Abstract
Mild cognitive impairment (MCI) is a stage between healthy aging and dementia. It is known that in this condition the connectivity patterns are altered in the resting state and during cognitive tasks, where an extra effort seems to be necessary to overcome cognitive decline. We aimed to determine the functional connectivity pattern required to deal with an internally directed cognitive state (IDICS) in healthy aging and MCI. This task differs from the most commonly employed ones in neurophysiology, since inhibition from external stimuli is needed, allowing the study of this control mechanism. To this end, magnetoencephalographic (MEG) signals were acquired from 32 healthy individuals and 38 MCI patients, both in resting state and while performing a subtraction task of two levels of difficulty. Functional connectivity was assessed with phase locking value (PLV) in five frequency bands. Compared to controls, MCIs showed higher PLV values in delta, theta, and gamma bands and an opposite pattern in alpha, beta, and gamma bands in resting state. These changes were associated with poorer neuropsychological performance. During the task, this group exhibited a hypersynchronization in delta, theta, beta, and gamma bands, which was also related to a lower cognitive performance, suggesting an abnormal functioning in this group. Contrary to controls, MCIs presented a lack of synchronization in the alpha band which may denote an inhibition deficit. Additionally, the magnitude of connectivity changes rose with the task difficulty in controls but not in MCIs, in line with the compensation-related utilization of neural circuits hypothesis (CRUNCH) model.
Keywords: Mild cognitive impairment, Internally directed cognitive state (IDICS), Functional connectivity, MEG, CRUNCH
Introduction
Mild cognitive impairment (MCI) is an intermediate state of the elderly brain between normal cognition and dementia. It is mainly characterized by objective evidence of memory impairment not yet encompassing the definition of dementia and which does not interfere with activities of daily living (Petersen et al. 2001). Prevalence of MCI ranges from 3 to 19 % in adults older than 65 years (Gauthier et al. 2006). Most MCI cases are due to Alzheimer’s disease (AD), since recent studies have found a high rate of progression from MCI to AD. For example, following Petersen’s criteria, the conversion rate from MCI to dementia is of about 12 % per year, while healthy controls convert at a 1–2 % rate (Petersen 2004).
When compared with demented patients and controls without cognitive impairment, individuals with MCI (hereafter known as MCIs) have intermediate amounts of AD pathological signs, such as amyloid deposition, tau-positive tangles in medial temporal lobes (Mufson et al. 1999), cholinergic dysfunction (Haense et al. 2012), gray matter loss (Chételat et al. 2002), and white matter lesions (Fellgiebel et al. 2005). These neuropathological features could be considered intermediate between normal aging and early AD (Petersen et al. 2006; He et al. 2009; Zhang et al. 2012).
AD has been considered as a “disconnection syndrome” (Delbeuck et al. 2003). At a structural level, anatomical links in AD patients are disrupted, and this has been associated with a neurofibrillary pathology (Braak and Braak 1991). At a functional level, a decrease in the brain interactions has been shown in several studies (Stam et al. 2006 Locatelli et al. 1998; Berendse et al. 2000; Stam and van Dijk 2002; see Jeong 2004 for a review; Koenig et al. 2005; Sanz-Arigita et al. 2010). The concept of functional connectivity has emerged to evaluate functional relations between brain regions. It quantifies statistical interdependencies between different physiological signals, providing information about functional interactions between the corresponding brain regions (Friston 2001) and was proven to be also altered in MCI (Bajo et al. 2010). Moreover, synchronization abnormalities seem to be related to the degree of dementia, being more accentuated in patients with a higher cognitive decline (Stam et al. 2003; Jeong 2004). Electrophysiological techniques such as magnetoencephalography (MEG) or electroencephalography (EEG) enable the assessment of this interaction in the time-frequency domain. MEG is a noninvasive technique based on the measurement of magnetic brain activity. Compared with fMRI which gives an indirect estimate of brain activity through hemodynamic responses, MEG provides a direct measure of electrophysiological activity, reflecting neuronal communication with great temporal resolution (Hämäläinen et al. 1993) and, therefore, allowing the investigation of several brain rhythms (1–100 Hz). Indeed, MEG oscillatory activity has been proven useful in measuring both spontaneous and task-induced brain rhythms in normal and pathological aging (Fernández et al. 2006; Osipova et al. 2006; Stam 2010; Leirer et al. 2011; Bajo et al. 2010; Zamrini et al. 2011).
Most resting state studies in MCI have shown an increase in low-frequency activity, which has been usually accompanied by a decrease in synchronization. For example, Moretti et al. (2008) reported a decline in intrahemispheric fronto-parietal coherence and an increase in temporal interhemispheric coherence in the MCI group when compared to elderly controls and also a reduced beta band functional connectivity. Gómez et al. (2009) found a decreased synchronization essentially in the beta band in MCI subjects. However, other resting state studies have failed to find functional connectivity differences between MCI and healthy controls (Stam et al. 2003; Jiang 2005; Zheng et al. 2007; Tao and Tian 2005).
By contrast, most task studies found an increased synchronization in MCIs when compared to healthy people during a cognitive task (i.e., memory task) (Pijnenburg et al. 2004; Jiang 2005; Zheng et al. 2007; Bajo et al. 2010). For instance, Jiang and Zheng (2006) reported a higher inter- and intrahemispheric EEG coherence in MCI in a working memory task. Bajo et al. (2010) found higher interhemispheric synchronization likelihood in MCI between left and right temporo-frontal sensors in most frequency bands during a short-term memory task and higher connectivity for those that later on developed dementia (Bajo et al. 2012). This increase in functional connectivity is usually interpreted as a compensatory mechanism and is associated with the risk for the progression to AD (Rossini et al. 2006). Indeed, it seems to indicate a loss of brain efficiency (Buldú et al. 2011).
This discrepancy between cognitive task and resting state studies could be due to the differences in the nature of brain state during resting and cognitive tasks. Most studies in cognitive functioning have been based on external stimuli. Others have focused on spontaneous or goal-directed internal mentation, such as future planning (Andrews-Hanna 2012). However, little is known about brain activity during internal cognitive tasks. This would be worthwhile, since during an internal cognitive task, subjects are doing a specific task without dealing with external stimuli.
The present paper evaluates functional connectivity patterns in MCIs and their age-matched controls during resting state and an internally governed task with two levels of difficulty. The novelty of this study lies in its task design, which is a combination between resting state and a cognitive state with no external stimuli. This condition could be named “internally directed cognitive state” (IDICS) and would allow us to explore how the brain works during a pure cognitive state. For this purpose, we chose a calculation task, since it can be used in an internal way and it is usually impaired in AD (Parlato et al. 1992; Rémy et al. 2004) and MCI patients (Zamarian et al. 2007b, a; Li et al. 2010). The performance of this task requires the knowledge of numbers and arithmetic (long-term memory), the ability to manipulate and update the information mentally (working memory), a high level of self-monitoring, and the capacity to inhibit distracting stimuli (executive functioning). Functional connectivity in different frequency bands was assessed with phase locking value (PLV), and connectivity patterns were compared across groups and conditions. To our knowledge, there are no EEG/MEG studies about internal cognitive processes in MCI patients.
The exploration of the neural networks involved during a pure resting state and IDICS in MCI and controls was proposed under the following hypotheses: (1) During resting state, the MCI group will show a decreased synchronization in higher frequency bands compared to the control group. (2) Our results will differ from those studies which have employed external stimuli since IDICS requires higher levels of internal information processing and therefore of top-down control. (3) MCI patients will show a higher inter- and intrahemispheric synchronization than the control group during the execution of the task. (4) In both groups, the overall connectivity will increase along with the difficulty of the task.
Materials and methods
Subjects
Eighty-nine subjects older than 65 years enrolled voluntarily in the study. They were all right-handed (Oldfield 1971) and native Spanish speakers. The whole sample comprised 34 healthy elders and 55 MCI patients. For the subsequent analysis, only 38 MCIs (18 males) and 32 controls (10 males) were used: 17 MCIs and 2 controls were excluded based on task execution (see “Experimental design”). Healthy individuals were recruited from the Seniors Center of the district of Chamartín, Madrid, and MCI patients from the Geriatric and Neurological Units of the Hospital Universitario San Carlos, Madrid and the Memory Decline Prevention Center, Madrid.
