Abstract
Background
Islet cell transplantation (ICT) is a promising approach to cure patients with type 1 diabetes. We have implemented a new immunosuppression protocol with antithymoglobulin plus antiinflammatory agents of anakinra and eternacept for induction, and tacrolimus plus mycophenolate mofetil for maintenance (T cell depletion with antiinflammatory [TCD-AI] protocol), resulting in successful single-donor ICT.
Methods
Eight islet recipients with type 1 diabetes reported adverse events (AEs) monthly. AEs were compared among three groups: first infusion with the TCD-AI protocol (TCD-AI-1st) and first and second infusion with the Edmonton-type protocol (Edmonton-1st and Edmonton-2nd).
Results
The incidence of symptomatic AEs within the initial 3 months in the TCD-AI-1st group was less than in the Edmonton-1st and -2nd groups, with a marginally significant difference (mean ± S.E.: 5.5±0.3, 7.5±0.5, and 8.3±1.3, respectively; p = 0.07). A significant reduction in liver enzyme elevation after ICT was found in the TCD-AI-1st group compared to the Edmonton-1st and -2nd groups (p < 0.05). Due to AEs, all patients in the Edmonton protocol eventually converted to the TCD-AI protocol, whereas all patients tolerated the TCD-AI protocol.
Conclusions
TCD-AI protocol can be tolerated for successful ICT although this study includes small cohort and large population trial should be taken.
Keywords: Adverse event, Sirolimus, Antithymocyte globulin, Anti-inflammatory agents, Type 1 diabetes
INTRODUCTION
Islet cell transplantation (ICT) offers a significant advantage in curing type 1 diabetic patients by replacing β cells in an intervention that is less invasive than whole organ pancreas transplantation (1, 2). The Edmonton protocol for ICT brought in the new era, resulting in insulin-independence, but only approximately 10% of the recipients remained insulin independent 5 years after ICT (3). Recent reports have demonstrated that the insulin-independent period can be prolonged by using immunosuppression regimens with T cell depletion or blockage of T cell stimulation (4–10). We have implemented a novel immunosuppression regimen with antiinflammatory agents, using antithymocyte globulin (ATG) as induction, with its double blockage of anti-interleukin (IL)-1β and tumor necrosis factor (TNF)-α, followed by tacrolimus plus mycophenolate mofetil (MMF) instead of sirolimus for maintenance immunosuppression, named as T cell depletion with antiinflammatory (TCD-AI) protocol in this report. This combination of drugs can lead to highly successful ICT (11).
One of the major issues in clinical ICT is the risk of adverse events (AEs) related to immunosuppressants (12). The administration of a calcineurin inhibitor in organ transplant recipients has been associated with renal dysfunction (13). Sirolimus, a maintenance immunosuppressant in the Edmonton protocol, damages islet endocrine function (14, 15) and causes notorious mouth ulcers. Malignant diseases possibly related to immunosuppressants were also reported in the Collaborative Islet Transplant Registry (CITR) (16). Newer immunosuppression protocols after Edmonton protocol have been developed to maximize graft function as well as to minimize AEs although limited information on AEs are available (Table 1). To position ICT as a feasible intervention and improve quality of life in islet recipients, benefits such as stabilization of glycemic controls and prevention of diabetic complications should outweigh the risks of AEs. However, the study on AEs in T cell depletion-type immunosuppression protocol with double blockage of IL-1β and TNF-α has not reported so far and is still questioned.
Table 1.
Newer immunosuppression protocol in ICT.
| Protocol Induction |
Maintenance | n | Reported adverse events | Reference | |
|---|---|---|---|---|---|
| ATG (basiliximab for 2nd Tx) + belatacept | Sirolimus ± MMF | 5 | (Both belatacept and efalizumab group) | Oral ulcer (many), nausea (3), diarrhea (4), neutropenia (3), thrombocytepenia (1),Rash or erythema (2) | 6, 7 |
| ATG (basiliximab for 2nd Tx) + efalizumab | Sirolimus ± MMF | 5 | (Efalizuma b group) | Rash/Erythema (2), partial portal vein thrombosis (1) | |
| ATG + etanercept | Cyclosporine + everolimus or MMF | 6 | Oral ulcer (6), leukopenia (6), transient elevations of liver enzymes (6), acute cholecystitis (2), increased serum creatinine (6), >25% decreased GFR (2), hypertension (1), hyperlipidemia (1) | 4 | |
| ATG + daclizumab + etanercept | MMF + sirolimus ± low-dose tacrolimus | 8 | Lymphopenia (5), transient neutropenia (5), oral ulcer (8) | 61 | |
| Alemtuzumab + etanercept | Sirolimus + tacrolimus/MMF | 3 | Leukopenia (3), lymphopenia (3), Skin rash (3) | 5 | |
| Daclitumab ± infliximab, | Sirolimus + tacrolimus | 16 | Aspication pneumonia (1), parvovirus infection (1), HES (1), short-term memory loss 1), renal dysfunction (1), exzema (1) and insomnnia/depression (1), Leukopenia/neutropenia (9), serum creatinine elevation (2), macroalbuminuria (5), mild hypertension (1), hyperlipidemia (14), mouth ulcer, peripheral edema, insomnia, tremor, anxiety, headache, gynecological abnormalities, bone marrow suppression, electrolyte abnormalities, proteinuria | 62 | |
| Daclitumab + etanercept + exenatide | Sirolimus + tacrolimus | 6 | Weight loss (6), nausea (6), vomitting (6), viral stomatitis (1), severe anemia (1), elevated creatinine (2), myonecrosis (1) | 63 | |
| Daclitumab + Efalizumab | MMF + tacrolimus | 4 | Anemia (2), elevation of liver enzymes (2), | 8 | |
The numbers in parentheses indicate number of patients with individual AE.
According to the annual report of CITR, the majority of AEs related to immunosuppression occur within the first year of follow-up (16). Therefore, reduction of AEs in the first follow-up year is important for quality of life in islet recipients. Herein the aim of this study is assessing the AEs within the first year after ICT using the TCD-AI protocol compared to the Edmonton-type protocol.
PATIENTS AND METHODS
The protocol and consent forms were approved by the institutional review board of Baylor Research Institute (Dallas, TX). Written consent forms were obtained from all participants.
Patients
Eight patients who received a total of 13 ICTs at our hospitals (Baylor University Medical Center at Dallas and Baylor All Saints Medical Center at Fort Worth, TX) from November 2005 to February 2010 were included in this study. The same islet staffs served for both two hospitals and there is no difference in the management of ICT between the two hospitals. Islets were isolated in the single facility (Baylor Research Institute, Dallas, TX) and shipped to the two hospitals. Islet preparations were performed as previously described (11, 17), except for the one transplantation where islets were isolated at a remote center (18). The islet recipients attended an outpatient clinic every month, and blood samples were taken to measure blood cells and biochemistry. Patients also reported the AEs at their clinic visits. Doppler ultrasonography and eye examinations were performed every 6 months during the study. The median follow-up period was 29.1 months (range, 12–56 months).
