Abstract
Background
Epiboly represents the process by which keratinocytes migrate to envelop a surface. We have been investigating a living bilayered skin construct (BSC) that is used in the treatment of lower extremity wounds due to venous insufficiency and diabetes. The construct demonstrates epiboly after injury and incubation in vitro, and this model may be useful for studying epidermal migration and the process of skin maturation. Punch biopsies of the construct in vitro were cultured and immunostained for specific keratins at baseline, and at 24–72 hours. For comparison, skin biopsy specimens from human chronic venous ulcers and acute healing wounds were similarly processed. We found that K1 and K10 were fully expressed in the epidermis of the fully epibolized surface on BSC. K1 was also present in the migrating edge of specimens, while K10 was not detectable. K16 and K6 were evident in normal skin and the epibolized area of the construct; K6 expression was very prominent in the migrating edge. Importantly, K17 was distinctly limited to the epibolized surface and the migrating edge, and its expression was very similar to that observed in healing human wounds. In conclusion, differential expression of keratins in this epiboly model closely reflects in vivo studies and supports keratin specificity in the processes of migration and differentiation of new epidermis. Therefore, these findings provide further and important validity for the study of epithelialization and the hope of developing prognostic markers for venous ulcer healing.
Keywords: epiboly, wound healing, keratin, leg ulcers, wound healing model, keratinocytes
BACKGROUND
Wound healing is recognized to occur through three stages: inflammation, tissue formation/cell proliferation, and remodeling.1 Reepithelialization usually begins through keratinocyte migration from stratified epithelia at the wounds’ edges, followed by further proliferation and differentiation of the keratinocytes.2 We have recently introduced the use of a living bilayered skin construct (BSC) as a model for studying keratinocyte migration in vitro. We have demonstrated that the epidermal layer of BSC injured and incubated in vitro migrates around the underlying dermis, a process known as epiboly. Epiboly can be considered a form of neoepidermis. Previous experiments have established the specificity of epiboly, which can be blocked by antibodies to certain cytokines and growth factors.3 We have proposed that the epiboly model may be helpful in understanding the roles of the various components involved in the process of epithelialization. In this study, for clinical purposes, we focused on the expression of certain keratins critical to epidermal migration and proliferation. Several studies have examined the expression of various keratin intermediate filaments in the process of keratinocyte migration during in vivo wound healing.4–7 Demonstrating a parallel pattern of keratin expression in wound healing model systems serves to strengthen their relevance to the actual wound healing process and increases a model’s validity for addressing mechanistic questions. Among other mechanistic studies and techniques, such studies have been employed to test various recent models of wound healing8, 9 Similarly, if the epiboly process in BSC demonstrates a similar pattern of keratin expression as the in vivo situation, then it, too, can be further qualified as a valid model.
Keratins are intermediate filament proteins found in epithelial cells. They are classified into type I and type II keratins, depending on their genomic structure and nucleotide sequence homology.10 Type I keratins, labeled K 9–20, are acidic and have low molecular weight, while type II keratins, labeled K 1–8, are basic and have high molecular weight. Keratins appear as heterodimers in a pattern that is tissue and differentiation-specific.11 Proliferating progenitor cells in the basal layer of the epithelium demonstrate K5 and K14 expression. As proliferation slows and differentiation increases in epidermal cells migrating upward into the suprabasal layers, K1 and K10 are more prominently expressed.6 Thus, K1 and K10 are considered to be markers of terminal differentiation.
Table 1 is provided as a summary of keratin expression, displaying if and in which layers specific keratins are present in normal skin, epiboly, and the migrating edge. Upon injury, keratinocytes are activated through an orchestrated response by a number of growth factors and other signal molecules. These events, in turn, lead to activation of transduction pathways that ultimately alter gene expression in the epidermal cells at the wound’s edges. K6, K16, and K17 are some of the keratins that may be expressed in the activated (i.e., proliferation, migration, etc.) epidermis.12 While there is an induction of these particular keratins, there is a concomitant downregulation of K1 and K10.10 K16 promotes a reorganization of the keratin filaments in the keratinocytes at the wound’s edges, inducing a cytoarchitectural change that prepares the cells for migration.6 K6 and K16 expression is not limited to post-injury events and wound healing, but has also been correlated with other physiological and pathological settings characterized by cell division, such as hyperproliferative cutaneous diseases including psoriasis or squamous cell carcinoma.10 The results presented here will add to the features of keratin expression shown in Table 1, particularly to the role of K17 in epidermal migration.
Table 1.