Subject’s characteristics are shown in Table 1. No statistical differences (p < 0.10) were found between controls and MCIs in age, sex, or educational level, but both groups did differ in Mini Mental State Examination (MMSE) and normalized hippocampal volume (p < 0.00001). Normalized hippocampal volume in MCI was significantly smaller than in controls, which is a biomarker that reflects neuronal injury (Albert et al. 2011).
Table 1.
Subject’s information. Characteristics given as mean ± standard deviation
| Control group ( n = 32) | MCI group ( n = 37) | p values | ||
|---|---|---|---|---|
| Age (years) | 71.8 ± 3.8 | 72.5 ± 4.5 | 0.50 | |
| Sex (M/F) | 10/22 | 18/20 | 0.22 | |
| Educational level | 3.8 ± 1.1 | 3.3 ± 1.3 | 0.12 | |
| MMSE score (points) | 29.3 ± 0.9 | 27.1 ± 2.2 | 3.9 × 10−5 | |
| Volume ratio: hippocampus/intracranial | (5.09 ± 0.62) × 10−3 | (4.17 ± 0.89) × 10−3 | 9.8 × 10−5 | |
| Number of clean 4-s segments | Resting | (27.6 ± 6.5) | (27.9 ± 5.6) | 0.83 |
| 1-subraction | (22.5 ± 6.8) | (21.7 ± 8.2) | 0.55 | |
| 3-subtraction | (21.2 ± 5.6) | (21.9 ± 8.2) | 0.94 | |
Educational level was grouped into five levels: 1. Illiterate, 2. Primary studies, 3. Elemental studies, 4. High school studies, and 5. University studies. p values for between-group differences were introduced. Wilcoxon-Mann-Whitney test was used for continuous variables (age, educational level, MMSE, volume ratios) and Fisher’s exact test for sex differences
M males, F females, MMSE Mini Mental State Examination score
Diagnostic criteria
All participants were rated with a variety of standardized diagnostic instruments that included the following: the Spanish version of the MMSE (Lobo et al. 1979), the Geriatric Depression Scale (GDS; Yesavage et al. 1982), the Global Deterioration Scale (GDS; Reisberg et al. 1982), the Hachinski Ischemic Score (HIS; Rosen et al. 1980), the Functional Assessment Questionnaire (FAQ; Pfeffer et al. 1982), the questionnaire for Instrumental Activities of Daily Living (IADL; Lawton and Brody 1969), and the Functional Assessment Staging (FAST; Auer and Reisberg 1997).
MCI diagnosis was established according to Petersen’s criteria (Petersen 2004; Grundman et al. 2004): (1) memory complaint, corroborated by an informant; (2) abnormal memory function, documented by delayed recall of one paragraph from the Logical Memory II subtest of the Wechsler Memory Scale—Revised (cutoff scores ≤16 for ≥16 years of education; ≤8 for ≥8–15 years of education); (3) normal general cognitive function, determined by a MMSE score greater than or equal to 24; (4) no or minimal impairment in activities of daily living (ADLs) revealed by the Lawton scale, as determined by a clinical interview with the patient and informant; and (5) not being sufficiently impaired, cognitively and functionally, to meet the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (NINCDS-ADRDA) (McKhann et al. 1984) for AD as judged by a clinician experienced in AD research. MCIs were classified as amnestic MCI, at the stage 3 of the GDS, and they did not meet the diagnostic criteria for dementia.
The age of the participants ranged from 65 to 85, since the incidence and prevalence of AD starts to rise around 65 years, and at the age of 80 and 90, it is nearly 50 % (Zamrini et al. 2011). All of them were in good health, with no significant cerebral vascular disease, and no history of psychiatric or neurological disorders. Other inclusion criteria included a modified Hachinski score less than or equal to 4, a Geriatric Depression Scale score lower than 9, and a computed tomographic or magnetic resonance imaging (MRI) brain scan within 12 months before MEG screening without indication of infection, infarction, or focal lesions. We performed on every participant complementary explorations (class II evidence level) to rule out possible causes of cognitive decline such as B12 vitamin deficit, diabetes, thyroid problems, syphilis, or human immunodeficiency virus. Besides, those subjects with alcoholism (>3 alcoholic beverages per day) or chronic use of medication such as anxiolytics were not included in the study. Finally, MCI patients were required to suspend the ingestion of drugs which could affect MEG activity (e.g., cholinesterase inhibitors) 48 h before the recordings.
Patients and controls also received a neuropsychological assessment, in order to explore their cognitive status in multiple cognitive functions such as memory, language, and executive functions. This included the Clock Drawing Test (Agrell and Dehlin 1998), Direct and Inverse digit Spam Test (Wechsler Memory Scale Revised, WMS-III; Wechsler 1987), Immediate and Delayed Recall (WMS-III; Wechsler 1987), Phonemic and Semantic Fluency (Controlled Oral Word Association Test, COWAT; Benton and Hamsher 1989), Ideomotor Praxis of Barcelona Test (Peña-Casanova 1990), rule shift cards (Behavioural Assessment of the Dysexecutive Syndrome, BADS; Norris and Tate 2000), Visual Object and Space Perception Test (VOSP; Warrington and James 1991), Boston Naming Test (BNT; Kaplan et al. 1983), and Trail Making Test A and B (TMTA and TMTB; Reitan 1958).
Before the MEG recording, all participants gave a written informed consent to participate in the investigation. The study was approved by the local ethics committee.
Experimental design
IDICS was based on a calculation mental task, which consisted of subtracting numbers mentally. Participants were told an initial number and instructed to repeatedly subtract a given amount from that number over the course of 1 min. After that minute, they were asked what number they had reached, and the researchers made a note of their answers. Participants were allowed to perform the subtraction at their own pace and were instructed to keep their eyes closed while performing the task. Counting aloud and finger counting was prohibited, and participants were instructed to continue the task regardless of any doubts of error.
The IDICS task had two levels of difficulty:
1-subtraction: Participants had to subtract 1 by 1 from a given fixed number.
3-subtraction: Participants had to subtract 3 by 3 from a given fixed number.
In each subtraction condition, four numbers were given: two two-digit and two three-digit. The same numbers were used for all the participants but their order was changed in order to counterbalance experimental conditions. So, for each subject, we registered 8 min of brain activity and eight behavioral responses, one for each given number. If a subject performed badly more than one subtraction exercise in any condition (1-subtraction of 3-subtraction), he/she was excluded from the present functional connectivity analysis. Following this criterion, 17 MCIs and 2 controls were excluded and only data from 70 subjects out of 89 recorded subjects were used. A subtraction exercise was classified as badly performed if (1) participants informed that they got lost or stopped performing the task correctly, (2) they summed instead of subtracting, or (3) the final number they reached seemed out of a normal range (either too big, meaning they had subtracted very slowly or stopped doing so at some point or too small meaning they had probably skipped some number intervals). As the task is not externally controlled except for the number they give after 1 min, the external assessment of the performance is rather qualitative, and the validity of the data relied to a great extent on the participants’ assessment of their own performance.
MEG recordings and preprocessing
Brain magnetic fields were measured while each subject sat in a magnetically shielded room (VacuumSchmelze GmbH, Hanau, Germany), using a 306 channel Vectorview system (Elekta Neuromag) at the Center for Biomedical Technology (Madrid, Spain). Fields were measured during a task-free, 3-min eyes-closed condition and during the execution of the IDICS task. Subjects were instructed to close their eyes in both conditions and move as little as possible.
The MEG system comprised 102 magnetometers and 204 planar gradiometers. Sampling frequency was 1,000 Hz and an online filtering of 0.1–330 Hz was applied. A head position indicator (HPI) system and a three-dimensional digitizer (Fastrak Polhemus) were used to determine the position of the head with respect to the sensor array. Four HPI coils were attached to the subject (one on each mastoid, two on the forehead), and their position with respect to the three fiducials (nasion and left and right pre-auricular points) was determined. To record vertical electrooculogram (EOG), two electrodes were attached above and below the left eye, and a third one to the left earlobe for electrical grounding.
External noise was removed from the MEG data using the temporal extension of Signal-Space Separation (tSSS) (Taulu and Kajola 2005) as implemented with the MaxFilter software (version 2.2, Elekta-Neuromag) with a 10-s raw data buffer and a subspace correlation limit of 0.9. Data was subsequently adjusted for head movement every 200 ms and transformed into a common space.
Data was then preprocessed with Fieldtrip (Oostenveld et al. 2011). The continuous time series (resting state and mental calculation task) were first separated into segments of 4 s. Then, jump, muscle, and ocular artifacts were located using all 306 sensors and the additional EOG channels. All segments containing artifacts were eliminated from the analysis. As a result, all subjects had at least N = 15 clean segments per condition. The amount of clean trials was similar across groups (Table 1).
