Abstract
The purpose of this study is to build a biological age (BA) equation combining telomere length with chronological age (CA) and associated aging biomarkers. In total, 139 healthy volunteers were recruited from a Chinese Han cohort in Beijing. A genetic index, renal function indices, cardiovascular function indices, brain function indices, and oxidative stress and inflammation indices (C-reactive protein [CRP]) were measured and analyzed. A BA equation was proposed based on selected parameters, with terminal telomere restriction fragment (TRF) and CA as the two principal components. The selected aging markers included mitral annulus peak E anterior wall (MVEA), intima-media thickness (IMT), cystatin C (CYSC), D-dimer (DD), and digital symbol test (DST). The BA equation was: BA = −2.281TRF + 26.321CYSC + 0.025DD − 104.419MVEA + 34.863IMT − 0.265DST + 0.305CA + 26.346. To conclude, telomere length and CA as double benchmarks may be a new method to build a BA.
Keywords: Telomere length, Biological age, Chronological age, Aging markers
Introduction
Functional decline is an inevitable consequence of aging progress (Tang et al. 2010; Mak 2013). The most common indicator to evaluate aging function is chronological age (CA). However, the aging process occurs gradually. Aging is a highly individual process and has a high degree of inter-individual and between-individual differences (AihieSayer et al. 1999). For example, some individuals at a CA of 85 years old have similar physiological conditions as younger individuals; however, physiological dysfunction in some individuals may occur before 60 years old (Gunn et al. 2009). Due to the large variation between individuals, CA does not accurately reflect the performance of the aging body (Sprott 2010).
Correct assessment of the functional status of aging is essential to study and treat aging-related diseases (Blagosklonny 2009). Biological aging is defined as a set of processes derived from the gradual reduction in the viability of the organism and gradual increase in the vulnerability (Mitnitski et al. 2013). Since CA is not an accurate indicator of biological aging (Simm and Johnson 2010), researchers have attempted to find an alternative biological indicator to measure age. The evaluation of biological aging uses indices to measure the extent of aging and to identify different individuals that have the same CA but different biological activity.
A number of representative parameters have been chosen to reflect the extent and rate of individuals to measure the aging process (Sprott 2010; Baker and Sprott 1988; Butler et al. 2004; Majkić-Singh 2011; Bae et al. 2008). However, studies have not found a single biomarker that fully reflects the degree of aging. Therefore, statistical methods have been developed to evaluate representative system/organ markers to build functional age equations (Park et al. 2009; Jee et al. 2012), such as vascular age (Cuende et al. 2010; Lloyd-Jones et al. 2004), skin age (Guinot et al. 2002), mental test score (Hodkinson 2012), dental age (Gupta et al. 2013; Hägg and Matsson 1985), skeletal Age (Gupta et al. 2013), perceived age (Rippon et al. 2013), and cognitive age (Hong et al. 2013). Other equations have been created to evaluate the overall function of the “functional age” concept, such as the biological age (Nakamura and Miyao 2007; Rippon et al. 2013), and Frailty index (Searle et al. 2008; Sternberg et al. 2011; Jones et al. 2004).
Selecting biomarkers of aging is closely related to parameters that reflect the biological aging process, as shown in Table 1 (Bai et al. 2010; Park et al. 2009; Nakamura et al. 1998; Nakamura and Miyao 2007; Ueno et al. 2003). CA does not accurately reflect the functional state of the body. Thus, CA may not be a suitable benchmark for selecting aging biomarkers. However, many biomarkers are chosen based on CA as a benchmark (Nakamura et al. 1994; Nakamura and Miyao 2007). In this study, we tried to explore a new age benchmark to select biomarkers of aging.
Table 1.
Criteria for biomarkers of aging (BoA)
| Standards | Specific content | References |
|---|---|---|
| Primary standards for BoA | Should reflect the basic biological processes of aging | (Reff et al. 1982; Baker and Sprott 1988; Mooradian 1990; McClearn 1997; Hodkinson 1972; López-Otín et al. 2013) |
| Should be quantitatively related to biological parameters | (Bai et al. 2010; Park et al. 2009; Nakamura et al. 1998; Nakamura and Miyao 2007; Ueno et al. 2003) | |
| Should be obtained in a healthy population | (Mooradian 1990; Johnson 2006; Sprott 2010;) | |
| Should reflect the aging rate in a short time | (Reff et al. 1982; Baker and Sprott 1988; Mitnitski et al. 2013; Mooradian 1990; Hodkinson 1972; McClearn 1997; Rattan 2013) | |
| Secondary standards for BoA | Should be minimally traumatic | (Hodkinson 1972; Reff et al. 1982; Baker and Sprott 1988; McClearn 1997) |
| Should be repeatable and reproducible | (Reff et al. 1982; Baker and Sprott 1988; Mooradian 1990; McClearn 1997; Hodkinson 1972; Nakamura et al. 1994) | |
| Should be representative | (Nakamura et al. 1994) |
Genetics play an important role in aging; however, genetic indicators are rarely used as biomarkers of aging for building BA equations (Sprott 2010). Telomere restriction fragment (TRF) is generally recognized as a genetic marker of aging at the cellular level (Mather et al. 2011; Sanders and Newman 2013; Saeed et al. 2012), appears to be associated with systemic aging (Zglinicki and Martin-Ruiz 2005; Mather et al. 2011; Bekaert et al. 2005), and reflects the dynamic aging process (Zglinicki and Martin-Ruiz 2005; Benetos et al. 2001). However, it is unclear whether TRF can serve as a biomarker of aging (Fossel 2012; Mather et al. 2011). Recent studies indicate that TRF reflects changes in functional aging (Boonekamp et al. 2013; López-Otín et al. 2013). In this study, we attempted to use TRF as a benchmark to select other biomarkers of aging to build a BA equation.
