Abstract
Cancer is an age-associated disease. Although the mechanisms of age-associated increase in cancer incidence are not completely understood, it is believed that the tumor stromal environment significantly influences epithelial malignancy. Fibroblasts are a major cell type in the stroma and, under normal conditions, fibroblasts reside in the quiescent state. Cellular quiescence is a reversible process where cells enter into the proliferative cycle and then exit back to quiescence. We have shown previously that quiescent fibroblasts lose their proliferative capacity as they age, and we defined this mode of cellular aging as chronological life span. Using conditioned media and co-culture experiments, results from this study show that normal human fibroblasts (NHFs) nearing the end of their chronological life span stimulate the proliferation of MB231 and MCF7 human breast epithelial cancer cells. Chemokine C-C motif ligand 5 (CCL5) expression was found to be approximately 8-fold higher in old compared to that in young quiescent NHFs, which correlated with an increase in the ERK1/2-cyclin D1 pro-proliferative pathway in MB231 cells. Conditioned media treated with anti-CCL5 antibody suppressed the activation of the ERK1/2-cyclin D1 pathway and proliferation of MB231 cells. Hydroxytyrosol, a dietary polyphenol and an active ingredient of olive, inhibited CCL5 expression in aging quiescent NHFs. This inhibition was associated with NHFs inability to activate the ERK1/2-cyclin D1 pathway and enhance proliferation of MB231 cells. These results show that fibroblasts nearing the end of their chronological life span promote proliferation of human breast epithelial cancer cells and dietary polyphenols inhibit this process.
Keywords: CCL5, Hydroxytyrosol, Tumor microenvironment, Aging, Quiescent fibroblasts
Introduction
The incidence of cancer increases with age. Although the mechanisms of age-associated increase in cancer are not completely understood, it is hypothesized that tumor microenvironment significantly influences cancer cell proliferation. Tumor microenvironment includes many cell types: cancer cells, innate and adaptive immune cells, endothelial cells, and fibroblasts. Fibroblasts are no longer considered passive bystanders of the tumor microenvironment. They contribute to the synthesis and remodeling of the extracellular matrix as well as the production of secretory and non-secretory factors that influence cancer cell proliferation in a stromal environment.
We have previously shown that quiescent normal human fibroblasts (NHFs) undergo age-associated changes including loss of proliferative capacity, mitochondrial injury, and an increase in oxidative stress (Sarsour et al. 2005, 2008, 2010, 2012). Because this mode of aging was distinct from the replicative life span that refers to limited number of cell division and telomere attrition, we termed it as “chronological life span” (Sarsour et al. 2005). Chronological life span is defined as the duration that quiescent (G0) normal cells retain their capacity to re-enter the proliferative cycle and transit back to quiescence. Overexpression of the nuclear encoded and mitochondria matrix localized manganese superoxide dismutase (MnSOD) suppressed age-associated mitochondrial abnormalities and extended chronological life span of fibroblasts (Sarsour et al. 2005, 2010). A similar extension in chronological life span was also observed in long-duration quiescent fibroblasts that were cultured in media supplemented with the phytochemical, hydroxytyrosol (Sarsour et al. 2012). Hydroxytyrosol (2-(3,4-dihydroxyphenyl) ethanol) is a hydrophilic polyphenol commonly found in olives (Schaffer et al. 2007). We have previously shown that hydroxytyrosol undergoes catechol-semiquinone-quinone redox cycling to generate superoxide that was associated with a significant increase in MnSOD expression, suggesting that hydroxytyrosol could impact chronological life span by activating MnSOD expression and suppressing mitochondrial oxidative stress in aging quiescent fibroblasts (Sarsour et al. 2010, 2012).
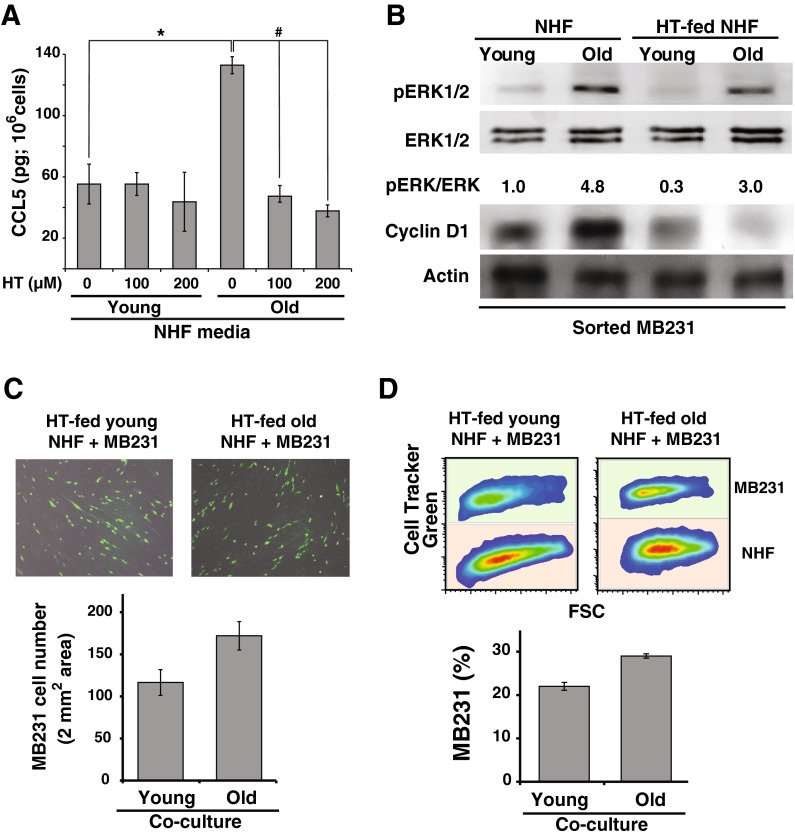
Consistent with the hypothesis of both secretory and non-secretory factors contributing to fibroblast-associated increases in cancer cell proliferation, our recent results showed a significant increase in the expression of the chemokine C-C motif ligand 5 (CCL5/RANTES) in quiescent human fibroblasts (Kumar et al. 2012). CCL5 functions primarily through binding to its receptor, CCR5. CCL5 can also bind to CCR1 and CCR3. CCRs belong to the G protein-coupled receptor family. CCL5-CCR5 interaction activates the MAPK signaling pathways promoting proliferation (Soriano et al. 2003; Wong et al. 2001; Turner et al. 1995, 1998; Bacon et al. 1995). Results from this study showed a significant increase in CCL5 expression in aged compared to young quiescent fibroblasts. Breast cancer cell proliferation in co-cultures of aged quiescent fibroblasts was significantly higher compared to co-cultures of young quiescent fibroblasts. CCL5 secreted from aged quiescent fibroblasts was associated with the activation of the ERK1/2-cyclin D1 pathway in MB231 cancer cells promoting their proliferation. These effects were blunted in co-cultures of MB231 and hydroxytyrosol-fed aged quiescent fibroblasts.