Edmonton-type Protocol
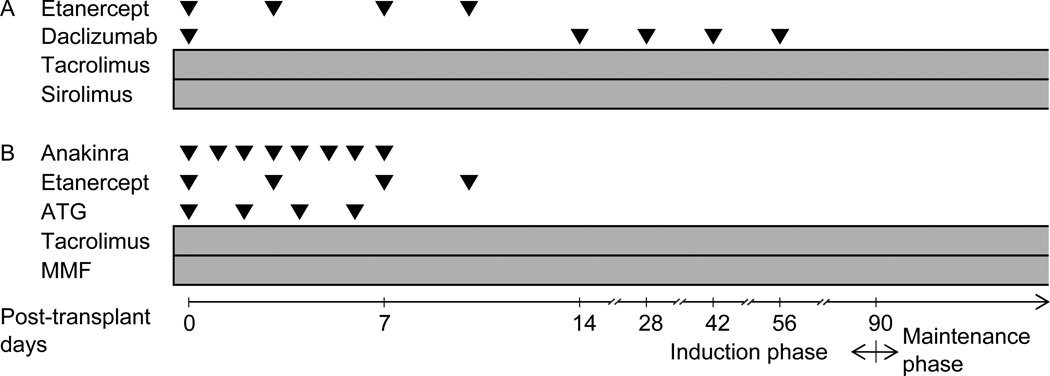
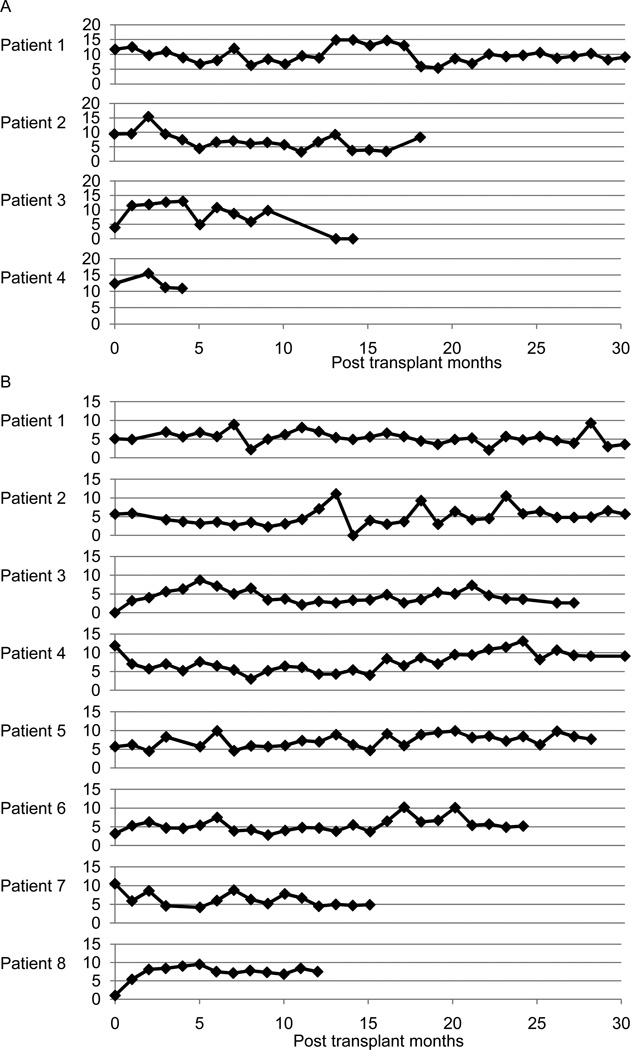
Based on the Edmonton protocol, immunosuppression was induced with daclizumab (Zenapax, Roche Pharmaceuticals, Nutley, NJ), with 1 mg/kg of subcutaneous injection on days 0, 14, 28, 42, and 56 (17, 19–20). Sirolimus (Rapamune, Wyeth Pharmaceuticals, Philadelphia, PA) and tacrolimus (Prograf, Astellas, Deerfield, IL) were administered to maintain immunosuppression (Fig. 1A). The doses of the maintenance immunosuppressants were adjusted to maintain trough levels of 3–6 ng/mL of tacrolimus and 12–15 ng/mL of sirolimus (Fig. 2). Etanercept (Enbrel, Immunex, WA) (50 mg) was given intravenously 1 hour before transplantation and 25 mg was administered subcutaneously on days 3, 7, and 10 posttransplant as an anti-inflammatory treatment.
Figure 1.
Immunosuppression protocols for ICT: (A) Edmonton-type protocol and (B) TCD-AI protocol. ATG indicates antithymoglobulin; MMF, mycophenolate mofetil.
Figure 2.
Serum trough levels (ng/mL) of (A) sirolimus and (B) tacrolimus.
TCD-AI Protocol
As the second-generation protocol, we used rabbit antithymocyte globulin (rATG, Thymoglobulin, Genzyme, Framingham, MA) at a concentration of 1.5 mg/kg on days 0, 2, 4, and 6 posttransplant for induction immunosuppression (11). Premedication of ATG included the administration of up to 500 mg of intravenous methylprednisolone, 650 mg of acetaminophen and 25–50 mg of diphenhydramine. Anakinra (Kineret, Amgen, Thousand Oaks, CA) (100 mg) was administered intravenously 1 hour pretransplant and subcutaneously for 7 days after transplantation as an anti-IL-1 β therapy (11). In addition, 50 mg of etanercept was delivered intravenously 1 hour before transplantation. A reduced 25-mg dose of etanercept was given subcutaneously on days 3, 7, and 10 posttransplant as an anti–TNF-α therapy. To maintain immunosuppression, we used mycophenolate mofetil (MMF, CellCept, Roche Laboratories, Nutley, NJ) (2 g/day orally) and tacrolimus (Prograf, Astellas, Deerfield, IL), aiming for trough levels of 5–10 ng/mL (Figs. 1B and 2).
Concomitant Medications
Oral valganciclovir (450 mg) was administered for 14 weeks irrespective of the donor’s and recipient’s cytomegalovirus serology status in order to prevent future lymphoproliferative disorders or graft loss. Trimethoprim-sulfamethoxazole (80 mg of trimethoprim and 400 mg of sulfamethoxazole per day) was used for standard Pneumocystis jirovecii prophylaxis during the study. The oral administration of enteric-coated aspirin (81 mg per day) was started on day 7. Vitamin A (25,000 IU per day), vitamin B6 (100 mg per day), and vitamin E (800 mg per day) were also administered orally after day 1 posttransplant.
The administration of exenatide (Byetta, Amylin Pharmaceuticals, San Diego, CA; 10 mg twice daily with subcutaneous injection) or sitagliptin (Januvia, Merck Pharmaceuticals, Whitehouse Station, NJ; 100 mg daily with oral administration) was allowed in our protocol.
Data Analysis of AEs
In our protocol, AEs were defined by any reactions, side effects, or other untoward medical occurrences that are temporally, but not necessarily causally, related to the ICT procedure or medications. According to the protocol descriptions, AEs were collected from monthly patient journals, physicians’ records and laboratory data.
All reported AEs were included in this study irrespective of their relationship with immunosuppression protocols. AEs and their grading were classified according to CITR guideline that was modified from the Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0 provided by the National Cancer Institute (21). AEs of grade 1 (mild) to 2 (moderate) were grouped as low grade, and those of grade 3 (severe) to 4 (life-threatening or disabling) as high grade for ease of reference to the CITR annual report where AEs of grade 3 or above were summarized (16, 21). For example, mild to moderate pain interfering with function, but not interfering with activity of daily living (ADL) was classified as low-grade pain whereas severe pain interfering ADL or disabling was grouped to high-grade.
Symptomatic AEs such as pain or vomiting were assessed with the incidence while asymptomatic AEs as laboratory data were evaluated using their numerical values. Hemorrhage and pain at the infusion site were categorized as infusion-related AEs. Estimated glomerular filtration rate (eGFR) was calculated with the four-variable Modification of Diet in Renal Disease equation (22).
Grouping
The AEs were categorized based on their association with the first ICT with the TCD-AI protocol (TCD-AI-1st) or the first and second ICT with the Edmonton-type protocol (Edmonton-1st and -2nd, respectively). AEs in the induction phase (initial 3 months after each infusion) and maintenance phase (the following 9 months) were separately evaluated. If the immunosuppression protocol was changed during the follow-up period, AEs after the modification were excluded from the analysis.