Functional connectivity analysis
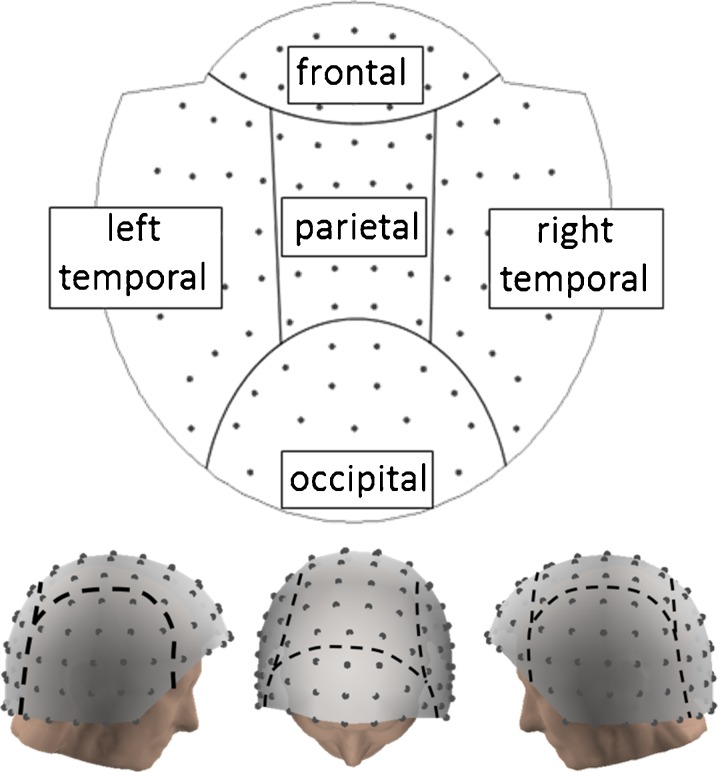
In order to provide additional information to the measures widely used in the study of functional connectivity in dementia (coherence and synchronization likelihood), PLV (Mormann et al. 2000) between pairs of sensors was computed. To avoid mixing signals of different nature and noise profiles, we used only magnetometers for this functional connectivity analysis (we note however that gradiometers were used in the temporal signal space separation step, so the resulting magnetometer data contain indirectly gradiometer information). We discarded eight inferior temporal sensors from the analysis for being noisy, keeping 94 magnetometers for the analysis. Figure 1 shows the distribution of these 94 channels in the MEG helmet in two and three dimensions and separates channels into different head regions for better understanding of the geometry.
Fig. 1.
MEG sensor layout. Magnetometer positions in 2D and 3D, separated into frontal, parietal, occipital, and left and right temporal regions
First, time series were filtered with a Finite Impulse Response filter of order 600 (which introduced a zero-phase distortion) into different frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz), yielding a narrow band signal xj(t) for each sensor j = 1… 102. Its phase φj(t) was extracted via a Hilbert transform.
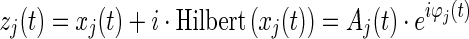 |
1 |
Then, the synchronization between a pair of phases φj(t) and φk(t) was assessed by computing the PLV.
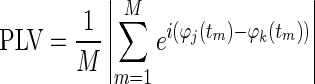 |
2 |
where M = 4,000 is the number of samples in the time series (4 s sampled at 1,000 Hz). This calculation was repeated for all pairs of sensors and averaged over segments belonging to the same condition, ending up with a symmetrical 94 × 94 connectivity matrix for each subject, condition (resting, 1- and 3-subtraction task), and frequency band.
Hippocampal volume
Hippocampal volumes were measured in order to provide an anatomical measure that quantifies the degree of brain atrophy. Indeed, a decrease in hippocampal volume has been related to MCI and Alzheimer’s disease (Dubois et al. 2007; Moretti et al. 2007). For each subject, a high-resolution T1-weighted magnetic resonance was acquired with a General Electric 1.5 T magnetic resonance (MR) scanner, using a high-resolution antenna and a homogenization PURE filter (Fast Spoiled Gradient Echo sequence, TR/TE/TI = 11.2/4.2/450 ms; flip angle 12°; 1 mm slice thickness, 256 × 256 matrix and FOV 25 cm). Freesurfer software (version 5.1.0) and its specialized tool for automated subcortical segmentation (Fischl et al. 2002) were used to segment the subject’s T1-weighted volume into different regions. Then, hippocampal volume was normalized with the overall intracranial volume to account for differences in head volume over subjects.
Statistical analysis
To compare connectivity values between conditions and groups (controls vs. MCIs), we employed nonparametric statistical tests: Wilcoxon signed rank tests for differences between conditions and independent samples and Wilcoxon-Mann-Whitney tests for differences between diagnoses. The results were corrected for multiple comparisons with a permutation technique that was introduced by Maris and Oostenveld (2007). Following this approach, elements from the two compared groups (e.g., condition 1 vs. condition 2 or diagnosis 1 vs. diagnosis 2) were shuffled randomly. Then, two subsets with the same size as the originals groups were created that contained the shuffled elements. A new statistical test (signed rank Wilcoxon or Wilcoxon-Mann-Whitney) was performed for these two randomly chosen groups. This procedure was repeated 5,000 times, yielding a set of surrogate p values. The final and corrected p value was then defined as the proportion of permutations with a p value lower than the one in the original test.
To assess the relationship between neurophysiological activity and cognitive performance, the average connectivity in significant links in the between-conditions analysis was correlated with several neuropsychological test scores in the whole sample (controls and MCIs). Additionally, hippocampal volumes were also included for correlations, in order to have an anatomical measure of the pathology. Tests were described in the diagnostic criteria section of this paper. Spearman’s r was calculated, along with the p value testing for the hypothesis of no correlation. This p value was corrected for multiple comparisons with a permutation approach, as explained in the previous paragraph.
Results
Behavioral performance
In order to check whether subjects were actually performing the task after every 1-min subtraction exercise, subjects announced the final number they had reached. Since all participants used the same original numbers in their countdown, the final number provides insight into the individual’s performance. The final numbers were compared between groups with a Wilcoxon-Mann-Whitney test. Significant differences in this final number would indicate a different execution level across groups. This comparison was done separately for all subtraction exercises (four exercises for each subtraction task).
There were no significant differences between controls and MCI in any of the eight answers, although the MCI group’s responses had higher standard deviation values than the responses from the control ones. This indicates that both groups were actively engaged in the task and had a similar performance (see Table 2).
Table 2.