Summary of Keratin Immunostaining/Expression within the Epidermis in Different Settings Associated with the Physiological Events of Epiboly and Wound Healing
| Keratin Type |
Biological Event or Feature |
Normal Skin Epidermal Localization |
Epidermal Expression During Epiboly |
Epidermal Expression at the Migrating Edge |
|---|---|---|---|---|
| K1 | Differentiation | Immediate suprabasal | Immediate suprabasal | Absent in basal cell layer |
| K10 | Differentiation | High suprabasal | High suprabasal | Absent in all layers |
| K16 | Proliferation | Immediate suprabasal | Immediate suprabasal | Immediate suprabasal location |
| K6 | Proliferation | Immediate suprabasal | Immediate suprabasal | Present in all epidermal layers |
| K17 | Tumors (BCC not SCC) | Generally absent | Markedly present | Present in all epidermal layers |
A previous study demonstrated the role of K17 in promoting contractility in the keratinocyte. Evidence for this finding lies in the detection of K17 in normal transitional and pseudostratified epithelia, such as in the urinary and respiratory tracts.12 Other studies have reported that K17 knockout mice embryos have delayed wound closure, and that K17 null keratinocytes have impaired protein synthesis and cell growth.5, 13 These findings further support the integral role K17 plays in the process of reepithelialization.
Human wound healing studies of biopsies from acute wounds at various stages of healing have demonstrated the differentiation-specific expression of keratins. One such study noted K5 and K14 in the basal cells of the normal epithelium and suprabasally in the wound’s edges. K10 was only expressed in the suprabasal layer of intact epidermis. Proliferative markers such as K16 were demonstrated in the upper suprabasal cells in newly developed epidermis; K17 was similarly distributed, although at higher levels and with more intense levels of immunostaining.4 Another study of in vivo wound healing strengthened these observations, such as K14 basal and K10 suprabasal immunostaining in normal epidermis. In this report, we demonstrate the dynamic expression of keratins in our epiboly model of an organotypic culture model, and provide useful validity and correlations with normal skin and venous ulcers wound edges. The results may also be applicable for investigating wound healing.
MATERIAL AND METHODS
Bioengineered skin and culture conditions
The living bilayered skin construct (BSC) used in these studies is a readily available bioengineered skin substitute (Organogenesis, Inc., Canton, MA). It was chosen because of the following: 1) it is approved by the FDA; 2) because of FDA regulation the BSC requires full quality control; 3) the BSC has been shown by our group to have specific biological and molecular properties, including epiboly in vitro. It should be noted that our clinical and research group has no conflict of interest with this construct and none of the authors has been or is a consultant. The BSC consists of an epidermal layer of keratinocytes over a dermal layer of fibroblasts. Both of these cell types are from neonatal foreskin.14 We have previously shown this skin construct has the ability to “heal’ itself after injury, and produces growth factors and cytokines in a profile that is very similar to what is observed during wound healing in vivo.15 The BSC sits on a nutrient agar plate separated by a porous nylon membrane and is stored at room temperature for up to ten days. In this study, the BSC was used within the first three days of obtaining it. Punch biopsies (6-mm) were taken from the BSC construct, and careful efforts were taken to ensure that the nylon membrane would stay behind while keeping the bilayered specimen intact. Specimens were immediately transferred to 6-well tissue culture plates, so that there were 2–3 specimens floating in 3 ml of serum-free medium per plate (Dulbecco’s Modified Eagle’s medium (DMEM), Gibco, Gaithersburg, MD).3 The BSC samples were incubated at 37°C and 5% CO2. Histological analysis was performed at baseline, 24, 48 and 72 hours; basic formalin fixation and histological processing were used.
Human wound biopsies
We obtained approval from the Institutional Review Board at our facility for studying keratin expression in acute and chronic wounds in human subjects. Three patients with chronic venous leg ulcers, unresponsive to treatment for more than a year, were selected for this study. On day one, two biopsies were taken; one of the wound edge of the ulcer and another of normal ipsilateral thigh skin, thus creating an acute wound on the thigh. On day three, a biopsy was taken of the edge of the healing acute thigh wound in the same patients. Specimens underwent identical processing to the BSC biopsies as described below. This protocol has been previously described.16, 17
Immunohistochemistry
Buffered formalin (10%) was used to fix the specimens (construct, normal skin, and venous ulcer edges) for 24 hours. Specimens were then routinely processed for paraffin embedding and cut to 4 µm sections on superfrost plus slides. Specimens were deparaffinized and then blocked for endogenous peroxide. For K1, K6, K16, and K17, antigen retrieval was done using 0.01M citrate buffer (pH 6.0), and monoclonal antibodies to these keratins were diluted 1:30 with antibody diluent (DAKO; Carpinteria, CA). For K10, Proteinase K was used in antigen retrieval and a 1:50 dilution of K10 monoclonal antibody was added. Incubation with respective primary antibodies was carried out for one hour at room temperature. All antibodies were obtained from Novocastra, Vision Biosystems (Norwell, MA). Washes were done with 1× PBS solution with 4% normal horse serum. The secondary antibody for all specimens was a biotinylated horse anti-mouse IgG (Vector, Burlingame, CA), diluted 1:200, and incubated for one hour at room temperature. Streptavidin/HRP (DAKO) was diluted 1:400 and incubated for 40 minutes at room temperature. Slides were stained with chromogen (DAKO AEC+) and counterstained with Mayer’s hematoxylin (DAKO) before mounting with DAKO ultramount.
