Abstract
Bivalve mollusks have several unique traits, including some species with exceptionally long lives, others with very short lives, and the ability to determine the age of any individual from growth rings in the shell. Exceptionally long-lived species are seldom studied yet have the potential to be particularly informative with respect to senescence-resistance mechanisms. To this end, we employed a range of marine bivalve mollusk species, with lifespans ranging from under a decade to over 500 years, in a comparative study to investigate the hypothesis that long life requires superior proteome stability. This experimental system provides a unique opportunity to study closely related organisms with vastly disparate longevities, including the longest lived animal, Arctica islandica.
Specifically, we investigated relative ability to protect protein structure and function, both basally and under various stressors in our range of species. We found a consistent relationship between species longevity, resistance to protein unfolding, and maintenance of endogenous enzyme (creatine kinase) activity. Remarkably, our longest-lived species, Arctica islandica (maximum longevity >500 years), had no increase in global proteome unfolding in response to several stressors. Additionally, the global proteome of shorter-lived species exhibited less resistance to temperature-induced protein aggregation than longer-lived species. A reporter assay, in which the same protein's aggregation properties was assessed in lysates from each study species, suggests that some endogenous feature in the cells of long-lived species, perhaps small molecular chaperones, was at least partially responsible for their enhanced proteome stability. This study reinforces the relationship between proteostasis and longevity through assessment of unfolding, function, and aggregation in species ranging in longevity from less than a decade to more than five centuries.
Keywords: Proteostasis, Bivalves, Longevity, Comparative aging, Arctica islandica, Unfolding, Aggregation
Introduction
The bivalve mollusks (clams, oysters, mussels) comprise an ancient group of marine and freshwater animals with approximately 10,000 living species. Two aspects of bivalve biology make them particularly attractive for aging research. First, many species exhibit annual growth rings in their shells, thus allowing the accurate assessment of chronological age and age-specific growth rate in large numbers of both living and dead individuals from natural populations (Richardson 2001). The ability to assess patterns of growth, development, reproduction, and longevity in natural populations has made bivalves particularly useful for examining questions about the evolution of life histories. It is known, for instance, that in general, longer-lived bivalve species grow slower, reach reproductive maturity later, and achieve a larger final body size than shorter-lived species (Ridgway et al. 2010). Second, bivalves contain a number of the longest-lived animal species, with known longevities of some species exceeding 500 years (Schöne et al. 2005; Wanamaker et al. 2008). By contrast, other bivalve species live no more than 1 year (Estabrooks 2007). This extraordinary range of longevities within the bivalves—many species of which have broadly similar ecologies and habitat preferences—facilitates the identification of senescence-resistance mechanisms, particularly because many species can also be maintained easily in the laboratory.
One of the putative mechanisms of senescence resistance is the ability to maintain an intact and highly functional proteome over time in the face of an inevitable diversity of internal and external stresses (Kikis et al. 2010; Koga et al. 2011). The idea that proteome stability, or proteostasis as it has come to be known, is a necessary and integral factor for longevity assurance is an intuitive and satisfying concept; it makes sense that compromised proteostasis would prevent exceptional longevity. Beyond this assumption, there is substantial evidence linking proteostasis and longevity. Life extending interventions such as caloric restriction have demonstrated increased proteostasis in mice (Chakravarti and Chakravarti 2007; Lass et al. 1998), as well as a decrease in the accumulation of carbonylated proteins (Dubey et al. 1996; Nagai et al., 2000; Radák et al. 2002; Sohal et al. 1994). Furthermore, genetic manipulations in Caenorhabditis elegans insulin-like signaling pathway that extend lifespan also decrease aggregation of reporter proteins (Cohen et al. 2006; Hsu et al. 2003; Morley et al. 2002). The reverse model is also notable. That is, pharmaceutical intervention with Thioflavin T to combat aggregation of polyglutamine and amyloid beta also extends the lives of C. elegans (Alavez et al. 2011). Thus, longevity manipulations affect proteostasis and proteostasis manipulations can affect longevity.
Damaged proteins have also been shown in many studies to accumulate with age, disrupting folding, function, and any downstream effects of that function (Koga et al. 2011; Morimoto and Cuervo 2009); (Koga et al. 2011; Morimoto and Cuervo 2009). Compromised protein structure can lead to the assembly of insoluble aggregates associated with normal aging as well as age-related diseases (David et al. 2010). Dysfunctional proteins can also disrupt cellular activity over time, ultimately deteriorating organismal health, evidenced as aging (Bokov et al. 2004; Martin et al. 1996; Nyström 2005; Sohal 2002; Terman and Brunk 2006).
As Arctica islandica, a key species in this study, can live in excess of 500 years, presumably they as well as other exceptionally long-lived species employ exemplary protein quality control mechanisms. Here, we investigate protein homeostasis in bivalve mollusks of a range of longevities, evaluating the hypothesis that their ability to prevent protein damage, maintain structure, and stabilize their proteins against aggregation is a significant determinate of longevity.
