Summary
Background
Many organisms, from bacteria to human hunter-gatherers, use specialized random walk strategies to explore their environment. Such behaviors are an efficient stratagem for sampling the environment and usually consist of an alternation between straight runs and turns that redirect these runs. Drosophila larvae execute an exploratory routine of this kind that consists of sequences of straight crawls, pauses, turns, and redirected crawls. Central pattern generating networks underlying rhythmic movements are distributed along the anteroposterior axis of the nervous system. The way in which the operation of these networks is incorporated into extended behavioral routines such as substrate exploration has not yet been explored. In particular, the part played by the brain in dictating the sequence of movements required is unknown.
Results
We report the use of a genetic method to block synaptic activity acutely in the brain and subesophageal ganglia (SOG) of larvae during active exploratory behavior. We show that the brain and SOG are not required for the normal performance of an exploratory routine. Alternation between crawls and turns is an intrinsic property of the abdominal and/or thoracic networks. The brain modifies this autonomous routine during goal-directed movements such as those of chemotaxis. Nonetheless, light avoidance behavior can be mediated in the absence of brain activity solely by the sensorimotor system of the abdomen and thorax.
Conclusions
The sequence of movements for substrate exploration is an autonomous capacity of the thoracic and abdominal nervous system. The brain modulates this exploratory routine in response to environmental cues.
Introduction
In many organisms, the rhythmic movements of locomotion are incorporated into extended behavioral routines that facilitate the exploration of an environment. Often these exploratory routines constitute some form of random walk, in which straight line movement alternates with redirection and the acquisition of a new trajectory [1–6]. Behavioral sequences of this kind are an effective stratagem for the complete exploration of an environment for an available food source [7].
At hatching, Drosophila larvae execute a search routine of this kind [8, 9]. It consists of two characteristic components: the repeated wave-like contractions of the body wall, which allow the larvae to crawl over the substrate [10], and a pause followed by a unilateral backward contraction of anterior segments, which, on the resumption of forward crawling, redirects the larva on a new trajectory. We have set out to investigate the organization of the neural networks that underlie this exploratory behavior.
In vertebrates and invertebrates like Drosophila, central pattern-generating networks that underlie the performance of rhythmic movements such as chewing, walking, and flying are distributed along the anterior posterior axis of the nervous system coinciding with the arrangement of the muscles and effectors (such as mouthparts and limbs) on which they operate [11, 12]. Thus it is likely (although this has been disputed [13]) that the central pattern generators required for larval locomotion are confined to the thoracic and abdominal segments of the nervous system, which innervate the muscles required for crawling. However, the way in which the operation of these networks is incorporated into extended behavioral routines such as substrate exploration has not yet been explored. In particular, the part played by the brain in dictating the sequence of movements required is unknown.
Here we report the use of a genetic method that allows the activity of the brain and suboesophageal ganglia (SOG) to be manipulated acutely in living animals during an ongoing behavioral sequence. We show that the brain and anterior segments of the nerve cord are not required for the normal performance of an exploratory routine. Substrate exploration through a random walk is an autonomous capacity of the thoracic and abdominal nervous system. Furthermore, although the brain is required for properly oriented chemo-tactic movements, light avoidance behavior can be mediated in the absence of brain activity solely by the sensorimotor system of the abdomen and thorax.
Results
The Brain and Subesophageal Segments of the Nervous System Are Not Required for Peristaltic Locomotion
Drosophila larvae move over the substrate by peristaltic crawling. In forward movement, a wave of muscle contractions passes along the body segments from posterior to anterior (Figure 2A) [9, 10]. Larvae usually move forward but may briefly move backward in response to sensory input from the head. In backward movement, the wave of contractions is reversed and passes from anterior to posterior. It is likely that the neuronal circuits that orchestrate repeated waves of peristaltic contractions in crawling larvae are located in the thoracic and abdominal segments of the nervous system but the role of more anterior segments including the brain is less clear. It has been reported that the brain may be required either to trigger [13] or to maintain [14] the rhythmically repeated movements of larval crawling.
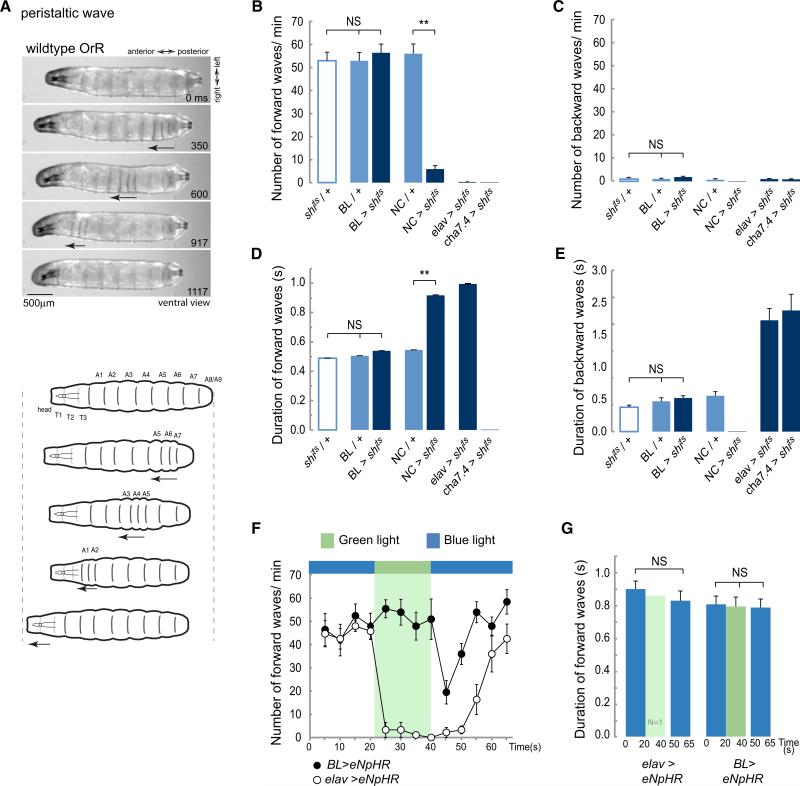
Figure 2. Inhibiting Synaptic Activity in the Brain Lobes and SOG Does Not Affect the Propagation of Peristaltic Contraction Waves.