IDICS performance in controls and MCIs. Mean values ± standard deviation of the responses given by the participants after 1 min of subtraction
| Numbers | Mean values ± standard deviation | p values | |
|---|---|---|---|
| Controls | MCIs | ||
| 75 | 14.67 ± 17.20 | 23.00 ± 20.06 | p = 0.192 |
| 99 | 39.50 ± 21.48 | 55.14 ± 34.81 | p = 0.226 |
| 158 | 102.12 ± 30.04 | 102.36 ± 37.96 | p = 0.594 |
| 246 | 184.92 ± 37.34 | 169.73 ± 51.33 | p = 0.639 |
| 89 | 24.65 ± 24.24 | 30.50 ± 24.69 | p = 0.349 |
| 97 | 27.58 ± 17.78 | 51.36 ± 26.37 | p = 0.051 |
| 174 | 121.00 ± 43.89 | 114.77 ± 36.54 | p = 0.983 |
| 237 | 183.28 ± 34.85 | 166.18 ± 45.46 | p = 0.186 |
Numbers of the 1-subtraction condition: 75, 99, 158, and 246. Numbers of the 3-subtraction condition: 89, 97, 174, and 237. The p values were obtained with a Mann-Whitney U test
MEG connectivity results
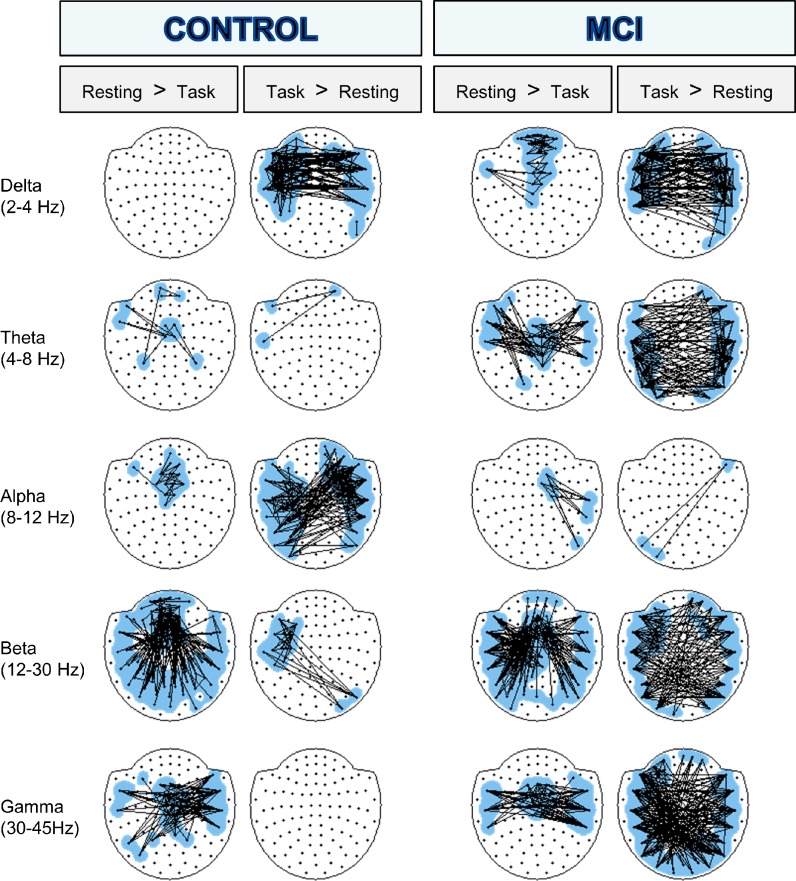
The result of the between-conditions analysis is summarized in Figs. 2 and 3. Figure 2 compares resting state and 3-subtraction task and shows the topography of the modified links. The topography of the connectivity change produced by the 1-subtraction task was not represented for the sake of simplicity. However, in general terms, it was similar than the one for the 3-subtraction task.
Fig. 2.
Statistical differences in connectivity between resting and 3-substraction task for controls and MCIs. Significantly altered links (p < 0.005) are shown for every frequency band. A line between two nodes represents a link that has been significantly altered and is either decreased (resting > task) or increased (task > testing) in the 3-substraction task. For clarity in visualization, only a selection of links was displayed: firstly, in order to reduce loose links, nodes participating with a single significant link were discarded, and secondly, a maximum of 150 links was plotted (if more links were statistically significant, 150 links with the lowest p value were chosen)
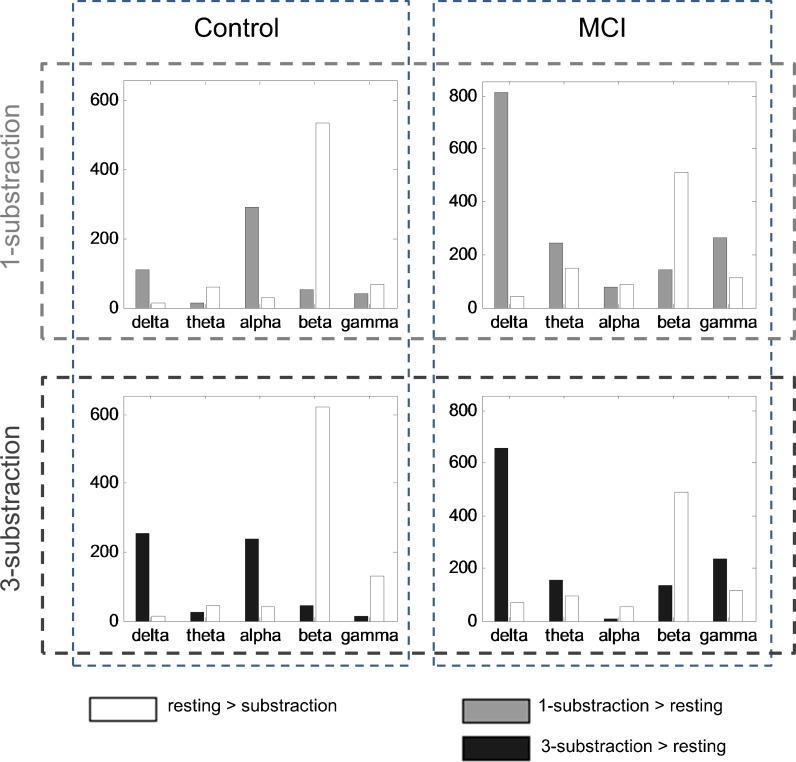
Fig. 3.
Number of significantly altered links when comparing resting and task for different groups and frequency bands. The first row shows differences between 1-substraction task (gray) and resting state (white), the second row considers 3-substraction (black) and resting. The two groups (controls and MCIs) are represented in different columns. Only links with p < 0.005 were considered
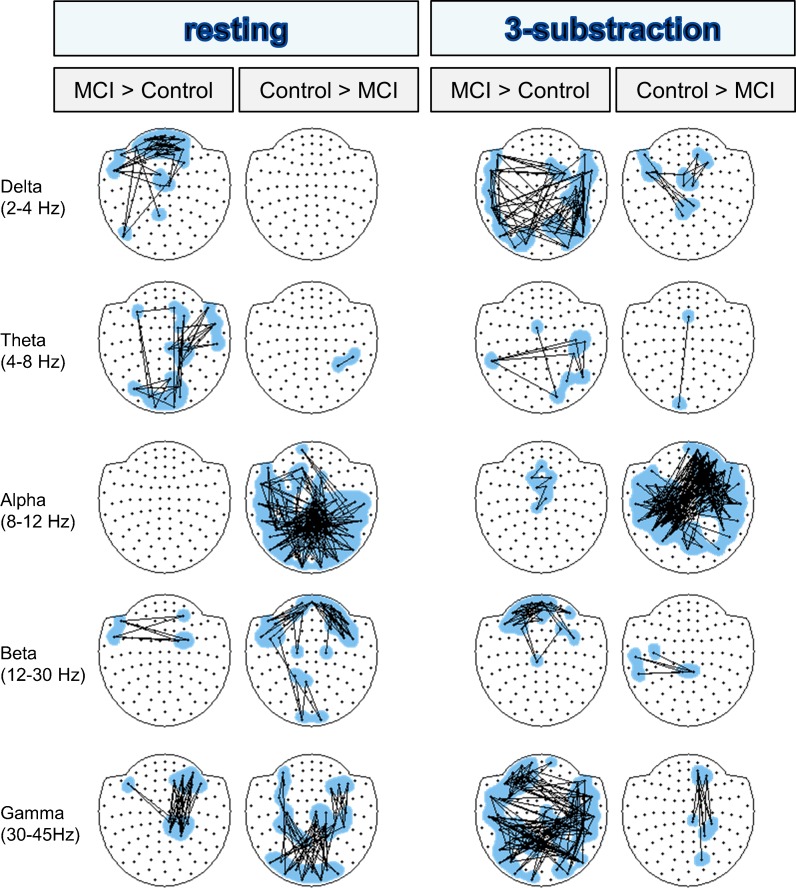
Figure 3 contrasts resting with 1- and 3-subtraction task, displaying the overall amount of altered links and enabling a comparison of the network change produced by both tasks. Finally, Fig. 4 represents differences between controls and MCI for resting state and for a resting-normalized 3-subtraction task separately, allowing a direct contrast between both groups. Results will be now described separately for every frequency band, focusing on connectivity differences and neuropsychology correlations.
Fig. 4.
Statistical differences in connectivity between controls and MCIs in resting state and 3-substraction task. Significantly altered links (p < 0.02) are shown for every frequency band. A line between two nodes represents a link that has been significantly altered and is either increased (MCI > control) or decreased (control > MCI) in the MCI group. As in Fig. 2, for clarity in visualization, loose links were removed and a maximum of 150 links was displayed
Delta band
During resting state, MCI patients exhibited higher values of connectivity fundamentally over frontal regions than controls (see Fig. 4). This increment was inversely correlated with MMSE (r = −0.28, p = 0.026), Immediate (r = −0.46, p < 0.0001) and Delayed Recall (r = −0.37, p = 0.002), rule shift cards (r = −0.3, p = 0.016), Phonemic (r = −0.26, p = 0.035) and Semantic Fluency (r = −0.35, p = 0.003), and Boston Naming Test (r = −0.41, p = 0.001) and directly correlated with TMTA time (r = 0.30, p = 0.016) and TMTB time (r = 0.30, p = 0.019).