Materials and methods
Overall experimental design
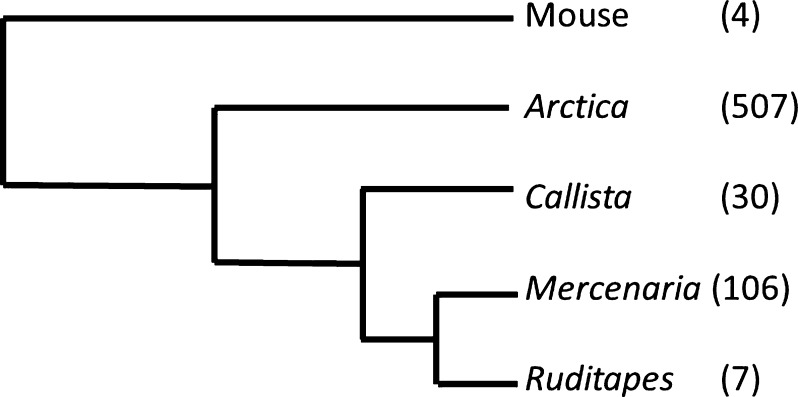
We focused our study on four species of bivalve mollusks—Ruditapes phillipanarum (maximum reported longevity, Lmax, 7 years), Callista chione (Lmax = 30 years), Mercenaria mercenaria (Lmax = 106 years), and A. islandica (Lmax = 507 years) (Fig. 1). These species have overlapping temperature preferences, the warmest preferring 8–24 °C (Ruditapes), the coolest preferring 5–15 °C (Arctica) and range in size from a maximum shell height of 60 mm (Ruditapes) to 120 mm (Mercenaria). In the laboratory, we maintained all species at ~10 °C. Note that there is no obvious phylogenetic relation to longevity, as the shortest-lived and second to longest-lived are the closest relatives (Fig. 1). In some experiments, we included mouse (C57BL/6) tissue for comparison with mammalian values.
Fig. 1.
Phylogeny and maximum longevities of the species. Arctica = Arctica islandica, longevity documented in (Butler et al. 2013); Callista = Callista chione, longevity documented in (Powell and Cummins 1985); Mercenaria = Mercenaria mercenaria, longevity documented in (Ungvari et al. 2011); Ruditapes = Ruditapes philippinarum, longevity documented in (Ponurovskii 2008). Phylogeny from (Giribet 2002; Taylor et al. 2007). In parentheses, maximum reported longevity in years
To investigate the relationship between longevity and proteostasis, these species were subjected to a barrage of protein homeostasis assays. These assays evaluated protein structure, function and aggregation in vitro, both basally and in response to several stressors. Basal responses alone are often less informative about the ability to maintain proteome stability over time than how the proteome responds to stress. The stressors chosen for these experiments were urea, tert-butyl hydroperoxide (TBHP), and high temperature. We took pains to use stressors that would not simply reflect adaptation of our species to their particular ecological conditions. Thus, urea was chosen as it should not be normally experienced by bivalves at any detectable levels. Urea acts as an unfolding agent, disrupting hydrogen bonding and weakening water structure around the protein (Bennion and Daggett, 2003), and at high concentrations can completely denature the protein. TBHP is a stable form of hydrogen peroxide and disrupts protein structure and function by oxidative damage. We also used high temperature as a general protein denaturant. While the consequences to protein structure are similar, the mechanisms differ from urea. Note that we used temperatures substantially higher than any of our species would experience in their natural environments.
The doses and durations of our stressors were chosen based on previous research using similar assays (Pérez et al., 2009; Salmon et al., 2009; Ungvari et al., 2011). See below for details.
Tissue sample preparation
Samples were obtained from wild caught bivalves and provided by collaborators from the Virginia Institute of Marine Science and the School of Ocean Sciences in Bangor, Wales. Individual ages were determined by shell sclerochronology (Richardson and Walker, 1991). All individuals from all species were relatively young, sexually mature, adults.
Tissues were dissected, flash frozen in liquid nitrogen, and maintained in cryogenic conditions. Tissues chosen include the foot muscle and adductor muscle, as these are critical to bivalve survival. The adductor provides the clams only defense against unforgiving aquatic predators, firmly holding the clam closed against intrusion. If this function is compromised, the clam becomes an effortless prey. These tissues are also large, easily dissected, and have the most comparable mammalian tissue equivalent. Skeletal muscle was used for the mouse test. Samples were homogenized on ice in the noted buffer with protease inhibitor cocktail to maintain sample integrity. Ultracentrifugation at 100,000 g isolates both the soluble cytosolic fraction, and an insoluble pellet fraction. Protein concentration was determined using the BCA assay and diluted to 1 mg/mL.