RESULTS
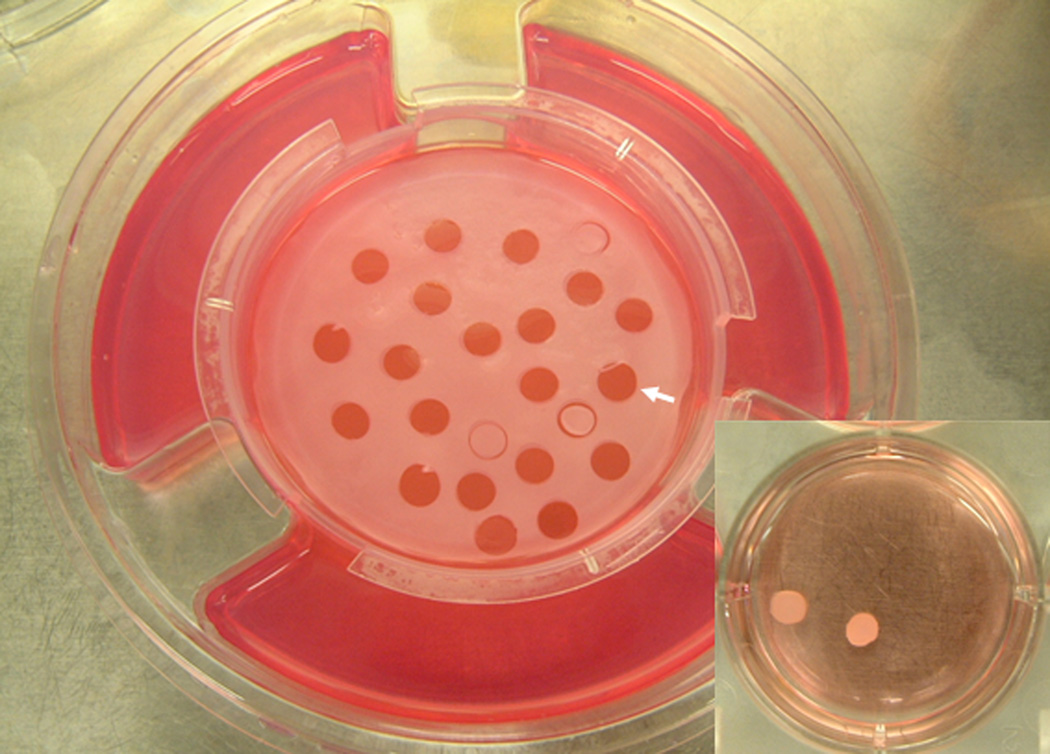
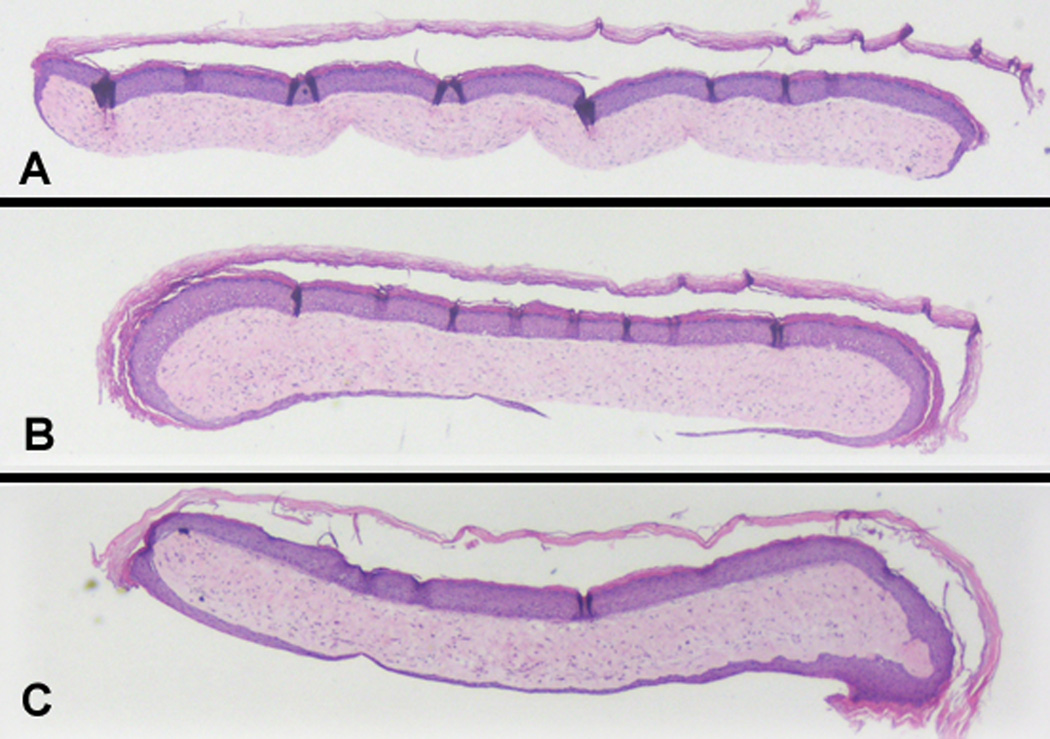
Figure 1 shows the bilayered skin construct (BSC) on its standard nutrient agar and the methodology used for obtaining samples from it. Punch biopsies (6-mm) were made into the construct, being careful to separate these punch biopsy samples from the underlying nylon membrane that divides the construct from the agar. These punch biopsy specimens were then removed from the rest of the construct and, as shown in the inset on the bottom right, placed in tissue culture plates containing serum-free media (DMEM). Figure 2 shows a representative example of the fully epibolizing construct occurring in a time-dependent extent after a specimen has been incubated in DMEM for up to 72 hours. Figure 2 illustrates a definite neoepidermis, developed through the process of epiboly, which has migrated to envelop the dermal side of the construct.3
Fig. 1. Harvesting of punch biopsy samples from the bilayered living skin construct (BSC).