(A) Description of a peristaltic wave. Video frames and diagrams showing a ventral view of a WT larva, OrR, at different stages during a peristaltic wave. The arrow highlights the segments contracting as the wave progress along the abdominal segments A8/9 to A1.
(B–E) Early third-instar larvae expressing shibirets were transferred to 36°C to block synaptic transmission and their behavior was evaluated. An average of 26 animals was tested for each one of the genotypes. (B) Average number of forward waves/min (±SEM). (C) Average number of backward waves/min (±SEM). (D) Average duration of forward waves in seconds (±SEM). (E) Average duration of backward waves (±SEM). See also Movies S1, S2, S3, and S4. (F and G) The peristaltic crawling of larvae expressing eNpHR evaluated under blue or green light. Green light selectively activates halorhodopsin and leads to membrane hyperpolarization. (F) Average number of forward waves/min (±SEM). (G) Average duration of forward waves (±SEM). A 10 s recovery phase was allowed before resuming the analysis at the end of the green light pulse. One elav > eNpHR animal executed a few forward waves under green light. It is probable that this animal was slightly bigger and that this affected the light penetration. Eight BL > eNpHR and 11 elav > eNpHR animals were evaluated. A one-way ANOVA with a Bonferroni post hoc test was performed to compare the treatments. **p < 0.01; NS means nonsignificant. See also Figure S1 and Movies S5 and S6.
BL refers to the driver directed to the brain lobes and SOG; NC, the driver directed to the nerve cord.
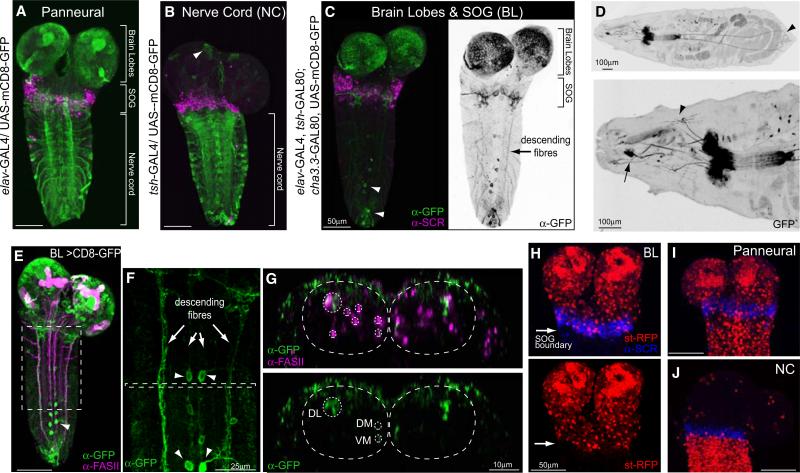
To resolve this question, we generated a line of flies (BL) with a combination of Gal4 drivers and repressors that targets expression specifically to the brain and suboesophageal ganglia as well as neurons whose axons descend posteriorly from these regions (Figure 1C). We used antibody staining to confirm that expression in the central nervous system (CNS) was confined to cells of the brain and SOG and that posteriorly it did not extend beyond the domain of Sex Combs Reduced (SCR) (Figures 1C and 1H). SCR is a Hox gene whose expression marks the labial segment of the CNS and hence defines the posterior boundary of the SOG [15]. No thoracic or abdominal sensory neurons are labeled (Figure 1D) and expression in descending axons is limited to three main pathways that correspond to the Fasciclin II-positive dorsolateral (DL), dorsome-dial (DM), and ventromedial (VM) tracts (Figures 1E–1G) [16]. For comparison, we used the teashirt Gal4 driver (tsh-Gal4), whose expression pattern is complementary, namely exclusively in the cells of the thoracic and abdominal nervous system (Figures 1B and 1J) [17]. We refer to this line as NC.
Figure 1. Driver Lines to Manipulate the Activity of Distinct Regions of the Nervous System.
(A–C) Expression patterns of the driver lines in the CNS of early third-instar larvae were assessed with UAS-mCD8-GFP and immunostaining with anti-GFP and anti-SCR antibodies. SCR labels the most posterior (labial) segment of the subesophageal ganglion. (A) The panneural driver line elav-Gal4. (B) The nerve cord (NC) driver line tsh-GAL4. (C) A driver line for the brain lobes, SOG, and descending neurons, called BL-Gal4, was generated by the combination of elav-Gal4 with two Gal80 repressor lines, one expressed in the NC, tsh-GAL80, and one in sensory neurons, cha3.3-GAL80. GFP expression is restricted to neurons located in the brain lobes and SOG. Descending fibers are labeled (arrow). A few scattered motorneurons in abdominal segments as well as a group of neurons in abdominal segment 9 (A9) are labeled (arrow head).
(D) GFP expression in a whole larva. Upper panel shows that most sensory neurons are excluded from the BL expression pattern. A group of neurons in A9 send processes to the anal plate (arrow head). Lower panel shows that a few anterior sensory neurons are seen in the BL expression pattern. These include a pair of multidendritic neurons (arrow head) and the Bolwig organs (arrow).
(E–G) Double staining with FasII to assess the positioning of the descending fibers. (E) Projection of whole CNS. (F) Inset from e showing descending fibers (arrows) and a few motoneurons (arrow heads). (G) Transverse view of inset from (F). The upper panel shows the FasII positive tracts, marked with a dashed line, as well as BL descending fibers. The lower panel shows that the BL neurons descend in the DL, DM, and VM tracts.
(H–J) Images showing boundaries of the driver lines. (H) Upper and lower panels show BL > st-RFP; RFP expression is confined to the brain lobes and SOG. Anti-SCR marks posterior boundary of expression. (I) elav > st-RFP. Expression levels in the brain were similar in the panneural and BL lines. (J) NC > st-RFP. Expression is confined to the region posterior to the SOG marked by anti-SCR. An average of 9 elav-Gal4, 18 BL-Gal4, and 9 NC-Gal4 animals was analyzed for each panel.