Protein unfolding assay
Changes in surface hydrophobicity are often employed to sensitively monitor structural changes in proteins associated with altered function. We employed a recently developed technique for screening conformational changes in complex protein mixtures. The fluorescent probe BisANS binds to hydrophobic pockets as they become exposed on the surface of unfolding proteins (Pierce et al., 2006). With UV cross-linking, this binding becomes covalent. Unbound probe is removed during SDS-PAGE. BisANS fluorescence increases with unfolding until the surface hydrophobic pockets themselves begin to lose their structure, at which point fluorescence declines.
The buffer used is 50-mM Tris, 1 mM MgSO4 at pH 7.4. After stressing the samples with urea for 1 h, 0.1 mM 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid, dipotassium salt (BisANS) is added on ice in the dark with UV light for 1 h. The samples are run on SDS-PAGE, and then scanned to quantify BisANS fluorescence in the entire lane. The gel is then stained with coomassie, washed, and rescanned to allow for loading corrections. Final values are expressed as the ratio of BisANS fluorescence to total protein, indicative of global protein surface hydrophobicity.
Creatine kinase activity
We wished to also monitor protein function when subjected to extrinsic stress. Creatine kinase (CK) is a convenient enzyme for activity assessment, which can be measured spectrophotometrically in an enzyme-coupled system (Tanzer and Gilvarg, 1959). This conventional assay was carried out at room temperature by adding 37 μL of a solution containing 8.5 mM ATP, 1.22 mM NADH, 2.0 mM phosphoenol pyruvate, 15 units/mL lactate dehydrogenase, 7 units/mL pyruvate kinase, 28 mM MgSO4, and 26 mM reduced glutathione at pH 7.4, combined with 117 mL of buffered creatine containing 0.4 M glycine, 53.2 mM creatine, and 62 mM potassium carbonate at pH 8.9. After adding 4 uL of tissue extract containing 1 mg of protein/mL in 5 mM glycine at pH 9.0, activity was measured by the decrease in 340-nm absorbance, indicative of NADH oxidation over 30 min every 33 s.
Temperature-induced protein aggregation
Exposure of hydrophobic regions of proteins during unfolding often leads to the sequestering of these regions within oligomeric structures, thereby forming protein aggregates. We evaluated the formation of endogenous protein aggregation in response to serial exposure to higher and higher temperatures to induce greater and greater protein unfolding. We included a mouse muscle sample in these assays to determine how temperature-induced protein aggregation compared in a short-lived mammal living normally at 37 °C with bivalves living at considerably colder temperatures.
Procedurally, we first isolated a pure soluble cytosolic fraction by 100,000 g ultracentrifugation of our homogenized tissue for 1 h and used only the supernatant for the following experiments. We diluted this supernatant to 1 mg/mL in 20 mM KPO4, 0.5 mM MgCl2, and 1 mM EDTA. Samples were then incubated at 42 °C for 1 h followed by 1 h of 100,000 g ultracentrifugation. The resulting pellet contained 42 °C stress-induced aggregations. We dissolved the pellet in 2 % SDS, 1 mM DTT, 0.5 % sodium deoxycholate, and 0.5 % IGEPAL CA-630. Protein concentration in the pellet and the remaining supernatant was determined by BCA assay. We then repeated this procedure with the remaining supernatant, this time exposing it to 52 °C for 1 h, centrifuged again at 100,000 g, and repeated the assessment of resulting pellet and supernatant. This pellet now contained all new aggregates induced by 52 °C temperature stress, but not the proteins that already aggregated out at only 42°. Finally, the remaining supernatant was heated to 95 °C for an hour, centrifuged again, and assessed as before.
In order to look at the aggregation properties under the most extreme temperature stress, we again took our original pure soluble cytosolic tissue fraction and boiled it for 15 min before centrifuging to separate soluble proteins from aggregates. Because aggregation was so extensive under this stress, we tried a variety of centrifugation strategies to see which resulted in separating the species best. Ultimately, for this experiment, we used 14,000 g for 5 min. Then, we quantified the protein in the pellet and supernatant as before.
We wished to investigate whether differences in proteome stability observed among species reflected some inherent property of their unique collective proteins versus whether it might reflect differentially effective protective mechanisms such as unique molecular chaperones or chaperones. To address this, we used the same protein—FITC-tagged bovine serum albumin (BSA)—as an aggregation reporter in all our species. This assay was performed similarly to the other temperature stress experiments except that 50 μg of FITC-BSA was added to the cytosolic fraction prior to stress. BSA was chosen due to its availability, stability, and lack of reactivity. The insoluble fraction isolated by ultracentrifugation was dissolved as before and equal volumes quantified for FITC fluorescence indicative of BSA aggregation.
Statistical analysis
Statistics were performed using JMP 9.0 software (SAS, Cary, NC, USA). Data are visualized as means, error bars are ±1 SEM. Differences among species were assessed by ANOVA. Bars not sharing a common letter are statistically different by post hoc testing using Tukey's HSD, p < .05.