Statistics
Statistical analysis was performed using PASW statistics 18.0.2 (SPSS Inc, Chicago, IL). Two-group comparisons were performed with the Fisher’s exact test for categorical values and the Mann-Whitney U test for continuous values. The incidence of AEs and the results of blood examinations were tested with the Kruskal-Wallis test nonparametrically. Multiple comparisons of the proportion of patients with AEs were employed using the chi-square test followed by Ryan correction (23). Values were expressed as mean ± S.E. Statistical significance was considered as a two-sided p value of <0.05 for all statistical tests.
RESULTS
Patient Characteristics and transplantation
There were no significant differences between the two groups in basic characteristics before transplantation (Table 2). Also no significant differences were observed in transplant conditions including islet yield per body weight, tissue volume, and initial/maximum/change of portal vain pressure (Table 3). The immunosuppression protocol in all patients in the Edmonton group was changed to the TCD-AI protocol after the second ICT since no patient could tolerate the gastrointestinal AEs (Fig. 3). There were no cases of malignant disease or death in our cohort.
Table 2.
Basic Patient Characteristics Before Transplant
| Variable | Edmonton-like protocol | TCD-AI protocol |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | ||
| Infusion (n) | 2a | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0.14c |
| Age | 46 | 57 | 55 | 51 | 38 | 53 | 50 | 35 | 0.15b |
| Sex | M | F | F | F | F | F | F | F | 1.0c |
| Weight (kg) | 79.3 | 59.1 | 80.1 | 65.5 | 54.9 | 75.9 | 52.1 | 54.2 | 0.08b |
| Body mass index | 21.2 | 23.4 | 29.9 | 26.6 | 20.7 | 27.9 | 19.1 | 21.2 | 0.19b |
| Duration of diabetes mellitus (yr) | 23 | 42 | 43 | 38 | 33 | 44 | 40 | 25 | 1.0b |
| Insulin requirement (unit)/day | 46 | 25.5 | 30 | 40 | 24 | 40 | 30 | 24 | 0.24b |
| Insulin requirement (unit)/kg of body weight/day | 0.58 | 0.43 | 0.37 | 0.61 | 0.44 | 0.53 | 0.56 | 0.44 | 1.0b |
| Diabetic complicationsd | None | R, N, K | None | R | R | R, N | None | None | – |
| Hemoglobin A1c (%) | 6.8 | 7.4 | 8.3 | 7.4 | 8.4 | 9.4 | 8.6 | 6.8 | 0.19b |
| SUITO index | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.6 | 0.32b |
| Hypoglycemic unawareness | Y | Y | Y | Y | Y | Y | Y | N | 1.0c |
| Glomerular filtration rate (mL/min) | 121 | 76 | 108 | 128 | 149 | 82 | 100 | 144 | 0.56b |
The islets isolated at the remote center were used for initial infusion.
Mann-Whitney U test.
Fisher’s exact test.
R, retinopathy; N, neuropathy; K, nephropathy.
Table 3.
Transplant conditions
| Edmonton-type protocol group | TCD-AI protocol 1st infusion (n=4) |
P value | ||
|---|---|---|---|---|
| 1st infusion (n=4) | 2nd infusion (n=4) | |||
| Islet yield per body weight (IEQ/kg) | 7881 ± 1753 | 8435 ± 2429 | 10953 ± 1015 | 0.48 |
| Tissue volume (mL) | 7.6 ± 1.6 | 5.9 ± 1.4 | 5.8 ± 1.0 | 0.62 |
| PVP (mmHg) | ||||
| Initial | 8.0 ± 1.1 | 6.8 ± 1.0 | 8.8 ± 1.3 | 0.49 |
| Maximum | 17.0 ± 2.9 | 12.5 ± 1.6 | 15.0 ± 1.2 | 0.33 |
| ΔChange | 9.0 ± 3.8 | 5.8 ± 1.9 | 6.3 ± 2.2 | 0.68 |
Abbreviation; PVP: portal vein pressure.
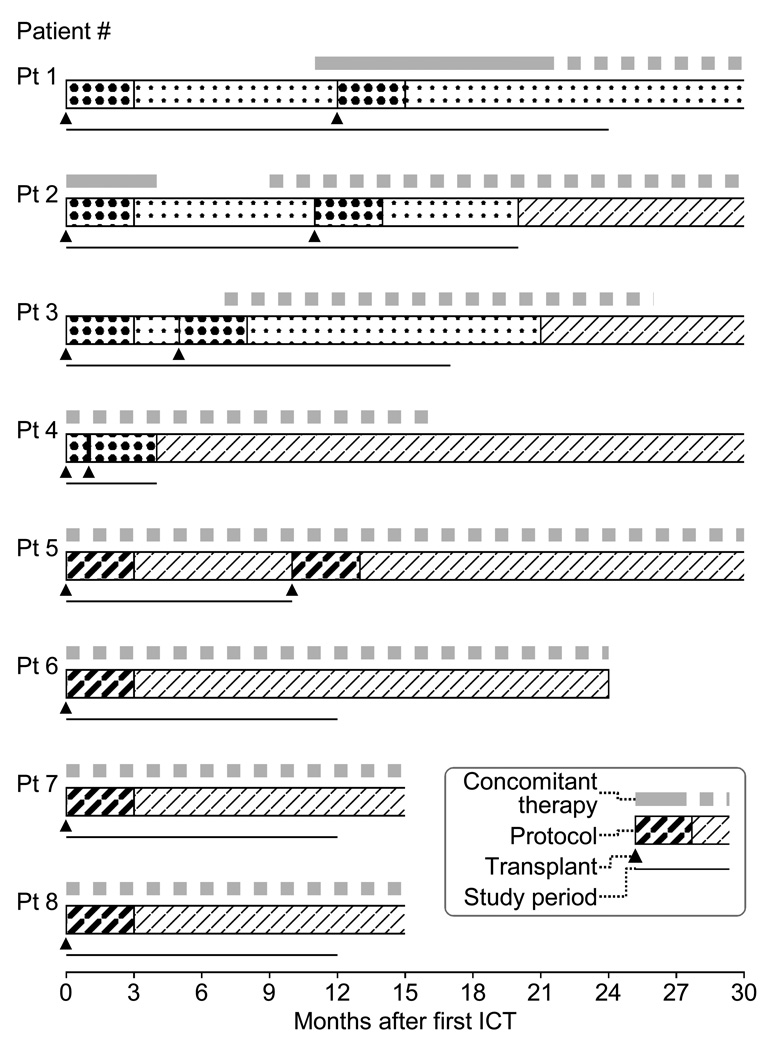
Figure 3.
Immunosuppression for each patient.
Clinical course of immunosuppression for each patient is shown as follows from upper row: concomitant therapy (exenatide; gray solid line and sitagliptin; gray dotted line), immunosuppression protocol (induction therapy using Edmonton-type protocol; densely dotted area, maintenance therapy using Edmonton-type protocol; lightly dotted area, induction therapy using TCD-AI protocol; densely slashed area and maintenance therapy using TCD-AI protocol; lightly slashed area), islet cell transplantation (triangle) and the period included in this study (black narrow line). Immunosuppression maintenance for patient 1 was changed to the TCD-AI protocol 34 months after the initial ICT.
Adverse Events Related to the Islet Infusion Procedure
Hemorrhage from the percutaneous transhepatic portal access was observed in four infusions: one low-grade bleed at the first infusion in the Edmonton-type group, one high-grade bleed at the second infusion in the Edmonton-type group, and two high-grade bleeds in the TCD-AI protocol group. All recipients suffered from pain at the infusion site immediately after ICT, but the pain was low grade. There was no portal vein thrombosis in this cohort.