Materials and methods
Cell culture
Human normal skin fibroblasts (AG01522D, isolated from a 3-day-old individual) were purchased from the Coriell Cell Repositories. MDA-MB-231 and MCF7 human mammary epithelial cancer cells were purchased from ATCC. Normal human fibroblasts (NHFs) were cultured in Dulbecco's modified Eagle's medium (Gibco, USA) supplemented with 10 % fetal bovine serum (HyClone, USA) and antibiotics. One million NHFs per T-25 tissue culture flask were cultured for different days with change in media every 3 days (Sarsour et al. 2005). Quiescence was achieved by contact inhibition. Cultures with less than 5 % S-phase cells were considered quiescent. Quiescent cultures of less than 20 days were considered young, while quiescent cultures of more than 50 days were considered old. The proliferative capacity of quiescent NHFs started to decline beginning 30 days of quiescence (Sarsour et al. 2005). We used distinct cellular and molecular parameters to group NHFs into “young” and “old” categories based on our previously published results (Sarsour et al. 2005, 2010, 2012). Quiescent NHFs that retain their proliferative capacity (i.e., these NHFs are able to re-enter the proliferative cycle and then exit back to the quiescent state) and exhibit a lower level of the cyclin D1/CDK4,6 inhibitor p16 (p16 inhibits cell cycle progression) are considered as “young.” Quiescent NHFs aged in culture that lose their proliferative capacity and have a higher level of p16 (at least 2-fold increase) are considered as “old.” Conditioned media collected from young and old quiescent NHF cultures were filtered (0.2 μm filter) and supplemented with 25 % DMEM media prior to their use in culturing cancer cells. Quiescent NHFs cultured in presence of hydroxytyrosol (3,4-dihydroxyphenyl ethanol; purity ≥98 %; Cayman Chemical Company, USA) were replenished every 3 days with fresh media containing hydroxytyrosol. NHFs of passages 7 and 8 were used in all experiments.
Co-culture of quiescent fibroblasts and breast cancer cells
Co-culture experiments were performed using DMEM-adapted NHFs and MB231 cells. MB231 cells were first incubated with CellTracker™ fluorescent green probe (Invitrogen, USA), and labeled cells were washed to remove un-incorporated dye. An equal number of CellTracker green-labeled MB231 cells was plated on lawns of young and old quiescent NHFs and co-cultured for 5 days prior to analysis. CellTracker green-positive (MB231) and negative (NHFs) cells were analyzed using Becton Dickinson Aria II flow cytometer, 488 nm excitation laser, and 530/30 band pass filter for emission. Flow cytometry-based aseptic cell sorting was performed to separate MB231 cells from co-cultures of NHFs. An aliquot of the sorted MB231 cells was cultured in regular media for 3 days, and cell numbers were counted using a Z1 Coulter Counter (Beckman Coulter, USA). The remainder of the sorted MB231 cells was used for western blot analysis.
Proliferation of MB231 cells in co-cultures of NHFs was also evaluated using a fluorescent microscope equipped with ultraviolet epi fluorescent source and 520 nm green long pass filter (Olympus, USA). The microscope is equipped with a high-resolution CCD camera (2,040 × 1,536 pixels) and 10× objective lens. Five random fields were captured for each sample, and five samples were included for each treatment. Cell density was determined by ImageJ (NIH, USA) using the particle counter software. CellTracker green MB231 cells were counted after the image was processed for green channel separation; threshold and particle size adjusted to minimal visible cell size in an area of 2 mm2.
Doubling time and plating efficiency
Cells were plated in 60 mm dishes and counted every 2 days. Monolayer cultures were trypsinized and cell number counted using a Z1 Coulter Counter (Beckman Coulter, USA). To calculate population doubling time, we applied regression plots of exponential growth and then used the equation Td = 0.693 t/ln(Nt/N0) where t represents time in days, Nt represents cell numbers at time (t), and N0 represents initial number of cells. For measurements of plating efficiency, monolayer cultures were trypsinized and replated at limited dilutions in 60 mm dishes. After 14 days of culture, cells were fixed in ethanol and stained with 0.05 % Coomassie blue. Colonies with 50 or more cells were counted and the plating efficiency was calculated, (number of colonies/number of cells plated) × 100.
CCL5 neutralization experiments were done using monoclonal human CCL5/RANTES antibody (R and D, USA). This antibody has a neutralization dose (ND50) of 100–400 ng/ml. Conditioned media collected from NHFs were incubated with the CCL5 antibody for 2 h prior to their use in culturing MB231 cells.
Protein analysis
Cell lysates were prepared by sonication of cell pellets that were resuspended in lysis buffer: phosphate buffer (pH 7.4), 2 % NP-40, DNAase (Zymo Research Corp., USA), phosphatase (PhosSTOP, Roche, USA), and protease inhibitor cocktail (Sigma, USA). Equal amount of proteins were separated by 12 % SDS-PAGE and transferred onto a nitrocellulose membrane using a semi-dry apparatus. Membranes were incubated with antibodies against cyclin D1 (1:1,000, BD, USA), ERK1/2 (1:1,000, Cell Signaling, USA), phosphorylated ERK1/2 (1:1,000, Cell Signaling, USA), and actin (1:1,000, Millipore, USA). Secondary antibodies used were the following: HRP-conjugated anti-mouse antibodies from sheep (1:5,000, GE Healthcare) and anti-rabbit antibodies from goat (1:10,000, GE Healthcare). Immunoreactive bands were developed by Pierce ECL 2 western blotting substrate (Thermo Scientific, USA), and Typhoon FLA 7000 (GE, USA) was used to visualize and quantitate results. Actin protein levels were used for loading correction.
CCL5 protein levels in media were measured by using a ready-made ELISA kit following the manufacture provided protocol (Invitrogen, USA). The assay is sensitive enough to detect 2 pg/ml of CCL5. Using this assay, we did not detect CCL5 in regular cell culture media. Therefore, we included regular cell culture media as a negative control for the assay. Different amounts of recombinant human CCL5 were added to regular cell culture media and a standard curve was generated. This standard curve was used to calculate CCL5 levels in conditioned media collected from NHFs, and results were calculated as picograms per one million cells.
Real-time PCR
Total cellular RNA was isolated using the Trizol reagent (Invitrogen, USA). RNA was quantified using a ND1000 nanodrop spectrophotometer (Nanodrop, USA). cDNA was synthesized using the high-capacity cDNA archive kit (Applied Biosystems, USA). Real-time PCR assay was performed using 2× Power SYBR green real-time master-mix (Applied Biosystems, USA) using the following set up: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min (StepOne plus Sequence Detection System, Applied Biosystems, USA). A threshold of amplification in the linear range was selected to calculate the cycle threshold (CT) for each sample. The relative mRNA levels were calculated as follows: ΔCT (sample) = CT (mRNA of interest) − CT (18S); ΔΔCT = ΔCT (post-treatment time point) − ΔCT (control); 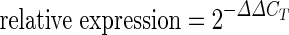 . Primer pairs (forward–reverse; amplicon size) used for amplification were as follows: CCL5 (GCAGCCCTCGCTGTCATCCT–AAGACGACTGCTGGGTTGGAGC; 176 bp); 18S (AACTTTCGATGGTAGTCGCCG–CCTTGGATGTGGTAGCCGTTT; 84 bp); CCR1 (AAGTCCCTTGGAACCAGAGAGAAG–TCCAACCAGGCCAATGACAAA; 182 bp); CCR3 (TGTTTCAGGAGTGGTGACGC–TTCACTTCTCCAATACAACTCAGCA; 235 bp); CCR5 (CTGGCATAGTATTCTGTGTAGTGGG–TGTTTCTTTTGAAGGAGGGTGGA; 202 bp).