Results
Protein unfolding
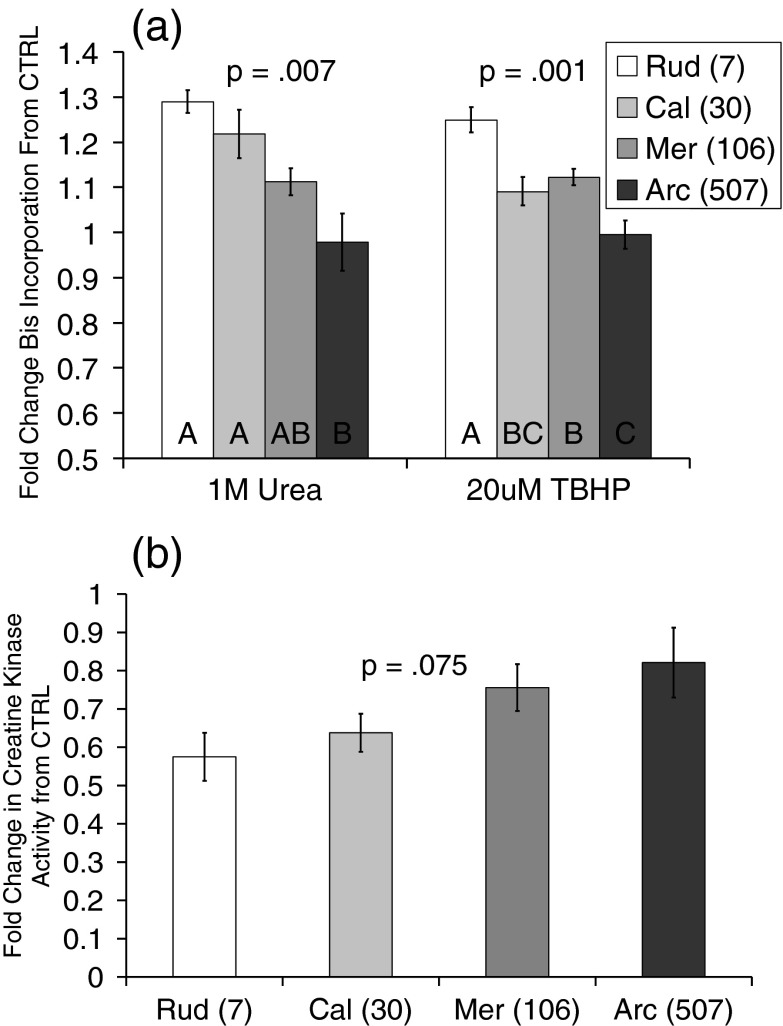
To assess the relationship between species longevity and resistance to protein unfolding, we measured the incorporation of the BisANS probe using urea and TBHP as diverse stressors. Given that BisANS is most useful at distinguishing differences when there is neither too little unfolding nor too much (in which case surface hydrophobic pockets begin losing integrity), we have presented data from only the most informative stressor concentrations. Data are expressed as the fold change from basal (= control) levels after stress (Fig. 2a). At these stress levels of both urea and TBHP, our longest-lived species, A. islandica, exhibited no increase in protein unfolding from basal levels and successively shorter-lived species displayed successively greater unfolding (p = .007 and .001 for urea and TBHP, respectively).
Fig. 2.
a Protein unfolding: BisANS incorporation indicative of global proteome surface hydrophobicity after 1 M urea or 20 μM TBHP stress as compared to basal levels. In both results, there are highly statistically significant differences among species. Bars not sharing a common letter are significantly different (p < .05) as assessed by Tukey's HSD after one-way analysis of variance. Species effects under the urea stress: F(3,8) = 8.73, p = .007 and TBHP: F(3,8) = 14.34, p = .001. b Enzyme activity: reduction in endogenous creatine kinase activity in response to stress with 1 M urea. Although the overall ANOVA is only marginally significant, the order of activity loss is, as expected, inversely related to species longevity. F(3,15) = 2.81, p = .075. Rud = Ruditapes, Cal = Callista, Mer = Mercenaria, Arc = Arctica. Numbers in parentheses are maximum species longevity in years
Creatine kinase activity
As protein function is inherently tied to its structure, we asked whether the unfolding demonstrated with the BisANS assay correlated with compromised enzyme activity. Creatine kinase activity was utilized as a representative enzyme with which to monitor protein function. Using the same 1-M urea stress as the BisANS assay, reduction in CK activity varied inversely according to species longevity (Fig. 2b). These differences were marginally statically significant (p = 0.075). It should be noted that other enzymes—exogenous lactate dehydrogenase and pyruvate kinase—are added to complete the enzyme system and assess CK activity. These enzyme functions would also likely be strained by the urea unfolding stress.