Adverse Events during the Induction Phase
Symptomatic AEs in the induction phase are summarized in Table 4. The incidence of overall symptomatic AEs in the TCD-AI group was less than that in the Edmonton-1st and -2nd group, with marginal statistical significance (TCD-AI-1st, Edmonton-1st, and Edmonton-2nd: 5.5 ± 0.3, 7.5 ± 0.5, and 8.3 ± 1.3, respectively; p = 0.07 between TCD-AI-1st versus both Edmonton-1st and -2nd groups). Whereas no significant difference was found in the three groups if gastrointestinal AEs were excluded (p = 0.27). One high-grade AE of cardiac ischemia occurred in the TCD-AI group on day 21 and was treated by percutaneous coronary intervention. The incidence of skin and gastrointestinal AEs was higher in the Edmonton-type group than in the TCD-AI group. Of note, all patients in the Edmonton-type group developed mouth ulcers, whereas no patients in the TCD-AI group did (p < 0.01). Marginally significant differences were also observed between the TCD-AI-1st and Edmonton-2nd groups for bruising and rash (p = 0.09 and 0.07, respectively).
Table 4.
Incidence of Symptomatic Adverse Events in the Induction Phase
| Category | Adverse event | Protocol-infusion | ||
|---|---|---|---|---|
| Edmonton- 1st (n = 4) |
Edmonton- 2nd (n = 4) |
TCD-AI- 1st (n = 4) |
||
| Allergy/immunology | Allergic rhinitis | 0 | 1 | 2 |
| Cardiac-general | Hypertension | 0 | 1 | 0 |
| Cardiac ischemia/infarction | 0 | 0 | 1a | |
| Constitutional symptoms | Fatigue | 3 | 2 | 3 |
| Insomnia | 1 | 1 | 2 | |
| Weight loss | 2 | 1 | 2 | |
| Rigors/chills | 0 | 1 | 0 | |
| Dermatology | Bruisingc | 2 | 3 | 0 |
| Flushing | 1 | 0 | 0 | |
| Pruritus | 0 | 1 | 0 | |
| Rashd | 1 | 3 | 0 | |
| Acne | 0 | 0 | 1 | |
| Alopecia | 1 | 0 | 0 | |
| Gastrointestinal | Anorexia | 0 | 0 | 1 |
| Mucositis (functional/symptomatic)—stomach | 3 | 1 | 1 | |
| Diarrhea | 2 | 2 | 0 | |
| Nausea | 3 | 3 | 1 | |
| Mouth ulcerb | 4 | 4 | 0 | |
| Vomiting | 2 | 1 | 3 | |
| Lymphatics | Edema: limb | 0 | 2 | 0 |
| Neurology | Mood alteration—anxiety | 0 | 0 | 1 |
| Dizziness | 0 | 0 | 1 | |
| Tremor | 0 | 1 | 0 | |
| Pain | Headache | 0 | 0 | 2 |
| Throat/pharynx/larynx | 1 | 1 | 0 | |
| Extremity-limb | 3 | 3 | 1 | |
| Renal-genitourinary | Urinary frequency/urgency | 0 | 1 | 0 |
High-grade adverse event.
p < 0.01 for TCD-AI-1st versus Edmonton-1st and -2nd groups.
p = 0.09 between TCD-AI-1st and Edmonton-2nd group.
p = 0.07 between TCD-AI-1st and Edmonton-2nd group.
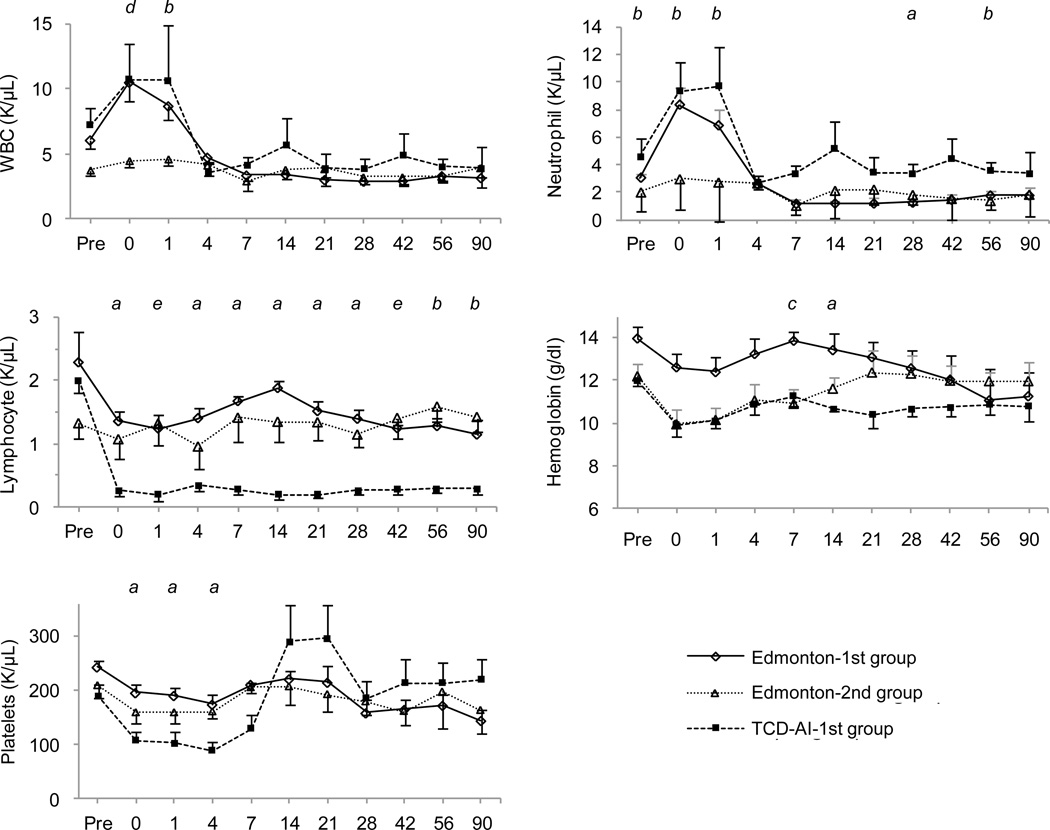
Regarding blood cell counts, the Edmonton-2nd group did not show an increase in white blood cells or neutrophils in the early phase of ICT, whereas the Edmonton-1st and TCD-AI-1st groups showed a doubling of these cells (Fig. 4). The number of lymphocytes in the TCD-AI-1st group significantly decreased compared to the Edmonton-type groups. Hemoglobin levels in the Edmonton-2nd and TCD-AI-1st groups, and the number of platelets in the TCD-AI-1st group, were lower compared to the others; however, platelet levels recovered 2 weeks after ICT.
Figure 4.
Impact of induction therapy on blood cell counts, with days after transplant shown on the X axis.
There were significant differences between the following pairs (p < 0.05): (a) Edmonton-1st vs. TCD-AI-1st; (b) Edmonton-2nd vs. TCD-AI-1st; (c) Edmonton-1st vs. Edmonton-2nd and TCD-AI-1st; (d) Edmonton-2nd vs. Edmonton-1st and TCD-AI-1st; and (e) TCD-AI-1st vs. Edmonton-1st and Edmonton-2nd. WBC indicates white blood cells.