The solid arrow points to the site where a punch biopsy has been removed from the BSC, which is still in place on its nutrient agar. Other punch biopsies have been made but the samples have not yet been lifted. Inset: punch biopsy samples that have been harvested and placed in serum-free media.
Fig. 2. Sequence of time-dependent epiboly in the bilayered skin construct (BSC) in vitro.
By 72 hours, the BSC has undergone fully epiboly, as evidenced by the migration of the original epidermis of the construct to completely envelop the BSC dermal component. The very margins of the epibolized (inferior) epidermal component show early evidence of acanthosis and stratum corneum formation, while the middle has not yet differentiated and has not yet formed a stratum corneum. 20×.
Figure 3 is provided as a positive control, and shows the immunostaining of K1 (Figure 3A) and K10 (Figure 3B) in the suprabasal layers of normal epidermis. K10 immunostaining is more apparent than that of its keratin partner, suggesting that K10 is more prominently expressed in terminally differentiated keratinocytes. Figure 3C shows K17 immunostaining markedly present in basal cell carcinoma, which provides evidence of its association with abnormal hyperproliferative epithelium. Previous studies have found that K17 is consistently present in these tumors.18
Fig. 3. Control immunostaining for K1, K10 and K17 in normal skin and basal cell carcinoma.
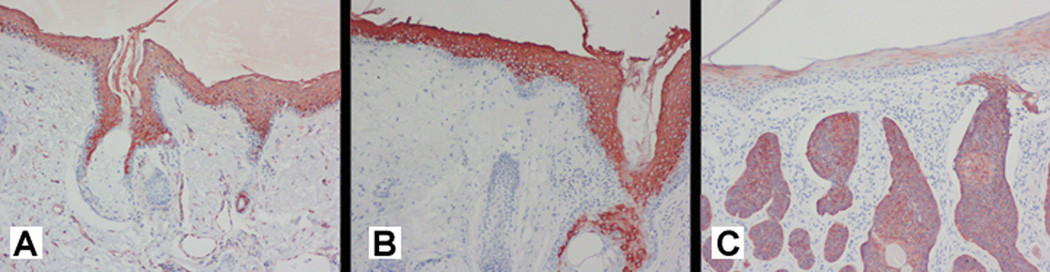
Intense immunostaining for (A) K1 and (B) K10 is present in the suprabasal keratinocytes of normal control skin (no immunostaining is detectable in the basal cell layer. 20×. (C) The dermal tumor aggregates of basal cell carcinoma show intense immunostaining for K17, while faint suprabasilar immunostaining is present in the overlying epidermis. 20×.