Temperature-induced protein aggregation
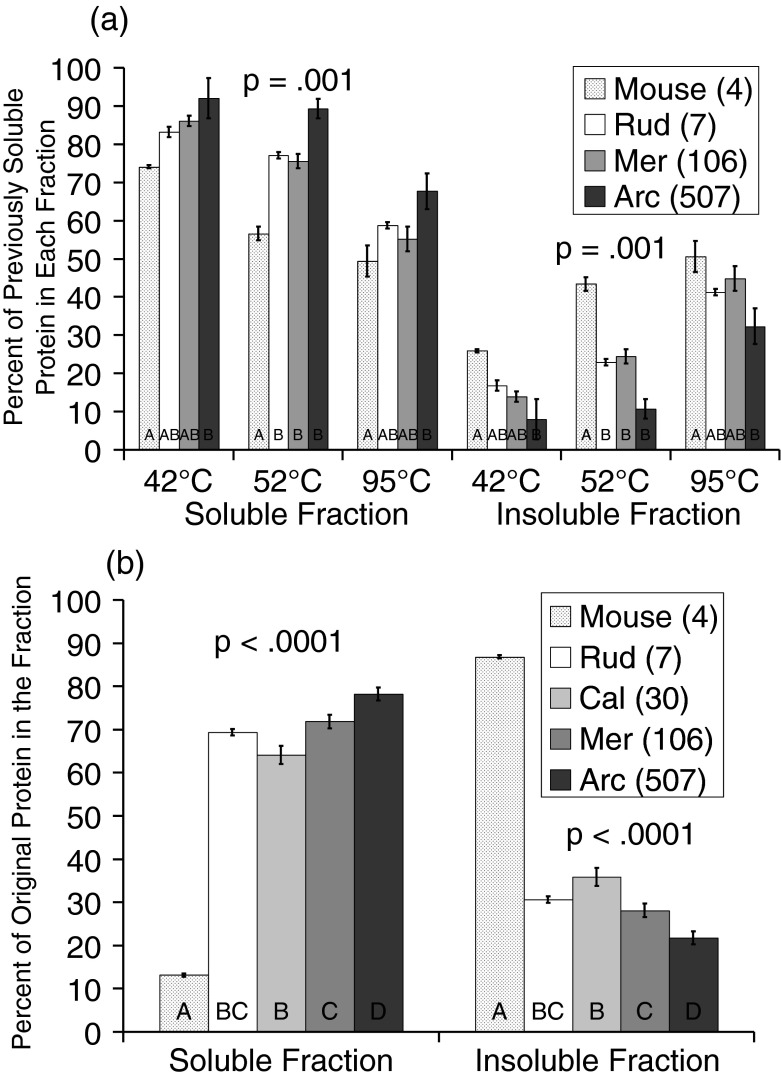
Unfolding of proteins is linked to protein aggregation as exposed hydrophobic regions will “stick” to one another forming multimeric aggregates of a variety of sizes. We examined temperature-induced aggregation of endogenous bivalve and mouse proteins by serially increasing temperature and monitoring the insoluble protein aggregates formed as well as the protein remaining in the soluble fraction after ultracentrifugation (Fig. 3a). After 42 °C temperature stress for 1 h, there is a clear and consistent relation between species longevity and protein aggregation—longer-lived species aggregate fewer proteins. Surprisingly, more mouse proteins became insoluble at 42° than any of the bivalves, even though 42° is much closer to the body temperature at which mice routinely live. Additional temperature stress (to 52°) aggregates additional proteins, but the same pattern remains. Arctica aggregates fewest proteins, mice most. This is true even at 95°.
Fig. 3.
a Serial temperature-induced aggregation: we partitioned pure soluble cytosolic lysates into soluble and insoluble fractions after temperature stress. The surviving soluble fraction is reused for each higher temperature, with insoluble temperature-induced aggregates removed each time by 100,000 g ultracentrifugation for 1 h. Note that fewer aggregates are seen in the longest-lived bivalve at each temperature, and most aggregates are seen in the mouse at each temperature. These differences were assessed by two-way analysis of variance, finding a main effect of species, F(3,24) = 7.04, p = .001, and of stress, F(2,24) = 20.40, p < .001. There was no significant interaction between species and stress levels, F(6,24) = 2.00, p = .105. b Denatured aggregation: we partitioned pure soluble cytosolic lysates into soluble and insoluble fractions after boiling for 15 min and 14,000 g centrifugation for 5 min. The results were assessed by one-way analysis of variance, F(4,15) = 353.99, p < .0001. Individual differences between species were assessed post hoc by Tukey's HSD, and bars not sharing a common letter are statistically different p < .05
To take this principle to its extreme, cytosolic samples were brought to 100 °C for 15 min and centrifuged at 14,000 g for 5 min. Given that so many proteins will be aggregated with this extreme stress, we tried a variety of centrifugation strategies to see if any of them separated our species adequately. In this experiment, we are therefore more likely comparing aggregate size rather than total number of aggregates. Surprisingly, the protein concentration in the resulting supernatant indicates that even after boiling, the long-lived species are superior at maintaining proteins in a soluble form (Fig. 3b). Mouse and Arctica are again at either end of the spectrum. In both the serial temperature and boiling assays, the middle-lived species cluster together between extremes. In the serial temperature assay, the shortest and longest are significantly different from each other, but are not statistically different from the middle cluster at every temperature (p = .001). For the boiling-induced aggregation assay, the longest and shortest were again significantly different from each other and the middle cluster (p < .0001) indicating there is a species effect for both serial temperature and boiling aggregation. It is important to note that the short-lived bivalve species in our study tend to live in somewhat shallower and warmer waters and may be adapted for greater temperature fluctuations than the deep dwelling Arctica. Yet despite this adaptation to a more variable environment, their proteins are less resistant to high temperatures. Longevity appears to be the key determinate for aggregation resistance, not environmental temperature range.