Delta band connectivity pattern was greatly altered by the counting task compared to resting state, showing a big increase of PLV interhemispheric connections in all groups, which were of higher magnitude in MCIs (see Figs. 2 and 4).
The interhemispheric increment of synchronization observed in the control group while performing the task was negatively correlated with the Inverse Digit Spam Test (r = −0.32, p = 0.010). Besides, a greater interhemispheric synchronization, as MCIs showed, was negatively correlated with hippocampal volume (r = −0.27, p = 0.049), Inverse Digit Spam Test (r = −0.27, p = 0.031), Semantic Fluency (r = −0.25, p = 0.043), and Immediate (r = −0.26, p = 0.037) and Delayed Recall (r = −0.27, p = 0.030), evidencing that a lower behavioral performance is associated with a higher connectivity value. Additionally, a decrease in short range delta connectivity between frontal sensors when performing the task was positively correlated with TMTA time (r = 0.26, p = 0.042) and inversely correlated with Semantic Fluency (r = −0.25, p = 0.043), Immediate (r = −0.36, p = 0.003) and Delayed Recall (r = −0.35, p = 0.005), and Boston Naming Test (r = −0.31, p = 0.014).
Theta band
MCIs showed an increase in synchronization over right parieto-temporal areas and between fronto-occipital regions during resting state compared to the control group (see Fig. 4). This hyperconnectivity was negatively related to the hippocampal volume (r = −0.35, p = 0.01) and to the scores in MMSE (r = −0.30, p = 0.015), Clock Drawing test (order subtest) (r = −0.39, p = 0.001), Immediate (r = −0.56, p < 0.0001) and Delayed Recall (r = −0.55, p < 0.0001), rule shift cards (r = −0.35, p = 0.004), Phonemic (r = −0.27, p = 0.028) and Semantic Fluency (r = −0.37, p = 0.002), and Boston Naming Test (r = −0.34, p = 0.002).
Theta band PLV distribution differed greatly in controls and MCIs during the performance of the task (see Fig. 2). In the control group, few differences were found between resting and the mental calculation state and were not correlated with neuropsychological or hippocampal measures. However, the MCI group showed both increases and decreases of synchronization during the IDICS execution. The interhemispheric desynchronization from central to lateral sensors was inversely correlated with Immediate (r = −0.246, p = 0.048) and Delayed Recall (r = −0.281, p = 0.025), and the excess of interhemispheric PLV was directly correlated with TMTA time (r = 0.277, p = 0.027).
Alpha band
There were clear differences in synchronization in resting state between both groups, since the controls showed higher connectivity values, mainly over central and posterior interhemispheric regions, than the MCI patients (see Fig. 4). In addition, this increment was positively correlated with several tests such as MMSE (r = 0.42, p = 0.001), Immediate (r = 0.40, p = 0.001) and Delayed Recall (r = 0.44, p < 0.0001), Phonemic (r = 0.29, p = 0.021) and Semantic Fluency (r = 0.35, p = 0.003), and Boston Naming Test (r = 0.30, p = 0.016).
In addition, alpha connectivity behaved very differently from the previous bands, and the changes in controls were bigger than in MCIs for this band during the IDICS task (see Fig. 2). Controls showed an important increase of interhemispheric PLV and a decrease in short-range coupling in middle frontal sensors during the task, while in the MCI group, far fewer links were altered in the transition from the resting to the cognitive state.
The interhemispheric increase in synchronization during the performance of the mental task was directly related to MMSE (r = 0.285, p = 0.021), Immediate (r = 0.372, p = 0.002) and Delayed Recall (r = 0.309, p = 0.013), Phonemic (r = 0.294, p = 0.018) and Semantic Fluency (r = 0.267, p = 0.029), Boston Naming Test (r = 0.285, p = 0.024), TMTA time (r = −0.428, p = 0.0003), and TMTB time (r = −0.369, p = 0.003). The decrease in synchronization in middle frontal regions was positively correlated with hippocampal volume (r = 0.35, p = 0.011), MMSE (r = 0.253, p = 0.042), Immediate (r = 0.270, p = 0.03) and Delayed Recall (r = 0.330, p = 0.008), Semantic Fluency (r = 0.388, p = 0.001), and Boston Naming Test (r = 0.377, p = 0.002) and negatively correlated with TMTA time (r = −0.268, p = 0.032) and TMTB time (r = −0.399, p = 0.001). These results point out the important relationship between this frequency band and the hippocampal volume and the cognitive status.
Beta band
Controls showed higher synchronization values over interhemispheric frontal areas and parieto-occipital regions during resting state than the MCI subjects, who exhibited an increase in connectivity between parietal and left temporal areas (see Fig. 4). The rise of synchronization over interhemispheric frontal areas and parieto-occipital regions was directly correlated with hippocampal volume (r = 0.5, p < 0.0001) and Immediate (r = 0.35, p = 0.004) and Delayed Recall (r = 0.37, p = 0.003) and inversely correlated to TMTA time (r = −0.26, p = 0.04), while the hypersynchronization between parietal and left temporal areas was negatively related to Clock Drawing test (order subtest) (r = −0.37, p = 0.002), Immediate (r = −0.4, p = 0.002) and Delayed Recall (r = −0.42, p = 0.001), Semantic Fluency (r = −0.4, p = 0.001), and Boston Naming Test (r = −0.42, p = 0.01) and positively correlated to TMTB time (r = 0.31, p = 0.015).
During the execution of the task, both groups exhibited an interhemispheric and anterior-posterior desynchronization, being higher in the case of the controls, and different patterns of increase of synchronization (see Fig. 2). The increase of connectivity observed in the control group between left anterior areas and right posterior regions was not correlated with anatomical and neuropsychological information, while the interhemispheric synchronization found in the MCI group was inversely correlated with Inverse Digit Spam (r = −0.249, p = 0.047) and Immediate Recall (r = −0.253, p = 0.042).
Although an increase in desynchronization was observed in both groups while performing the task, the pattern of the controls was positively correlated with Clock Drawing Test (order subtest) (r = 0.414, p = 0.001) and inversely correlated with TMTB time (r = −0.280, p = 0.029), while the pattern observed in the MCI group was negatively correlated with Inverse Digit Spam (r = −0.332, p = 0.007) and Immediate (r = −0.344, p = 0.005) and Delayed Recall (r = −0.266, p = 0.033). Connectivity differences between both groups were mainly located in frontal regions where connectivity was higher in controls in resting state and higher in MCI during the task, indicating a difficulty for MCIs to desynchronize during the task (see Fig. 4).
Gamma band
In resting state, controls presented higher connectivity in parieto-occipital sensors than MCIs, while these last showed higher PLV values between right fronto-temporo-parietal areas (see Fig. 4). The increase observed in the control group was positively correlated with MMSE (r = 0.3, p = 0.015), Immediate (r = 0.5, p = 0.0001) and Delayed Recall (r = 0.44, p = 0.0001), Semantic Fluency (r = 0.4, p = 0.001), and hippocampal volume (r = 0.5, p = 0.0001), whereas the hypersynchronization between right fronto-temporo-parietal areas was inversely correlated with Clock Drawing Test (order subtest) (r = −0.28, p = 0.021), Direct Digit Spam (r = −0.32, p = 0.009), Immediate (r = −0.41, p = 0.001) and Delayed Recall (r = −0.34, p = 0.006), Semantic Fluency (r = −0.33, p = 0.006), and shift cards (r = −0.3, p = 0.018).
During the execution of IDICS, both groups presented a central to lateral desynchronization within the gamma band. The desynchronization pattern observed in the control group was directly correlated with the Boston Naming Test (r = 0.279, p = 0.027), Ideomotor Praxis (r = 0.26, p = 0.04), and Immediate (r = 0.312, p = 0.011) and Delayed Recall (r = 0.352, p = 0.004), while the desynchronization pattern found in the MCI group did not correlate with hippocampal atrophy and cognitive performance.
Only the MCI group showed an increase of interhemispheric synchronization that was inversely correlated with Direct (r = −0.302, p = 0.014) and Inverse Digits Spam (r = −0.396, p = 0.001) and Immediate (r = −0.267, p = 0.032) and Delayed Recall (r = −0.341, p = 0.006). This suggests that hypersynchronization at higher frequencies could be associated with worse cognitive performance in memory tasks.
