Abstract
Introduction
Oral submucous fibrosis (OSMF) is a chronic insidious disease mainly associated with fibroelastic change of the oral mucous membrane, leading to progressive trismus and oral burning sensation. The management of OSMF is empirical, depends on staging of the condition and is combination of conservative/medical/surgical interventions. Management of moderate OSMF is more challenging as conservative and medical treatments are not effective while surgical techniques involving fibrotomy and reconstruction of resultant defect are excessive. Lasers can provide an alternative and better means for surgical fibrotomy in moderate OSMF as they are minimally invasive and have the advantage of short operating time, less hemorrhage, faster healing, less morbidity, less surgical-site scarring and relapse. Laser fibrotomy in moderate OSMF have been done under general anesthesia.
Materials and Methods
A case series of 16 cases of moderate OSMF treated with Erbium Chromium Yttrium Scandium Gallium Garnet (ErCr:YSGG) laser fibrotomy under local anesthesia in combination with cessation of habits, topical steroids, lycopene and oral physiotherapy is presented.
Results
The mean increase in mouth opening achieved at 1 year was 17.5 mm. The mean difference in the preoperative and 1 year mouth opening was found to be statistically significant. The mean difference in the preoperative and six-month Visual Analogue Scale scores for oral burning sensation and Oral Health Impact Profile-14 scores for assessment of oral health-related quality of life was statistically significant implying improvement.
Conclusions
ErCr:YSGG laser fibrotomy under local anesthesia is a minimally invasive, cost effective, chair-side procedure and an useful adjunct in management of moderate OSMF.
Keywords: Oral submucous fibrosis, Trismus, Laser surgery, Burning mouth, Oral health-related quality of life
Introduction
Oral submucous fibrosis (OSMF) is a chronic debilitating disease of oral cavity, with multifactorial etiology but predominantly caused by areca/betel nut, a habit commonly practiced in South East Asia and India. A condition similar to OSMF has been reported in ancient Indian literature as “Vidari”. Joshi (1953) first used the term OSMF to describe this condition [1]. The prevalence varies according to ethnicity and geographical distribution and varies from 0.1 to 3.4 % [2–4]. It is also a potentially malignant condition and rate of malignant transformation over a period of 17 years has been reported as 7.6 % and the relative risk is as high as 397.3 [5]. It is characterized by progressive fibrosis of the lamina propria and deeper connective tissues and juxta epithelial inflammatory reaction of the oral mucosa, oropharynx and frequently upper third of the esophagus. OSMF is associated with a high rate of morbidity as the disease causes oral burning sensation, progressive inability to open the mouth resulting in difficulty in eating, swallowing, nutritional deficiency, poor oral hygiene and impaired ability to speak [6]. The functional limitations and oral pain and discomfort may also have psychological and behavioral impact on the patient although this has not been studied as yet.
It has been classified clinically into four groups depending on the severity of trismus and extent of fibrosis of oral mucosa (Table 1) [7]. The management is empirical, depends on the staging of the disease and mainly comprises of various combinations of conservative/medical or surgical interventions [8]. The treatment outcomes in OSMF have been mainly assessed with respect to improvement in trismus and oral burning sensation. There is no absolute cure for OSMF and all current interventions have been found to be of little benefit. Early and mild disease like Group I and II OSMF are mainly managed conservatively by cessation of habit, oral physiotherapy, anti-oxidant therapy, nutritional, vitamin and iron supplements along with topical corticosteroids [9, 10]. Moderate (Group III) OSMF, in addition to conservative therapy has been managed by adjuvant medical treatment like intralesional injections of corticosteroids, hyaluronidase or placentrex, systemic corticosteroids, immunomodulators and pentoxyfilline [11]. Conservative and medical management alone have been useful only in early and mild disease but the benefits derived in moderate and advanced disease are quite limited, achieved after prolonged treatment and associated with relapse. The long duration of the medical treatment also increases the expense, patient non-compliance and likelihood of adverse systemic effects [11]. Surgical management for trismus has been used in moderate and severe OSMF (Groups III and IV) with some success. Conventional surgical techniques aimed at excision of fibrous bands are associated with increased intra operative bleeding, which is difficult to manage in presence of trismus. Secondary healing seen after simple excision of fibrous band leads to further fibrosis, disability and relapse. Electro surgical techniques, though associated with reduced bleeding ends up in deep tissue damage, which in turn leads to increased post-operative fibrosis [12–15]. Aggressive surgical interventions are usually reserved for advanced disease like Group IV OSMF where a sufficient mouth opening can only be achieved by complete release of fibrotic bands and reconstruction of resultant defect with split thickness skin graft, buccal fat pad, micro-vascular free radial forearm flap, tongue flap, or naso-labial flap [16]. Sometimes these have to be supplemented with coronoidectomy and temporalis muscle myotomy. Currently the management of moderate OSMF (Groups II and III) is more challenging as they do not show much benefit with conservative and medical therapy and aggressive surgical approach is not warranted. Lasers can be used as an alternative technique for surgical fibrotomy in OSMF as they have many advantages over current techniques. They can provide a bloodless surgical area due to less hemorrhage, are minimally invasive and associated with less post-operative fibrosis in surgical area. Recently different types of lasers (Diode, KTP-532) have been used for surgical management of trismus especially in Group II, III and in some Group IV OSMF cases. They have used the lasers for fibrotomy only without reconstruction with local flaps with promising results [12–15]. Most of the studies with lasers in OSMF have been done under general anesthesia which not only increases the risk for the patient but is also time consuming, expensive and requires post-operative hospitalization for recovery. Erbium Chromium Yttrium Scandium Gallium Garnet ErCr:YSGG laser has many applications in dentistry but its use in surgical management of OSMF has not been studied in a case series [17–20].