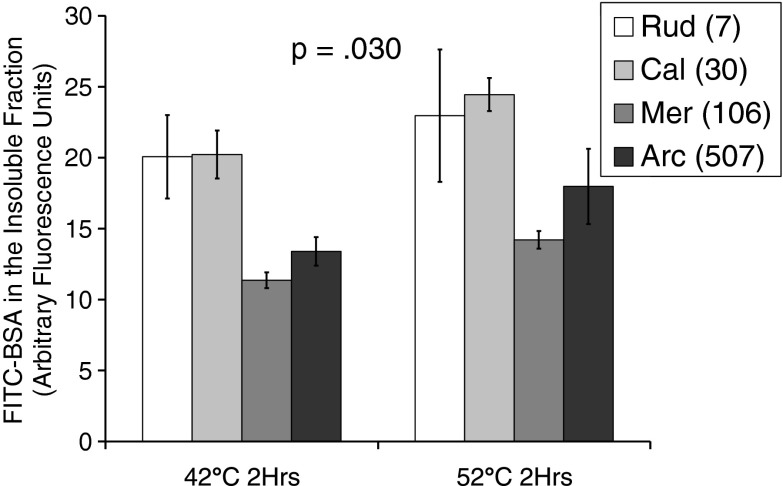
To investigate whether the proteome resistance to aggregation is a characteristic of a particularly protective cellular environment as contrasted with some inherent property of each species unique proteome, we repeated the aggregation assay using a fluorescently tagged reporter protein, BSA. We introduced 50 μg of FITC-tagged BSA into pure soluble lysates from each of our species and determined their aggregation properties at both 42 and 52 °C. Fluorescence in the resulting pellets indicated that BSA-specific aggregation was higher in the two shortest-lived species, while the two longest-lived species were lower (Fig. 4), indicating a longevity effect on reporter aggregation (p = .03).
Fig. 4.
FITC-tagged BSA aggregation: using the same reporter protein in the lysates of our various species allowed us to investigate the potential role of molecular chaperones and co-chaperones in the protein stability properties of our species. Data represents BSA aggregates in the pellet after 2-h temperature stress at 42 or 52 °C and 1-h ultracentrifugation at 100,000 g. The two shorter-lived species clustered together and aggregated more BSA than the two longer-lived species, which also clustered together. Results were assessed by two-way analysis of variance, indicating a significant main effect of species, F(3,15) = 3.90, p = .030, but no difference between 42 and 52 °C, F(1,15) = .788, p = .389. There was also no interaction effect, F(3,15) = .068, p = .976
Discussion
Bivalve mollusks provide examples of some of the longest-lived animals on earth (Abele et al., 2008; Ridgway et al., 2010). Therefore, these must have exceptional protection against the damaging processes of aging. However, there are also relatively short-lived bivalves. In this paper, we described experiments investigating whether exceptional resistance to loss of proteome homeostasis might play a role in bivalve longevity. We found that in response to unfolding stress, longer-lived species maintained enzyme function and resisted unfolding better than short-lived species. The global proteome of longer-lived species also resisted temperature-induced aggregation better than did shorter-lived species. Finally, a reporter assay, in which the aggregation properties of the same protein was examined in the lysate of the study species indicated that some intrinsic property of the cells of long-lived species seemed at least partially responsible for the aggregation resistance of their proteome.
The organisms chosen in this study feature the longest-lived animal, A. islandica. This species must maintain proper protein structure and function for the entirety of its five century maximum lifespan. As the cellular functional components, proteins carry out much of the necessary activity, including repair or removal of damaged cellular machinery. Thus maintaining a functional proteome is critical for functional cell maintenance, and is in turn critical for organismal health. Indeed, the exceptional stress resistance of Arctica has also been demonstrated in response to oxidative stress in vivo (Ungvari et al., 2011) as well as some, but not all, genotoxic stressors (Ungvari et al. 2012).
Other comparative studies using exceptionally long-lived models have implicated proteostasis as a major determinant of lifespan, especially when exposed to exogenous stressors (Austad 2001). For instance, while the short-lived mouse has a large increase in protein unfolding, carbonylation and cysteine oxidation when exposed to stress, the naked mole rat, which survives an astounding 28 years (Buffenstein and Jarvis 2002), does not (Buffenstein 2008; Pérez et al. 2009). Similar results were found using bats (Salmon et al. 2009) with an approximate lifespan of 12 years. These species exhibit unique ecologies and behavior, drastically reducing extrinsic mortality, and likely contribute to the evolution of mechanisms to extend lifespan.
