Abstract
Mild cognitive impairment (MCI) has been described as an intermediate stage between normal aging and dementia. Previous studies characterized the alterations of brain oscillatory activity at this stage, but little is known about the differences between single and multidomain amnestic MCI patients. In order to study the patterns of oscillatory magnetic activity in amnestic MCI subtypes, a total of 105 subjects underwent an eyes-closed resting-state magnetoencephalographic recording: 36 healthy controls, 33 amnestic single domain MCIs (a-sd-MCI), and 36 amnestic multidomain MCIs (a-md-MCI). Relative power values were calculated and compared among groups. Subsequently, relative power values were correlated with neuropsychological tests scores and hippocampal volumes. Both MCI groups showed an increase in relative power in lower frequency bands (delta and theta frequency ranges) and a decrease in power values in higher frequency bands (alpha and beta frequency ranges), as compared with the control group. More importantly, clear differences emerged from the comparison between the two amnestic MCI subtypes. The a-md-MCI group showed a significant power increase within delta and theta ranges and reduced relative power within alpha and beta ranges. Such pattern correlated with the neuropsychological performance, indicating that the a-md-MCI subtype is associated not only with a “slowing” of the spectrum but also with a poorer cognitive status. These results suggest that a-md-MCI patients are characterized by a brain activity profile that is closer to that observed in Alzheimer disease. Therefore, it might be hypothesized that the likelihood of conversion to dementia would be higher within this subtype.
Keywords: Mild cognitive impairment, Subtypes, MEG, Relative power, Neuropsychological performance
Introduction
The definition of mild cognitive impairment (MCI) underwent a notable evolution during the last two decades. In essence, the concept of MCI was coined to define a clinical condition where an objective cognitive decline can be detected, but this decline is still not severe enough to be considered a full-blown dementia. Petersen and the Mayo Clinic group (Petersen et al. 1999) offered one of the first operational definitions of MCI, which was subsequently accepted by a majority of the scientific community. In the first characterization of Petersen et al. (2001), MCI was a predominantly amnestic problem. MCI was also characterized as a “transitional state.” Thus, this clinical condition would be an intermediate state or a “boundary” between normal aging and dementia, since a number of studies demonstrated that patients with a diagnosis of MCI are at higher risk of developing Alzheimer’s disease (AD) when compared with the healthy aged population (Farias et al. 2005; Petersen 2005; Shah et al. 2000).
Following up this evolution, two articles represented a key modification of MCI concept (Petersen 2004; Winblad et al. 2004). The original notion of a predominant amnestic problem was substituted by a new perspective where several clinical subtypes of MCI were described. This new perspective classified patients according to two orthogonal axes. In the first axis, patients with a predominant memory defect are classified as “amnestic MCI” (a-MCI), while those with a predominant impairment in cognitive domains such as language, executive functions, or visuospatial skills are classified as “nonamnestic MCI” (na-MCI). In the second axis, patients are categorized according to the number of affected cognitive domains. Those with only one affected domain (e.g., memory or language) are categorized as “single domain” MCIs (sd-MCI), while those with more than one affected domain (e.g., memory plus language) are categorized as “multidomain” MCIs (md-MCI). The combination of these axes gives rise to the nowadays more broadly utilized classification of MCI subtypes: amnestic single domain MCI (a-sd-MCI), amnestic multidomain MCI (a-md-MCI), nonmanestic single domain MCI (na-sd-MCI), and nonamnestic multidomain MCI (na-md-MCI).