. Primer pairs (forward–reverse; amplicon size) used for amplification were as follows: CCL5 (GCAGCCCTCGCTGTCATCCT–AAGACGACTGCTGGGTTGGAGC; 176 bp); 18S (AACTTTCGATGGTAGTCGCCG–CCTTGGATGTGGTAGCCGTTT; 84 bp); CCR1 (AAGTCCCTTGGAACCAGAGAGAAG–TCCAACCAGGCCAATGACAAA; 182 bp); CCR3 (TGTTTCAGGAGTGGTGACGC–TTCACTTCTCCAATACAACTCAGCA; 235 bp); CCR5 (CTGGCATAGTATTCTGTGTAGTGGG–TGTTTCTTTTGAAGGAGGGTGGA; 202 bp).
Statistical analysis
Statistical significance was determined using one-way analysis of variance (ANOVA) with post hoc analyses using the Tukey's honestly significant difference test. Tests are used to compare and determine differences between and within data groups depending on the means and standard deviation values of each variable. Homogeneity of variance was assumed at 95 % confidence interval. Results with p < 0.05 were considered significant.
Results
Aging quiescent fibroblasts promote breast cancer cell proliferation
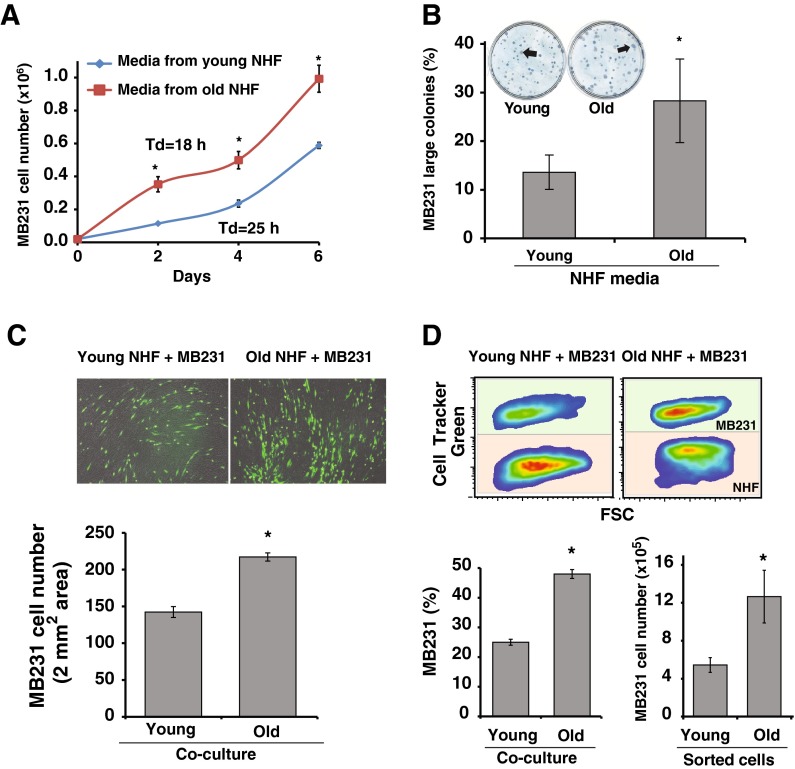
To determine if aged quiescent fibroblasts influence epithelial cancer cell proliferation, we used two different approaches: a paracrine approach using conditioned media and a cell-to-cell co-culture method. An equal number of MB231 cells were cultured in conditioned media collected from young and old quiescent cultures of NHFs. Conditioned media were supplemented with 25 % regular media prior to use. Cell numbers were counted at 2 days interval and doubling time calculated. MB231 cells cultured in conditioned media collected from old quiescent NHFs showed consistently a higher cell number in all time points compared to cell numbers in MB231 cultures that were incubated in conditioned media collected from young quiescent NHFs (Fig. 1a). Aging quiescent fibroblast-induced increase in MB231 proliferation was also evident from these cells exhibiting a lower doubling time: 18 h compared to 25 h in MB231 cells cultured in conditioned media collected from young quiescent NHFs (Fig. 1a). The difference in MB231 cell doubling time was also apparent from an increase in the colony size of MB231 cells cultured in conditioned media collected from old vs. young quiescent NHFs (Fig. 1b).
Fig. 1.
Aging quiescent fibroblast promotes breast cancer cell proliferation. a MB231 human breast epithelial cancer cells were cultured in conditioned media that were collected from young and old quiescent normal human fibroblasts (NHFs). Cell numbers were counted at 2 days interval, and cell doubling time was calculated from the exponential portion of the growth curve. Asterisks represent statistical significance between young and old cultures; n = 3, p < 0.05. b MB231 cells were plated at limited dilution for single cell colony assay. Cells were cultured in conditioned media for 14 days, fixed, and stained with Coomassie blue for visualization. Colonies with more than 150 cells were considered large colonies. Asterisks represent statistical significance between young and old cultures; n = 3, p < 0.05. c Upper panel: representative microscopy images of 4-day co-cultures of CellTracker green-labeled MB231 cells cultured on top of monolayers of young and old quiescent NHFs. Images of green fluorescence and bright field pictures were combined for the final image. Lower panel: NIH Image J software was used to count cells in 2 mm2 area. Asterisks represent statistical significance between young and old cultures; n = 5, p < 0.05. d Flow cytometry analysis of co-cultures. Upper panel: representative pseudo-color density plots of 5-day co-cultures of CellTracker green-labeled MB231 cells cultured on top of monolayers of young and old quiescent NHFs. Lower left panel: percentage of MB231 cells in co-cultures. Asterisks represent statistical significance between young and old cultures; n = 5, p < 0.05. Lower right panel: MB231 cells were sorted from co-cultures of NHFs by flow cytometry and cultured in regular media for 3 days prior to counting cells. Asterisks represent statistical significance between young and old cultures; n = 3, p < 0.05
Aged quiescent fibroblast-induced increase in MB231 proliferation was also observed in co-culture experiments. An equal number of CellTracker green-labeled MB231 cells was layered on lawns of young and old quiescent NHFs, and co-cultures were continued for 5 days. MB231 proliferation in co-cultures was measured by using fluorescent microscopy imaging (Fig. 1c) and flow cytometry (Fig. 1d). Results from the microscopy imaging showed more than 50 % increase in MB231 cell density in co-cultures of old vs. young quiescent NHFs: 217 cells/2 mm2 in old compared to142 cells/2 mm2 in co-cultures of young quiescent NHFs (Fig. 1c bottom panel). Results from the flow cytometry analysis showed approximately 50 % MB231 cells in co-cultures of old quiescent NHFs compared to 25 % MB231 cells in co-cultures of young quiescent NHFs (Fig. 1d, left lower panel). Furthermore, aseptic sorting of MB231 cells from co-cultures of quiescent NHFs followed by 3 days of culturing of sorted MB231 cells also showed a significant increase in the number of MB231cells that were sorted from co-cultures of old quiescent NHFs compared to MB231 cells sorted from co-cultures of young quiescent NHFs (Fig. 1d, right lower panel). These results demonstrate that aged quiescent fibroblasts promote cancer cell proliferation, and this effect persists even after cancer cells were separated from co-cultures of fibroblasts.