Many methods have been used to build BA equation. Compared with multiple regression analysis, principal component analysis (PCA) or factor analysis (FA) is relatively stable and superior for building BA equations (Nakamura et al. 1988; Park et al. 2009). In this study, we used the FA method to a BA equation based on TRF and CA, together with a number of indices independently associated with either telomere length or CA.
Methods
Screening of healthy volunteers
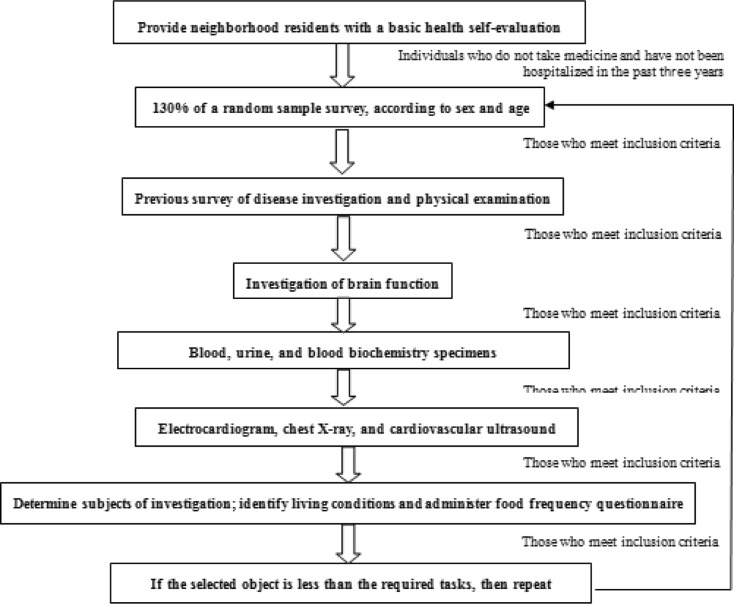
In 2011, 139 healthy volunteers, aged 35 to 91 years, were recruited from the Han population in Beijing city. The volunteers were not taking any medications and had not been hospitalized in the past 3 years. All participants signed an informed consent form. The study inclusion criteria and a flow chart for the study enrollment process are shown in Fig. 1. This research was approved by the Human Ethics Committee of Chinese PLA General Hospital in Beijing and was conducted in accordance with the Declaration of Helsinki and subsequent amendments.
Fig. 1.
Flow diagram for the screening of healthy volunteers
Measurements of various indicators (Annex 1)
Indicators of cardiovascular, kidney, and brain functions, as well as clinical, inflammatory, oxidative stress, genetic, psychological, and lifestyle habit factors were assessed in all the volunteers. A total of 105 indicators were assessed as potential age-related indices, which are described in Appendix. Briefly, all subjects completed a life habit survey concerning their smoking and alcohol habits, dietary patterns, frequency of physical activity, and other lifestyle factors. Blood pressure and body weight measurements were obtained. Cardiovascular function was assessed through ultrasound and electrocardiogram (ECG) measurements. Blood biochemistry was examined through routine blood analysis and urinalysis procedures.
Genomic DNA was isolated using a cell tissue genomic DNA extraction kit (Beijing TIANGEN, Co., Ltd). TRF length was measured using the Telo TTAGGG telomere length assay (Roche, Germany). Briefly, genomic DNA (1.5 μg) was digested with the restriction enzymes, Rsa I and Hinf I, for 2 h at 37 °C. The resulting DNA fragments were separated in a 0.8 % agarose gel by electrophoresis, then were denatured, neutralized, and transferred to a nylon membrane and cross-linked with UV light (UVP, USA). Blotted DNA fragments were then incubated with a telomeric probe (digoxigenin (DIG) 3′-end labeled 5′-[CCCTAA]3) at 42 °C for 3 h, followed by incubation with a DIG-specific antibody covalently coupled to alkaline phosphatase. Binding sites of the telomere probe were visualized using a highly sensitive chemiluminescence substrate that metabolizes alkaline phosphatase. TRF lengths were compared with molecular weight markers (Roche, Germany) (Tsuji et al. 2002) observed on X-ray film, and mean TRF lengths were estimated using Quantity One software (BioRad) (Tsuji et al. 2002).
Selection of aging biomarkers
A pair-wise correlation analysis was performed between the candidate indicators with CA and TRF (p < 0.05, r > 0.15), followed by a redundancy analysis to reduce the dimensionality (p < 0.05, r > 0.7 or p > 0.05). FA variables with high factor loading were selected as aging markers.
Construction of the BA formula
The marker coefficients were calculated by FA. An integral formula for the biological age score (BAS) was derived by FA. Each biomarker was subjected to data standardization using the following formula: X1 = (X − mean) / SD, where X is the original value, and SD is the standard deviation. BA was determined from the BAS equation and CA with the following formula: BA = BAS (standard CA) + mean CA. The corrected BA was determined with the following formula: Corrected BA = BA + Z (Dubina et al. 1984), where Z = (yi − y) (1 − b), yi is the CA of an individual, y is the average CA of all samples, and b is the coefficient of the simple linear regression between BA and CA (Park et al. 2009).