Table 1.
Staging of oral submucous fibrosis [7]
| Staging | Criteria |
|---|---|
| Group I (early) | Inter incisal distance greater than 35 mm |
| Group II (mild) | Inter incisal distance of 26–35 mm |
| Group III (moderate) | Inter incisal distance of 15–25 mm. Fibrotic bands are visible at the soft palate, pterygomandibular raphe and anterior pillars of fauces |
| Group IV (advanced) IVA | Inter incisal distance of less than 15 mm with extensive fibrosis of mucosa all over |
| IVB | Premalignant and malignant changes throughout the mucosa (histologically) |
Khanna and Andrade [7] staging of OSMF
The treatment outcomes in OSMF have been mostly assessed with relief of trismus and oral burning sensation but psychosocial impact of disease on patient also needs to be assessed. Oral health impact profile-14 (OHIP-14) is a questionnaire containing 14 items that address oral functional limitations, oral pain and discomfort and psychological and behavioral impacts of oral conditions. It is short, simple, understood by the patient and easy to score. It can be therefore used as a tool to measure the oral health-related quality of life outcome in management of OSMF [21, 22].
A case series of 16 patients with moderate OSMF treated with ErCr:YSGG laser fibrotomy under local anesthesia in combination with cessation of habits, topical steroids, lycopene and oral physiotherapy is presented. The assessment of treatment outcomes with respect to mouth opening, oral burning sensation and oral health-related quality of life is also presented and the results are compared with the findings of other studies that have used lasers in the management of OSMF.
Materials and Methods
The study sample was derived from patients reporting in the outpatient department of our institute, who had a clinical and histological diagnosis of OSMF. The trismus in Group I OSMF is not very severe so an invasive procedure like laser fibrotomy is not required. The degree of trismus in Group IV OSMF is such that accessibility to surgical area for laser fibrotomy with ErCr: YSGG laser under local anesthesia is very difficult.
The inclusion criteria therefore selected were:
The patients had not undergone any previous medical or surgical treatment for OSMF.
They were categorized as belonging to Group II and Group III (moderate) OSMF according to Khanna and Andrade’s clinical classification
There was no evidence of moderate/severe dysplasia or malignancy on histopathological examination.
Sixteen patients were included in the study for duration of 6 months. Ethical clearance from our institution ethics committee and informed written consent was obtained from the patients before inclusion in the study. The history and clinical details including pre treatment mouth opening measurements, oral burning sensation and OHIP-14 scores were recorded in a prepared performa for each patient. The mouth opening was recorded as inter incisal distance using calipers and measurement scale. Burning sensation was assessed by using visual analogue scale (VAS) which was enumerated as a line marked from 0 (no burning sensation) to 10 (strongest burning sensation) and the patient was asked to assess his symptom and objectivize it as a number on the scale. Oral health-related quality of life was assessed using OHIP-14 questionnaire with a reference period of 6 months (Table 2). Routine hematological, and serological investigations were done at baseline (prior to incisional biopsies) and at 3rd and 6th month in all patients. Conservative therapy common to all patients included cessation of all oral habits, topical application of steroids, antioxidant (Cap Lycopene 8 mg in divided doses) and oral physiotherapy (Table 3). All the patients were enrolled in the tobacco cessation clinic at our institute before the commencement of treatment. Sources of chronic intraoral trauma like sharp teeth, calculus and appliances were also removed in all patients before treatment.
Table 2.