CCL5 secreted from aging quiescent fibroblasts promotes breast cancer cell proliferation
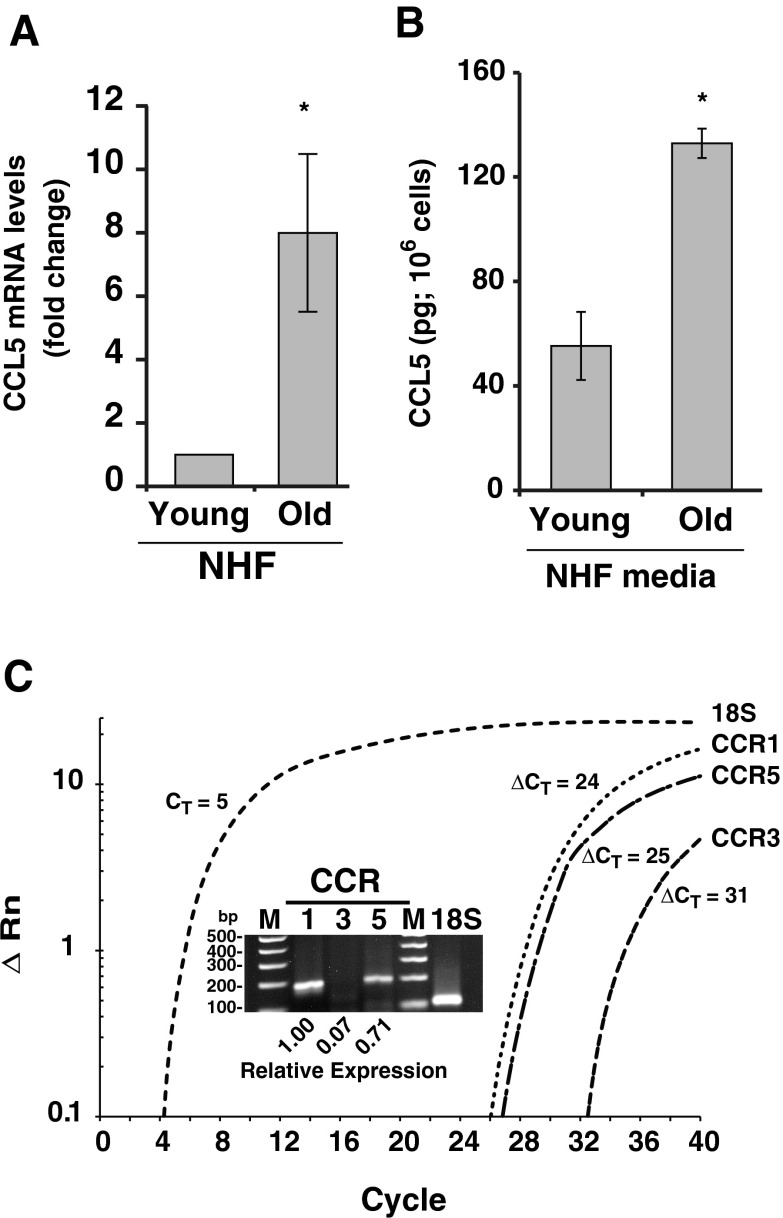
We have recently shown that CCL5 expression is higher in quiescent compared to proliferating cells (Kumar et al. 2012). To determine if CCL5 regulates aging quiescent fibroblast-induced increase in cancer cell proliferation, a Q-RT-PCR assay was performed to measure CCL5 mRNA levels in young and old quiescent NHFs. CCL5 mRNA levels increased approximately 8-fold in old quiescent NHFs compared to young quiescent NHFs (Fig. 2a). Consistent with these results, CCL5 protein levels in the media of old quiescent NHFs increased approximately 3-fold compared to CCL5 protein levels in media of young quiescent NHFs, 132 and 55 pg/106 cells, respectively (Fig. 2b). These results showed that CCL5 expression increases as quiescent NHFs age in culture.
Fig. 2.
Age-associated increase in CCL5 expression in quiescent NHFs. a Real-time PCR analysis of CCL5 mRNA levels in young and old quiescent NHFs. Fold change was calculated relative to CCL5 mRNA levels in young NHFs. Asterisks represent statistical significance between young and old cultures; n = 3, p < 0.05. b An ELISA-based assay was used to measure CCL5 protein levels in media collected from young and old quiescent NHFs. Asterisks represent statistical significance between young and old cultures; n = 3, p < 0.05. c Real-time PCR analysis of CCR1, CCR3, and CCR5 mRNA levels in MB231 cells. Insert: PCR amplified products were analyzed by agarose gel electrophoresis and visualized by staining with ethidium bromide
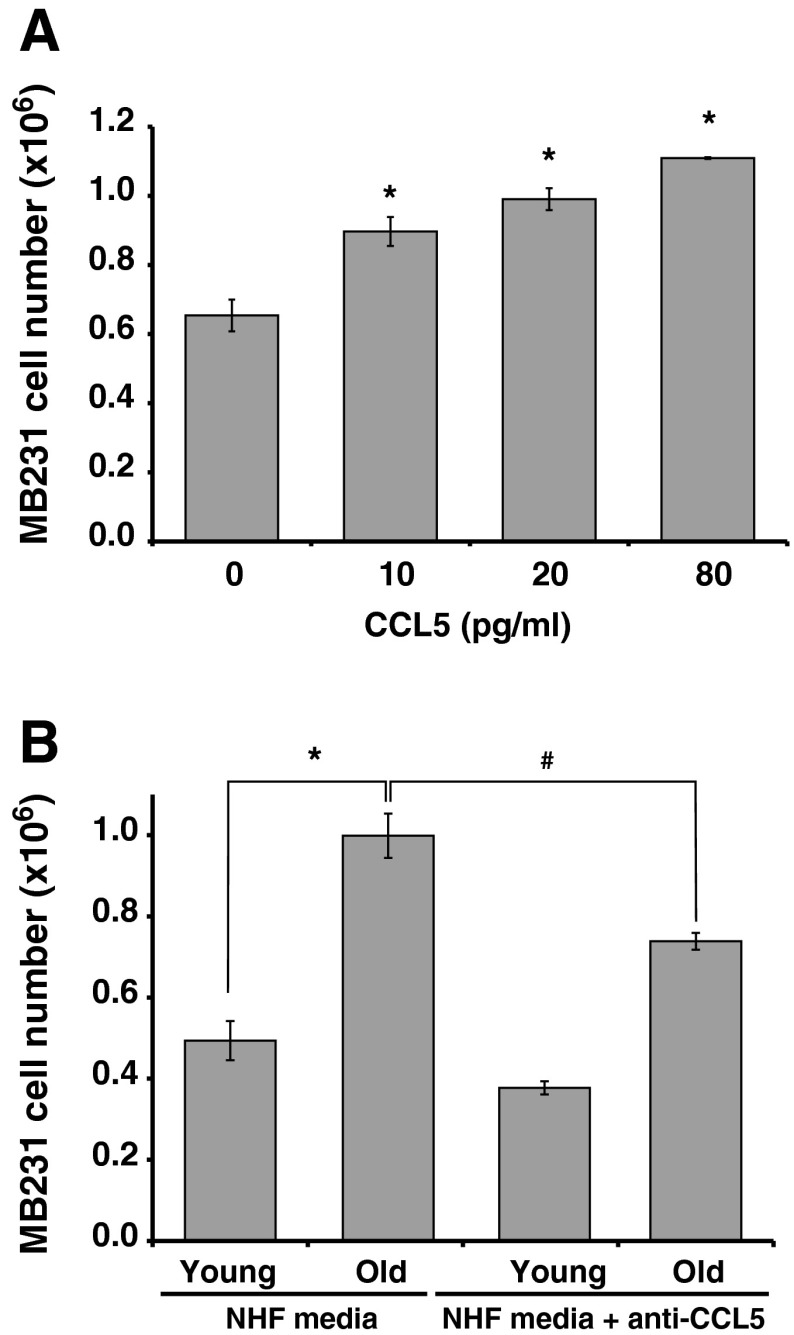
CCL5 functions through its interaction with CCR5. It can also bind to CCR1 and CCR3 receptors. To determine the growth promoting properties of CCL5, initially we performed a Q-RT-PCR assay to measure CCR1, CCR3, and CCR5 mRNA levels in MB231 cells. Results showed that all three receptors are expressed in MB231 cells. Whereas, CCR1 and CCR5 expression was comparable, CCR3 is minimally expressed in MB231 cells (Fig. 2c). MB231 cells were incubated with human recombinant CCL5 (R and D Systems, USA) and cell number counted 6 days after the addition of CCL5. Treatment with CCL5 showed a dose-dependent increase in MB231 cell numbers (Fig. 3a). The specificity of CCL5 stimulating MB231 proliferation was further tested by incubating the conditioned media with monoclonal antibody against human CCL5 (R and D