1- and 3-subtraction tasks
In general, the connectivity patterns in 1- and 3-subtraction tasks were alike. Since topography in both conditions was similar, the overall amount of altered links between resting and 1- and 3-subtraction tasks is shown in Fig. 3, which allows the comparison between the network changes induced by both tasks. In particular, if the transition from resting to 1- and then to 3-subtraction task was linear, one would expect that the differences between resting and task would be much bigger for the 3- than for the 1-subtraction task. And this could be seen through a higher amount of altered links in the 3- than in the 1-subtraction task. For controls, such a tendency was found in delta and beta and to a lesser extent in the gamma band. On the contrary for MCIs, there was rather a small decrease in the number of links in the 3-subtraction task when compared to the 1-subtraction task.
Discussion
The aim of this study was twofold: to investigate how functional connectivity changes between resting state and IDICS with increasing processing demands and to consider how the presence of cognitive impairments in aging modifies these synchronization patterns. To this end, we measured MEG signals in healthy elderly adults and in patients diagnosed with MCI, and we employed an internal mental calculation task with two levels of difficulty, 1-subtraction and 3-subtration. As expected, task load modulated connectivity patterns, which differed between both groups, and MCI displayed hypersynchronization in most frequency bands and a lack of synchronization in the alpha band.
Resting vs. IDICS task
Delta results are in line with most calculation task studies, which have mainly used EEG and analyzed power distributions (Harmony et al. 1996, 2004; Dimitriadis et al. 2010; Giannitrapani 1971). Delta band activity is considered to increase during cognitive tasks requiring attention to internal processing and to decrease under conditions which demand attention to the external environment. Additionally, the magnitude of the increment rises with the difficulty of the task (Harmony et al. 1996; Dimitriadis et al. 2010). Here we showed that in both groups, delta connectivity was greater during mental calculation than in resting state, a finding in line with the previous literature. This increased connectivity between the left and right anterior regions indicates the necessity of the interaction between both hemispheres for the performance of mental calculation and could be engaged in updating of arithmetic operations and manipulation of information (Krueger et al. 2011).
No important differences in the theta band could be ascertained in the control group. Some studies using EEG to investigate cognition during arithmetic and mental tasks have described an increase in theta band activity in the frontal midline areas of the brain, although in these cases, spectral power was being considered instead of connectivity (Sasaki et al. 1996; Ishii et al. 1999; Onton et al. 2005; Yener et al. 2013). For MCIs, bigger connectivity changes between task and resting were needed to deal with the subtraction task. These results could be explained for two possible reasons: First, individuals in the MCI group found the task more difficult to complete and thus displayed more theta band activity (theta band activity being attributed to memory load, complex tasks, and attention) (Gevins et al. 1997) and second because the MCI group usually shows an increase in both theta power and connectivity during the execution of cognitive tasks (Jiang 2005; Jiang et al. 2008; Aurtenetxe et al. 2013).
In the alpha band, the control group showed an increase in connectivity during the subtraction task. These results are consistent with recent studies which consider that the alpha band is associated with internal tasks (Jensen et al. 2002; Cooper et al. 2003; Sauseng et al. 2005; Palva and Palva 2007; Benedek et al. 2011), such as mental calculation, and that may reflect an internal information processing which involves top-down control and may be related to an inhibition of external interfering input (Jensen and Mazaheri 2010). Controls also displayed a desynchronization over frontal brain areas, which could be related to the processing of semantic information such as mathematical knowledge (Klimesch et al. 2007). For MCIs, few connectivity changes were found in this band, suggesting that this group had less cognitive control. It could be speculated that the excess synchronization in delta, theta, beta, and gamma bands compensated for the lack of connectivity changes in the alpha band.
In the beta band, long distance connections between left anterior region and right posterior areas increased in the control group. Conversely, there was a broad desynchronization in both groups in the IDICS task compared to resting state. This may reflect the activation of areas needed for calculation—such as the fronto-parietal network (Krueger et al. 2011)—or the deactivation of others that are able to adapt to the cognitive difficulty. Each factor could play a role in the successful execution of cognitive tasks. These results could be related to the Default Mode Network (DMN) (Raichle et al. 2001), which is an antero-posterior network that deactivates when performing a task. So the DMN might behave in the same way in internal and external cognitive tasks, although further studies would be needed to test this hypothesis. Finally, our results suggest that the main differences from group to group were found in the anterior regions of the DMN. These regions were found to be more desynchronized in the control group when compared to MCIs, indicating that MCIs were not able to sufficiently deactivate this network to perform the task.
Similar to the beta band findings, a desynchronization of the resting state networks of both groups was noted during task performance when looking at results in the gamma band. In this line, Park et al. (2012) found higher gamma event-related desynchronization in elderly controls when compared to a group of MCI patients, which was related to cognitive performance.
Control group vs. MCI group
As expected, differences in resting state connectivity between control and MCI were found. The MCI group presented a higher synchronization than the control group in low-frequency bands (e.g., frontal delta and antero-posterior right theta) and this increase was related to hippocampal atrophy (i.e., theta band) and to a lower global cognitive status (i.e., MMSE) and worse performance in attention, memory, language, and executive functioning. On the contrary, they exhibited lower connectivity values in the alpha band, especially interhemispheric and in central and posterior regions, as well as the beta band in frontal areas. The desynchronization observed in the MCI group was related to poorer performance in multiple cognitive domains, such as memory or executive function, and also with a smaller hippocampal volume. Finally, MCIs showed a higher synchronization in the gamma band between right anterior and central areas, which was related to a poorer performance in attention, memory, and executive function, while in the control group, this increase was localized in more posterior regions and was directly correlated with a greater hippocampal volume and higher scores in MMSE, Immediate and Delayed Recall, and Semantic Fluency. Most of these findings, and especially the decrease in synchronization in alpha and beta bands, are in agreement with those found in MCI and AD studies (Berendse et al. 2000; Jelic et al. 2000; Koenig et al. 2005; Stam et al. 2003; Moretti et al. 2008; Gómez et al. 2009), suggesting that MCI could be considered as the beginning of the “disconnection syndrome” (Delbeuck et al. 2003).
For the subtraction task, MCIs showed an increase of synchronization in most frequency bands during the execution of the task when compared to the resting state. This points out that the MCIs were calculating in a rather inefficient way, since they needed more brain connections than the control group to perform the same task. These findings are in agreement with many studies which employed cognitive tasks in the MCI population (Pijnenburg et al. 2004; Jiang 2005; Jiang and Zheng 2006; Bajo et al. 2010). In the alpha band, however, MCIs were desynchronized during the task. This could mean that MCI showed an impairment in the mechanisms related to top-down control and inhibition of external inputs (Klimesch et al. 2007), which would indicate that MCIs struggled with centering on the task. On the whole, these synchronization/desynchronization profiles could be used as a potential biomarker of the MCI disease.
To further investigate the meaning of the hyper/hyposynchronization found during the execution of the IDICS task and their relation to brain atrophy and cognitive behavior, connectivity changes were correlated with hippocampal volumes and neuropsychological tests. In the MCI group, the increases and decreases found in synchronization in delta, theta, beta, and gamma bands during the execution of the task correlated with a lower performance in most of the neuropsychological measures employed, including semantic and episodic memory, attention, and executive functioning. Additionally, higher PLV values in delta band during IDICS were related to lower hippocampal volume, indicating that delta hypersynchronization during the execution of the task relates to anatomical and functional deterioration. It is noteworthy that in the control group, an interhemispheric increase in the alpha band was directly correlated with global cognitive status (i.e., MMSE), semantic and episodic memory, and executive functioning, while a frontal decrease in connectivity was also related to the hippocampal volume. These results indicate that the ability to modulate the alpha band is essential for a good cognitive functioning.
Task load
In delta, beta and, to a lesser extent, in gamma frequency bands, the number of altered links was higher in the 3- than the 1-subtraction task when compared against the resting state, indicating that task load modulates connectivity changes. Other studies have described how brain activity increases with task difficulty or cognitive load in aging (Rypma et al. 2007; Zarahn et al. 2007; Morcom et al. 2007). Palva et al. (2010) found that an increase in working memory load resulted in a strengthened brain synchrony. Micheloyannis et al. (2005) suggested that increasing the complexity of arithmetic tasks by increasing the demand for additional operations is associated with a more widespread synchronization pattern. However, this increase of altered connections with task difficulty was not present in MCI. This effect could be interpreted under the framework of the compensation-related utilization of neural circuits hypothesis (CRUNCH) model (Reuter-Lorenz and Cappell 2008), which was introduced to explain how cortical circuits adapt to increasing task load. When the old and young subjects are compared, the older cohort recruit more neural resources to acquire a similar performance at low levels of task demand, leading to higher levels of neural activity. Regardless, they have fewer resources available to meet the processing requirements of more demanding task (as a resource ceiling is reached) and their performance then declines. A similar effect seems to occur in this study, when elders with and without cognitive impairment were compared. In the control group, the synchronization changes rose with the difficulty of the task (i.e., delta band), while in the MCI group, more resources were needed to achieve the same level of performance in the 1-subraction task, but a decline in synchronization changes was noted in the 3-subtraction condition. However, further studies with increasing load are needed to verify this effect in both normal and pathological aging.