The exceptional longevities in these organisms are especially significant given their small body size, which has been correlated to short lifespan (Speakman 2005). Yet none are as successful as the ocean quahog, A. islandica, with a maximum lifespan of 500 years. Closely related bivalves cover a range of longevities down to 1 year. The lifespans of the species chosen have no correlation with phylogeny, and across Bivalvia, lifespan is independent of ocean climate zone (Philipp et al. 2006; Scourse et al. 2006). These characteristics combine to make an ideal model for comparative biology of aging and to investigate the relationship between longevity and proteostasis. Our results reinforce this relationship.
The preservation of protein structure should also prevent aggregation, as insoluble aggregates are the only possible fate for severely unfolding proteins in vitro, as there is neither autophagy nor proteasome-dependent degradation to remove them. Indeed, longer-lived bivalves resisted both endogenous aggregation and reporter aggregation, indicating protective machinery is in place to stabilize proteins regardless of their source. This feature is also notable in the creatine kinase enzyme system, as exogenous enzymes are used to complete the system and must also remain functional despite the addition of stressors. Again, long-lived bivalves were superior in protecting enzyme function as compared to the shorter-lived.
The likely factor contributing to this stability would be a superior chaperone system, capable of indiscriminately stabilizing unfolding proteins regardless of their origin. A robust and adaptive suite of chaperones present in long-lived lysate would explain both their remarkably stable proteome and ability to protect exogenous proteins. In particular, we suspect small heat shock proteins may play a large role in our results, as they function independent of ATP (Jakob et al. 1993; Kampinga and Garrido 2012), which we did not include in the aggregation or unfolding buffers. Unfortunately, it has proven difficult to probe standard chaperone levels due to variations in antibody affinity across the species. Furthermore, only gross analysis of large chaperone families would be possible, and novel chaperones would be missed. Identification of the specific macromolecules Arctica is utilizing to stabilize bait proteins are underway utilizing mass spectrometry techniques, with the recently published genome providing an invaluable resource.
Combined with the similar results in other exceptionally long-lived models, our data strongly suggest that protein homeostasis is a critical determinate for longevity. The truly remarkable performance of bivalves, and A. islandica in particular, as compared to C57BL/6 mice suggests there is room to improve mammalian proteostasis. The mechanisms underlying this performance could yield medically relevant targets, potentially reducing age-related losses in proteostasis and aggregation diseases in particular, such as Alzheimer's disease.
Acknowledgments
This study was supported by R01 AG035327 as part of the NIA/BBSRC—Partnering Awards to Support Collaborative Research on the Biology of Aging Program and the Barshop Institute for Longevity and Aging Studies. We thank Mark Luckenbach for providing us samples, and Rochelle Wei for technical assistance.
References
- Abele D, Strahl J, Brey T, Philipp EER. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008;42:474–480. doi: 10.1080/10715760802108849. [DOI] [PubMed] [Google Scholar]
- Alavez S, Vantipalli MC, Zucker DJS, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. An experimental paradigm for the study of slowly aging organisms. Exp Gerontol. 2001;36:599–605. doi: 10.1016/S0531-5565(00)00229-1. [DOI] [PubMed] [Google Scholar]
- Bennion BJ, Daggett V. The molecular basis for the chemical denaturation of proteins by urea. Proc Natl Acad Sci U S A. 2003;100:5142–5147. doi: 10.1073/pnas.0930122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B: Biochem, Syst, Environ Physiol. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Jarvis JUM. The naked mole rat–a new record for the oldest living rodent. Sci Aging Knowl Environ. 2002;2002:pe7. doi: 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- Butler PG, Wanamaker AD Jr, Scourse JD, Richardson CA, Reynolds DJ (2013) Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeogr Palaeoclimatol Palaeoecol 373:141–151
- Chakravarti B, Chakravarti DN. Oxidative modification of proteins: age-related changes. Gerontology. 2007;53:128–139. doi: 10.1159/000097865. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Estabrooks SL. The possible role of telomeres in the short lifespan of the bay scallop, Argopecten irradians (Lamarck 1819) J Shellfish Res. 2007;26:307–313. doi: 10.2983/0730-8000(2007)26[307:TPROTI]2.0.CO;2. [DOI] [Google Scholar]
- Giribet GW. On bivalve phylogeny: a high-level analysis of the Bivalvia (Mollusca) based on combined morphology and DNA sequence data. Invertebr Biol. 2002;121:271–324. doi: 10.1111/j.1744-7410.2002.tb00132.x. [DOI] [Google Scholar]