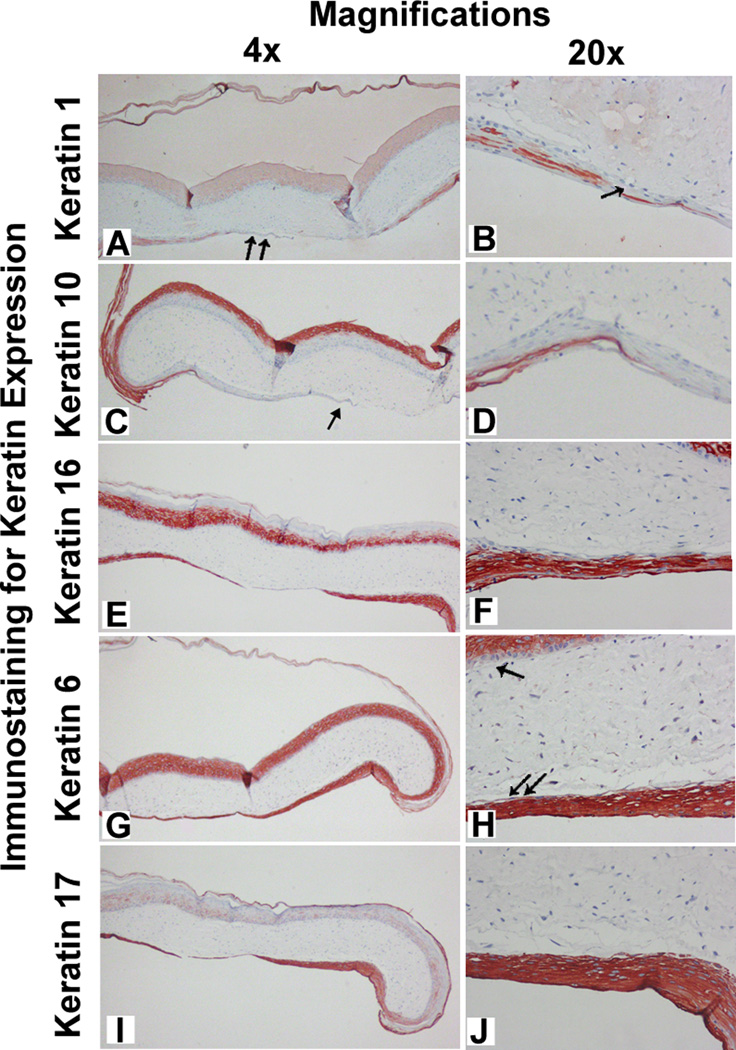
Figure 4 represents the immunostaining in BSC for five different keratins at 72 hours during the process of epiboly. Each row shows immunostaining for one specific keratin at two levels of microscopic magnification (4× and 10×, respectively). Immunostaining of epiboly at 72 hours showed differential keratin expression. K1 was detected in the suprabasal cell layer both in the epibolized surface (double arrow in Figure 4A) and its migrating edge (single arrow in Figure 4B). As in normal control skin, K10 may be a much more prominent marker of cell differentiation, as its immunostaining at 72 hours during epiboly is greater than that observed with its K1 partner. Interestingly, K10 detection during epiboly is localized in the high suprabasal layers (Figure 4D) but is distinctly absent in the migrating edge (single arrow in Figure 4C). K16 is expressed in the suprabasal layer of the epibolized surface as well as at the migrating edge. However, K6 gas a differential localization, in that is expressed only suprabasally in the original epidermis of the construct (single arrow in Figure 4H), but is present in all the layers of the migrating edge (double arrow in Figure 4H). Importantly, K17 immunostaining selectively highlights the epibolized epidermis and, therefore, may serve as a marker for epiboly. This latter finding may important in future analysis of and prognosis for chronic wounds.
Fig. 4. Immunostaining during epiboly of BSC for differential keratin expression in vitro.
The BSC has been incubated for 72 hours. The differential expression of keratins in rows (keratin type) and the two columns (4× and 20× magnification, respectively). The epiboly process is shown inferiorly in all sections. (Panel A) Completion of epiboly (double arrow) immunostained with K1 antibodies; (Panel B) Diminished or absent K1 expression in the basal cell layer of the migrating edge (single arrow); (Panel C) Complete apparent absence of K10 expression in the migrating epidermal edge (single arrow); (Panel D) Diminished K10 expression in the basal layer of the epibolized (inferior) surface; (Panels E and F) K16 is expressed throughout the epidermis of BSC and its epibolozied surface and migrating tongue; (Panel G) K6 is pecularly localized in the suprabasilar layers of the starting (superior, single arrow) epidermis of the construct, but is expressed throughout (Panel H, double arrow) in the migrating edge, including the basal cells; (Panels I and J). There is very marked localization of K17 restricted to the migrating edge of the construct (inferior portion of Panel I and thick epiboly migrating edge of panel J).