We used the BL and NC driver lines to express a dominant-negative temperature-sensitive form of Shibire, UAS-shits, in crawling early third-instar larvae. shibire encodes Dynamin, which is essential for the recycling of synaptic vesicles, and at the restrictive temperature (~36°C) the function of Shibirets is blocked leading, rapidly and reversibly, to almost complete vesicle depletion and arrested synaptic transmission [18–20]. We assayed larval behavior in an arena consisting of an agar-coated Peltier device that provided a uniform, homogeneous environment [21]. Larvae were free to crawl over this surface.
At the restrictive temperature, control larvae (carrying a single copy of either the transgene UAS-shits (shits/+) or the driver lines BL (BL/+) or NC (NC/+) crawled forward. Occasionally they would produce a backward wave (Figures 2B and 2C; see also Movie S1 available online). There was no difference in the speed of wave propagation (forward or backward) among any of the controls that we tested (Figures 2D and 2E).
Not surprisingly, when synaptic transmission was blocked throughout the thoracic and abdominal nervous system (NC > shits where > means -GAL4/UAS-) or in two positive controls where all neurons (elav > shits) (Figures 1A and 1I) or all cholinergic neurons (cha > shits) were targeted, the larvae were almost completely paralyzed (Figures 2B and 2C) with only occasional and highly aberrant muscle contractions (Figures 2D and 2E; Movies S2 and S3).
We now used the BL line to target the expression of shits specifically to the brain, SOG, and descending axons. When synaptic transmission was blocked at the restrictive temperature, these larvae continued to crawl actively, with forward waves of peristaltic contraction (with occasional backward waves) (Movie S4). When we analyzed these movements in detail, we found that there was no significant difference from any of the controls (shits/+ and BL/+) either in the timing of contractions or in the number of forward and backward waves (Figures 2B–2E). We conclude that normal patterns of peristaltic locomotion continue in the absence of synaptic transmission in the brain and SOG.
To confirm this finding, we used targeted expression of Halorhodopsin (UAS-eNpHR) to block neuronal activity in the brain and SOG of crawling larvae. When Halorhodopsin is activated by exposure to light of ≈580 nm, Cl− ions enter the cell, hyperpolarizing those cells that express it [22]. This has the advantage of blocking all transmission, chemical or electrical, thus silencing the brain and SOG (Figure S1).
To allow for any behavioral changes that might occur in response to the intense light (0.214 mW/mm2) necessary for Halorhodopsin activation, we evaluated crawling behavior under nonactivating blue light conditions at the same intensity before comparing it to behavior under activating green light conditions. Control larvae with panneural expression of Halorhodopsin (elav > eNpHR) immediately ceased to move on exposure to activating light and remained paralyzed (Movie S5). After a 10 s recovery phase under blue light, the larvae resumed crawling and after 20 s their peristaltic movements were indistinguishable from those before the activity block (Figures 2F and 2G). When the same protocol was applied to larvae carrying BL > eNpHR, the result was very different. Shifting the light from blue to green did not produce any change either in the number of waves (Figure 2F) or the timing of contractions during peristaltic waves (Figure 2G; Movie S6). On return to blue light there was an abrupt decrease in the frequency of contraction waves, probably as a result of rebound firing in BL neurons [23, 24] but after 10 s peristaltic crawling resumed with the same characteristics as before.
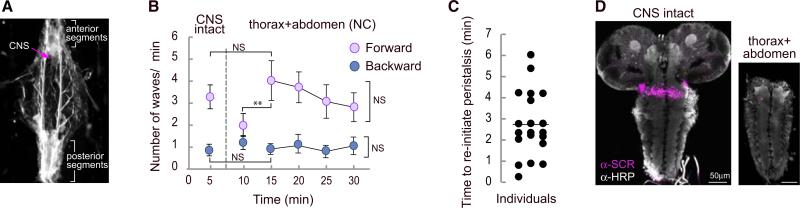
We supplemented these experiments by using a semi-intact preparation to investigate the effects of surgically removing the brain and SOG on forward and backward waves of peristaltic muscle contractions (Figure 3A). Partially dissected larvae with intact CNS perform forward and backward peristaltic contraction waves (Figure 3B). When the brain and SOG are severed from the thoracic and abdominal neuromeres (Figure 3D), peristaltic movement stops and is replaced by uncoordinated segment contractions that are probably caused by tonic activation from damaged neurons. This phase of uncoordinated movement persists for up to 6 min, but then coordinated contractions resume spontaneously (Figures 3B and 3C), once again indicating that activity in the brain, the SOG, and axons descending from these areas is not necessary to initiate or maintain the movements of peristaltic crawling. In all cases, the number of forward and backward peristaltic waves returned to a frequency similar to that of animals with an intact CNS and persisted for at least a further 15 min (Figure 3B). In our preparation and unlike those reported by others, even though the wave frequency decreased as a result of the dissection, it was not necessary to use drug application to initiate or maintain these rhythmic waves of contractions [14].
Figure 3. The Brain Lobes and SOG Are Not Required to Sustain and Initiate Peristaltic Waves.
(A) Larvae were pinned down loosely and filleted without damage to anterior and posterior segments. The CNS was exposed and the number of peristaltic waves was quantified by observing the muscle field.
(B) Average number of forward and backward peristaltic waves (±SEM) when the CNS was intact (first 5 min) and after the ablation of the brain and SOG (next 25 min).
(C) Time to reinitiate peristalsis after ablation of brain lobes and SOG. The transverse line represents the average time to resume peristalsis.
(D) Representative immunostaining against HRP and SCR to evaluate the precision of the surgery. The lack of SCR staining indicates that the SOG was removed. Twenty individuals were analyzed. A one-way ANOVA with Bonferroni post hoc test comparing the data for 5, 10, and 15 min was performed. An ANOVA for repeated measures was employed for 15 to 30 min. **p < 0.01.
Taking all of these findings together, we conclude that circuitry present in the thoracic and abdominal segments of the nervous system is sufficient to sustain well-coordinated movements of peristaltic crawling and to permit alternations between forward and backward waves of contraction like those seen in controls.