Results
Study subjects
As shown in Table 2, a total of 139 healthy Han individuals (aged 35–92, mean age 60.29 ± 14.33) were divided into five groups.
Table 2.
Group characteristics
| Age group | Male | Female | Total |
|---|---|---|---|
| 30–44 | 14 | 13 | 27 |
| 45–54 | 13 | 13 | 26 |
| 55–64 | 13 | 14 | 27 |
| 65–74 | 15 | 16 | 31 |
| >75 | 14 | 14 | 28 |
| Total | 69 | 70 | 139 |
Selected aging markers
A positive correlation was found between TRF and CA (r = 0.314, p < 0.01). The correlation analysis between each index and TRF or CA was performed, and the results are shown in Tables 3 and 4, respectively. The indices, which were independently related to telomere length and CA, were considered as aging biomarkers. R > 0.7 (p < 0.05) was used as the standard threshold to exclude the redundant indices. The remaining indicators were mitral annulus peak E anterior wall (MVEA), intima-media thickness (IMT), Cystatin C (CYSC), D-dimer (DD), and digital symbol test. A summary of the selected markers is shown in Table 5.
Table 3.
Correlation of each index with telomere length (TRF)
| Index | AID | MVEI | MVEA | TMD | IMD | IMT | EDVmax | CYSC | DD | DST | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TRF | R | −0.203 | 0.225 | 0.199 | −0.153 | −0.220 | −0.249 | −0.182 | −0.193 | −0.187 | 0.252 |
| P | 0.017 | 0.008 | 0.019 | 0.071 | 0.012 | 0.004 | 0.040 | 0.023 | 0.036 | 0.005 | |
| AID | R | −0309 | −0.336 | 0.330 | 0.189 | 0.121 | 0.011 | 0.341 | 0.138 | −0.178 | |
| P | 0.001 | 0.001 | 0.001 | 0.031 | 0.170 | 0.905 | 0.001 | 0.124 | 0.049 | ||
| MVEI | R | 0.654 | −0.278 | −0.198 | −0.406 | −0.136 | −0.322 | −0.160 | 0.223 | ||
| P | 0.001 | 0.001 | 0.024 | 0.001 | 0.125 | 0.001 | 0.073 | 0.014 | |||
| MVEA | R | −0.244 | −0.250 | −0.326 | −0.086 | −0.304 | −0.225 | 0.208 | |||
| P | 0.004 | 0.004 | 0.001 | 0.333 | 0.001 | 0.011 | 0.022 | ||||
| TMD | R | 0.506 | 0.194 | 0.001 | 0.261 | 0.094 | −0.058 | ||||
| P | 0.001 | 0.026 | 0.990 | 0.002 | 0.298 | 0.522 | |||||
| IMD | R | 0.381 | −0.033 | 0.208 | 0.099 | −0.027 | |||||
| P | 0.001 | 0.714 | 0.017 | 0.283 | 0.773 | ||||||
| IMT | R | 0.091 | 0.291 | 0.225 | −0.076 | ||||||
| P | 0.309 | 0.001 | 0.014 | 0.419 | |||||||
| EDVmax | R | 0.175 | 0.190 | −0.117 | |||||||
| P | 0.049 | 0.041 | 0.213 | ||||||||
| CYSC | R | 0.263 | −0.108 | ||||||||
| P | 0.003 | 0.234 | |||||||||
| DD | R | −0.192 | |||||||||
| P | 0.038 |
Table 4.
Correlation of each index and CA
| Index | AID | MVEI | MVEA | TMD | IMD | IMT | EDVmax | CYSC | DD | DST | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | R | 0.421 | −0.630 | −0.670 | 0.335 | 0.335 | 0.464 | 0.230 | 0.405 | 0.326 | −0.356 |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.009 | 0.001 | 0.001 | 0.001 | |
| AID | R | −0.309 | −0.336 | 0.330 | 0.189 | 0.121 | 0.011 | 0.341 | 0.138 | −0.178 | |
| P | 0.001 | 0.001 | 0.001 | 0.031 | 0.170 | 0.905 | 0.001 | 0.124 | 0.049 | ||
| MVEI | R | 0.654 | −0.278 | −0.198 | −0.406 | −0.136 | −0.322 | −0.160 | 0.223 | ||
| P | 0.001 | 0.001 | 0.024 | 0.001 | 0.125 | 0.001 | 0.073 | 0.014 | |||
| MVEA | R | −0.244 | −0.250 | −0.326 | −0.086 | −0.304 | −0.225 | 0.208 | |||
| P | 0.004 | 0.004 | 0.001 | 0.333 | 0.001 | 0.011 | 0.022 | ||||
| TMD | R | 0.506 | 0.194 | 0.001 | 0.261 | 0.094 | −0.058 | ||||
| P | 0.001 | 0.026 | 0.990 | 0.002 | 0.298 | 0.522 | |||||
| IMD | R | − | − | 0.381 | −0.033 | 0.208 | 0.099 | −0.027 | |||
| P | 0.001 | 0.714 | 0.017 | 0.283 | 0.773 | ||||||
| IMT | R | 0.091 | 0.291 | 0.225 | −0.076 | ||||||
| P | 0.309 | 0.001 | 0.014 | 0.419 | |||||||
| EDVmax | R | − | 0.175 | 0.190 | −0.117 | ||||||
| P | 0.049 | 0.041 | 0.213 | ||||||||
| CYSC | R | 0.263 | −0.108 | ||||||||
| P | 0.003 | 0.234 | |||||||||
| DD | R | −0.192 | |||||||||
| P | 0.038 |
Table 5.