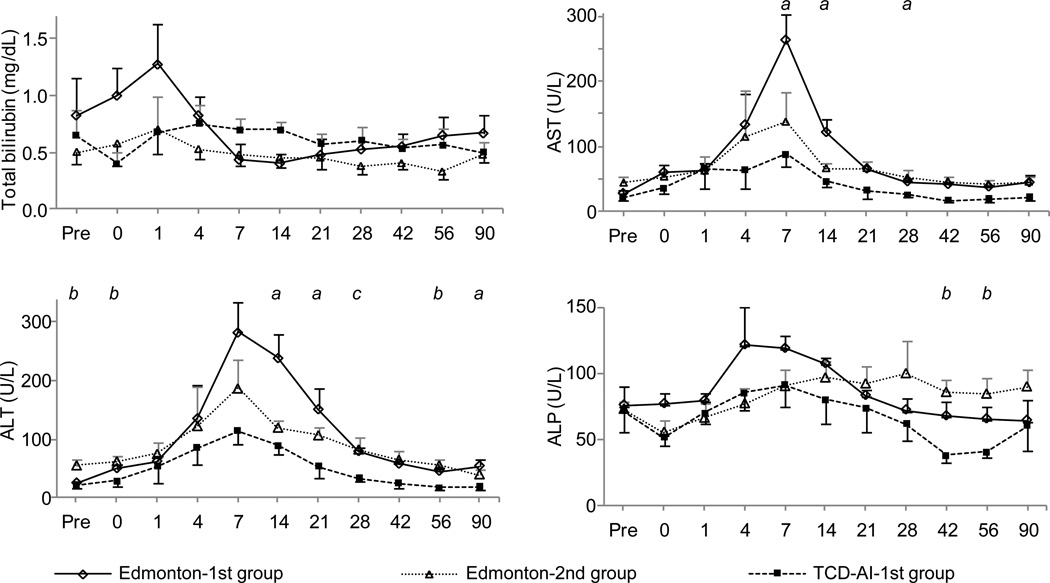
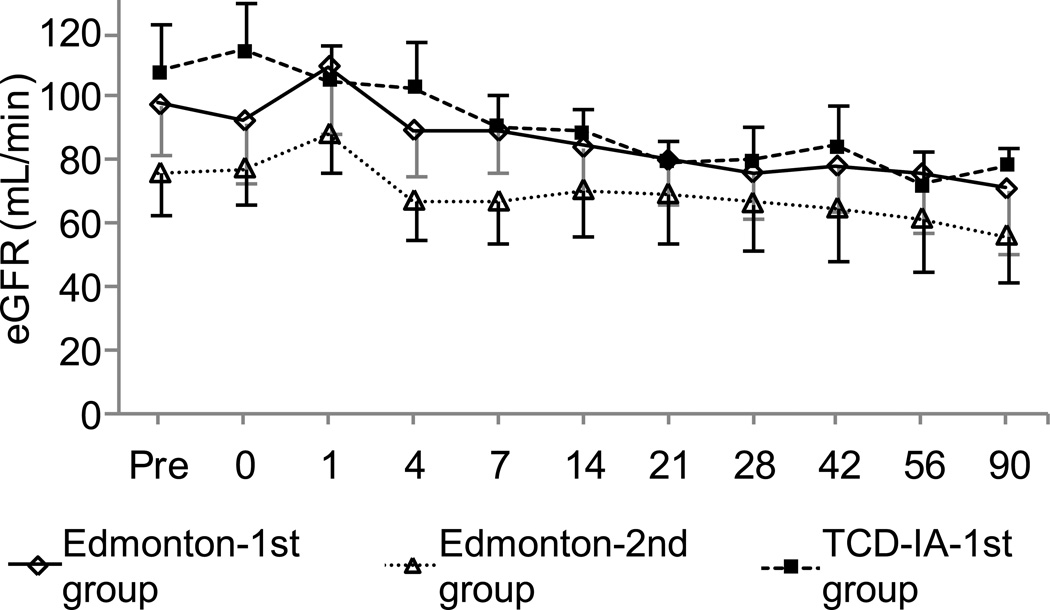
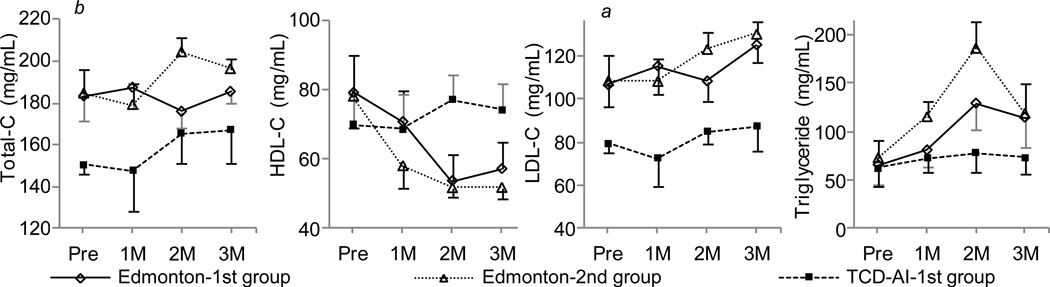
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels 1 and 2 weeks after ICT were significantly lower in the TCD-AI-1st group than in the Edmonton-1st group (p < 0.05) (Fig. 5). The TCD-AI-1st group and the Edmonton-1st group showed a similar pattern of estimated glomerular filtration rate, whereas the Edmonton-2nd group had lower values; the difference was not significant (Fig. 6). The TCD-AI protocol group also showed lower levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides than the other two groups, but no significant differences were observed after ICT (Fig. 7).
Figure 5.
Impact of induction therapy on liver function tests, with the X axis indicating days after transplantation.
There were significant differences between the following pairs (p < 0.05): (a) Edmonton-1st vs. TCD-AI-1st; (b) Edmonton-2nd vs. TCD-AI-1st; and (c) TCD-AI-1st vs. Edmonton-1st and Edmonton-2nd. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
Figure 6.
Estimated glomerular filtration rate (eGFR) in the induction phase, with the X axis indicating days after transplantation.
Figure 7.
Impact of induction therapy on lipid profiles, with the X axis indicating months after transplantation.
There were significant differences between the following pairs (p < 0.05): (a) Edmonton-1st vs. TCD-AI-1st; and (b) Edmonton-2nd vs. TCD-AI-1st. Total-C indicates total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Adverse Events in Maintenance Phase
The Edomontol-1st and -2nd groups included 3 patients for each since patient #4 in Figure 3 was excluded due to no period of maintenance phase after first ICT and maintenance with TCD-AI protocol after the second ICT (Table 5). Symptomatic AEs in the maintenance phase totaled 8.0 ± 2.5 in the Edmonton-1st group, 8.7 ± 1.5 in the Edmonton-2nd group, and 5.3 ± 0.5 in the TCD-AI-1st group, a difference that was not statistically significant (p = 0.21) (Table 5). Two high-grade AEs were observed in the maintenance phase: severe diarrhea was developed in the Edmonton-2nd group and diagnosed as Crohn’s disease, and ileus in the TCD-AI-1st group was recovered by temporal non-per-oral nutrition (NPO) with a 2-day admission. All patients in the Edmonton-type group developed mouth ulcers, while no patients in the TCD-AI group did (p = 0.03). Also, all patients experienced extremity edema in the Edmonton group, and only one patient had edema in the TCD-AI group, showing a marginally significant difference (p = 0.09). No symptoms suggested opportunistic infection and no progressed retinopathy were reported compared with baseline, and no changes of portal flow assessed by Doppler ultrasonography were found during the study period. No changes in the eye examinations were observed for both groups throughout the study.
Table 5.