Turning Behavior Does Not Require the Brain and Subesophageal Segments of the Nervous System
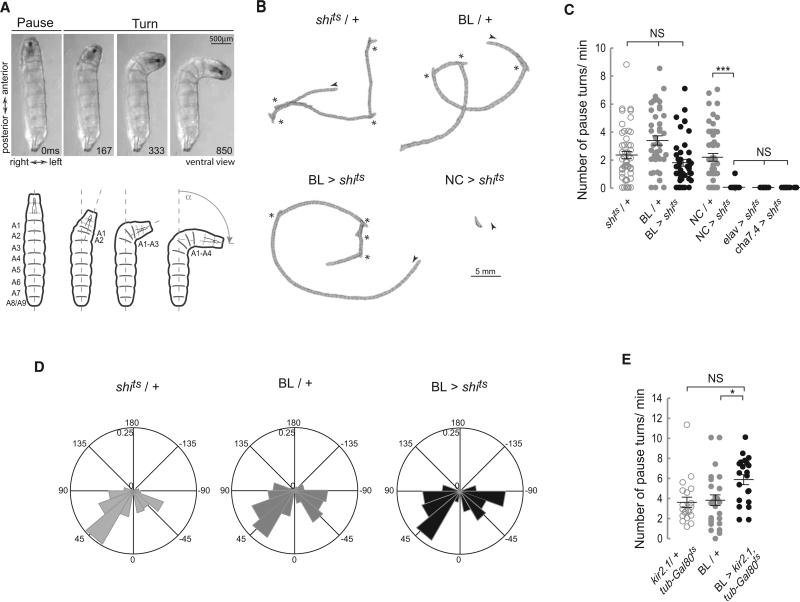
The exploratory behavior of wild-type (WT) larvae consists of straight crawls interspersed with turns followed by a redirected crawling trajectory. Turns are initiated by a pause at the end of a forward wave of peristaltic contractions followed by a unilateral and sequential backward contraction of the most anterior segments, which proceeds as far as abdominal segment 4 and has the effect of throwing the anterior of the animal into a curve (Figure 4A; Figure S2). At this point, if a forward wave is initiated posteriorly, then the crawl will proceed on the redirected trajectory. Alternatively, the unilateral contraction on one side may be followed by a further contraction on the opposite side, which swings the head in the opposite direction, and there may be a series of such swings before the larva resumes its crawl in a new direction. These pause turn events have been described as decision-making points in which larvae explore olfactory or temperature gradients and select a new direction for movement [25, 26].
Figure 4. Blocking Synaptic Transmission in the BL Does Not Alter the Number and Angle of Pause Turns.
(A) Description of a pause turn. Video frames and diagram showing a ventral view of a WT larva, OrR, at different stages during a pause turn. A unilateral wave of muscle contraction moves backward from A1 to A4 on the left hand side. Segments A5 to A9 remain relaxed on both sides of the animal. See also Figure S2.
(B–D) Crawling behavior was recorded on video and analyzed. (B) Representative crawling patterns depicted by perimeter stacks from a 2 min crawling episode at 1 frame/s. An arrow head marks the beginning of each track, while asterisks mark pause turn events. (C) Scatter plot and average number of pause turns/min (±SEM). Blocking activity in the BL did not affect the number of turns as confirmed by a Kruskal-Wallis and a Dunn's Multiple Comparison test. An average of 46 animals was tested. **p < 0.01; ***p < 0.001. (D) Polar histogram of turning angles frequency. The frequency distribution of turning angles (α) see (A) for both controls and the BL > shits was nonsignificantly different. A Watson's U2 test for nonparametric two-samples test was performed: shits /+ versus BL > shits U20.05,197,137 = 0.12; BL/+ versus BL > shits U20.05,269,137 = 0.06.
(E) Scatterplot including average number of pause turns/min (±SEM). Hyperpolarizing the BL domain with Kir2.1 does not affect the number of turns. A one-way ANOVA with a Bonferroni post hoc test was used for comparison between genotypes, *p < 0.05.
If pause turns are indeed decision-making points, then it is highly likely that they would be regulated by higher centers in the brain. However, it is not clear whether the brain itself is required for the execution of these pause turns and their integration into the behavioral routine of exploration by crawling. To investigate this, we compared the behavior of control larvae with those where synaptic activity was blocked either in the brain and SOG or elsewhere. Paralyzed positive controls, elav > shits, cha > shits and NC > shits did not perform pause turns (Figures 4B and 4C). However, when we blocked synaptic transmission in the brain and SOG (BL > shits), there was no significant alteration in the number of pause turns as compared to controls (shits/+ and BL/+) (Figures 4B and 4C), indicating that the normal exploratory program of runs interspersed with pause turns operates autonomously in the absence of input from the brain. To corroborate this finding, we decided to silence neurons by hyperpolarizing them using targeted misexpression of the inward rectifying K+ channel kir2.1 [27]. To restrict the effects of this manipulation to larval stages, we antagonized Gal4 activity with tubulin-GAL80ts during development. Hyperpolarizing the neurons of the brain and SOG in larvae with kir2.1 confirmed that pause turns occurred normally even when these regions of the nervous system were inactivated (Figure 4E). The lack of brain activity also had no significant effect on the frequency or the angle of turns (Figure 4D) in the homogeneous environment provided by our test arena.
Our results suggest that (1) the neuronal control for pause turns is located in the thoracic and abdominal segments of the nervous system and (2) the normal integration of these events into an exploratory locomotor program of crawls interspersed with pause turns is an autonomous property of the thoracic and abdominal nervous system that can operate with or without input from the brain.