Systems, USA) for 2 h prior to culturing MB231 cells. Cell number was counted 6 days after addition of control and anti-CCL5-treated conditioned media. As shown before (Fig. 1a), the number of MB231 cells increased approximately 2-fold in cultures incubated with conditioned media collected from old compared to young quiescent NHFs (Fig. 3b). It is interesting to note that this increase in cell number was significantly reduced in cultures incubated with anti-CCL5 antibody treated-conditioned media collected from old quiescent NHFs (Fig. 3b). These results demonstrate that CCL5 regulates aging quiescent fibroblast-associated increase in the MB231 human breast cancer cell proliferation.
Fig. 3.
CCL5 secreted by old quiescent NHFs stimulates MB231 breast cancer cell proliferation. a MB231 cells were cultured in presences of different amounts of recombinant human CCL5 and cell number counted after 6 days of the addition of CCL5. Asterisks represent statistical significance compared to cell numbers in untreated control cultures; n = 3, p < 0.05. b Conditioned media treated with anti-human CCL5 monoclonal antibody (0.4 μg/ml) was used to culture MB231 cells and cell number counted at 6 days after plating. Asterisks represent statistical significance compared to cultures grown in conditioned media collected from young quiescent NHFs; n = 3, p < 0.05
CCL5 activates the ERK1/2 and cyclin D1 signaling pathway
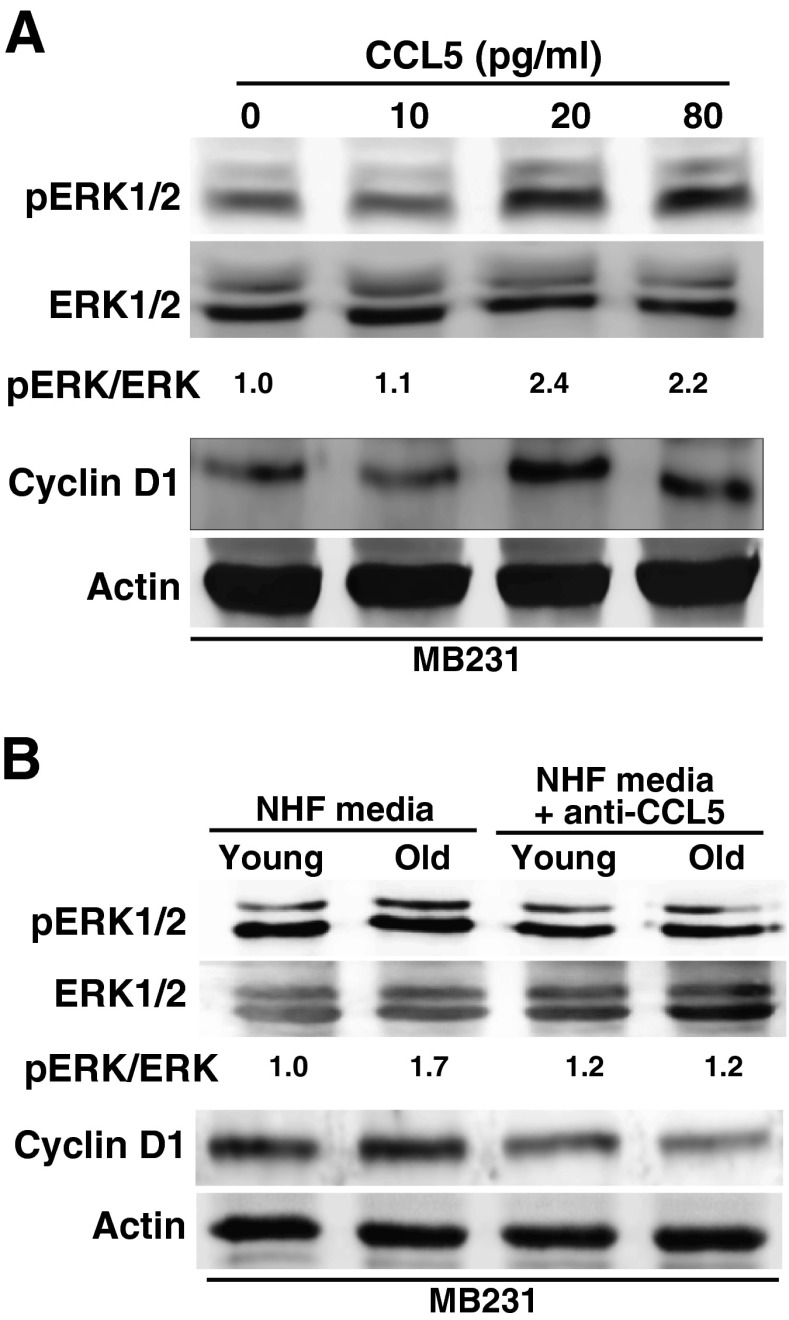
To determine if CCL5 activates the ERK1/2-cyclin D1 pro-proliferative pathway, initially MB231 cells were incubated with CCL5 (0–80 pg/ml) for 3 days and cells were harvested for western blot analysis. Results showed a dose-dependent increase in ERK1/2 phosphorylation and cyclin D1 protein levels (Fig. 4a). A regulatory role of CCL5 activating the ERK1/2-cyclin D1 pro-proliferative pathway is also evident from results shown in Fig. 4b. Total cellular proteins were isolated from MB231 cells incubated with un-treated and anti-CCL5 antibody-treated conditioned media collected from young and old quiescent NHFs. ERK1/2 phosphorylation and cyclin D1 protein levels were significantly suppressed in MB231 cells that were cultured in anti-CCL5 antibody-treated conditioned media (Fig. 4b). These results suggest that CCL5 secreted from aged quiescent fibroblasts activates the ERK1/2-cyclin D1 pro-proliferative pathway in MB231 cancer cells, which was associated with an increase in MB231 proliferation.