General summary of selected biomarkers of age (BoA)
| BoA | Mean | Std. deviation |
|---|---|---|
| TRF | 6.272 | 1.507 |
| CYSC | 0.769 | 0.144 |
| DD | 426.786 | 165.420 |
| MVEA | 0.135 | 0.0415 |
| IMT | 0.641 | 0.115 |
| DST | 35.670 | 12.978 |
Construction of the BAS formula
TRF, MVEA, IMT, CYSC, DD, and DST underwent FA. The results are shown in Table 6. The proportion of each index was greater than 0.5 (0.540–0.682).
Table 6.
Component matrix and component score coefficient matrix of biomarkers of age (BoA)
| BoA | Component matrix | Component score coefficient matrix |
|---|---|---|
| TRF | −0.540 | −0.240 |
| CYSC | 0.596 | 0.265 |
| DD | 0.665 | 0.295 |
| MVEA | −0.682 | −0.303 |
| IMT | 0.635 | 0.282 |
| DST | −0.542 | −0.241 |
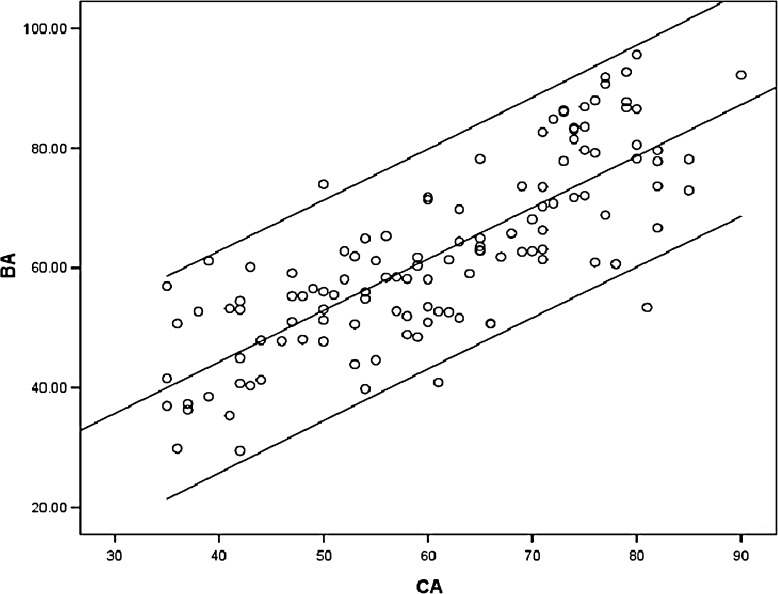
The score coefficient of each biomarker is shown in Table 6. BAS was derived via FA and was as follows: BAS = −0.240TRF + 0.265CYSC − 0.241DST + 0.282IMT − 0.303MVEA + 0.295DD. The calculated BA using the BAS formula and CA was as follows: BA = −2.281TRF + 26.321CYSC + 0.0256DD − 104.419MVEA + 34.863IMT − 0.265DST + 44.734. The final corrected BA formula was as follows: Corrected BA = −2.281TRF + 26.321CYSC + 0.0256DD − 104.419MVEA + 34.863IMT − 0.265 DST + 0.305CA + 26.346 (Table 7). The scatter plot of the corrected BA formula with CA is shown in Fig. 2.
Table 7.
The linear correlation results of chronological age and biological age
| Model | Unstandardized coefficients | Standardized coefficients | t | p value | |
|---|---|---|---|---|---|
| B | Std. error | Beta | |||
| Constant | 18.58 | 3.764 | 4.937 | 0.001 | |
| Age | 0.695 | 0.061 | 0.699 | 11.442 | 0.001 |
Fig. 2.
Scatter plot of the corrected BA formula with CA
Discussion
The base of building a BA equation is the selection of biomarkers of aging, which are closely related with biological aging parameters. In the past, CA has served as a benchmark for selecting aging biomarkers; however, CA does not accurately reflect aging conditions. Thus, in this study, we used an alternative benchmark to select the aging biomarkers.
Genetic components play an important role in aging (Guarente and Kenyon 2000), and 20–50 % of the variation in lifespan is caused by genetic differences (Yashin et al. 1999; Mitchell et al. 2001; Skytthe et al. 2003). Telomeres are the cap structure of eukaryotic chromosome ends and play an essential role in preventing chromosome degradation, fusion, or recombination, in maintaining the stability and integrity of the chromosome and to ensure a complete copy of the genetic information (Blackburn 1991). Telomeres shorten with each cell division, ultimately leading to critically short telomeres in normal somatic cells. Telomere shortening is an inherent mechanism of cellular senescence and reflects the changes in functional aging. Thus, telomere length is considered to be a marker of biological age (Sanders and Newman 2013; Boonekamp et al. 2013; López-Otín et al. 2013). In this study, we attempted to use TRF as a benchmark to select other biomarkers of aging to build a BA equation. Considering that this is the first study to use the TRF as a benchmark, we also included CA as the conventional benchmark to maintain consistency with previous studies. We found that all the indices related to CA were related to TRF. Surprisingly, the indices correlated better with TRF than with CA, suggesting that TRF is a more rigorous benchmark than CA. The aging markers in this study were MVEA, IMT, CYSC, DD, and DST.