The Alternation between Crawls and Turns Is an Intrinsic Property of the Abdominal and/or Thoracic Network for Locomotion
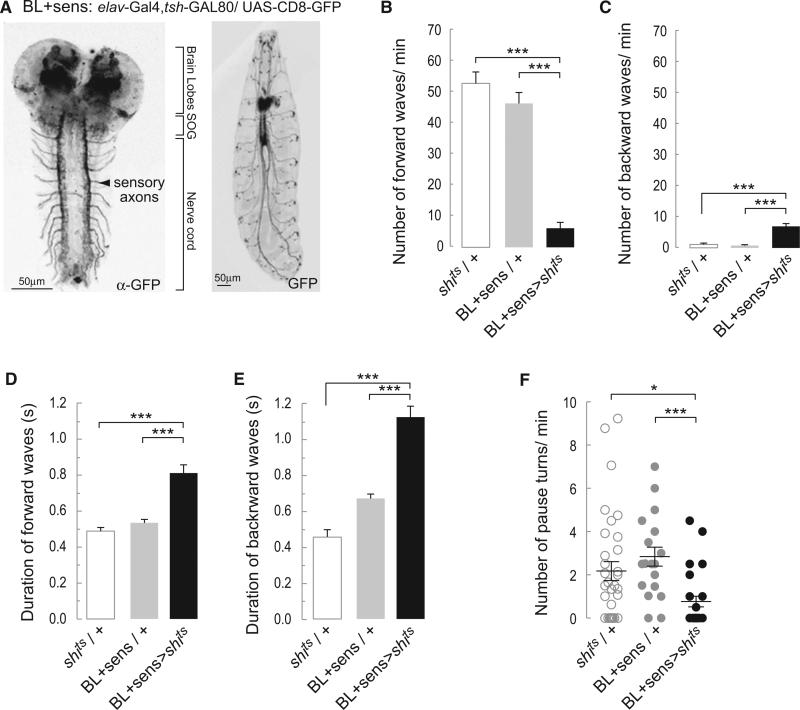
It is not clear, however, whether the alternation of crawls and pause turns is intrinsic to networks within the central nervous system or depends on some form of sensory input. To resolve this question, we generated a driver line BL+sens (elav-GAL4, tsh-GAL80) that is expressed in the brain, the SOG, and all sensory neurons (Figure 5A). Blocking synaptic transmission by overexpressing Shibirets in the BL+sens pattern (BL+sens > shits) caused crawling defects. The larvae performed significantly fewer forward peristaltic waves (Figure 5B) and more backward waves (Figure 5C). Forward and backward waves were slow (Figures 5D and 5E). This phenotype is similar to that described when transmission was blocked with UAS- shits in all sensory neurons [28]. The BL+sens > shits larvae were able to perform pause turns, but their frequency was reduced compared to controls (BL+sens/+ and shits/+) (Figure 5F). These results were confirmed using the line BL+sens > kir2.1, tubulin-GAL80ts. Larvae of this genotype performed few slowly propagating peristaltic waves, alternating with pause turns (data not shown). In all these instances, crawling was profoundly disturbed and the larvae barely progressed over the substrate. Nonetheless, they still executed waves of peristaltic contractions and alternated these with turns.
Figure 5. Blocking Synaptic Transmission in the Brain and Peripheral Nervous System Does Not Preclude the Alternation between Peristalsis and Turns.
(A) Representative confocal images revealing the pattern of expression of BL+sens driver line with UAS-mCD8-GFP. Left shows the immunostaining against GFP highlights strong expression in the sensory terminals in the nerve cord. Right shows that the peripheral nervous system can be observed in the whole animal.
(B) Average number of forward peristaltic waves/min (±SEM).
(C) Average number of backward peristaltic waves/min (±SEM).
(D) Average duration of forward peristaltic waves in s (±SEM).
(E) Average duration of backward peristaltic waves in s (±SEM). A one-way ANOVA with Bonferonni post hoc test was performed to compare the different treatments in (B)–(E).
(F) Average number (±SEM) of pause turns performed per min. A Kruskal-Wallis test was performed followed by a Dunn's multiple comparison. * means p < 0.05; **p < 0.01; and ***p < 0.001. An average of 26 animals was tested for each of the genotypes.
We conclude that the alternation between crawling and pause turn events is an intrinsic property of central networks in the thoracic and abdominal nervous system that can operate independently of input from either the brain or the sensory system.
The Brain Modulates Locomotion during Chemotaxis
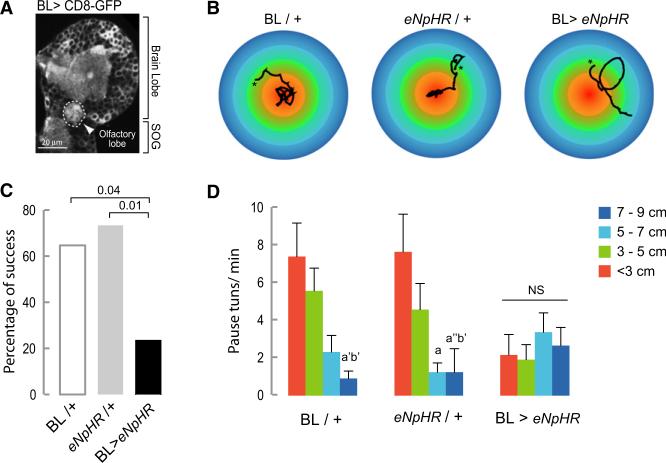
We expect that this autonomous output of the thoracic and abdominal nervous system will be modified both by sensory input and by descending inputs from the brain. During a food search in a highly heterogeneous environment (unlike the uniform environment of our test arena), the frequency and direction of turns is likely to be modified by the integration in the brain of olfactory and other cues with information such as the metabolic state of the larva. To test this prediction, we evaluated the exploratory behavior of larvae in the presence of an attractive olfactory stimulus (the odor of yeast presented as an inaccessible drop over the center of the agar coated arena). Control larvae aggregate in the central area, but if activity is blocked with Halorhodopsin in the brain (including the olfactory lobes, Figure 6A) and SOG, the percentage of larvae able to reach the central area of the plate, which is the peak of the olfactory gradient, is drastically reduced (Figures 6B and 6C). This suggests that such larvae, unlike controls, were unable to modify their exploratory locomotor routine in response to the olfactory stimulus. It is known that when larvae are near the source of an attractive odorant they locate it and stay in its proximity by performing frequent turns [26]. In agreement with this, we found that BL/+ and eNpHR/+ controls showed increased numbers of pause turns on reaching the central area of the plate (Figures 6B and 6D). No such increase in the rate of turns as a function of the distance to the odorant source was observed in BL > eNpHR animals with the result that, unlike controls, they failed to aggregate in the region of the food source (Figures 6B and 6D).
Figure 6. Chemotaxis-Dependent Change in Frequency of Pause Turns Is Prevented when Synaptic Activity Is Inhibited in the BL.
(A) The BL driver line is expressed in the olfactory lobe as labeled with anti-GFP.