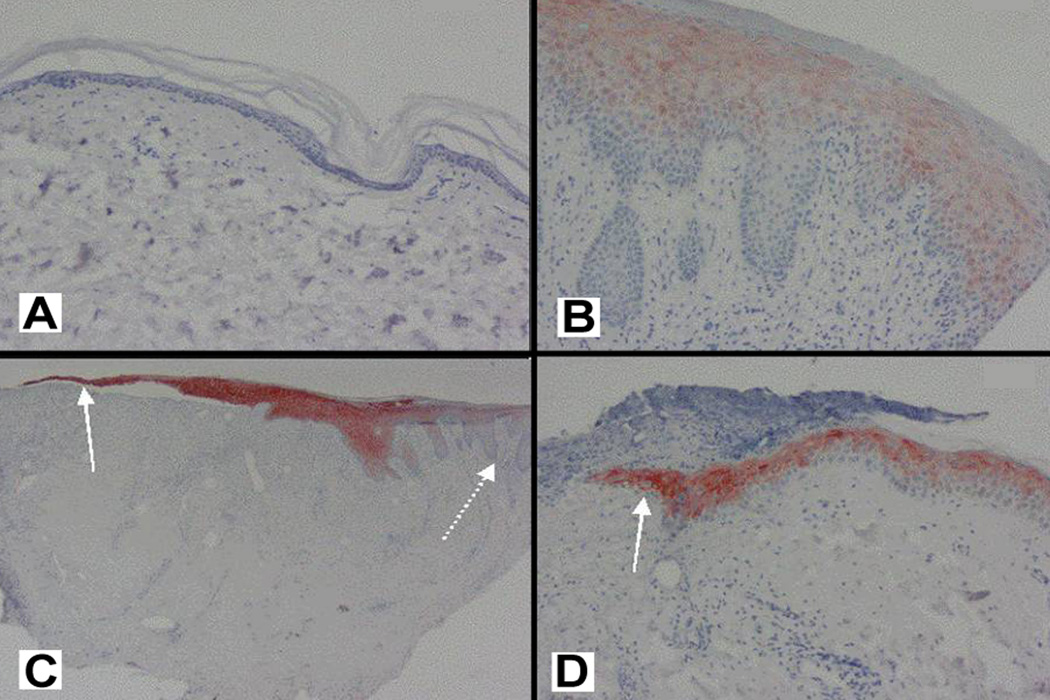
Figure 5 illustrates representative findings of K17 expression in both acute and chronic non-healing human venous ulcers. K17 is completely undetectable in normal skin (Figure 5A), and is restricted in a diffuse pattern to the suprabasal layer of the chronic wound edge (Figure 5B). These finding are in sharp contrast to the marked immunostaining of K17 in the thin basal layer of the migrating edge in both a chronic (Figure 5C) and acute wound (Figure 5D). However, once epithelialization has occurred, the new epidermis over the healed acute wound demonstrates K17 immunostaining suprabasally. This finding suggests that mature keratinocytes which have migrated and repopulated the wound bed may no longer be required for further migration, and are therefore able to differentiate and move suprabasally.
Fig. 5. Representative K17 immunostaining in human acute and chronic wounds.
(A) K17 is absent in normal epidermis at baseline, before acute injury. 20×. (B) K17 is diffusely detectable in the chronic wound edge and restricted to the suprabasal layers. 20×. (C) The migrating edge of the chronic wound exhibits strong K17 immunostaining (solid arrow), while the acanthotic margin exhibits decreased K17 expression (dashed arrow). 10×. (D) Marked K17 immunostaining is clearly observed in the migrating edge (solid arrow) of the acute wound, which in this case is migrating under the overlying cellular-filled crust of the wound
DISCUSSION
In this study, we characterized that keratin immunostaining in an epiboly model of a living bilayered skin construct (BSC) mirrors closely the expression and localization seen in vivo with human wounds, both acute or secondary to venous insufficiency. The construct displayed a pattern of differential keratin pattern in the newly epibolized skin as well as the migrating edge. These findings were consistent with the understanding of keratin specificity with regard to differentiation and migration in vivo. Of particular notice were the following immunostaining results: 1) high K1 and K10 levels as well as K16 and K17, in the fully epibolized surface of BSC; 2) K1 but not K10 evidence in the migrating edge of BSC and wounds; 3) K6 levels high in the migrating edge; 4) distinct K17 prominence in the migrating edge of human wounds and BSC, and restricted to epibolized surfaces. Taken together, our findings strongly indicate the validity of this epiboly model for studying keratinocyte differentiation and migration. It may lead to specific prognostic factors in predicting healing of human wounds.
Keratin levels by immunostaining do not by themselves provide the entire characterization and correlation between BSC and human wounds; actual localization and suprabasilar expression are also highly informative. Thus, although K1 and K10 are molecular dimeric partners, they differ in the level and localization of suprabasal expression. K1 is found in the immediate suprabasal cell layer and is only faintly expressed in normal skin and the epibolized surface. However, K10 is limited to the high suprabasal cell layers and is much more dramatically expressed. If we equate a greater level of differentiation to further distance from the basal keratinocytes (as an hypothesis), then K10 could be a more specific marker for epidermal differentiation. This hypothesis is further supported by the absence of K10 immunostaining in all the epidermal layers of the migrating edge, while K10 is clearly detectable in the newly epibolized epidermis. Therefore, K10 may not be expressed until the migrating edge has further differentiated itself. In contrast, K1 is apparent in the advancing epidermal cells at the edge.