Oral health impact profile-14 questionnaire with scoring system [21]
| 1 | Trouble pronouncing words |
| 2 | Sense of taste worse |
| 3 | Painful aching in mouth |
| 4 | Uncomfortable to eat foods |
| 5 | Been self conscious |
| 6 | Felt tense |
| 7 | Difficult to relax |
| 8 | Been embarrassed |
| 9 | Felt life less satisfying |
| 10 | Diet been unsatisfactory |
| 11 | Had to interrupt meals |
| 12 | Been irritable with others |
| 13 | Difficulty doing usual jobs |
| 14 | Totally unable to function |
| Response format scoring All the time—5 Very often—4 Fairly often—3 Sometimes—2 Seldom—1 Never—0 | |
Table 3.
Treatment protocol
| Tab Amoxicillin with clavulanic acid 625 mg thrice daily, Tab Ibuprofen 400 mg thrice daily, Tab Paracetamol 500 mg four times daily (post-operatively for 5 days) |
| Chlorhexidine gel 0.2 % for topical application post-operatively on the intra oral wound four times daily for 1 month |
| Cessation of all oral habits, oral prophylaxis, oral hygiene instructions |
| Oral physiotherapy (cheek ballooning and mouth opening exercises) four times daily for 5 min. |
| Topical steroids (triamcinolone acetonide gel 0.1 % as topical application thrice daily) for 6 months |
| Antidel# oxidant (Cap Lycopene 4 mg two times daily) for 6 months |
Laser fibrotomy with ErCr:YSGG laser (Waterlase C100 Biolase) was done under local anesthesia at power of 1.5 W, water 10 % and air 13 % using a sapphire tip (G6, 600 μm in diameter, 6 mm in length) in non-contact mode. An inverted Y-shaped incision, with a depth of 2 mm was made with the two arms extending from the retromolar area to the premolar area on the buccal mucosa attempting to cut through all palpable fibrotic bands. The incisions were extended anteriorly up to the commissures in those cases where fibrotic bands were present anteriorly (Fig. 1). Finger palpation was used to break the fibrous bands and increased mouth opening was achieved with a mouth gag intra operatively. Only unilateral incisions were made in patients with unilateral disease while bilateral incisions were made in patients with bilateral disease. Intra operative mouth opening measurements were recorded. The laser fibrotomies was performed in all the patients by a single, trained, senior oral and maxillofacial surgeon. All the patients were followed every week in the first month during the healing phase after laser fibrotomy and then every month for 6 months. Oral physiotherapy and oral habit cessation counseling was reinforced at every follow-up visit. Mouth opening was measured intra-operatively, at day 15, 1, 2, 3, 4, 5, 6, 12 months, post-operatively (Fig. 2). The mean increase in the mouth opening was determined over a period of 1 year. Oral burning sensation was evaluated post-operatively at day 15, 1, 2, 3, 4, 5, 6, 12 months using VAS scale. Oral health-related quality of life was assessed at 6 months post-operatively using OHIP-14. The mean pre operative and post-operative mouth opening, VAS scores and OHIP-14 scores were analyzed using the paired t test for significance. The results were compared with other studies that have used lasers in the surgical management of OSMF. The patients are being reviewed every 3 months for surveillance against malignant transformation.
Fig. 1.
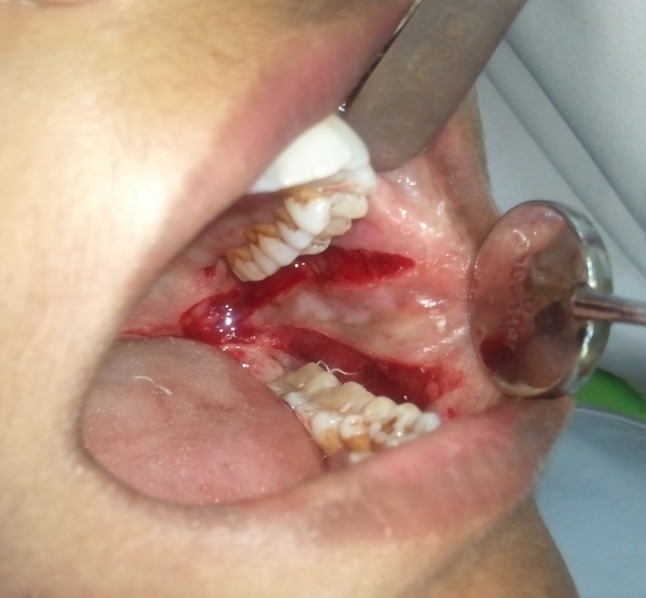
Inverted “Y” shaped incision with laser
Fig. 2.