(B) Representative tracks of larvae crawling under green light in an arena with a central olfac-tory cue. The asterisk indicates the beginning of the track.
(C) Percentage of success. The percentage of larvae that reached the central area of the plate defined by a circle with a 3 cm diameter was quantified. A Fisher's exact test was performed to evaluate differences between the genotypes.
(D) The number of pause turns/min was quantified in concentric areas of different diameters. Thirteen animals of each control group and 17 of the experimental were tested. A Kruskal-Wallis test with Dunn's multiple comparison was performed to evaluate the significance between the different diameters in each of the treatments. a’ means p < 0.05, a” p < 0.001 comparing with < 3cm; b means p < 0.01 and b’ p < 0.05 comparing with 3–5 cm. NS, nonsignificant.
Thoracic and Abdominal Circuitry Integrates Sensory Information and Initiates Changes in Crawling Pattern
To show whether locomotor routines can be redirected by inputs other than those coming from the brain, we decided to evaluate the light avoidance response induced by the photoreceptors tiling the body wall of the larva [29] in the absence of brain function. When high intensity light is presented to the anterior part of the animal, it induces an avoidance response, which consists of an interruption to forward crawling and either the initiation of backward movements or a turn away from the light. This switch from a forward crawl to either a turn or a backward crawl provides us with the opportunity to test whether circuits located in the nerve cord have the potential to integrate sensory information and direct a switch in motor outputs without the intervention of the brain.
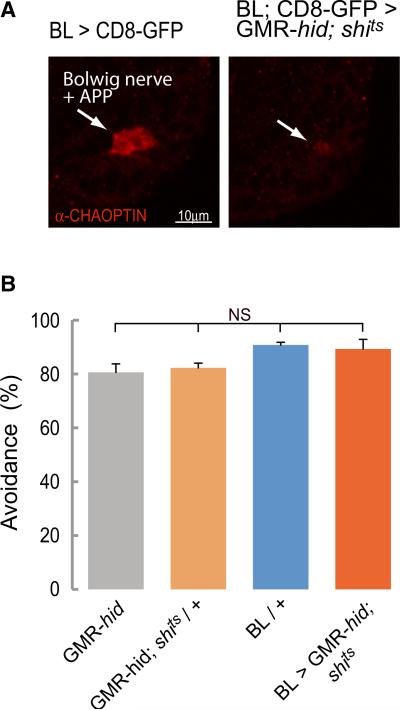
We carried out our experiments on larvae where synaptic transmission in the brain and SOG had been blocked with shits and whose visual organs connected to the brain (the Bolwig organs) had been genetically ablated (GMR-hid/+ background larvae, Figure 7A; [30]). When controls (GMR-hid or BL/+ or GMR-hid; UAS-shits/+ at 36°C) were exposed to a 2.2 mm diameter spot of blue light (480 ± 10 nm) at an intensity of 0.905 mW/ mm2, they made an avoidance response that removed the anterior of the larva from the light within 5 s (Figure 7B). The avoidance response typically consisted of backward peristaltic movements, abrupt head turns, or a combination of the two. One GMR-hid animal produced the stereotyped rolling defensive behavior [31]. The response of larvae in which the activity of the brain and SOG was blocked (BL/GMR-hid;UAS-shits) was not significantly different from controls (Figure 7B). These larvae too moved away from the light source by changing their forward crawling behavior to backward crawling or turning.
Figure 7. The Photoavoidance Response Does Not Require the Brain.
(A) The ability of larvae to escape from a spot of intense blue light in 5 s was assessed. Immunostaining showing that photoreceptors are ablated in BL > GMR-hid; shits third-instar larvae. Arrows indicate Bolwig's nerve and developing adult photoreceptor projection (APP), labeled with anti-chaoptin, in controls and residual staining in BL > GMR-hid; shits.
(B) Percentage of animals avoiding the blue light (±SEM). There were no significant differences between genotypes as tested with a Kruskal-Wallis test followed by a Dunn's multiple comparison. An average of 43 animals was tested for each genotype.
We conclude that in response to a sensory stimulus such as light, the thoracic and abdominal segments of the nervous system are independently capable of organizing a well-coordinated avoidance sequence that terminates forward crawling and initiates an alternative motor pattern, namely a turn or a backward crawl.
Discussion
An important initial step in understanding the relationship between neural circuits and patterns of behavior is to pinpoint those parts of the network that are necessary and sufficient for the particular behavior that is being studied. For example, the presence of spinal circuits sufficient for generating rhythmic locomotor movements was first demonstrated using surgical techniques to disconnect the spinal cord from both descending inputs from the brain and feedback from the periphery [32]. To our knowledge, the work reported here is the first time that it has been possible to remove brain function reversibly from a freely moving animal and to study the effects of this manipulation on patterns of behavior.
Like many other animals, Drosophila larvae use a random walk strategy to explore their environment. This exploratory behavior, which is already present in newly hatched larvae, consists of forward crawls that are interrupted by pauses, turns, and redirected forward crawls, and this enables the larva to make a comprehensive survey of any substrate over which it moves. The length of forward crawls and the frequency of pause turns varies: for example, immediately after hatching there is an increased frequency of turns [33], whereas larvae that are starved perform long bouts of crawling uninterrupted by turns. This stratagem, known as area restricted search, assumes an internal state sensor that regulates the turning frequency and allows the animal to visit distant sites when food resources become unavailable [34, 35].
In addition, the search routine is modified (by altering the frequency and direction of turns) in response to external cues during behaviors such as chemo- and thermotaxis [25, 26, 36]. These behavioral adjustments occur in response to input from sense organs on the head and suggest that the brain has an important role in modifying exploratory behavior in response to environmental cues. Interestingly, our results show that one component of the underlying mechanism is a free-running behavioral program for exploration that can operate independently of the brain and SOG. This autonomous exploratory capacity of more posterior segments of the nervous system is revealed when brain input is acutely removed when the larva is freely crawling in a homogeneous environment. Under these circumstances, exploratory crawling continues with characteristics (run lengths, pause turn frequency) that remain unchanged. This is very different from the behavior that has been observed in other insects whose brains have been removed by decapitation. In the absence of a brain, cockroaches, mantis, or adult Drosophila become very sluggish, and although they can be stimulated to produce short episodes of walking, they usually remain motionless [37–39]. Similarly, in the absence of reticulospinal drive, semirestrained zebrafish larvae cannot sustain autonomous activity in the swimming central pattern generator for more than a few seconds [24]. The contrasting observation in Drosophila that the presence or absence of the brain has no effect on the characteristics of crawling in a homogeneous environment allows us to conclude that the fundamental structure of the exploratory crawling program is intrinsic to the thoracic and abdominal ganglia and that the role of the brain is to adjust the performance of this routine to prevailing environmental conditions.
