization of latent α1-antitrypsin. J Biol Chem. 1995;270:5282–5288. doi: 10.1074/jbc.270.10.5282. [DOI] [PubMed] [Google Scholar]
- 29.Chang W-SW, Wardell MR, Lomas DA, Carrell RW. Probing serpin reactive loop conformations by proteolytic cleavage. Biochem J. 1996;314:647–653. doi: 10.1042/bj3140647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988;336:257–258. doi: 10.1038/336257a0. [DOI] [PubMed] [Google Scholar]
- 31.Sidhar SK, Lomas DA, Carrell RW, Foreman RC. Mutations which impede loop/sheet polymerization enhance the secretion of human α1-antitrypsin deficiency variants. J Biol Chem. 1995;270:8393–8396. doi: 10.1074/jbc.270.15.8393. [DOI] [PubMed] [Google Scholar]
- 32.Elliott PR, Lomas DA, Carrell RW, Abrahams J-P. Inhibitory conformation of the reactive loop of α1-antitrypsin. Nat Struct Biol. 1996;3:676–681. doi: 10.1038/nsb0896-676. [DOI] [PubMed] [Google Scholar]
- 33.Elliott PR, Abrahams J-P, Lomas DA. Wildtype α1-antitrypsin is in the canonical inhibitory conformation. J Mol Biol. 1998;275:419–425. doi: 10.1006/jmbi.1997.1458. [DOI] [PubMed] [Google Scholar]
- 34.Cruz M, Molina JA, Pedrola D, Muñoz-López F. Cirrhosis and heterozygous α1-antitrypsin deficiency in a 4 year old girl. Helv Paediatr Acta. 1975;30:501–507. [PubMed] [Google Scholar]
- 35.Campra JL, Craig JR, Peters RL, Reynolds TB. Cirrhosis associated with partial deficiency of alpha-1-antitrypsn in an adult. Ann Intern Med. 1973;78:233–238. doi: 10.7326/0003-4819-78-2-233. [DOI] [PubMed] [Google Scholar]
- 36.Fagerhol MK, Tenfjord OW. Serum Pi types in some European, American, Asian and African populations. Acta Pathol Microbiol Scand. 1968;72:601–608. doi: 10.1111/j.1699-0463.1968.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 37.Graham A, et al. Molecular characterisation of three alpha-1-antitrypsin variants: proteinase inhibitor (Pi) nullcardiff (Asp256→Val); Pi Mmalton (Phe52→deletion) and Pi I (Arg39→Cys) Hum Genet. 1989;84:55–58. doi: 10.1007/BF00210671. [DOI] [PubMed] [Google Scholar]
- 38.Baur X, Bencze K. Study of familial alpha-1-proteinase inhibitor deficiency including a rare proteinase inhibitor phenotype (IZ) Respiration. 1987;51:188–195. doi: 10.1159/000195201. [DOI] [PubMed] [Google Scholar]
- 39.Selvin PR. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- 40.Carrell RW, Stein PE, Fermi G, Wardell MR. Biological implications of a 3Å structure of dimeric antithrombin. Structure. 1994;2:257–270. doi: 10.1016/s0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 41.Koloczek H, Banbula A, Salvesen GS, Potempa J. Serpin α1-proteinase inhibitor probed by intrinsic tryptophan fluorescence spectroscopy. Protein Sci. 1996;5:2226–2235. doi: 10.1002/pro.5560051109. [DOI] [PMC free article] [PubMed] [Google Scholar]