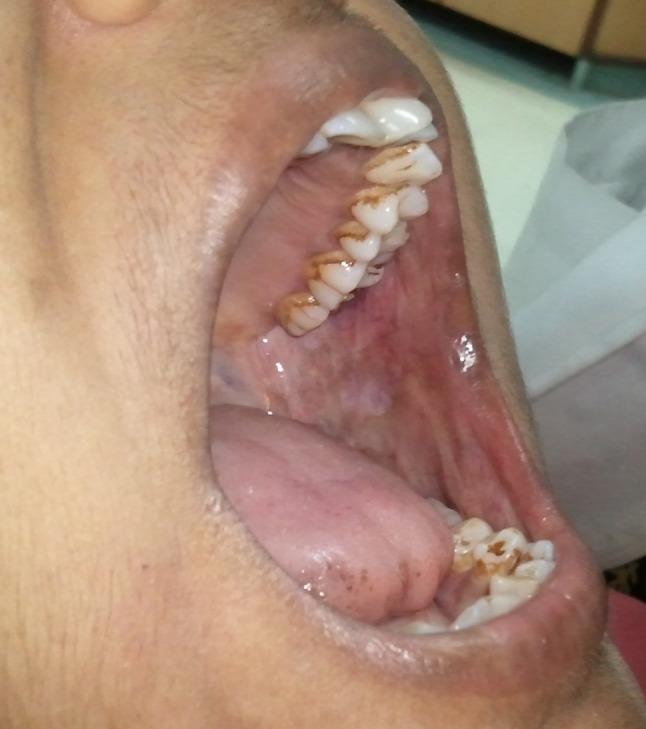
Increased mouth opening with minimal scarring in surgical site at 12 months (same patient as in Fig. 1)
Results
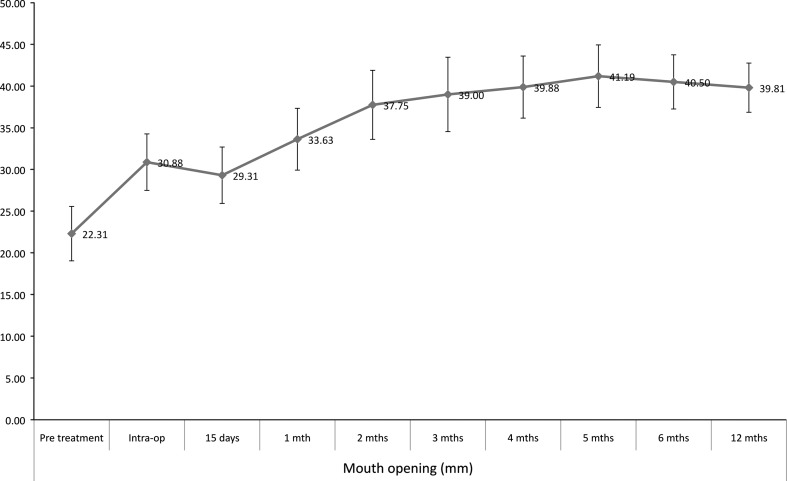
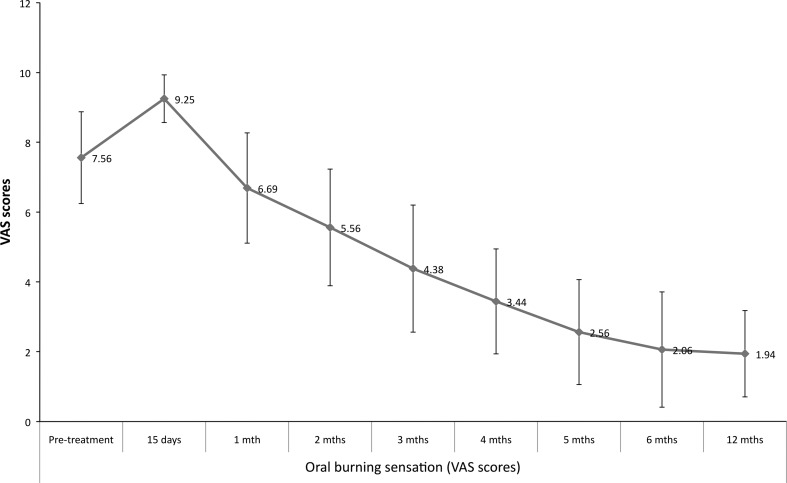
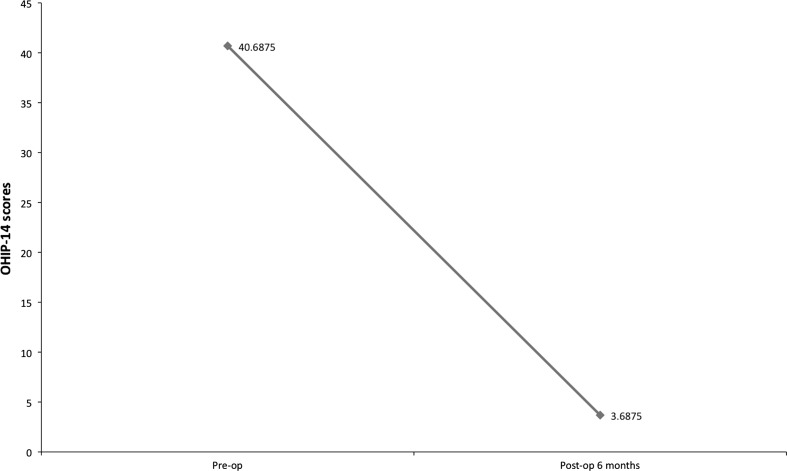
The age of the patients ranged from 20–46 years (mean 32.3 years) with a preponderance of males (male:female—5:3). Gutka habit (commercial preparation of betel nut) was most commonly seen in 87.5 % (14/16) of the cases. Bilateral involvement was most common, seen in 81.3 % of the cases. Fourteen cases were in Group III and 2 were in Group II as per OSMF classification. The mean mouth opening measurements over a period of 12 months is shown in Fig. 3. The mouth opening achieved intra operatively tends to decrease in the immediate post-operative period till the first month and then increases progressively. The increase in the mouth opening at 12-month follow-up was found to be statistically significant (paired t test, t = −18.724, p < 0.001). The oral burning sensation increased in the immediate post-operative period but improved after the first month (Fig. 4). Oral burning sensation improved from severe to mild during the 6 months follow-up and the decrease in the mean VAS scores was found to be statistically significant (paired t test, t = 12.298, p < 0.001). Decline in mean OHIP-14 scores over a period of 6 months showed improvement in oral health-related quality of life and this was found to be statistically significant (paired t test, t = 14.259, p < 0.001) (Fig. 5). Malignant transformation has not been noted in any of the study cases that are still under review.
Fig. 3.
Mean mouth opening measurements in mm over a period of 12 months
Fig. 4.
Mean VAS score for oral burning sensation over a period of 12 months
Fig. 5.
Mean OHIP-14 scores over 6 months interval
Laser fibrotomy was done as a chair-side procedure under local anesthesia with an average operating time of 20 min for each side. The size of the hand piece and surgical tips provided good access to the surgical site despite reduced mouth opening in these patients. Other post-operative complications following laser fibrotomy like hemorrhage, post-operative infection, wound dehiscence, nerve damage, collateral damage to adjacent tissues and teeth was not recorded in any case. The mouth opening achieved at 6 months was sustained at 12-month review in 50 % of cases while others relapsed by 1–2 mm.
The current study was compared to other studies that have used lasers in the surgical management of OSMF (Table 4) [12–15]. All the previous studies have used lasers in surgical management of OSMF (Group III mostly) under general anesthesia. None of the studies have assessed burning sensation or oral health related quality of life during the course of treatment. The results of our study were better than the results achieved by Shah et al. [12], Kameshwaran et al. [13] and Talsaniya et al. [14] with an average increase in mouth opening of 17.5 mm.
Table 4.
| Parameters | Shah et al. [12] | Kameshwaran et al. [13] | Talsania et al. [14] | Nayak et al. [15] | Our study 2012 |
|---|---|---|---|---|---|
| Number of cases | 10 | 15 | 08 | 09 | 16 |
| Age (average in years) | 27 | 29 | 27 | 35.8 | 32.3 |
| Male/female | NA | 15/0 | 8/0 | 9/0 | 10/6 |