In summary, controls exhibited connectivity changes in all frequency bands to deal with the IDICS. These changes were related to the task difficulty, especially in delta and beta frequency bands, according to the CRUNCH model. MCIs showed different connectivity profiles than controls, pointing out that the MEG could contribute to the assessment of the MCI diagnosis, in addition to that of the biomarkers commonly used in clinical practice (Molinuevo and Rami 2013). MCIs showed a higher synchronization increase to perform the task, except from the alpha band. This increment did not correlate positively with the task load and was associated with a lower behavioral cognitive function. In fact, this hyperconnectivity in MCI has been found previously in working memory tasks with external stimuli (Bajo et al. 2010) and was used as a marker for prediction of conversion to dementia (Bajo et al. 2012). It can be speculated that the profile of hypersynchronization found here in the MCI patients may be related to the pathological process of AD. This view of synchronization as pathological, not as compensatory, would be in agreement with the hypothesis proposed by de Haan et al. (2012). They postulated that the increase in connectivity reported in MCIs might be due to higher amyloid accumulation over the most connected regions (hubs), which are especially vulnerable to AD. In this line, hyperactivity has been associated with the release of beta amyloid protein into interstitial fluid and neuronal hyperexcitability (Cirrito et al. 2008). In fact, recent findings indicate that in the vicinity of the beta amyloid plaque, there is a decrease in the number of gabergic neurons (Garcia-Marin et al. 2009) which could increase hyperexcitability. Thus, the greater the excitability, the greater the likelihood of neuronal synchronization and, as a consequence, a profile of widespread connectivity as is the case of the MCI patients.
The internal cognitive task introduced here differs from the most commonly employed cognitive paradigms, because of the absence of external stimuli, which requires inhibition from both the environment and internal thoughts. This would suggest that the MCIs had difficulties with top-down control processes during the calculation task, since they failed to synchronize in the alpha band. Impairment in inhibitory control mechanisms may affect the performance of daily living activities, contributing to the patient’s progressive inability to adapt to the environment and to control for internal emotional stimuli (Fujie et al. 2008). Thus, the IDICS allows a better comparison with the resting state condition than usual external tasks, as it resembles the resting condition more and provides relevant information regarding the brain network impairment in MCI.
Limitations of the study
It is important to note that this study present some limitations. First of all, the IDICS task employed here is advantageous and novel in that it enables the study of brain alterations in MCI independently from the effect of external stimuli. However, it has a clear drawback: as an internally driven task, its performance is difficult to assess externally. The consideration of the final number that was reached from the subjects after a subtraction exercise provides some insight into the task execution, but it is not a direct metric of performance. Second, we analyzed functional connectivity in classically defined frequency bands, with fixed frequency limits: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz). However, we did not examine power spectra. A future spectral analysis could provide insight on how brain rhythms change with the IDICS task and with the MCI disease. Third, the analysis was performed in sensor space and, therefore, lacks spatial accuracy. Future studies in source space could locate the brain areas and the networks, as the DMN, involved in functional connectivity, changes in MCI. Fourth, as the calculation task used requires different cognitive processes, factors such as attention could be affecting the task execution. Fifth, to propose the MEG as a new potential biomarker, it should be compared with the established biomarkers in dementia research such as cerebrospinal fluid (CSF) and positron emission tomography with Pittsburgh compound B (PET-PIB) (Dubois et al. 2010).
Acknowledgments
This study was supported by two projects PSI2009-14415-C03-01 and PSI2012-38375-C03-01 from the Spanish Ministry of Science and Economy, a predoctoral fellowship from the Ministry of Education (FPU AP-2008-00175), a PICATA predoctoral fellowship of the Moncloa Campus of International Excellence (UCM-UPM), a predoctoral fellowship from the Spanish Ministry of Science and Innovation (BES-2010-036469), and a predoctoral fellowship from the Basque Government.
Footnotes
María Eugenia López and Pilar Garcés have contributed equally to this work.
Contributor Information
María Eugenia López, Phone: +34-91-3367307, FAX: +34-91-3366828, Email: meugenia.lopez@ctb.upm.es.
Pilar Garcés, Phone: +34-91-3364632, Email: pilar.garces@ctb.upm.es.
Pablo Cuesta, Phone: +34-91-3364632, Email: pablo.cuesta@ctb.upm.es.
Nazareth P. Castellanos, Phone: +34-91-3364632, Email: nazareth.castellanos@ctb.upm.es
Sara Aurtenetxe, Phone: +34-91-3364632, Email: sara.aurtenetxe@ctb.upm.es.
Ricardo Bajo, Phone: +34-628909785, Email: ricardo.bajo@ctb.upm.es.
Alberto Marcos, Email: amarcosdolado@gmail.com.
Mercedes Montenegro, Email: montejop@madrid.es.
Raquel Yubero, Email: rayubpan@gmail.com.
Francisco del Pozo, Email: francisco.delpozo@ctb.upm.es.
Miguel Sancho, Phone: +34-91-3944388, Email: msancho@ucm.es.
Fernando Maestú, Phone: +34-91-3364632, Email: fernando.maestu@ctb.upm.es.
References
- Agrell B, Dehlin O. The clock-drawing test. Age Ageing. 1998;27:399–403. doi: 10.1093/ageing/27.3.399. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr. 1997;9(Suppl 1):167–171. doi: 10.1017/S1041610297004869. [DOI] [PubMed] [Google Scholar]
- Aurtenetxe S, Castellanos NP, Moratti S, et al. Dysfunctional and compensatory duality in mild cognitive impairment during a continuous recognition memory task. Int J Psychophysiol. 2013;87:95–102. doi: 10.1016/j.ijpsycho.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Bajo R, Maestú F, Nevado A, et al. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J Alzheimers Dis. 2010;22:183–193. doi: 10.3233/JAD-2010-100177. [DOI] [PubMed] [Google Scholar]
- Bajo R, Castellanos NP, Cuesta P, et al. Differential patterns of connectivity in progressive mild cognitive impairment. Brain Connect. 2012;2:21–24. doi: 10.1089/brain.2011.0069. [DOI] [PubMed] [Google Scholar]
- Benedek M, Bergner S, Könen T, et al. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia. 2011;49:3505–3511. doi: 10.1016/j.neuropsychologia.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A, Hamsher K. Multilingual aplasia examination. 2. Iowa City: AJA Associates; 1989. [Google Scholar]
- Berendse H, Verbunt JP, Scheltens P, et al. Magnetoencephalographic analysis of cortical activity in Alzheimer’s disease: a pilot study. Clin Neurophysiol. 2000;111:604–612. doi: 10.1016/S1388-2457(99)00309-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buldú JM, Bajo R, Maestú F, et al. Reorganization of functional networks in mild cognitive impairment. PLoS One. 2011;6:e19584. doi: 10.1371/journal.pone.0019584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, De La Sayette V, et al. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang J-E, Lee J, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJJ, et al. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol. 2003;47:65–74. doi: 10.1016/S0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- De Haan W, Mott K, van Straaten ECW, et al. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol. 2012;8:e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M, Collette F. Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev. 2003;13:79–92. doi: 10.1023/A:1023832305702. [DOI] [PubMed] [Google Scholar]
- Dimitriadis SI, Laskaris NA, Tsirka V, et al. What does delta band tell us about cognitive processes: a mental calculation study. Neurosci Lett. 2010;483:11–15. doi: 10.1016/j.neulet.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Müller MJ, Wille P, et al. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fernández A, Hornero R, Mayo A, et al. MEG spectral profile in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2006;117:306–314. doi: 10.1016/j.clinph.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Brain function, nonlinear coupling, and neuronal transients. Neuroscientist. 2001;7:406–418. doi: 10.1177/107385840100700510. [DOI] [PubMed] [Google Scholar]
- Fujie S, Namiki C, Nishi H, et al. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:432–439. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- Garcia-Marin V, Blazquez-Llorca L, Rodriguez J-R, et al. Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front Neuroanat. 2009;3:28. doi: 10.3389/neuro.05.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M et al (2006) Mild cognitive impairment. Lancet 1262–1270 [DOI] [PubMed]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Giannitrapani D. Scanning mechanisms and the EEG. Electroencephalogr Clin Neurophysiol. 1971;30:139–146. doi: 10.1016/0013-4694(71)90274-4. [DOI] [PubMed] [Google Scholar]