It is possible to infer the likely distribution of the underlying circuitry within the thorax and abdomen from other aspects of the larva's behavior. Of the two components to the exploratory behavior of the Drosophila larva, the crawl and the pause turn, the pause turn can be elicited during crawling, as an avoidance response to mechanical stimulation of anterior segments [40] or, as our experiments show, in the absence of a brain or visual organs as a response to input from light sensitive body wall receptors. Thus, at any point during a crawl, a stimulus to anterior segments is sufficient to elicit a pause turn. However, our findings show that the network that generates the pause turn operates in a time-dependent fashion during exploratory behavior. The underlying process appears to operate autonomously in a homogeneous environment but is subject to modulation by the brain in the presence of olfactory or other cues. The nature of the time-dependent process is unknown, but it is likely that the network that generates the pause turn either in response to a stimulus or stochastically during exploration is located in the thoracic part of the thoracic abdominal nervous system. It is already known that these segments behave differently from those of the abdomen during crawling and it is these segments that initiate the movements of the pause turn and inputs to these segments that provoke the avoidance response to stimulation [9]. It should now be possible to test the suggestion that circuitry for the two elements of exploratory crawling is located in different segments of the nervous system using a similar approach to the one described here and to investigate the time dependent process that regulates the occurrence of a pause turn during spontaneous crawling and how this is modulated by the descending inputs from the brain during goal-directed behavior.
Experimental Procedures
Exploratory Behavior Analysis
Eggs were collected from flies kept on apple juice agar plates supplemented with yeast paste. Third-instar larvae were washed to remove traces of food and allowed to crawl for 2 min on a clean, dry plate. They were then transferred to the arena and a movie was recorded after 15 sec acclimation to the plate. The behavior was tested in at least three independent repetitions of the experiment. Larval locomotion was monitored as previously described [21]. The arena consists of a 5 × 5 cm Peltier device (TE Technology, USA) coated with 2 ml of 0.9% agarose containing 0.6% black Indian ink. A 0–30V variable DC Power Supply Unit (PSU) (Rapid Electronics Ltd., UK) powered the device. During the experiment, the temperature was monitored with a thermocouple probe attached to a multimeter (Uni-Trend UT60-E, Uni-Trend Group Limited, China). Larval movements were recorded with a JVC TKC1380 camera mounted on a Leica M420 microscope.
For UAS-shits experiments, larvae were grown on yeast paste at 22°C for 4 days [18] and early third-instar larvae were analyzed at 36°C (temperatures over 34°C produced paralysis in the positive controls elav > shits and cha7.4 > shits animals).
For halorhodopsin (UAS-eNpHR-YFP) experiments, larvae were grown at 22°C in yeast supplemented with 1 mM of transretinal. Locomotion of early third-instar larvae was first recorded under blue light with an excitation filter BP 480/40 (GFP2 Leica). For halorhodopsin activation, animals were exposed to green light of a bandwidth 530–560 nm (DsRed filter BP 545/30 Leica, Germany) with an intensity of 0.214 mW/mm2. The light intensity was measured with a PM100 optical power meter attached to a sensor S130A, 400–1,100 nm (Thorlabs Karlsfeld, Germany). Movies were recorded with a DFC420 C digital camera on a Leica MZ16 F Fluorescence Stereomicroscope.
For UAS-EGFP-kir2.1, tubulin-GAL80ts experiments larvae were raised at 18°C until late first instar and transferred to 29°C for 72 hr. Under these conditions only 5% ± 3% of the elav-GAL4/ UAS-EGFP-kir2.1, tubulin-GAL80ts larvae survived, guaranteeing that the level of expression of KIR2.1 is sufficient to produce a phenotype. The size of the BL+sens-GAL4/ UAS-EGFP-kir2.1, tubulin-GAL80ts larvae was very variable, but all analyzed animals had reached third instar.
To analyze the number of peristaltic waves and their duration, we captured 30 s movies at 30 frames per second. The movements of the abdominal segments were evaluated frame by frame and quantified with the open source software VCode 1.2.1 http://social.cs.uiuc.edu/projects/vcode.html.
To analyze the number of turns and their angle, we used a VGA webcam (Logitech, Logitech Europe S.A., EEU) to capture 2 min movies at a rate of 15 frames/s. Data analysis was performed using Dynamic Image Analysis Software (DIAS) 3.4.2 (Sholl technologies, USA).
Olfactory Test
We placed 20 ml of a 20% yeast solution inside a plastic cup located in the center of the lid of a 10 cm petri dish. One third-instar larva was placed 2.25 cm from the edge on a plate coated with 2 ml of 0.9% agarose. The lid was closed and the larva was allowed to crawl for 5 min under green light. We confirmed that the treatment blocked neuronal activity during 5 min with elav > eNpHR animals, which remained paralyzed. The plate was then photographed and the length of the track and number of pause-turns was quantified with ImageJ (National Institutes of Health).
Light Avoidance Assay
The assay was performed as previously described [29]. Early third-instar larvae raised at 22°C were transferred to a 10 cm Petri dish coated with 0.9% agarose. The plate was then transferred to the surface of a water bath at 36°C. A shutter (Sutter Instruments) triggered by an external stimulator (Grass s88) delivered a 5 s pulse of a 2.2 mm in diameter spot of green light (0.905 mW/mm2) from a mercury short arc lamp HBO 50W/AC L1 (OSRAM) at a 115× magnification. No change in the temperature was associated with illumination.