| OSMF groupa | II, III | II, III | III, IVA | III | II, III |
| Type of laser | Diode | KTP 532 | Diode | KTP 532 | ErCr:YSGG |
| Type of anesthesia | General | General | General | General | Local |
| Type of incision | Inverted “Y” shape | “Z” shape | Linear | Multiple parallel | Inverted “Y” shape |
| Site of incision | Retromolar area, buccal mucosa | Pterygomandibular raphe, faucial pillars | Anterior faucial pillar, retromolar trigone, buccal mucosa | Anterior faucial pillar, retromolar trigone, soft palate, buccal mucosa | Retromolar area, buccal mucosa |
| Adjunctive treatment | Oral physiotherapy, | Oral physiotherapy, antioxidants, intralesional steroids | Oral physiotherapy, antioxidants | Oral physiotherapy, antioxidants | Oral physiotherapy, antioxidants, topical steroids |
| Average increase in mouth opening (mm) | 15 (Group II), 17 (Group III) | 12.6 | 15 | 23.7 | 17.5 |
| Follow up (months) | 3 | 12 | 18–36 | 12 | 12 |
OSMF oral submucous fibrosis
aKhanna and Andrade staging of OSMF [7]
Discussion
The etiological factors in OSMF other than betel/areca nuts are capsaicin, hypersensitivity, autoimmunity, genetic predisposition and chronic iron and vitamin B-complex deficiency. Various commercial preparations of mixture of areca nut and tobacco referred to as gutka, pan masala or mawa are increasingly being used by young people in India and are cause for increased incidence of OSMF. We also found gutka habit to be more common in our study sample. In OSMF the mucosa losses its pliability and becomes hard and rigid leading to progressive trismus. The most common sites are the buccal mucosa followed by palate, retromolar region, faucial pillars and pharynx. The fibrosis in OSMF is generally irreversible even after the cessation of the habits and removal of other etiologic factors. Various therapeutic agents used in OSMF are inadequate in improving trismus and decreasing fibrosis. With lasers, oral surgeons are provided with a new minimally invasive modality for surgical management of OSMF. When tissues interact with laser, the effect is influenced by the emission wavelength, tissue optical properties, time of exposure, energy used, and absorption of the laser energy into the tissues. The amount of laser energy needed to produce the desired results varies depending on the tissue involved, and also the wavelength used [23]. ErCr:YSGG Laser works on 2,780 μm wavelength, which has a high affinity for water and hydroxyapatite. The uniqueness of this system lies in the presence of air/water spray, which has dual role to assist in the cutting as well as serving as coolant to keep the surface temperature low, thus eliminating any detrimental thermal side effect. On soft tissue, the ErCr:YSGG laser works in a different manner, typically utilizing a very small amount of water and air as coolant. The cutting of the tissue is the result of direct laser energy seeking out the abundance of water molecules contained in the soft tissue. The photo-thermal action results in disruption of the tissue, using sufficient deposition of heat to vaporize the tissue. It has been shown that the ErCr:YSGG laser energy is selectively absorbed in the target tissue and may result in either a direct tissue cut (cold cut) or vaporization of the water within a cell causing rupture (thermal cut), a process known as thermal–mechanical tissue ablation. The thermal–mechanical tissue ablation limits the amount of collagen damage to as little as 5 μm (approximately 2 cell widths), leaving the extracellular collagen matrix unaffected. There is also reportedly less histamine release in tissues treated with laser, which accounts for the minimal post-operative pain and inflammation [17–19].