In current and our previous studies, IMT was selected as a biomarker of aging in healthy individuals. Carotid IMT has been associated with many cardiovascular outcomes such as systemic endothelial function (Halcox et al. 2009). It was proposed as a predictor of vascular events in the IMPROVE study (Baldassarre et al. 2013) and as a predictor of stroke in a multi-ethnic study of atherosclerosis (Polak et al. 2011). D-dimer levels increase with age (Tita-Nwa et al. 2010) and have served as a marker of atherothrombotic risk in healthy postmenopausal women (Pradhan et al. 2004). In addition, fibrin D-dimer associated with increased risk of stroke in aged males may be a useful marker to identify hypertensive patients with a high risk for stroke (Wannamethee et al. 2012). Increased levels of D-dimer in endothelial dysfunction have also been identified (Quinn et al. 2011). Serum cystatin C is a marker of glomerular filtration rate (GFR) and serves as a more sensitive marker of kidney function than serum creatinine in measuring normal kidney function (Dharnidharka et al. 2002). In addition, cystatin C can predict all-cause mortality and cardiovascular disease (Emberson et al. 2010; Lee et al. 2010; Bansal et al. 2012) as well as acute kidney injury (Zhang et al. 2011).
Our study found that the correlation coefficient of TRF and CA was −0.314, similar to the average level of the 124 cross-sectional studies reviewed by Müezzinler et al. (2013).
In this study, we found a correlation between telomere length and the indicators of cardiovascular function, including MVEA and IMT, but correlations were not found between telomere length and ECG parameters. This is consistent with previous studies (De Meyer et al. 2009; O’Donnell et al. 2008; Fitzpatrick et al. 2007). The reason may be that ECG is an indirect indicator for heart diseases but is not age dependent. Interestingly, in previous studies, blood pressure (BP) indices (MacDonald et al. 2004) such as systolic BP (Nakamura and Miyao 2007, 2008; Park et al. 2009; Hollingsworth et al. 1965; Bae et al. 2008), diastolic BP (Bae et al. 2008; Yashin et al. 2010), and pulse pressure (Zhang et al. 2014; Bai et al. 2010; Yashin et al. 2010) were selected as biomarkers of aging. Telomere length is closely associated with BP and may replace BP indices in building BA equations (Benetos et al. 2001).
A study including 38 female patients of dementia or dysfunction showed that TRF shortening was independently associated with declining episodic memory and learning (p = 0.032), non-verbal recognition memory (p = 0.007), and working memory capacity (p = 0.003) (Valdes et al. 2010). In this study, we found that DST had a high correlation coefficient with CA and TRF as compared to the trail making test (TMT) (Zhang et al. 2014), which reflects brain function. Previous studies have shown that TRF does not correlate with age-related decreases of lung function (Harris et al. 2006; Mather et al. 2010) or bone mineral density (Sanders et al. 2009; Tang et al. 2010; Valdes et al. 2007). Thus, in this study, we did not select lung or bone indicators.
Both diseases and aging can lead to TRF shortening (Sanders and Newman 2013). Thus, we selected healthy subjects in this study to avoid the interference of disease factors (Rattan 2013). In order to compare with previous studies, we used both TRF and CA as benchmarks. Interestingly, we found that all TRF-associated markers also correlated with CA, but not vice versa, suggesting that TRF is a more rigorous benchmark than CA. Note that all the aging markers are disease markers; however, not all disease markers are aging markers, indicating an intrinsic link between age and disease processes.
The number of samples analyzed in the present study was limited. This cross-sectional study needs to be validated by longitudinal studies. Nevertheless, the proposed BA equation, which uses both CA and TRF as benchmarks, consists of both genetic and physiological characteristics of aging and may serve as a better tool to assess biological age than previous biological age which only consist by vital organ function indexes.
Acknowledgments
We are grateful for those who participated in this research. This work was supported by the National Basic Research Program of China (No. 2103CB530800) and the National Key Technology R&D Program (No. 2011BAI10B00).
Appendix All indices (total 105)
Life habits survey (20)
All subjects received a survey concerning their smoking and alcohol habits, dietary patterns, frequency of physical activity, and other lifestyle factors. Daily living conditions were assessed with the following general indicators: educational extent, marital status, occupation, number of family members, relationship between family members, housing situation, annual income, self-assessment of economic status, method of staying healthy, frequency of performing daily activities, participation in interest groups, daily living conditions, smoking status, smoking age, smoking amount, smoking cessation time, number of smokers in the household, number of smokers in the workplace, frequency of exercise for >30 min, and overall mental state over the last year.
Blood pressure and body weight measurements (5)
Measurements were made in a quiet environment after the subject had rested for >15 min. Measurements were made according to the Krotkoff 5 method. The pulse pressure was calculated as PP = systolic blood pressure (SBP) − diastolic blood pressure (DBP). The body mass index (BMI) and waist-to-hip ratio (WHR) were measured simultaneously.