Consistent with the present findings, studies in human cutaneous wounds have shown that K1 and K10 exhibit reduced immunostaining at the edges of both acute and chronic wounds.2, 10 More recent studies also demonstrated that, while still detectable at the wound edge, K10 is absent or diminished in the keratinocytes that have just migrated over the wound center.4 The reduced histological levels of K1 and K10 provide further support for the validity of the BSC epiboly as a model for epidermal migration during wound healing. K1 and K10 immunostaining were largely absent in rapidly dividing cells at the epiboly migrating edge.
After acute injury, there is an upregulation of specific keratins at the wound edge, including K6, K16, and K17.10 These keratins have also been found upregulated at the edge of chronic venous ulcers.19 The readily detectable levels of these keratins, especially K16, has been shown to induce cytoarchitectural changes in the keratinocyte.6 These cytoarchitectural changes correspond most likely to an activated state that prepares the keratinocyte for migration and reepithelialization.12 The epiboly model shown in the present study supports this reported expression pattern of K6 and K16 in the migrating edge. We found that K16 was only expressed in the suprabasal layers, in agreement with previous in vivo studies.4 Similarly, we found K6 expressed throughout all the layers of the migrating edge, but not in the basal layer, once the migrating edge has established a newly epibolized epidermis. This observation suggests that keratinocytes displaying K6 are differentiating and moving upward in the new epidermis after migration is complete.
It is important to note that K6 expression is not clearly essential to the state of keratinocyte proliferation. Studies in knockout mice using K6 null embryos do not exhibit delayed wound healing, although increased skin fragility in these animals was noted after wounding.5, 20 In addition, while K6 and K16 enhanced immunostaining has been associated with squamous cell carcinoma (SCC), blocking the expression of these two keratins does not reduce the in vitro proliferation of neoplastic cells within SCC.21 Thus, there is no absolute proof that the expression of specific keratins plays a causative role in proliferation. We can only cautiously infer that K6 and K16 expression in areas such as the migrating edge and epiboly may be associated with hyperproliferative conditions. This hypothesis is strengthened by transgenic mouse studies demonstrating that forced human K16 expression leads to normal proliferation but alters other keratinocyte processes such as adhesion, differentiation, and migration.22 More recent investigations also support a possible role of K16 in keratinocyte migration by demonstrating that overexpression of K16 delays wound closure;23 this may be a consequence of aberrant heterodimerization with K6, or possibly, K16 homodimerization.24
We found K17 is expressed in the migrating edge of human samples, from both acute and chronic wounds and in agreement with previous wound studies.4 K17 is markedly expressed in all the epidermal layers of epiboly and the migrating wound edge, while remaining largely absent in normal skin. The role of K17 in keratinocyte migration has been explored thoroughly. One study showed K17 confers the contractility necessary for keratinocyte migration.12 Therefore, blocking the migratory capability of keratinocytes would delay wound closure. This hypothesis is supported by the findings of delayed wound closure in K17 null mice embryos.5 A possible mechanism for the delayed wound closure is the previously unexpected role played by K17 in protein synthesis.13 K17 expression is not limited to regenerating tissue, but is also observed in abnormal hyperproliferative skin diseases such as epithelial cysts, skin tumors, psoriasis, and neoplasms.12, 25
We recently reported how various growth factors play a role in the epiboly process. Blocking antibodies to factors such as EGF, IL1 α, and vitronectin decreased the process of epiboly. These factors and mediators are similarly known to affect epithelialization, demonstrating the sensitivity and specificity of the epiboly model.3 In this study, we addressed how keratin expression changes throughout the processes of keratinocyte migration and differentiation. Our results are in agreement with expression patterns found in vivo, and further validate epiboly as a model for studying the process of wound healing.
Studies will continue to analyze the similarities between the morphology of neoepidermis grown from bioengineered skin compared to human skin. The repertoire of keratin expression, among other biomarkers, needs to be investigated for mechanistic and prognostic reasons. One will need to test the performance of bioengineered skin relative to normal human skin during reepithelialization.26 Newer modifications of living constructs using complete human components have also been validated for their proliferative and differentiatial behavior using keratin expression patterns.27
ACKNOWLEDGEMENTS
This work was supported by funds from the following National Institutes of Health Grants (V. Falanga as he Principal Investigator): NIH NIAMS R01 grant, Award #AR060342; NIH NCRR Center of Biomedical Research Excellence (COBRE), Award #P20RR018757; NIH NCRR COBRE Administrative and Imaging Cores, Award #P20RR018757.