The advantages of ErCr:YSGG laser are that it can be used for fibrotomy in moderate OSMF as a chair side procedure under local anesthesia, due to short operating time, bloodless surgery, faster healing (minimal collateral/thermal damage), less morbidity (as it is minimally invasive 2 cell width thick), and spontaneous epithelialization of raw area [17]. Healing area remains in direct vision with no issues of donor site morbidity, graft failure or hospital stay hence site is available for repeat/secondary procedure. There is early restoration of function and relief from trismus as compared to conventional treatment. The only drawback is the high cost of the equipment and surgical tips.
The current study was compared to other studies that have used lasers in the surgical management of OSMF (Table 4) [12–15]. All the previous studies have used lasers in surgical management of OSMF (Group III mostly) under general anesthesia whereas we have used ErCr:YSGG laser in the same group of OSMF under local anesthesia. The type of the incision and site can affect the degree of mouth opening achieved by laser fibrotomy. Multiple parallel incisions used by Nayak et al. [15] achieved better results in relieving trismus. They also excised the fibrous bands in the soft palate in addition to those in the buccal mucosa, anterior faucial pillars and retromolar trigone, which may have contributed to the final outcome, although their procedures were done under general anesthesia [15]. We did not attempt to excise the fibrous band in the soft palate as our procedure was done under local anesthesia. It is difficult to comment if any particular type of laser is better in relieving trismus in OSMF for lack of comparative studies at present. The results of our study were better than the results achieved by Shah et al. [12], Kameshwaran et al. [13] and Talsaniya et al. [14] with an average increase in mouth opening of 17.5 mm. The mouth opening achieved was also comparable to results obtained by Sharma et al. [24] with conventional surgical fibrotomy along with reconstruction with buccal fat pad in group III OSMF. In our experience laser fibrotomy is a very good alternative to conventional surgical methods in OSMF and has a positive influence on treatment outcome with conservative and medical therapy.
Increase in oral burning sensation in the immediate post-operative period after laser fibrotomy was seen in our study. This worsening of symptoms is probably due to the open wound in the buccal mucosa, which heals, by secondary intention. The oral burning sensation improves once the wound undergoes epithelialization. There was a statistically significant decline in mean VAS scores and OHIP-14 scores after a period of 6 months. This aspect could not be compared with previous studies that have used lasers in management of OSMF as they have not assessed burning sensation or oral health related quality of life during the course of treatment.