Fig. 4.
CCL5 activates the ERK1/2-cyclin D1 pro-proliferative pathway in MB231 cells. Western blot analysis of phosphorylated ERK1/2, ERK1/2, cyclin D1, and actin protein levels in MB231 cells cultured in a regular media with and without recombinant CCL5 and b conditioned media from young and old quiescent NHFs that were treated with and without CCL5 antibody
Hydroxytyrosol inhibits CCL5 accumulation in aged quiescent fibroblasts and prevents aging fibroblast-induced increases in MB231 cancer cell proliferation
We previously showed that hydroxytyrosol protects quiescent NHFs from age-associated mitochondrial injury and extends fibroblasts chronological lifespan (Sarsour et al. 2012). To determine if hydroxytyrosol inhibits age-associated accumulation of CCL5, quiescent NHFs were cultured in presence of hydroxytyrosol for 20 (young) and 60 (old) days. Media was replenished every 3 days with fresh media containing hydroxytyrosol. An ELISA-based assay was performed to measure CCL5 protein levels in media. Results showed that hydroxytyrosol treatment suppressed age-associated increase in CCL5 protein levels (Fig. 5a). CCL5 protein levels in hydroxytyrosol-treated old quiescent NHFs were comparable to its levels in young quiescent NHFs.
Fig. 5.
Hydroxytyrosol suppresses age-associated increase in CCL5 accumulation in quiescent NHFs and inhibits their ability to activate the ERK1/2-cyclin D1 pro-proliferative pathway in MB231 cells. a An ELISA-based assay was used to measure CCL5 protein levels in conditioned media collected from hydroxytyrosol fed young and old quiescent NHFs. Asterisks and hashtag indicate statistical significance between corresponding treatments; n = 3, p < 0.05. b Western blot analysis of phosphorylated ERK1/2, ERK1/2, cyclin D1, and actin protein levels in MB231 cells separated from co-cultures of quiescent NHFs. Quiescent NHFs were cultured in presence and absence of hydroxytyrosol for 20 days (young) and 60 days (old) and used in co-culture experiments. c Left panels: representative microscopy images of 4-day co-cultures of CellTracker green-labeled MB231 cells and quiescent NHFs that were cultured in presence of hydroxytyrosol prior to the co-culture experiments (upper panel). MB231 cell numbers in 2 mm2 area were scored using NIH ImageJ (lower panel); n = 5. d Right panels: Flow cytometry measurements of CellTracker green-labeled MB231 cells in co-cultures of hydroxytyrosol-fed young and old quiescent NHFs (upper panel). The percentage of MB231 cells in 5-day co-culture of MB231 cultured on lawns of young and old quiescent NHFs (lower panel); n = 5
Next, we investigated whether hydroxytyrosol-induced suppression of CCL5 accumulation in old quiescent NHFs inhibits activation of the ERK1/2-cyclin D1 pro-proliferative pathway in cancer cells. MB231 cells were layered on top of young and old quiescent NHFs that were cultured in absence and presence of hydroxytyrosol prior to co-culture. Co-cultures were continued in regular media without hydroxytyrosol for 5 days. Aseptic flow cytometry sorting was performed to separate MB231 cells from co-cultures of NHFs. ERK1/2 and cyclin D1 protein levels in sorted MB231 cells were analyzed by western blotting. As shown before (Fig. 4b), phosphorylated ERK1/2 and cyclin D1 protein levels in sorted MB231 cells were higher in co-cultures of old vs. young quiescent NHFs (Fig. 5b). It is interesting to note that the old quiescent NHFs-induced increases in phosphorylated ERK1/2 and cyclin D1 protein levels were suppressed in MB231 cells that were co-cultured with hydroxytyrosol-fed old quiescent NHFs (Fig. 5b, compare lane 2 and lane 4). To determine if suppression of phosphorylated ERK1/2 and cyclin D1 protein levels impact MB231 proliferation, MB231 cell number was determined in co-cultures using microscopy and flow cytometry assays. Results from the microscopy assay showed that the number of MB231 cells was comparable in co-cultures of MB231 and hydroxytyrosol-fed young and old quiescent NHFs (Fig. 5c). Similar results were also obtained from the flow cytometry assay (Fig. 5d). The percentage of MB231 cells in co-cultures of hydroxytyrosol-fed young and old NHFs was approximately 20 and 25 %, respectively. Overall, these results showed that hydroxytyrosol inhibits age-associated accumulation of CCL5 in quiescent NHFs, which correlated with a suppression of phosphorylated ERK1/2 and cyclin D1 protein levels in MB231 and decrease in MB231 proliferation.
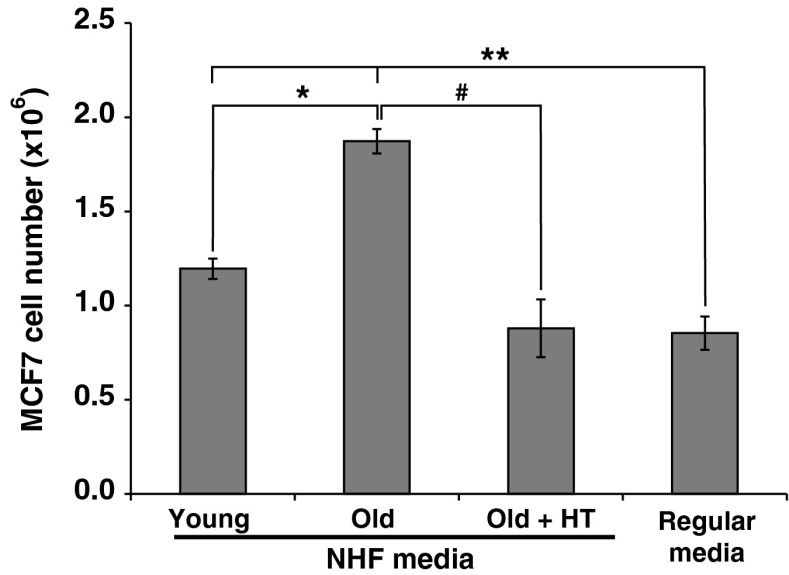
Aging fibroblasts-induced proliferation of breast cancer cells was also observed in MCF7 human breast epithelial cancer cells. MCF7 cells were cultured in conditioned media collected from young and old NHFs. MCF7 cells cultured in regular media were included for comparison. MCF7 cells cultured in conditioned media collected from old NHFs showed approximately 2-fold increase in cell number compared to MCF7 cells cultured in conditioned media collected from young NHFs (Fig. 6). This increase in cell number was significantly suppressed in MCF7 cells cultured in conditioned media collected from hydroxytyrosol-fed old NHFs. These results demonstrate that aging fibroblasts promote breast cancer cell proliferation.
Fig. 6.