Incidence of Symptomatic Adverse Events in the Maintenance Phase
| Category | Adverse event | Protocol-infusion | ||
|---|---|---|---|---|
| Edmonton- 1st (n = 3) |
Edmonton- 2nd (n = 3) |
TCD- AI-1st (n = 4) |
||
| Allergy/immunology | Allergic rhinitis | 0 | 1 | 2 |
| Cardiac-general | Hypertension | 0 | 0 | 1 |
| Constitutional symptoms | Fatigue | 1 | 2 | 2 |
| Insomnia | 1 | 0 | 1 | |
| Weight loss | 0 | 0 | 1 | |
| Weight gain | 0 | 1 | 0 | |
| Rigors/chills | 0 | 1 | 0 | |
| Fever | 0 | 2 | 0 | |
| Dermatology | Bruising | 2 | 0 | 0 |
| Pruritus | 0 | 1 | 0 | |
| Rash | 1 | 2 | 1 | |
| Acne | 1 | 1 | 0 | |
| Wound complication, noninfection | 1 | 1 | 0 | |
| Hypopigmentation | 1 | 0 | 0 | |
| Gastrointestinal | Anorexia | 2 | 1 | 1 |
| Mucositis (functional/symptomatic)–stomach | 0 | 2 | 0 | |
| Diarrhea | 2 | 1a | 1 | |
| Nausea | 1 | 2 | 0 | |
| Mouth ulcerb | 3 | 2 | 0 | |
| Ileus | 0 | 0 | 1a | |
| Infection | Upper respiratory infection | 1 | 0 | 1 |
| Lymphatics | Edema: limbc | 3 | 3 | 1 |
| Neurology | Mood alteration–anxiety, depression | 1 | 0 | 1 |
| Dizziness | 1 | 0 | 1 | |
| Tremor | 0 | 1 | 1 | |
| Numbness | 0 | 1 | 1 | |
| Pain | Headache | 0 | 0 | 1 |
| Extremity-limb | 2 | 1 | 3 | |
| Sexual reproductive function | Irregular menses | 0 | 0 | 1 |
High-grade adverse event.
p = 0.03 for TCD-AI-1st vs. Edmonton-1st groups.
p = 0.09 for TCD-AI-1st vs. Edmonton-1st and -2nd groups.
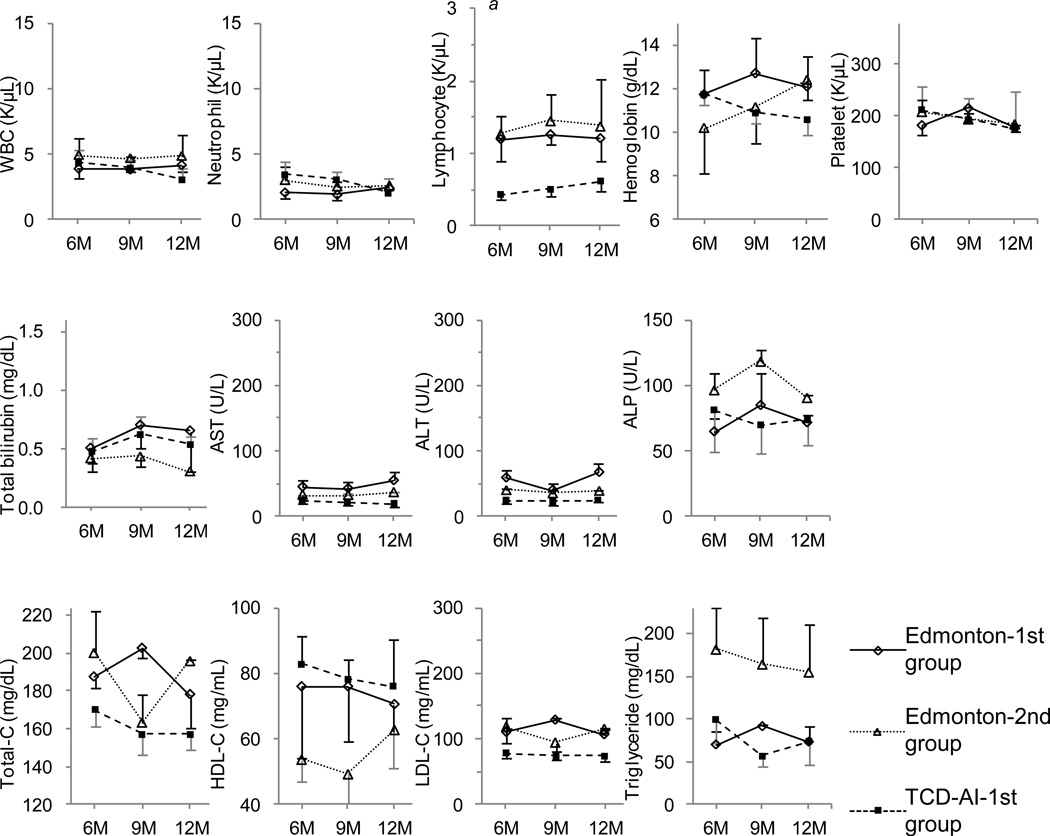
The number of lymphocytes in the TCD-AI-1st group in month 6 was significantly lower than that in the Edmonton-1st group, while in months 9 and 12, the number slightly recovered and no significant differences were found (Fig. 8). No significant differences were observed in liver function tests and lipid profiles between the groups during this period. Creatinine clearance tests at 1 year after ICT showed 99.7 ± 11.1 mL/min in the Edmonton-1st group, 63.0 ± 24.1 in the Edmonton-2nd group, and 78.0 ± 15.7 in the TCD-AI-1st group (not significant).
Figure 8.
Impact of immunosuppression on laboratory tests in the maintenance phase, with the X axis indicating months after transplantation.
(a) There was a significant difference between the Edmonton-1st group and the TCD-AI-1st group. WBC indicates white blood cells; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; Total-C, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
DISCUSSION
This report focused on the safety of ICT immunosuppression, comparing between the Edmonton-type protocol and the TCD-AI protocol. The TCD-AI protocol has been shown to be highly successful in single-donor ICT (11). In this study, the TCD-AI protocol also had the advantage of less symptomatic AEs in the induction phase with marginal significance level, especially for dermatology and gastrointestinal events, as well as significant reduction of liver enzyme elevations. In addition, gastrointestinal AEs and extremity edema in the maintenance phase were less frequent in the TCD-AI protocol. Of note, the possible bias that gastrointestinal AEs might be influenced by concomitant diabetic therapy of exenatide in Edmonton-type protocol group also should be considered. A persistently decreased number of lymphocytes up to month 6 was observed in the TCD-AI protocol group, although there was no opportunistic infection. Overall, the TCD-AI protocol had less AEs than the Edmonton-type protocol for ICT in this study with 1-year follow-up although preliminary results shown in this study should be cautiously interpreted due to limited number of cohort and multi-medication regimen.
Four bleedings from the infusion site were observed in this cohort, although all patients recovered without sequelae after blood transfusion and/or laparoscopic surgery. One case of a false aneurysm of a hepatic artery branch controlled by selective embolization was already reported (24). The portal vein cannulation is the only invasive procedure in ICT, and the related complications should be minimized. Real-time guidance of ultrasonography with an attachment probe (25), effective plugging in the catheter tract (26), and laparoscopic catheterization (27, 28) are helpful in preventing bleeding.