- Gómez C, Stam CJ, Hornero R, et al. Disturbed beta band functional connectivity in patients with mild cognitive impairment: an MEG study. IEEE Trans Biomed Eng. 2009;56:1683–1690. doi: 10.1109/TBME.2009.2018454. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Haense C, Kalbe E, Herholz K, et al. Cholinergic system function and cognition in mild cognitive impairment. Neurobiol Aging. 2012;33:867–877. doi: 10.1016/j.neurobiolaging.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, et al. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. doi: 10.1103/RevModPhys.65.413. [DOI] [Google Scholar]
- Harmony T, Fernández T, Silva J, et al. EEG delta activity: an indicator of attention to internal processing during performance of mental tasks. Int J Psychophysiol. 1996;24:161–171. doi: 10.1016/S0167-8760(96)00053-0. [DOI] [PubMed] [Google Scholar]
- Harmony T, Fernández T, Gersenowies J, Galán L, Fernández-Bouzas A, Aubert E, Díaz-Comas L (2004) Specific EEG frequencies signal general common cognitive processes as well as specific task processes in man. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology 53(3):207–16. doi:10.1016/j.ijpsycho.2004.04.006 [DOI] [PubMed]
- He J, Farias S, Martinez O, et al. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66:1393–1399. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Jelic V, Johansson S-E, Almkvist O, et al. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Study on EEG power and coherence in patients with mild cognitive impairment during working memory task. J Zhejiang Univ Sci B. 2005;6:1213–1219. doi: 10.1631/jzus.2005.B1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zheng L. Inter- and intra-hemispheric EEG coherence in patients with mild cognitive impairment at rest and during working memory task. J Zhejiang Univ Sci B. 2006;7:357–364. doi: 10.1631/jzus.2006.B0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zheng L, Yu E-Y. EEG coherence characteristics at rest and during a three-level working memory task in normal aging and mild cognitive impairment. Med Sci Monit. 2008;14:515–524. [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005;26:165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Krueger F, Landgraf S, van der Meer E, et al. Effective connectivity of the multiplication network: a functional MRI and multivariate Granger Causality Mapping study. Hum Brain Mapp. 2011;32:1419–1431. doi: 10.1002/hbm.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Leirer VM, Wienbruch C, Kolassa S, et al. Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav. 2011;5:222–228. doi: 10.1007/s11682-011-9126-3. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Feng L, Meng Q. Early event-related potentials changes during simple mental calculation in Chinese older adults with mild cognitive impairment: a case-control study. Neurosci Lett. 2010;475:29–32. doi: 10.1016/j.neulet.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Lobo A, Ezquerra J, Gómez Burgada F, et al. Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients) Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1979;7:189–202. [PubMed] [Google Scholar]
- Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G (1998) EEG coherence in Alzheimer’s disease. Electroen Clin Neuro 106(3):229–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9743281 [DOI] [PubMed]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Sakkalis V, Vourkas M, et al. Neural networks involved in mathematical thinking: evidence from linear and non-linear analysis of electroencephalographic activity. Neurosci Lett. 2005;373:212–217. doi: 10.1016/j.neulet.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Rami L. Applying the IWG research criteria in clinical practice: feasibility and ethical issues. Med Clin North Am. 2013;97:477–484. doi: 10.1016/j.mcna.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Miniussi C, Frisoni GB, et al. Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clin Neurophysiol. 2007;118:2716–2729. doi: 10.1016/j.clinph.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Frisoni GB, Pievani M, et al. Cerebrovascular disease and hippocampal atrophy are differently linked to functional coupling of brain areas: an EEG coherence study in MCI subjects. J Alzheimers Dis. 2008;14:285–299. doi: 10.3233/jad-2008-14303. [DOI] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, Elger CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D Nonlinear Phenom. 2000;144:358–369. doi: 10.1016/S0167-2789(00)00087-7. [DOI] [Google Scholar]
- Mufson EJ, Chen EY, Cochran EJ, et al. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Norris G, Tate RL. The Behavioural Assessment of the Dysexecutive Syndrome (BADS): ecological, concurrent and construct validity. Neuropsychol Rehabil. 2000;10:33–45. doi: 10.1080/096020100389282. [DOI] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Rantanen K, Ahveninen J, et al. Source estimation of spontaneous MEG oscillations in mild cognitive impairment. Neurosci Lett. 2006;405:57–61. doi: 10.1016/j.neulet.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Lee KS, An SK, et al. Gamma oscillatory activity in relation to memory ability in older adults. Int J Psychophysiol. 2012;86:58–65. doi: 10.1016/j.ijpsycho.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Parlato V, Lopez OL, Panisset M, et al. Mental calculation in mild Alzheimer’s disease: a pilot study. Int J Geriatr Psychiatry. 1992;7:599–602. doi: 10.1002/gps.930070810. [DOI] [Google Scholar]
- Peña-Casanova J. Programa Integrado de Exploración Neuropsicológica- Test Barcelona. Masson: Protocolo; 1990. [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Ski. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Rémy F, Mirrashed F, Campbell B, Richter W. Mental calculation impairment in Alzheimer’s disease : a functional magnetic resonance imaging study. Neurosci Lett. 2004;358:25–28. doi: 10.1016/j.neulet.2003.12.122. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. doi: 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Del Percio C, Pasqualetti P, et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143:793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/S0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, et al. Loss of “small-world” networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Tsujimoto T, Nishikawa S, et al. Frontal mental theta wave recorded simultaneously with magnetoencephalography and electroencephalography. Neurosci Res. 1996;26:79–81. doi: 10.1016/0168-0102(96)01082-6. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, et al. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp. 2005;26:148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ. Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci. 2010;289:128–134. doi: 10.1016/j.jns.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Dijk BW (2002) Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D: Nonlinear Phenomena 163(3–4):236–251. doi:10.1016/S0167-2789(01)00386-4
- Stam CJ, van der Made Y, Pijnenburg YAL, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003;108:90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Manshanden I, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage. 2006;32:1335–1344. doi: 10.1016/j.neuroimage.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Tao H-Y, Tian X. Coherence characteristics of gamma-band EEG during rest and cognitive task in MCI and AD. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2747–2750. doi: 10.1109/IEMBS.2005.1617040. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M. Presentation of electromagnetic multichannel data: the signal space separation method. J Appl Phys. 2005;97:124905. doi: 10.1063/1.1935742. [DOI] [Google Scholar]
- v d Pijnenburg YA, Made Y, van Cappellen van Walsum AM, et al. EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol. 2004;115:1332–1339. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Warrington E, James M. The visual object and space perception battery. Bury St. Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler memory scale-revised (manual) San Antonio: The Psycho; 1987. [Google Scholar]
- Yener GG, Kurt P, Emek-Savaş DD, et al. Reduced visual event-related delta oscillatory responses in amnestic mild cognitive impairment. J Alzheimers Dis. 2013;37:759–767. doi: 10.3233/JAD-130569. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Semenza C, Domahs F, et al. Alzheimer’s disease and mild cognitive impairment: effects of shifting and interference in simple arithmetic. J Neurol Sci. 2007;263:79–88. doi: 10.1016/j.jns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Stadelmann E, Nürk H-C, et al. Effects of age and mild cognitive impairment on direct and indirect access to arithmetic knowledge. Neuropsychologia. 2007;45:1511–1521. doi: 10.1016/j.neuropsychologia.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Zamrini E, Maestu F, Pekkonen E, et al. Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:280289. doi: 10.4061/2011/280289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, et al. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sachdev PS, Wen W, et al. Gray matter atrophy patterns of mild cognitive impairment subtypes. J Neurol Sci. 2012;315:26–32. doi: 10.1016/j.jns.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Zheng L, Jiang Z, Yu E. Alpha spectral power and coherence in the patients with mild cognitive impairment during a three-level working memory task. J Zhejiang Univ Sci B. 2007;8:584–592. doi: 10.1631/jzus.2007.B0584. [DOI] [PMC free article] [PubMed] [Google Scholar]