- Hsu A-L, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Kampinga HH, Garrido C. HSPBs: small proteins with big implications in human disease. Int J Biochem Cell Biol. 2012;44:1706–1710. doi: 10.1016/j.biocel.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Kikis EA, Gidalevitz T, Morimoto RI. Protein homeostasis in models of aging and age-related conformational disease. Adv Exp Med Biol. 2010;694:138–159. doi: 10.1007/978-1-4419-7002-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med. 1998;25:1089–1097. doi: 10.1016/S0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol Ser A: Biol Sci Med Sci. 2009;64A:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Takahashi R, Goto S. Dietary restriction initiated late in life can reduce mitochondrial protein carbonyls in rat livers: Western blot studies. Biogerontology. 2000;1:321–328. doi: 10.1023/A:1026590819033. [DOI] [PubMed] [Google Scholar]
- Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp E, Brey T, Heilmayer O, Abele D, Pörtner HO. Physiological ageing in a polar and a temperate swimming scallop. Mar Ecol Prog Ser. 2006;307:187–198. doi: 10.3354/meps307187. [DOI] [Google Scholar]
- Pierce A, deWaal E, Van Remmen H, Richardson A, Chaudhuri A. A novel approach for screening the proteome for changes in protein conformation†. Biochemistry. 2006;45:3077–3085. doi: 10.1021/bi052031i. [DOI] [PubMed] [Google Scholar]
- Ponurovskii SK. Population structure and growth of the Japanese littleneck clam _Ruditapes philippinarum_ in Amursky Bay, Sea of Japan. Russ J Mar Biol. 2008;34:329–332. doi: 10.1134/S1063074008050106. [DOI] [Google Scholar]
- Powell EN, Cummins H. Are molluscan maximum life spans determined by long-term cycles in benthic benthos? Oecologia. 1985;67:177–182. doi: 10.1007/BF00384281. [DOI] [PubMed] [Google Scholar]
- Radák Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002;37:1423–1430. doi: 10.1016/S0531-5565(02)00116-X. [DOI] [PubMed] [Google Scholar]
- Richardson CA. Molluscs as archives of environnmental change. Oceanogr Mar Biol Annu Rev. 2001;39:103–164. [Google Scholar]
- Richardson CA, Walker P. The age structure of a population of the hard-shell clam Mercenaria mercenaria from Southhampton Water, England, derived from acetate peel replicas of shell sections. ICES J Mar Sci. 1991;48:229–236. doi: 10.1093/icesjms/48.2.229. [DOI] [Google Scholar]
- Ridgway ID, Richardson CA, Austad SN. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J Gerontol Ser A: Biol Sci Med Sci. 2010;66A:183–190. doi: 10.1093/gerona/glq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Leonard S, Masamsetti V, Pierce A, Podlutsky AJ, Podlutskaya N, Richardson A, Austad SN, Chaudhuri AR. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–2326. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne BR, Fiebig J, Pfeiffer M, Gleβ R, Hickson J, Johnson ALA, Dreyer W, Oschmann W. Climate records from a bivalved Methuselah (Arctica islandica, Mollusca; Iceland) Palaeogeogr Palaeoclimatol Palaeoecol. 2005;228:130–148. doi: 10.1016/j.palaeo.2005.03.049. [DOI] [Google Scholar]
- Scourse J, Richardson C, Forsythe G, Harris I, Heinemeier J, Fraser N, Briffa K, Jones P. First cross-matched floating chronology from the marine fossil record: data from growth lines of the long-lived bivalve mollusk Arctica islandica. The Holocene. 2006;16:967–974. doi: 10.1177/0959683606hl987rp. [DOI] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/S0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-X. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Tanzer ML, Gilvarg C. Creatine and creatine kinase measurement. J Biol Chem. 1959;234:3201–3204. [PubMed] [Google Scholar]
- Taylor JD, Williams ST, Glover EA, Dyal P. A molecular phylogeny of heterodont bivalves (Mollusca: Bivalvia: Heterodonta): new analyses of 18S and 28S rRNA genes. Zool Scr. 2007;36:587–606. doi: 10.1111/j.1463-6409.2007.00299.x. [DOI] [Google Scholar]
- Terman A, Brunk UT. Oxidative stress, accumulation of biological “garbage”, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Ridgway I, Philipp EER, Campbell CM, McQuary P, Chow T, Coelho M, Didier ES, Gelino S, Holmbeck MA, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol Ser A: Biol Sci Med Sci. 2011;66A:741–750. doi: 10.1093/gerona/glr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Mason JB, Gruber H, Lee SW, Schwartz TS, Brown MK, Storm NJ, Fortney K, Sowa J, et al. Resistance to Genotoxic Stresses in Arctica islandica, the Longest Living Noncolonial Animal: Is Extreme Longevity Associated With a Multistress Resistance Phenotype? Sci: J. Gerontol.A Biol. Sci. Med; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanamaker AD, Heinemeier J, Scourse JD, Richardson CA, Butler PG, Eiriksson J, Knudsen KL. Very long-lived mollusks confirm 17th Century AD Tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008;50:399–412. [Google Scholar]