Cardiovascular ultrasound measurements (25)
Cardiovascular ultrasound measurements included the following parameters: left ventricular ejection fraction (LVEF); mitral early and mitral late diastolic peak flow velocity (MVE and MVA, respectively); ratio of the peak velocity of early filling to the peak velocity of atrial filling (E/A); mitral valve annulus lateral wall, anterior wall, inferior wall, and ventricular septum of the peak velocity of early filling (MVEL, MVEA, MVEI, and MVES, respectively); mitral valve annulus lateral wall, anterior wall, inferior wall, and ventricular septum of the peak velocity of atrial filling (MVAL, MVAA, MVAI, and MVAS, respectively); maximum and minimum peak systolic velocity (SPVmax and SPVmin, respectively); maximum and minimum carotid artery end-diastolic velocity (EDVmax and EDVmin, respectively); maximum and minimum internal diameter of the carotid artery (Dmax and Dmin, respectively); maximum and minimum carotid artery intimal-medial thickness (IMTmax and IMTmin, respectively); and heart rate (HR).
Blood biochemistry measurements (13)
Blood biochemistry was examined through routine blood analysis and urinalysis procedures. Serum or urine levels of urea (UR), creatinine (Cr), triglyceride, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), alanine aminotransferase, alanine transaminase, total protein (TP), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), and glucose (Glu) were measured.
Brain function measurement (7)
To assess brain function, the following factors were assessed: clock drawing test (CDT); stroop response time; stroop mistake number; trail making test (TMT), forward and backward digit span tasks (FDST and BDST, respectively); and mini-mental state examination (MMSE).
Genetics indicators (1)
Terminal telomere restriction fragment (TRF).
Urine (4)
The pH, specific gravity, and conductivity of urine were analyzed.
Routine blood (18)
Routine blood analyses included measurements of the following parameters: white blood cell count (WBC), lymphocytes, granulocytes (GRAN), red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell volume distribution width (RDW), platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW), platelet hematocrit (PCT), monocytes (MON), and the relative percentage of lymphocytes (LRR%), granulocytes (RPR%), and monocytes (MPR%).
Special index (6)
Other tested indices included Cystatin C (CysC), interleukin 6 (IL-6), C-reactive protein (CRP), D-dimer (DD), fibrinogen (Fib), and GFR (dual GFR).
ECG index (7)
ST, T, QRS, QT, QTC, PR, and heart rate.
Footnotes
Wei-Guang Zhang and Shu-Ying Zhu contributed equally to this work.
Contributor Information
Xue-Feng Sun, Email: xfssun@126.com.
Xiang-Mei Chen, Phone: +86-10-66937463, FAX: +86-10-68130297, Email: xmchen301@126.com.
References
- AihieSayer A, Osmond C, Briggs R, Cooper C. Do all systems age together? Gerontology. 1999;45(2):83–86. doi: 10.1159/000022068. [DOI] [PubMed] [Google Scholar]
- Bae C-Y, Kang YG, Kim S, Cho C, Kang HC, Yu BY, Lee S-W, Cho KH, Lee DC, Lee K. Development of models for predicting biological age (BA) with physical, biochemical, and hormonal parameters. Arch Gerontol Geriatr. 2008;47(2):253–265. doi: 10.1016/j.archger.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Bai X, Han L, Liu Q, Shan H, Lin H, Sun X, Chen X. Evaluation of biological aging process—a population-based study of healthy people in China. Gerontology. 2010;56(2):129–140. doi: 10.1159/000262449. [DOI] [PubMed] [Google Scholar]
- Baker GT, 3rd, Sprott RL. Biomarkers of aging. Exp Gerontol. 1988;23(4–5):223–239. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, Smit AJ, Giral P, Kurl S, Mannarino E. Progression of carotid intima-media thickness as predictor of vascular events results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013;33(9):2273–2279. doi: 10.1161/ATVBAHA.113.301844. [DOI] [PubMed] [Google Scholar]
- Bansal N, Whooley MA, Regan M, McCulloch CE, Ix JH, Epel E, Blackburn E, Lin J, C-y H. Association between kidney function and telomere length: the heart and soul study. Am J Nephrol. 2012;36(5):405–411. doi: 10.1159/000343495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25(4):3011–3021. [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2):381–385. doi: 10.1161/01.HYP.37.2.381. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging. 2009;1(3):281. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell. 2013;2(2):330–332. doi: 10.1111/acel.12050. [DOI] [PubMed] [Google Scholar]
- Butler RN, Sprott R, Warner H, Bland J, Feuers R, Forster M, Fillit H, Harman SM, Hewitt M, Hyman M. Biomarkers of aging: from primitive organisms to humans. J Gerontol Ser A. 2004;59:560–567. doi: 10.1093/gerona/59.6.B560. [DOI] [PubMed] [Google Scholar]
- Cuende JI, Cuende N, Calaveras-Lagartos J. How to calculate vascular age with the SCORE project scales: a new method of cardiovascular risk evaluation. Eur Heart J. 2010;31(19):2351–2358. doi: 10.1093/eurheartj/ehq205. [DOI] [PubMed] [Google Scholar]
- De Meyer T, Rietzschel ER, De Buyzere ML, Langlois MR, De Bacquer D, Segers P, Van Damme P, De Backer GG, Van Oostveldt P, Van Criekinge W. Systemic telomere length and preclinical atherosclerosis: the Asklepios Study. Eur Heart J. 2009;30(24):3074–3081. doi: 10.1093/eurheartj/ehp324. [DOI] [PubMed] [Google Scholar]