Supplementary Material
Acknowledgments
We thank J. Casal, B. Hedwig, M. Landgraf, P.A. Lawrence, and E. Merlo for useful discussions and for making constructive comments on the manuscript. We are grateful to L. Prieto Godino for invaluable discussions and Karl Deisseroth and Paul Garrity for sharing plasmids. We thank the Bloomington Stock Center for fly stocks and the Developmental Studies Hybridoma Bank for antibodies. J.B. is a Henslow Research Fellow from Downing College supported by the Cambridge Philosophical Society. S.R.P. was a Newton International Fellow from the Royal Society. M.B. is supported by an emeritus fellowship from the Leverhulme Trust.
Footnotes
Supplemental Information
Supplemental Information includes two figures, Supplemental Experimental Procedures, and six movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2012.07.048.
References
- 1.Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 2.Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds AM, Frye MA. Free-flight odor tracking in Drosophila is consistent with an optimal intermittent scale-free search. PLoS ONE. 2007;2:e354. doi: 10.1371/journal.pone.0000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J. Neurophysiol. 1997;78:214–228. doi: 10.1152/jn.1997.78.1.214. [DOI] [PubMed] [Google Scholar]
- 5.Humphries NE, Queiroz N, Dyer JRM, Pade NG, Musyl MK, Schaefer KM, Fuller DW, Brunnschweiler JM, Doyle TK, Houghton JDR, et al. Environmental context explains Lévy and Brownian movement patterns of marine predators. Nature. 2010;465:1066–1069. doi: 10.1038/nature09116. [DOI] [PubMed] [Google Scholar]
- 6.Brown C, Liebovitch L, Glendon R. Lévy Flights in Dobe Ju/'hoansi Foraging Patterns. Hum. Ecol. 2007;35:129–138. [Google Scholar]
- 7.Viswanathan GM, Buldyrev SV, Havlin S, da Luz MG, Raposo EP, Stanley HE. Optimizing the success of random searches. Nature. 1999;401:911–914. doi: 10.1038/44831. [DOI] [PubMed] [Google Scholar]
- 8.Suster ML, Bate M. Embryonic assembly of a central pattern generator without sensory input. Nature. 2002;416:174–178. doi: 10.1038/416174a. [DOI] [PubMed] [Google Scholar]
- 9.Lahiri S, Shen K, Klein M, Tang A, Kane E, Gershow M, Garrity P, Samuel AD. Two alternating motor programs drive navigation in Drosophila larva. PLoS ONE. 2011;6:e23180. doi: 10.1371/journal.pone.0023180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit R, Vijayraghavan K, Bate M. Hox genes and the regulation of movement in Drosophila. Dev. Neurobiol. 2008;68:309–316. doi: 10.1002/dneu.20589. [DOI] [PubMed] [Google Scholar]
- 11.Wigglesworth VB. The Principles of Insect Physiology. Seventh Edition Chapman and Hall Ltd; London: 1977. [Google Scholar]
- 12.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 13.Pereanu W, Spindler S, Im E, Buu N, Hartenstein V. The emergence of patterned movement during late embryogenesis of Drosophila. Dev. Neurobiol. 2007;67:1669–1685. doi: 10.1002/dneu.20538. [DOI] [PubMed] [Google Scholar]
- 14.Cattaert D, Birman S. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J. Neurobiol. 2001;48:58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Arias A, Ingham PW, Scott MP, Akam ME. The spatial and temporal deployment of Dfd and Scr transcripts throughout development of Drosophila. Development. 1987;100:673–683. doi: 10.1242/dev.100.4.673. [DOI] [PubMed] [Google Scholar]
- 16.Landgraf M, Sánchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev. Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 17.Fasano L, Röder L, Coré N, Alexandre E, Vola C, Jacq B, Kerridge S. The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell. 1991;64:63–79. doi: 10.1016/0092-8674(91)90209-h. [DOI] [PubMed] [Google Scholar]
- 18.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 19.Thum AS, Knapek S, Rister J, Dierichs-Schmitt E, Heisenberg M, Tanimoto H. Differential potencies of effector genes in adult Drosophila. J. Comp. Neurol. 2006;498:194–203. doi: 10.1002/cne.21022. [DOI] [PubMed] [Google Scholar]
- 20.Koenig JH, Saito K, Ikeda K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J. Cell Biol. 1983;96:1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berni J, Muldal A, Pulver S. Using Neurogenetics and the Warmth-Gated Ion Channel TRPA1 to Study the Neural Basis of Behavior in Drosophila. The Journal of Undergraduate Neuroscience Education. 2010;9:A5–A14. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 23.Inada K, Kohsaka H, Takasu E, Matsunaga T, Nose A. Optical dissection of neural circuits responsible for Drosophila larval locomotion with halorhodopsin. PLoS ONE. 2011;6:e29019. doi: 10.1371/journal.pone.0029019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc. Natl. Acad. Sci. USA. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L, Gershow M, Rosenzweig M, Kang K, Fang-Yen C, Garrity PA, Samuel AD. Navigational decision making in Drosophila thermotaxis. J. Neurosci. 2010;30:4261–4272. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Marin A, Stephens GJ, Louis M. Active sampling and decision making in Drosophila chemotaxis. Nat Commun. 2011;2:441. doi: 10.1038/ncomms1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 31.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham Brown T. The Intrinsic Factors in the Act of Progression in the Mammal. Proc. R. Soc. Lond. 1911;84:308–319. [Google Scholar]
- 33.Suster ML. PhD thesis. University of Cambridge; Cambridge, UK.: 2000. Neural Control of Larval Locomotion in Drosophila melanogaster. [Google Scholar]
- 34.Grunbaum D. Using Spatially Explicit Models to Characterize Foraging Performance in Heterogeneous Landscapes. Am. Nat. 1998;151:1–19. doi: 10.1086/286105. [DOI] [PubMed] [Google Scholar]
- 35.Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat. Neurosci. 2008;11:187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- 37.Ridgel AL, Ritzmann RE. Effects of neck and circumoesophageal connective lesions on posture and locomotion in the cockroach. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005;191:559–573. doi: 10.1007/s00359-005-0621-0. [DOI] [PubMed] [Google Scholar]
- 38.Roeder KD. The control of tonus and locomotor activity in the praying mantis (Mantis religiosa L.). J. Exp. Zool. 1937;76:353–374. [Google Scholar]
- 39.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.