Hydroxytyrosol suppresses aging quiescent fibroblasts-induced MCF7 breast cancer cell proliferation. MCF7 human breast epithelial cancer cells were cultured in conditioned media that were collected from young, old, and hydroxytyrosol-fed old quiescent fibroblasts. MCF7 cells cultured in regular media were included for comparison. Cell numbers were counted after 3 days of adding the conditioned media. Asterisk indicates statistical significance between the number of MCF7 cells cultured in conditioned media collected from young fibroblasts compared to the number of MCF7 cells cultured in conditioned media collected from old fibroblasts; hashtag indicates statistical significance between the number of MCF7 cells cultured in conditioned media collected from old fibroblasts compared to the number of MCF7 cells cultured in conditioned media collected from hydroxytyrosol-fed old fibroblasts; double asterisks represent statistical significance of the number of MCF7 cells cultured in regular culture media compared to the number of MCF7 cells cultured in conditioned media collected from young and old fibroblasts; n = 3, p < 0.05
Discussion
Age is a significant risk factor for cancer because cancer incidence increases exponentially with age (Dix 1989). Recent evidence also suggests that the tumor stromal environment is a critical regulator of cancer progression (Cook et al. 2004; van Kempen et al. 2003; Weber and Kuo 2012). Fibroblasts are an integral part of the tumor stromal environment (Kalluri and Zeisberg 2006). In this study, we used an in vitro co-culture approach to determine if aging of quiescent fibroblasts promotes breast cancer cell proliferation and whether a phytochemical approach to delay aging of quiescent fibroblasts inhibits their ability to enhance breast cancer cell proliferation. Our results showed that CCL5 secreted from aged quiescent fibroblasts activates the ERK1/2-cyclin D1 pro-proliferative pathway in breast cancer cells coinciding with an increase in their proliferation. Hydroxytyrosol treatments inhibit fibroblast age-associated accumulation of CCL5 and their ability to enhance the ERK1/2-cyclin D1 pathway and proliferation of breast cancer cells.
The major cell types in the tumor stroma include epithelial cancer cells, innate and adaptive immune cells, endothelial cells, and fibroblasts (Kalluri and Zeisberg 2006). The stroma is a collagen-rich structure near the basement membrane that the epithelial cells use for their structural support and functions. In addition to their primary role in collagen production and remodeling of the extracellular matrix, fibroblasts are also known to produce secretory and non-secretory factors that contribute to cancer progression in the stromal environment (Kalluri and Zeisberg 2006). Under normal conditions, fibroblasts reside in quiescent state. However, they are activated and recruited to the proliferative cycle in response to wound healing and fibrosis (Kalluri and Zeisberg 2006). These activated fibroblasts were originally described by Gabbiani as myofibroblasts (Gabbiani et al. 1972). Cancer cells are also well known to activate fibroblasts that are known as cancer associated fibroblasts (Xing et al. 2010). While some genetic signatures were identified in activated compared to normal resident fibroblasts (Bauer et al. 2010), their role in cancer progression is not completely understood. Besides, the complexity of the tumor stromal environment also makes it difficult to dissect the mechanisms regulating each cell type’s role in cancer progression.
Resident fibroblasts under normal conditions reside in the quiescent state. We used an in vitro cell culture approach to determine if aging of normal quiescent fibroblasts influences breast cancer cell proliferation. We have previously shown that quiescent fibroblasts aged in culture lose their capacity to enter back into the cell cycle and proliferate. We defined this mode of cellular aging as chronological life span (Sarsour et al. 2005, 2012). Quiescent NHFs were aged in culture for 20 days (young) and 50–60 days (old) with change in media at 3 days interval. MB231 cells cultured in conditioned media collected from old quiescent NHFs exhibit a doubling time of 18 h compared to 25 h in cells cultured in conditioned media collected from young quiescent fibroblasts (Fig. 1a). The acceleration of MB231 proliferation in conditioned media collected from old quiescent NHFs was also evident from an increase in the percentage of larger colonies (Fig. 1b). Aged quiescent fibroblast-induced increase in MB231 cell proliferation was also observed in co-culture experiments. Results from microscopy and flow cytometry assays showed a higher percentage of MB231 cells co-cultured with old quiescent NHFs (Fig. 1c, d). Surprisingly, the growth advantage properties of MB231 cells co-cultured with old quiescent NHFs were retained even after the separation of MB231 cells from co-cultures of fibroblasts (Fig. 1d, lower right panel). These results suggest that aging of quiescent fibroblasts has an enduring effect on cancer cell proliferation. Our results are comparable to recent reports of fibroblasts promoting breast and prostate cancer cell proliferation (Giannoni et al. 2010; Martens et al. 2003). Because these previous studies used cancer associated fibroblasts, the role of resident quiescent fibroblasts contributing to cancer cell proliferation was not clearly addressed. Our results support the hypothesis that in a stromal environment, quiescent resident fibroblasts nearing the end of their chronological life span promote cancer cell proliferation.
The observation of conditioned media stimulating MB231 proliferation (Fig. 1a, b) suggests that a paracrine effect contributes to aged quiescent fibroblast’s ability to enhance cancer cell proliferation. Consistent with this hypothesis, our results show a significant increase in CCL5 expression in old quiescent NHFs (Fig. 2). While the exact mechanisms regulating age-associated increase in CCL5 expression are unknown, oxidative stress has been shown to induce CCL5 expression (Kumar et al. 2012; Satriano et al. 1997). We have previously shown that aging quiescent fibroblasts exhibit mitochondrial injury, loss of cristae, and increase in ROS levels (Sarsour et al. 2005, 2010, 2012). Overexpression of MnSOD and treatment with hydroxytyrosol suppressed the age-associated mitochondrial injury (Sarsour et al. 2005, 2010, 2012). Our results also showed that oxidative stress induced by ionizing radiation significantly increased CCL5 expression (Kumar et al. 2012). Murine mesangial cells incubated with xanthine oxidase and hypoxanthine increased CCL5 expression, suggesting that superoxide could induce CCL5 expression (Satriano et al. 1997). In a separate study, mitochondrial ROS have also been suggested to enhance CCL5 expression (Kim et al. 2007). We postulate that an age-associated oxidative stress presumably due to an imbalance in mitochondrial ROS levels induces CCL5 expression in old quiescent fibroblasts.
The growth promoting property of CCL5 is evident from results shown in Fig. 3. CCL5 added to the media showed a dose-dependent increase in MB231 proliferation, while conditioned media incubated with anti-CCL5 antibodies suppressed MB231 proliferation (Fig. 3). MB231 cells express CCL5 receptors, CCR5 and CCR1 (Fig. 2c). Therefore, the growth promoting properties of CCL5 could be mediated by its interaction with CCR5 and CCR1 leading to the activation of the pro-proliferative signaling pathways. CCL5-CCR5 interaction has been shown to increase cyclin D1 translation correlating with an increase in proliferation of the MCF7 human breast cancer cells (Murooka et al. 2009; Soria and Ben-Baruch 2008). Consistent with these results, we have observed a significant increase in phosphorylated ERK1/2 and cyclin D1 protein levels in MB231 cells cultured in the presence of recombinant CCL5 (Fig. 4a). It is interesting to note that whereas MB231 cells cultured in conditioned media collected from old quiescent fibroblasts showed an increase in phosphorylated ERK1/2 and cyclin D1 protein levels, pretreatment of conditioned media with anti-CCL5 antibodies blunted these effects (Fig. 4b). These results indicate that CCL5 secreted from aging quiescent fibroblasts activates the ERK1/2-cyclin D1 pro-proliferative pathway in MB231 cells resulting in their enhanced proliferation.