Most symptomatic AEs in the induction phase as well as the maintenance phase in the TCD-AI protocol group related to dermatology and gastrointestinal AEs, which may link to the difference in the immunosuppression regimen—i.e., the elimination of daclizumab and sirolimus. Daclizumab was initially used in renal transplantation for immunosuppression induction, demonstrating significant reduction of acute rejection (29). Nasopharyngitis (14.6%) and pyrexia (10.7%) were reported as frequent AEs of daclizumab in a randomized, double-blind, placebo-controlled trial for patients with ulcerative colitis (30). In our cohort, AEs attributed to daclizumab were limited to one patient with allergic rhinitis. Sirolimus was originally identified as an antifungal agent, and studies demonstrated its antitumor and immunosuppressive effects (31). Due to its mechanism of inhibiting the mammalian target of rapamycin (mTOR)-induced cell cycle, sirolimus can influence immune cells such as T cells and B cells as well as multiple cell lines including hematopoietic, vascular, and bronchial smooth muscle cells (32–34). Subsequently, multiple AEs were reported in clinical trials with sirolimus. Commonly reported drug toxicities include leukopenia, thrombocytopenia, anemia, hyperlipidemia, hypercholesterolemia, diarrhea, wound complications, extremity edema, mouth ulcer, and acne (35–38). Similar events were also observed in our cohort, with a high frequency of AEs in the dermatology and gastrointestinal categories among the Edmonton-type protocol group. The number of white blood cells in the Edmonton-type second infusion group was lower than in the first infusion group, suggesting myelosuppression induced by sirolimus. The lipid profile trend in the Edmonton-type protocol group showing higher total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels compared with the TCD-AI protocol group might also be associated with sirolimus.
Of note, exenatide was administered in two patients in the Edmonton-type protocol group. We initially administered exenatide concomitantly, evident by excellent ICT results from the University of Miami group (39). However, the two patients developed more frequent gastrointestinal AEs of nausea and vomiting after the administration, resulting in the discontinuation of exenatide. In the previous articles, gastrointestinal AEs such as nausea and vomiting are frequently reported with exenatide (40–42); therefore, gastrointestinal AEs in the two patients might be associated with exenatide administration. After that, sitagliptin was alternatively used since it was shown that sitagliptin could prolong islet graft survival in mice model (43).
There were significant differences in elevations of liver enzymes in our cohort although significant differences were not found in patient background as well as transplant condition. Similar findings were also found in report from Emory University group (8). Therefore, we think that there is a possibility of immunosuppression protocol’s contribution to liver enzyme elevation.
Compared with the Edmonton-type protocol, rATG, anakinra, and MMF were newly added in the TCD-AI protocol. In renal transplantation trials, the major AEs of rATG were infusion reactions such as fever, gastrointestinal disorder, leukopenia, and anemia (44, 45). T cell depletion is a part of the mechanism of rATG, and lymphopenia was also observed in our cohort up to 6 months after ICT. Interestingly, no patients in this study had an infusion reaction. Anakinra is known to be a powerful anti-inflammatory agent, which can prevent fever (46–48). A previous study on ATG treatment with etanercept for patients with myelodysplastic syndrome (MDS) reported infusion reaction as a major AE even steroids were administered before the use of ATG (49). These findings suggest that the double blockage of TNF-α and IL-1β might contribute the inhibition of infusion reaction. The injection site reaction has been known as an AE by anakinra for rheumatoid arthritis (50, 51), but in our cohort, the reaction was unremarkable.
The reactivation of latent tuberculosis (TB) is a primary concern when the combination therapy of eternacept and anakinra is administered since anti-TNF-α agents can interrupt granuloma formation and stabilization for the prevention of TB infection (52, 53) and a series of TB infection after eternacept treatment for rheumatoid arthritis (RA) was reported (54). The US Centers for Disease Control and Prevention (CDC) announced the guideline for TNF-α therapy in 2005 and our protocol includes purified protein derivative (PPD) test and chest X-ray in screening phase as well as monthly clinical evaluation for opportunistic infection according to the CDC guideline (55). Thus, careful follow-up for TB infection is needed for the use of antiinflammatory protocol although our protocol limits the period of antiinflammatory administration as within initial 10 days after transplant, allowing reducing the risk of TB infection by long-term use of antiinflammatory agents such as RA treatments. No TB infection was reported in the double-blind randomized controlled trial of double blockage with etanercept and anakinra for RA treatment (56). Of note, the University of Alberta group demonstrated that the short-term combination therapy of etanercept and anakinra could significantly improve islet engraftment as well as viability using human islets in immunodeficient mice, suggesting clinical feasibility of TCD-AI protocol (57).
As common AEs of MMF, gastrointestinal events such as diarrhea, nausea, esophagitis, and gastritis have been reported in randomized clinical trials for renal transplantation (58–60). In our cohort, fewer AEs of the gastrointestinal category were observed in the TCD-AI protocol compared to the Edmonton-type protocol. Since the combination of immunosuppressive medications can result in a higher risk of opportunistic infections, careful long-term follow-up is needed.
From the perspective of tolerability, the frequent AEs in the Edmonton-type protocol resulted in the conversion from sirolimus to MMF in all four patients. Conversion or reduction of sirolimus was also reported in recent ICT cases (6–8). Immunosuppression protocols with sirolimus could lead to successful ICT (19, 20), but the long-term tolerability of the protocols might be limited due to multiple AEs.
Another possible bias is improvement of islet transplant team by increasing the experiences of ICTs since Edmonton-type protocol was performed in early era of our clinical study whereas TCD-AI protocol has been implemented since 2008 to date. The differences of clinical results between institutions with different number of ICT were reported even the unified protocol was used (20). Therefore, the randomized multicenter trial is required to justify the efficacy and safely of TCD-AI protocol.
In summary, we implemented a novel immunosuppression protocol with ATG for induction and tacrolimus and MMF for maintenance plus anti-inflammatory agents. Reported AEs in the TCD-AI protocol were fewer than in the Edmonton-type protocol, especially for the dermatology and gastrointestinal categories. Lymphocyte counts were significantly decreased in the TCD-AI group for the initial 6 months, but no opportunistic infections were observed. Importantly, no patients withdrew from the TCD-AI protocol, while all patients in the Edmonton protocol group switched their maintenance therapy to the TCD-AI protocol due to frequent AEs. Overall, the TCD-AI protocol is considered safe and tolerable in the long term for successful ICT. Since this report includes small number of patients at single institute, further investigation with a multicenter, large cohort study should be undertaken to draw the final conclusion.
ACKNOWLEDGMENTS
This study was partially supported by All Saints Health Foundation. The authors thank Ms. Yoshiko Tamura and Ms. Ana M. Rahman for technical support and Ms. Cynthia Orticio for manuscript editing.
Footnotes
DISCLOSURES: None of the authors have conflicts of interest related to this manuscript.