This clinical classification is extremely relevant because each subtype is linked to a presumed etiology. According to Petersen’s group, the amnestic subtypes (including single and multidomain) represent a prodromal form of AD, although vascular dementia may be also considered (Petersen 2004). Nonamnestic subtypes might be at higher risk of conversion to Lewy–body or frontotemporal dementias (Petersen 2004; Winblad et al. 2004). Implicitly, this categorization still assumes that MCI is a “predementia” stage, and most patients would convert to some type of dementia if they are followed up for enough time. However, investigations such as the population-based PAQUID study (Larrieu et al. 2002) demonstrated that this implicit assumption was not totally true. On the contrary, MCI emerged as an unstable clinical condition, with some patients progressing to different dementias, some patients remaining clinically stable over time, and notably some patients reverting to a “normal” clinical situation (40 % in the PAQUID study for example). As a consequence of this new scenario, recent proposals of MCI diagnostic criteria (Albert et al. 2011) claim the utilization of a very strict terminology (i.e., “MCI due to AD”), since different etiologies that yield different outcomes (including the reversion to a “normal” condition) might be underlying the observed cognitive deterioration.
Despite of the new consideration of MCI as an unstable condition, there is still solid evidence supporting the higher risk of conversion to dementia (especially AD) in this group of patients (Bennett et al. 2005; Gauthier et al. 2006; Morris et al. 2001). Therefore, the early detection of MCI is still a critical issue regarding the development of interventions to prevent or delay the process of neurodegeneration (Jelic et al. 2005). In order to attain such goal, it is important to bear in mind that clinical subtypes of MCI are not only associated with different etiologies but also with a more or less rapid conversion to dementia (Brodaty et al. 2012; Tabert et al. 2006). Considering this fact, a great effort has been devoted to investigate biological markers that may characterize the clinical subtypes of MCI.
Neurophysiological techniques such as electroencephalography (EEG) or magnetoencephalography (MEG) played a significant role in this line of research. The most stable pattern of EEG activity in MCI patients is defined by an increase in theta accompanied by a decrease of alpha and beta power, which correlates with APOE genotype, and hippocampal volumes (Babiloni et al. 2006b, c, 2009; Grunwald et al. 2002; Prichep et al. 2006). When the progression to AD was investigated, alpha and theta relative power in left temporo-occipital derivation correctly classified 85 % of MCI subjects who would suffer dementia (Jelic et al. 2000). Huang et al. (2000) demonstrated that a more anterior localization of theta and alpha activity was the best predictor of future conversion to AD within a MCI sample. Of note, the investigation of differential neurophysiological patterns in MCI subtypes is scarce. This issue was indirectly addressed by Babiloni et al. (2009) within the background of a research on the relationship between hippocampal volumes and alpha rhythms. The authors assessed a potential influence of the clinical subtype (i.e., a-MCI vs. na-MCI) and found no significant differences. In a subsequent study (Babiloni et al. 2010), the issue was explicitly investigated by comparing EEG rhythms in a-MCIs, na-MCIs, and aged subjects with subjective memory complaints. Results showed increased occipital theta and reduced alpha activity in a-MCI, as compared to na-MCI. No distinctions were made in terms a single or multidomain affectation.
Considering the scarcity of studies within this field and its potential clinical relevance, we conducted a MEG study where an exhaustive spectral analysis was carried out in a-sd-MCIs, a-md-MCIs, and healthy aged controls. Recently, a-md-MCI has been associated with increased levels of AD pathology and elevated risk of conversion to AD when compared with a-sd-MCI (Zhang et al. 2012; Brodaty et al. 2012; Wolk et al. 2009). As a consequence, we not only expect the typical pattern of increased theta and reduced alpha in the MCI groups. Furthermore, we hypothesize that a-md-MCI patients will exhibit a spectral pattern more proximate to the typical AD profile, including increased power within the delta and theta frequency ranges, and reduced activity in the high-frequency range as compared with a-sd-MCIs and healthy controls. Finally, bearing in mind that mesial–temporal atrophy is one of the more important markers within the normal aging-AD spectrum (see, for example, Jack et al. 1999; Martin et al. 2010), hippocampal volumes were calculated in order to assess the relationship between structural and functional information. To the best of our knowledge, this is the first neurophysiological study that combines spectral activity, neuropsychological performance, and hippocampal atrophy information to characterize the two amnesic clinical subtypes of MCI.