Dietary polyphenols (e.g., hydroxytyrosol in olives, curcumin in turmeric, epi-gallocatechin-gallate in green tea, quercetin in fruits and vegetables, resveratrol in grapes, etc.) are a class of phytoalexins normally produced by plants to fight infection (Queen and Tollefsbol 2010). Whereas, the mechanisms by which dietary polyphenols confer their beneficial effects are not completely understood, they are gaining widespread attention because of their preventive and therapeutic potentials in mitigating age-associated and oxidative stress-related health issues. Hydroxytyrosol is a hydrophilic polyphenol commonly found in olives. The use of olive oil in the diet is believed to be associated with a lower incidence of cancer and cardiovascular diseases in the Mediterranean population (Fabiani et al. 2008; Visioli et al. 1998). Activation of longevity proteins (SIRT and FoxO) in heart cells of hydroxytyrosol-fed rats suggests that hydroxytyrosol could impact aging (Mukherjee et al. 2009). We have shown earlier that hydroxytyrosol inhibits age-associated increase in mitochondrial injury and extends chronological life span of fibroblasts (Sarsour et al. 2012). Results from this study showed that age-associated accumulation of CCL5 is significantly suppressed in quiescent fibroblasts that were cultured in presence of hydroxytyrosol (Fig. 5a). Hydroxytyrosol-fed old quiescent fibroblasts neither enhanced MB231 cells ERK1/2-cyclin D1 pro-proliferative pathway nor their proliferation (Fig. 5b–d). These results demonstrate that aging of normal quiescent fibroblasts is a significant factor stimulating breast cancer cell proliferation. This premise is also supported by results presented in Fig. 6. MCF7 cells cultured in conditioned media collected from old quiescent NHFs showed a higher cell number compared to MCF7 cells cultured in conditioned media collected from young quiescent NHFs. Conditioned media collected from hydroxytyrosol-fed old NHFs were unable to enhance MCF7 cell proliferation (Fig. 6). In summary, our results demonstrate that CCL5 secreted from aging fibroblasts promotes human breast cancer cell proliferation by activating the ERK-cyclin D1 pathway in cancer cells and hydroxytyrosol treatment of fibroblasts blunted these effects. We speculate that dietary polyphenol-based approach to suppress age-associated decline in fibroblast function may delay progression of epithelial malignancy in a stromal environment.
Acknowledgments
We thank Mr. Justin Fishbaugh at the Flow Cytometry Core facility for assisting with the flow cytometry methods, and Mr. Tom Moninger of the Central Microscopy Research facility for assistance with the microscopy methods. Funding from NIH 2R01 CA111365 (PCG, EHS, and ALK), NIH T32CA078586 (JL), and Iowa Center for Research by Undergraduates Scholarship (MG) supported this work.
Financial support
Funding from NIH 2R01 CA111365 supported PCG, EHS, and ALK; NIH T32CA078586 supported JL; and Iowa Center for Research by Undergraduates Scholarship supported MG.
Conflicts of interest
None.
Abbreviations
- CCL5
Chemokine C-C motif ligand 5
- CCR
Chemokine C-C motif ligand receptor
- ERK1/2
Extracellular-signal-regulated kinases 1 and 2
- MnSOD
Manganese superoxide dismutase
- NHFs
Normal human fibroblasts
- ROS
Reactive oxygen species
References
- Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269(5231):1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- Bauer M, Su G, Casper C, He R, Rehrauer W, Friedl A. Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene. 2010;29(12):1732–1740. doi: 10.1038/onc.2009.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14(3):259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Dix D. The role of aging in cancer incidence: an epidemiological study. J Gerontol. 1989;44(6):10–18. doi: 10.1093/geronj/44.6.10. [DOI] [PubMed] [Google Scholar]
- Fabiani R, Rosignoli P, De Bartolomeo A, Fuccelli R, Servili M, Montedoro GF, Morozzi G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J Nutr. 2008;138(8):1411–1416. doi: 10.1093/jn/138.8.1411. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kim JS, Lee JY, Kim YJ, Youn HJ, Kim IY, Chee YJ, Oh YK, Kim N, Jung HC, Song IS. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-kappaB activation. Infect Immun. 2007;75(7):3373–3381. doi: 10.1128/IAI.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MG, Patel NM, Nicholson AM, Kalen AL, Sarsour EH, Goswami PC. Reactive oxygen species mediate microRNA-302 regulation of AT-rich interacting domain 4a and C-C motif ligand 5 expression during transitions between quiescence and proliferation. Free Radic Biol Med. 2012;53(4):974–982. doi: 10.1016/j.freeradbiomed.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JW, Sieuwerts AM, Bolt-deVries J, Bosma PT, Swiggers SJ, Klijn JG, Foekens JA. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost. 2003;89(2):393–404. [PubMed] [Google Scholar]
- Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46(5):573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387(2):381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Queen BL, Tollefsbol TO. Polyphenols and aging. Curr Aging Sci. 2010;3(1):34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem. 2005;280(18):18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Venkataraman S, Kalen AL, Oberley LW, Goswami PC. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell. 2008;7(3):405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour EH, Goswami M, Kalen AL, Goswami PC. MnSOD activity protects mitochondrial morphology of quiescent fibroblasts from age associated abnormalities. Mitochondrion. 2010;10(4):342–349. doi: 10.1016/j.mito.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Kalen AL, Goswami M, Buettner GR, Goswami PC. MnSOD activity regulates hydroxytyrosol-induced extension of chronological lifespan. Age (Dordr) 2012;34(1):95–109. doi: 10.1007/s11357-011-9223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriano JA, Banas B, Luckow B, Nelson P, Schlondorff DO. Regulation of RANTES and ICAM-1 expression in murine mesangial cells. J Am Soc Nephrol. 1997;8(4):596–603. doi: 10.1681/ASN.V84596. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Podstawa M, Visioli F, Bogani P, Muller WE, Eckert GP. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J Agric Food Chem. 2007;55(13):5043–5049. doi: 10.1021/jf0703710. [DOI] [PubMed] [Google Scholar]
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Soriano SF, Serrano A, Hernanz-Falcon P, Martin de Ana A, Monterrubio M, Martinez C, Rodriguez-Frade JM, Mellado M. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33(5):1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- Turner L, Ward SG, Westwick J. A role for phosphoinositide 3–kinase in RANTES induced chemotaxis of T lymphocytes. Biochem Soc Trans. 1995;23(2):283S. doi: 10.1042/bst023283s. [DOI] [PubMed] [Google Scholar]
- Turner SJ, Domin J, Waterfield MD, Ward SG, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2alpha. J Biol Chem. 1998;273(40):25987–25995. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82(11):539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- Visioli F, Bellomo G, Galli C. Free radical-scavenging properties of olive oil polyphenols. Biochem Biophys Res Commun. 1998;247(1):60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- Weber CE, Kuo PC. The tumor microenvironment. Surg Oncol. 2012;21(3):172–177. doi: 10.1016/j.suronc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Wong M, Uddin S, Majchrzak B, Huynh T, Proudfoot AE, Platanias LC, Fish EN. Rantes activates Jak2 and Jak3 to regulate engagement of multiple signaling pathways in T cells. J Biol Chem. 2001;276(14):11427–11431. doi: 10.1074/jbc.M010750200. [DOI] [PubMed] [Google Scholar]
- Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]