The increased mouth opening obtained with laser fibrotomy will not be sustained without rigorous daily oral physiotherapy by the patient [12–15]. Patient counseling and motivation is very important to ensure patient compliance with regards to oral physiotherapy and cessation of habit. There were no patient dropouts in this study, which can be attributed to their frequent counseling sessions in the tobacco cessation clinic. Medical therapies like antioxidants, nutritional supplements and corticosteroids are essential adjuncts as they reduce the oxidative stress, improve nutritional status and are anti-inflammatory and immunosuppressive which plays a significant role in achieving treatment goals in OSMF [25]. Lycopene is a common red colored carotenoid found predominantly in tomatoes but also in other fruits and vegetables and is found to be beneficial in the management of oral premalignant lesions. Lycopene was selected as an antioxidant in this study as it is has been reported to be less toxic, improves oral burning sensation and improves the mouth opening by 1–4 mm in OSMF in therapeutic doses. It may also inhibit abnormal fibroblasts and has an anti-inflammatory response in OSMF [26–28]. The mean mouth opening at 6 months was found to be more than the mean mouth opening achieved intraoperatively which reflects the effect of oral physiotherapy and supportive therapy in the treatment outcome. Intraoral traumatic factors can cause chronic inflammation of oral mucosa and concentrate carcinogens, which on deeper penetration can lead to malignant transformation [29]. Long-term surveillance for malignant transformation in OSMF must include removal of intraoral traumatic factors.
ErCr:YSGG laser fibrotomy under local anesthesia is not feasible in Group IV due to limited accessibility to surgical site but is a viable option in Group II and III OSMF. Small sample size and lack of substantive monitoring methods for habit cessation and oral physiotherapy are limitations in this study. The increased mouth opening and thereby improved oral function is achieved almost immediately and is sustained over a long period of time. The amount of scarring is very less which is evident by the minimal relapse seen at one-year follow-up. There are no associated risks of general anesthesia or donor site morbidity for reconstruction. It is also less time consuming and cost effective as there is no extra financial burden of hospital admission, and post-operative care on the patient. This is of special significance as most of these patients in Indian scenario are from lower socio-economic strata and laser fibrotomy offers an affordable adjunctive treatment option for them. ErCr:YSGG laser fibrotomy is a minimally invasive, cost effective procedure under local anesthesia that can be done in a clinical setting on a dental chair or a minor operating room. Further comparative or randomized controlled clinical studies with larger sample size are required to substantiate our findings.
Conflict of interest
None.
Contributor Information
Zainab Chaudhry, Phone: +91-124-4376057, Phone: +91-9654700956, FAX: +091-112-3217081.
Shalini R. Gupta, Email: shalinigupta@hotmail.com
References
- 1.Joshi SG (1953) Fibrosis of the palate and pillars. Indian J Otolaryngol 4:1
- 2.Rajendran R. Oral submucous fibrosis: etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–996. [PMC free article] [PubMed] [Google Scholar]
- 3.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–152. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 4.Bathi RJ, Parveen S, Burde K. The role of gutka chewing in oral submucous fibrosis: a case-control study. Quintessence Int. 2009;40:e19–e25. [PubMed] [Google Scholar]
- 5.Murti PR, Bhonsle RB, Pindborg JJ, Daftary DK, Gupta PC, Mehta FS. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol. 1985;13:340–341. doi: 10.1111/j.1600-0528.1985.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 6.Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: revisited. Oral Maxillofac Surg. 2011;15:1–9. doi: 10.1007/s10006-010-0219-8. [DOI] [PubMed] [Google Scholar]
- 7.Khanna JN, Andrade NN. Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases. Int J Oral Maxillofac Surg. 1995;24:433–439. doi: 10.1016/S0901-5027(05)80473-4. [DOI] [PubMed] [Google Scholar]
- 8.Hasan S, Sherwani O, Ahmed S, Khan MA. Oral submucous fibrosis turning into malignancy: a case report and review of literature. J Orofac Sci. 2011;3:30–36. [Google Scholar]