REFERENCES
- 1.de Kort H, de Koning EJ, Rabelink TJ, Bruijn JA, Bajema IM. Islet transplantation in type 1 diabetes. BMJ. 2011;342:d217. doi: 10.1136/bmj.d217. [DOI] [PubMed] [Google Scholar]
- 2.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN, Papas KK, Sutherland DE, Moran A, Hering BJ. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froud T, Baidal DA, Faradji R, Cure P, Mineo D, Selvaggi G, Kenyon NS, Ricordi C, Alejandro R. Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes. Transplantation. 2008;86:1695. doi: 10.1097/TP.0b013e31819025e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, Bluestone JA, Stock PG. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10:1870. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, McElroy J, Ramos MD, Kerlan RK, Fong L, Vincenti F, Bluestone JA, Stock PG. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90:1595. doi: 10.1097/TP.0b013e3181fe1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, Adams AB, Sears MH, Bowen PH, Kirk AD, Pearson TC, Larsen CP. Experience with a novel efalizumabbased immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10:2082. doi: 10.1111/j.1600-6143.2010.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro AM. State of the Art of Clinical Islet Transplantation and Novel Protocols of Immunosuppression. Curr Diab Rep. 2011;11:345. doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 10.Bellin MD, Barton FB, Heitman A, Harmon J, Balamurugan AN, Kandaswamy R, Sutherland DE, Alejandro R, Hering BJ. Potent Induction Immunotherapy Promotes Long-Term Insulin Independence After Islet Transplantation in Type 1 Diabetes. Am J Transplant. 2012 Apr 11; doi: 10.1111/j.1600-6143.2011.03977.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N, Naziruddin B, Levy MF. Improving efficacy of clinical islet transplantation with iodixanol based islet purification, thymoglobulin induction and blockage of IL-1 beta and TNF alpha. Cell Transplant. 2011;20:1641. doi: 10.3727/096368910X564058. [DOI] [PubMed] [Google Scholar]
- 12.Khan MH, Harlan DM. Counterpoint: clinical islet transplantation: not ready for prime time. Diabetes Care. 2009;32:1570. doi: 10.2337/dc09-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 14.Niclauss N, Bosco D, Morel P, Giovannoni L, Berney T, Parnaud G. Rapamycin impairs proliferation of transplanted islet β cells. Transplantation. 2011;91:714. doi: 10.1097/TP.0b013e31820c10c8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006;55:2429. doi: 10.2337/db06-0173. [DOI] [PubMed] [Google Scholar]
- 16.CITR Coordinating Center; the EMMES Corporation. The seventh annual report. Rockville, MD: 2011. Retrieved from https://web.emmes.com/study/isl/reports/01062012_7thAnnualReport.pdf. [Google Scholar]
- 17.Matsumoto S, Noguichi H, Shimoda M, Ikemoto T, Naziruddin B, Jackson A, Tamura Y, Olson G, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, Levy M. Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant. 2010;19:291. doi: 10.3727/096368909X481773. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Sakuma Y, Pileggi A, Fraker C, Alvarez A, Montelongo J, Szust J, Khan A, Inverardi L, Naziruddin B, Levy MF, Klintmalm GB, Goss JA, Alejandro R, Ricordi C. Shipment of human islets for transplantation. Am J Transplant. 2007;7:1010. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Islet Transplant Registry. Terminology criteria for adverse events (TCAE) in trials of adult pancreatic islet transplantation, Version 4.0. Rockville, MD: EMMES Corporation; 2008. [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ryan TA. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull. 1960;57:318. doi: 10.1037/h0044320. [DOI] [PubMed] [Google Scholar]
- 24.Onaca N, Naziruddin B, Randall HB, Meler JD, Sanchez EQ, Matsumoto S, Noguchi H, Jackson A, Diamond NG, Klintmalm GB, Levy MF. False aneurysm of a hepatic artery branch complicating intrahepatic islet transplantation. Transpl Int. 2009;22:663. doi: 10.1111/j.1432-2277.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 25.Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27:1166. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 26.Villiger P, Ryan EA, Owen R, O'Kelly K, Oberholzer J, Al Saif F, Kin T, Wang H, Larsen I, Blitz SL, Menon V, Senior P, Bigam DL, Paty B, Kneteman NM, Lakey JR, Shapiro AM. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5:2992. doi: 10.1111/j.1600-6143.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 27.Movahedi B, Keymeulen B, Lauwers MH, Goes E, Cools N, Delvaux G. Laparoscopic approach for human islet transplantation into a defined liver segment in type-1 diabetic patients. Transpl Int. 2003;16:186. doi: 10.1007/s00147-002-0517-7. [DOI] [PubMed] [Google Scholar]
- 28.Papalois A, Bonatsos G, Birbas C, Toutouzas K, Golematis B. Development of a laparoscopic approach for islet transplantation. Transplant Proc. 1997;29:2099. doi: 10.1016/s0041-1345(97)00250-9. [DOI] [PubMed] [Google Scholar]
- 29.Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J. Daclizumab Triple Therapy Study Group. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338:161. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 30.Van Assche G, Sandborn WJ, Feagan BG, Salzberg BA, Silvers D, Monroe PS, Pandak WM, Anderson FH, Valentine JF, Wild GE, Geenen DJ, Sprague R, Targan SR, Rutgeerts P, Vexler V, Young D, Shames RS. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut. 2006;55:1568. doi: 10.1136/gut.2005.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehgal SN. Immunosuppressive profile of rapamycin. Ann N Y Acad Sci. 1993;696:1. doi: 10.1111/j.1749-6632.1993.tb17136.x. [DOI] [PubMed] [Google Scholar]
- 32.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 33.Cole OJ, Shehata M, Rigg KM. Effect of SDZ RAD on transplant arteriosclerosis in the rat aortic model. Transplant Proc. 1998;30:2200. doi: 10.1016/s0041-1345(98)00590-9. [DOI] [PubMed] [Google Scholar]
- 34.Salminen US, Alho H, Taskinen E, Maasilta P, Ikonen T, Harjula AL. Effects of rapamycin analogue SDZ RAD on obliterative lesions in a porcine heterotopic bronchial allograft model. Transplant Proc. 1998;30:2204. doi: 10.1016/s0041-1345(98)00591-0. [DOI] [PubMed] [Google Scholar]
- 35.Gaber AO, Kahan BD, Van Buren C, Schulman SL, Scarola J, Neylan JF. Sirolimus High-Risk Study Group. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation. 2008;86:1187. doi: 10.1097/TP.0b013e318187bab0. [DOI] [PubMed] [Google Scholar]
- 36.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 37.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, Nyberg SL, Ishitani MB, Prieto M, Velosa JA. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006;6:514. doi: 10.1111/j.1600-6143.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 38.Patel SJ, Elliott EN, Knight RJ, Gaber LW, Gaber AO. Considerations in sirolimus use in the early and late post-transplant periods. Expert Opin Drug Saf. 2009;8:421. doi: 10.1517/14740330903037156. [DOI] [PubMed] [Google Scholar]
- 39.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86:1658. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD. Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev. 2004;20:411. doi: 10.1002/dmrr.499. [DOI] [PubMed] [Google Scholar]
- 41.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type-2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 42.Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26:837. doi: 10.1111/j.1464-5491.2009.02790.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim SJ, Nian C, Doudet DJ, McIntosh CH. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes. 2008;57:1331. doi: 10.2337/db07-1639. [DOI] [PubMed] [Google Scholar]
- 44.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 45.Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, Hurault de Ligny B, Touchard G, Moulin B, Le Pogamp P, Reigneau O, Guignard M, Rifle G. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002;2:48. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 46.Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roldan R, Ruiz AM, Miranda MD, Collantes E. Anakinra: new therapeutic approach in children with familial Mediterranean fever resistant to colchicine. Joint Bone Spine. 2008;75:504. doi: 10.1016/j.jbspin.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott BL, Ramakrishnan A, Fosdal M, Storer B, Becker P, Petersdorf S, Deeg HJ. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149:706. doi: 10.1111/j.1365-2141.2010.08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, Hanrahan PS, Kraishi MM, Patel A, Sun G, Bear MB 990145 Study Group. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63:1062. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 52.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 53.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 1997;27:3182. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 54.Mohan AK, Coté TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- 55.Winthrop KL, Siegel JN, Jereb J, Taylor Z, Iademarco MF. Tuberculosis associated with therapy against tumor necrosis factor alpha. Arthritis Rheum. 2005;52:2968. doi: 10.1002/art.21382. [DOI] [PubMed] [Google Scholar]
- 56.Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, Bekker P 20000223 Study Group. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 57.McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12:322. doi: 10.1111/j.1600-6143.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 58.European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321. [PubMed] [Google Scholar]
- 59.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 60.The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61:1029. [PubMed] [Google Scholar]
- 61.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 62.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037–2046. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 63.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]