REFERENCES
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999 Sep 2;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Kurokawa I, Mizutani H, Kusumoto K, Nishijima S, Tsujita-Kyutoku M, Shikata N, et al. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006 Jan-Feb;14(1):38–45. doi: 10.1111/j.1743-6109.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 3.Falanga V, Butmarc J, Cha J, Yufit T, Carson P. Migration of the epidermal over the dermal component (epiboly) in a bilayered bioengineered skin construct. Tissue Eng. 2007 Jan;13(1):21–28. doi: 10.1089/ten.2006.0148. [DOI] [PubMed] [Google Scholar]
- 4.Patel GK, Wilson CH, Harding KG, Finlay AY, Bowden PE. Numerous keratinocyte subtypes involved in wound re-epithelialization. J Invest Dermatol. 2006 Feb;126(2):497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- 5.Mazzalupo S, Wong P, Martin P, Coulombe PA. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn. 2003 Feb;226(2):356–365. doi: 10.1002/dvdy.10245. [DOI] [PubMed] [Google Scholar]
- 6.Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996 Feb;132(3):381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005 Sep-Oct;13(5):468–479. doi: 10.1111/j.1067-1927.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 8.Coolen NA, Vlig M, van den Bogaerdt AJ, Middelkoop E, Ulrich MM. Development of an in vitro burn wound model. Wound Repair Regen. 2008 Jul-Aug;16(4):559–567. doi: 10.1111/j.1524-475X.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 9.Geer DJ, Swartz DD, Andreadis ST. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng. 2004 Jul-Aug;10(7–8):1006–1017. doi: 10.1089/ten.2004.10.1006. [DOI] [PubMed] [Google Scholar]
- 10.Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997 Jul 18;236(2):231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- 11.O'Guin WM, Galvin S, Schermer A, Sun TT. Patterns of keratin expression define distinct pathways of epithelial development and differentiation. Curr Top Dev Biol. 1987;22:97–125. doi: 10.1016/s0070-2153(08)60100-3. [DOI] [PubMed] [Google Scholar]
- 12.Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001 May;116(5):633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006 May;441(18)(7091):362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 14.Falanga VJ. Tissue engineering in wound repair. Adv Skin Wound Care. 2000 May-Jun;13(2 Suppl):15–19. [PubMed] [Google Scholar]
- 15.Falanga V, Isaacs C, Paquette D, Downing G, Kouttab N, Butmarc J, et al. Wounding of bioengineered skin: cellular and molecular aspects after injury. J Invest Dermatol. 2002 Sep;119(3):653–660. doi: 10.1046/j.1523-1747.2002.01865.x. [DOI] [PubMed] [Google Scholar]
- 16.Butmarc J, Yufit T, Carson P, Falanga V. Human beta-defensin-2 expression is increased in chronic wounds. Wound Repair Regen. 2004 Jul-Aug;12(4):439–443. doi: 10.1111/j.1067-1927.2004.12405.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. J Cell Physiol. 2003 Jun;195(3):331–336. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 18.Proby CM, Churchill L, Purkis PE, Glover MT, Sexton CJ, Leigh IM. Keratin 17 expression as a marker for epithelial transformation in viral warts. Am J Pathol. 1993 Dec;143(6):1667–1678. [PMC free article] [PubMed] [Google Scholar]
- 19.Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008 Dec;12(6B):2675–2690. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003 Oct 27;163(2):327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopan R, Fuchs E. The use of retinoic acid to probe the relation between hyperproliferation-associated keratins and cell proliferation in normal and malignant epidermal cells. J Cell Biol. 1989 Jul;109(1):295–307. doi: 10.1083/jcb.109.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wawersik M, Coulombe PA. Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes. Mol Biol Cell. 2000 Oct;11(10):3315–3327. doi: 10.1091/mbc.11.10.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trost A, Desch P, Wally V, Haim M, Maier RH, Reitsamer HA, et al. Aberrant heterodimerization of keratin 16 with keratin 6A in HaCaT keratinocytes results in diminished cellular migration. Mech Ageing Dev. 2010 May;131(5):346–353. doi: 10.1016/j.mad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Trost A, Costa I, Jakab M, Ritter M, Haim M, Hintner H, et al. K16 is a further new candidate for homotypic intermediate filament protein interactions. Exp Dermatol. 2010 Aug;19(8):e241–e250. doi: 10.1111/j.1600-0625.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 25.Broekaert D, Goeman L, Ramaekers FC, Van Muijen GN, Eto H, Lane EB, et al. An investigation of cytokeratin expression in skin epithelial cysts and some uncommon types of cystic tumours using chain-specific antibodies. Arch Dermatol Res. 1990;282(6):383–391. doi: 10.1007/BF00372089. [DOI] [PubMed] [Google Scholar]
- 26.Mirastschijski U, Bugdahl R, Rollman O, Johansson BR, Agren MS. Epithelial regeneration from bioengineered skin explants in culture. Br J Dermatol. 2006 Jan;154(1):42–49. doi: 10.1111/j.1365-2133.2005.06997.x. [DOI] [PubMed] [Google Scholar]
- 27.El Ghalbzouri A, Commandeur S, Rietveld MH, Mulder AA, Willemze R. Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials. 2009 Jan;30(1):71–78. doi: 10.1016/j.biomaterials.2008.09.002. [DOI] [PubMed] [Google Scholar]