Methods
Subjects
MEG signals were obtained from 105 subjects older than 65 years of age, classified in three groups: 36 healthy controls, 33 a-sd-MCI patients, and 36 a-md-MCI patients. All of them were right-handed (Oldfield 1971) and native Spanish speakers. No significant differences were found in education, gender, or age among groups (see Table 1). MCI patients were recruited from the Geriatrics and Neurology Units of the “Hospital Universitario San Carlos” and the “Memory Decline Prevention Center,” both in Madrid, Spain. Healthy volunteers were recruited from the “Seniors Center of Chamartin District,” Madrid.
Table 1.
Mean values (± standard deviation) of the demographic and clinical characteristics of a-sd-MCI, a-md-MCI, and controls
| Control (n = 36) | a-sd-MCI (n = 33) | a-md-MCI (n = 36) | p value | |
|---|---|---|---|---|
| Age (years) | 72.36 ± 4.75 | 74.15 ± 6.07 | 73.94 ± 3.70 | p = 0.22 |
| Gender (M/F) | 11/25 | 13/20 | 13/23 | p = 0.12 |
| MMSE score | 29.14 ± 0.96 | 27.63 ± 2.47 | 25.65 ± 2.66 | p < 0.01 |
| Education (years) | 4.39 ± 1.23 | 4.27 ± 1.31 | 3.78 ± 1.24 | p = 0.48 |
| LH_ICV | 0.002610 ± 0.0003583 | 0.002148 ± 0.0004235 | 0.002062 ± 0.0005280 | p < 0.0001 |
| RH_ICV | 0.002608 ± 0.0002964 | 0.002133 ±0.0005079 | 0.002082 ± 0.0003512 | p < 0.0001 |
An ANOVA test was used for the comparisons
M male, F female, MMSE Mini-mental state examination score
Diagnostic criteria
All participants were screened by means of a variety of standardized diagnostic instruments that included: the Spanish version of the Mini Mental State Examination (MMSE; Lobo et al. 1979), the Global Deterioration Scale (GDS; Reisberg et al. 1982), the Functional assessment questionnaire (FAQ; Pfeffer et al. 1982), the Geriatric Depression Scale (GDS; Yesavage et al. 1982), the Hachinski Ischemic Score (Rosen et al. 1980), the questionnaire for Instrumental Activities of Daily Living (Lawton and Brodie 1969), and the Functional Assessment Staging (FAST; Auer and Reisberg 1997).
MCI diagnosis was established according to the criteria of Petersen (2004) and Grundman et al. (2004). Thus, MCI patients should fulfill the following requirements: (1) memory complaint, corroborated by an informant; (2) abnormal memory function, documented by delayed recall of one paragraph from the Logical Memory II subtest of the Wechsler Memory Scale—Revised (cutoff scores ≤ 16 for ≥16 years of education; ≤8 for ≥8–15 years of education); (3) normal general cognitive function, as determined by a MMSE score ≥24; (4) total absence or minimal impairment in activities of daily living revealed by the Lawton scale, as determined by a clinical interview with the patient and informant; and (5) not demented according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria as judged by an experienced clinician (McKhann et al. 1984). MCIs did not fulfill the diagnostic criteria for dementia (i.e., all were classified at the stage 3 of the Global Deterioration Scale) and performed at least 1 SD below average for their age and education on neuropsychological tests representing one or more areas of cognition (Jelic et al. 1996). Patients and controls were free of significant medical, neurological and/or psychiatric diseases (other than MCI). These inclusion criteria encompassed the absence of significant cerebral–vascular disease (i.e., modified Hachinski score ≤ 4) or depressive symptomatology (i.e., Yesavage’s Depression Scale scores > 9). Participants were not using drugs, which could affect MEG activity (including cholinesterase inhibitors).