- 9.Fedorowicz Z, Chan Shih-Yen E, Dorri M, Nasser M, Newton T, Shi L. Interventions for the management of oral submucous fibrosis. Cochrane Database Syst Rev. 2008;8:CD007156. doi: 10.1002/14651858.CD007156.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Angadi PV. Little evidence that current interventions can benefit patients with OSMF. Evid Based Dent. 2011;12:43. doi: 10.1038/sj.ebd.6400789. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Niranjan HS, Mehrotra R, Sharma D, Gupta SC. Efficacy of hydrocortisone acetate/hyaluronidase vs triamcinolone acetonide/hyaluronidase in the treatment of oral submucous fibrosis. Indian J Med Res. 2010;131:665–669. [PubMed] [Google Scholar]
- 12.Shah A, Raj S, Rasaniya V, Patel S, Vakade M. Surgical management of oral submucous fibrosis with the “Opus-5” Diode laser. J Oral Laser Appl. 2005;5:37–43. [Google Scholar]
- 13.Kameshwaran M, Raghavan D, Kumar ARS, Murali S. Surgical management of trismus due to oral submucous fibrosis—Lysis of fibrotic bands with the KTP-532 laser. Indian J Otolaryngol Head Neck Surg. 2006;58:229–231. doi: 10.1007/BF03050825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talsania JR, Shah UB, Shah AI, Singh NK. Use of diode laser in oral submucous fibrosis with trismus: prospective clinical study. Indian J Otolaryngol Head Neck Surg. 2009;61(Suppl 1):22–25. doi: 10.1007/s12070-009-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak DR, Mahesh SG, Aggarwal D, Pavithran P, Pujary K, Pillai S. Role of KTP-532 laser in management of oral submucous fibrosis. J Laryngol Otol. 2009;123:418–421. doi: 10.1017/S0022215108003642. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra D, Pradhan R, Gupta S. Retrospective comparison of surgical treatment modalities in 100 patients with oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e1–e10. doi: 10.1016/j.tripleo.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Ishizaki NT, Suzuki N, Kimura Y, Matsumoto K. Morphological changes of bovine mandibular bone irradiated by Er, Cr:YSGG laser: An in vitro study. J Clin Laser Med Surg. 2002;20:245–250. doi: 10.1089/10445470260420740. [DOI] [PubMed] [Google Scholar]
- 18.Eversole LR, Rizoiu IM. Preliminary investigations on the utility of an erbium, chromium YSGG laser. J Calif Dent Assoc. 1995;23:41–47. [PubMed] [Google Scholar]
- 19.Rizoiu IM, Eversole LR, Kimmel AI. Effects of an erbium, chromium yttrium, scandium, gallium, garnet laser on mucocutaneous soft tissues. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:386–395. doi: 10.1016/S1079-2104(96)80302-7. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary Z, Verma M, Tandon S. Treatment of oral submucous fibrosis with ErCr:YSGG laser. Indian J Dent Res. 2011;22:472–474. doi: 10.4103/0970-9290.87073. [DOI] [PubMed] [Google Scholar]
- 21.Slade GD. Derivation and validation of a short form oral health impact profile. Community Dent Oral Epidemiol. 1997;25:284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 22.Locker D, Matear D, Stephens M, Lawrence H, Payne B. Comparison of the GOHAI and OHIP-14 as measures of the oral health: related quality of life of the elderly. Community Dent Oral Epidemiol. 2001;29:373–381. doi: 10.1034/j.1600-0528.2001.290507.x. [DOI] [PubMed] [Google Scholar]
- 23.White JM, Chaudhry SI, Kudler JJ, Sekandari N, Schoelch ML, Silverman S., Jr Nd:YAG and CO2 laser therapy of oral mucosal lesions. J Clin Laser Med Surg. 1998;16:299–304. doi: 10.1089/clm.1998.16.299. [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, Thapliyal GK, Sinha R, Menon PS. Use of buccal fat pad for treatment of oral submucous fibrosis. J Oral Maxillofac Surg. 2012;70:228–232. doi: 10.1016/j.joms.2011.02.089. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Reddy MVR, Harinath BC. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J Clin Biochem. 2004;19:138–141. doi: 10.1007/BF02872409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:207–213. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Lu R, Dan H, Wu R, Meng W, Liu N, Jin X, Zhou M, Zeng X, Zhou G, Chen Q. Lycopene : features and potential significance in the oral cancer and precancerous lesions. J Oral Pathol Med. 2011;40:361–368. doi: 10.1111/j.1600-0714.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 28.Gowda BBK, Yathish TR, Sinhasan Sankappa P, Kumar Naik H, Somayaji P, Anand D. The response of oral submucous fibrosis to Lycopene—A carotenoid antioxidant: a clinicopathological study. J Clin Diagn Res. 2011;5:882–888. [Google Scholar]
- 29.Dayal RR, Anuradha Bhat K. Malignant potential of oral submucous fibrosis due to intaoral trauma. Indian J Med Sci. 2000;54:182–187. [PubMed] [Google Scholar]