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- Dubina TL, Mints AYa, Zhuk, EV (1984) Biological age and its estimation. III. Introduction of a correction to the multiple regression model of biological age in cross-sectional and longitudinal studies. Exp Gerontol 19(2):133–143 [DOI] [PubMed]
- Emberson J, Haynes R, Dasgupta T, Mafham M, Landray M, Baigent C, Clarke R. Cystatin C and risk of vascular and nonvascular mortality: a prospective cohort study of older men. J Intern Med. 2010;268(2):145–154. doi: 10.1111/j.1365-2796.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Fossel M. Use of telomere length as a biomarker for aging and age-related disease. Curr Transl Geriatr Gerontol Rep. 2012;1(2):121–127. doi: 10.1007/s13670-012-0013-6. [DOI] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408(6809):255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Guinot C, Malvy DJ-M, Ambroisine L, Latreille J, Mauger E, Tenenhaus M, Morizot F, Lopez S, Le Fur I, Tschachler E. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol. 2002;138(11):1454. doi: 10.1001/archderm.138.11.1454. [DOI] [PubMed] [Google Scholar]
- Gunn DA, Rexbye H, Griffiths CE, Murray PG, Fereday A, Catt SD, Tomlin CC, Strongitharm BH, Perrett DI, Catt M. Why some women look young for their age. PLoS ONE. 2009;4(12):e8021. doi: 10.1371/journal.pone.0008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Divyashree R, Abhilash P, Bijle MNA, Murali K. Correlation between chronological age, dental age and skeletal age among monozygoyic and dizygotic twins. J Int Oral Health: JIOH. 2013;5(1):16. [PMC free article] [PubMed] [Google Scholar]
- Hägg U, Matsson L. Dental maturity as an indicator of chronological age: the accuracy and precision of three methods. Eur J Orthod. 1985;7(1):25. doi: 10.1093/ejo/7.1.25. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119(7):1005–1012. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Hodkinson H. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972;1(4):233–238. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- Hodkinson H. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 2012;41(suppl_3):iii35–iii40. doi: 10.1093/ageing/afs148. [DOI] [PubMed] [Google Scholar]
- Hollingsworth JW, Hashizume A, Jablon S. Correlations between tests of aging in Hiroshima subjects—an attempt to define "physiologic age". Yale J Biol Med. 1965;38(1):11. [PMC free article] [PubMed] [Google Scholar]
- Hong S-J, Lui CSM, Hahn J, Moon JY, Kim TK (2013) How old are you really? Cognitive age in technology acceptance. Decis Support Syst 56:122–130
- Jee H, Jeon BH, Kim YH, Kim H-K, Choe J, Park J, Jin Y. Development and application of biological age prediction models with physical fitness and physiological components in Korean adults. Gerontology. 2012;58(4):344–353. doi: 10.1159/000335738. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41(12):1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Saver JL, Huang W-H, Chow J, Chang K-H, Ovbiagele B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations a meta-analysis. Circ: Cardiovasc Qual Outcome. 2010;3(6):675–683. doi: 10.1161/CIRCOUTCOMES.110.957696. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: findings from the Victoria longitudinal study. Gerontology. 2004;50(2):64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- Majkić-Singh N. What is a biomarker? From its discovery to clinical application. J Med Biochem. 2011;30(3):186–192. [Google Scholar]
- Mak K (2013) The normal physiology of aging. Colorectal cancer in the elderly. Springer, Heidelberg
- Mather KA, Jorm AF, Milburn PJ, Tan X, Easteal S, Christensen H. No associations between telomere length and age-sensitive indicators of physical function in mid and later life. J Gerontol A: Biol Med Sci. 2010;65(8):792–799. doi: 10.1093/gerona/glq050. [DOI] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A: Biol Med Sci. 2011;66(2):202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Biomarkers of age and aging. Exp Gerontol. 1997;32(1–2):87–94. doi: 10.1016/S0531-5565(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Hsueh WC, King TM, Pollin TI, Sorkin J, Agarwala R, SchaÈffer AA, Shuldiner AR. Heritability of life span in the Old Order Amish. Am J Med Genet. 2001;102(4):346–352. doi: 10.1002/ajmg.1483. [DOI] [PubMed] [Google Scholar]
- Mitnitski A, Song X, Rockwood K (2013) Assessing biological aging: the origin of deficit accumulation. Biogerontology 14(6):709–717 [DOI] [PMC free article] [PubMed]
- Mooradian AD. Biomarkers of aging: do we know what to look for? J Gerontol. 1990;45(6):B183–B186. doi: 10.1093/geronj/45.6.B183. [DOI] [PubMed] [Google Scholar]
- Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A: Biol Med Sci. 2007;62(10):1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. Sex differences in human biological aging. J Gerontol A: Biol Med Sci. 2008;63(9):936–944. doi: 10.1093/gerona/63.9.936. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K, Ozeki T. Assessment of biological age by principal component analysis. Mech Ageing Dev. 1988;46(1):1–18. doi: 10.1016/0047-6374(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Cutler RG, Ingram DK. Evaluating measures of hematology and blood chemistry in male rhesus monkeys as biomarkers of aging. Exp Gerontol. 1994;29(2):151–177. doi: 10.1016/0531-5565(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: further evaluation of hematology and blood chemistry data from a calorie restriction study in rhesus monkeys. Exp Gerontol. 1998;33(5):421–443. doi: 10.1016/S0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, D’Agostino RB, Wolf PA, Polak J, Cupples LA. Leukocyte telomere length and carotid artery intimal medial thickness the Framingham heart study. Arterioscler Thromb Vasc Biol. 2008;28(6):1165–1171. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Cho B, Kwon H, Lee C. Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in Korean men. Arch Gerontol Geriatr. 2009;49(1):7–12. doi: 10.1016/j.archger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42(11):3017–3021. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, LaCroix AZ, Langer RD, Trevisan M, Lewis CE, Hsia JA, Oberman A, Kotchen JM, Ridker PM. Tissue plasminogen activator antigen and D-dimer as markers for atherothrombotic risk among healthy postmenopausal women. Circulation. 2004;110(3):292–300. doi: 10.1161/01.CIR.0000134965.73212.A6. [DOI] [PubMed] [Google Scholar]
- Quinn T, Gallacher J, Deary I, Lowe G, Fenton C, Stott D. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta‐analyzes. J Thromb Haemost. 2011;9(8):1475–1482. doi: 10.1111/j.1538-7836.2011.04403.x. [DOI] [PubMed] [Google Scholar]
- Rattan SI (2013) Healthy ageing, but what is health? Biogerontology 14(6):673–677 [DOI] [PubMed]
- Reff ME, Schneider EL, Health E-UNIo (1982) Biological markers of aging. Dept. of Health and Human Services
- Rippon I, Kneale D, de Oliveira C, Demakakos P, Steptoe A. Perceived age discrimination in older adults. Age Ageing. 2013 doi: 10.1093/ageing/aft146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M, Berlin RM, Cruz TD. Exploring the utility of genetic markers for predicting biological age. Legal Med. 2012;14(6):279–285. doi: 10.1016/j.legalmed.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35(1):112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Cauley JA, Boudreau RM, Zmuda JM, Strotmeyer ES, Opresko PL, Hsueh WC, Cawthon RM, Li R, Harris TB. Leukocyte telomere length is not associated with BMD, osteoporosis, or fracture in older adults: results from the health, aging and body composition study. J Bone Miner Res. 2009;24(9):1531–1536. doi: 10.1359/jbmr.090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm A, Johnson TE. Biomarkers of ageing: a challenge for the future. Exp Gerontol. 2010;45(10):731–732. doi: 10.1016/j.exger.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Pedersen NL, Kaprio J, Stazi MA, Iachine I, Vaupel JW, Christensen K. Longevity studies in GenomEUtwin. Twin Res. 2003;6(5):448–454. doi: 10.1375/136905203770326457. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Biomarkers of aging and disease: introduction and definitions. Exp Gerontol. 2010;45(1):2–4. doi: 10.1016/j.exger.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Clarfield AM. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- Tang N, Woo J, Suen E, Liao C, Leung J, Leung P. The effect of telomere length, a marker of biological aging, on bone mineral density in elderly population. Osteoporos Int. 2010;21(1):89–97. doi: 10.1007/s00198-009-0948-4. [DOI] [PubMed] [Google Scholar]
- Tita-Nwa F, Bos A, Adjei A, Ershler WB, Longo DL, Ferrucci L. Correlates of D-dimer in older persons. Aging Clin Exp Res. 2010;22(1):20. doi: 10.1007/BF03324810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A, Ishiko A, Takasaki T, Ikeda N. Estimating age of humans based on telomere shortening. Forensic Sci Int. 2002;126(3):197–199. doi: 10.1016/S0379-0738(02)00086-5. [DOI] [PubMed] [Google Scholar]
- Ueno LM, Yamashita Y, Moritani T, Nakamura E. Biomarkers of aging in women and the rate of longitudinal changes. J Physiol Anthropol Appl Hum Sci. 2003;22(1):37–46. doi: 10.2114/jpa.22.37. [DOI] [PubMed] [Google Scholar]
- Valdes A, Richards J, Gardner J, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector T. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007;18(9):1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- Valdes A, Deary I, Gardner J, Kimura M, Lu X, Spector T, Aviv A, Cherkas L. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol Aging. 2010;31(6):986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Whincup PH, Lennon L, Rumley A, Lowe GD. Fibrin D-dimer, tissue-type plasminogen activator, von Willebrand factor, and risk of incident stroke in older men. Stroke. 2012;43(5):1206–1211. doi: 10.1161/STROKEAHA.111.636373. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Iachine IA, Harris JR. Half of the variation in susceptibility to mortality is genetic: findings from Swedish twin survival data. Behav Genet. 1999;29(1):11–19. doi: 10.1023/A:1021481620934. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Akushevich I, Arbeeva L, Kravchenko J, Il’yasova D, Kulminski A, Akushevich L, Culminskaya I, Wu D (2010) Dynamic determinants of longevity and exceptional health. Curr Gerontol Geriatr Res. doi:10.1155/2010/381637 [DOI] [PMC free article] [PubMed]
- Zglinicki T, Martin-Ruiz C. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5(2):197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58(3):356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- Zhang WG, Bai XJ, Sun XF, Cai GY, Bai XY, Zhu SY, Zhang M, Chen XM (2014) Construction of an integral formula of biological age for a healthy chinese population using principle component analysis. J Nutr Health Aging. 18(2):137–142 [DOI] [PubMed]