Patients and controls received an exhaustive neuropsychological assessment in order to establish their performance level in multiple cognitive domains. The assessment included clock drawing test (Agrell and Dehlin 1998), direct and inverse digit span tests (Wechsler Memory Scale Revised, WMS-III; Wechsler 1987), immediate and delayed recall (WMS-III; Wechsler 1987), phonemic and semantic fluency (Controlled Oral Word Association Test; Benton and Hamsher 1989), ideomotor praxis of Barcelona test (Peña-Casanova 1990), rule shift Cards (BADS; Norris and Tate 2000), visual object and space perception test (VOSP; Warrington and James 1991), Boston naming test (BNT; Kaplan et al. 1983), and trail making test A and B (TMTA and TMTB; Reitan 1958). The TMT subtest A and B offer two different scores. The first one (i.e., TMT “accuracy”) denotes the number of correct responses. The second score (i.e., TMT “time”) denotes the time subjects need to complete the task, with a limit of 200 s in TMTA and 400 s in TMTB.
According to their clinical and neuropsychological profile, patients were further divided in two groups: (1) the a-md-MCI group where patients showed a memory deficit accompanied by various degrees of impairment in cognitive domains such as language, executive function, and/or visuospatial skills and (2) the a-sd-MCI group where patients exhibited an isolated memory impairment (Petersen 2004).
Prior to the MEG recording, all subjects signed an informed consent that explained the technical and ethical considerations of the investigation. The study was approved by the local ethics committee.
MEG recordings
MEGs were acquired (Fig.1, step 1) with a 306-channel Vectorview system (ElektaNeuromag), which combines two orthogonal, planar gradiometers, and one magnetometer. Only MEG signals derived from magnetometers (i.e., 102 channels) were submitted for further analyses. The MEG system was placed in a magnetically shielded room (VacuumSchmelze GmbH, Hanua, Germany) at the “Laboratorio UPM-UCM de Neurociencia Cognitiva y Computacional” (Madrid, Spain). Subjects were in an awake but resting state with their eyes closed and under vigilance control during the recording. They were asked to avoid making movements. For each subject, 3 min of MEG signal were acquired at a sampling frequency of 1,000 Hz (online bandpass filtering at 0.1–330 Hz).
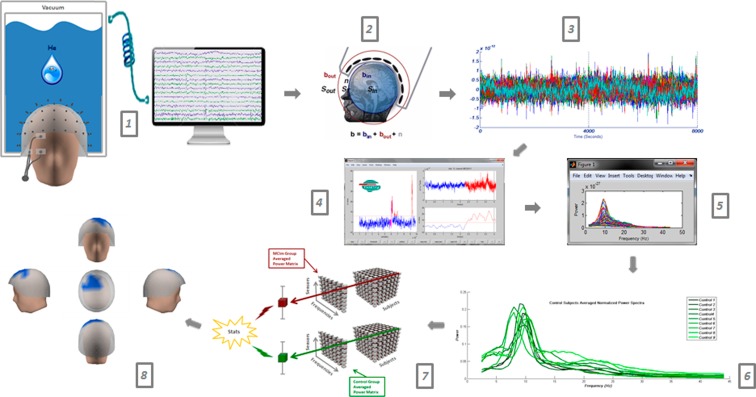
Fig. 1.
Workflow: resting state MEG recordings (1), Elekta software spatial filtering (2), signal segmentation (3), Fieldtrip artifact processing (4), visual trial selection through power spectra (5), power spectra averaging and normalization (6), statistics (7), and results (8)
The head movement was controlled by means of a head-position indicator (HPI) with coils attached to the scalp. HPI coils position and subject head shape were defined using a three-dimensional digitizer (FastrakPolhemus) referenced to three anatomical (fiducial) locations: the nasion and the left and right preauricular points. Blinks were monitored by two bipolar electrodes attached above and below the left eye and one electro attached to the lower cheek (ground). Recordings were offline filtered (Fig. 1, step 2) and corrected for head movements with a temporal signal space separation with movement compensation (Tsss-mc) (Taulu and Kajola 2005, Maxfilter 2.2 software); correlation threshold = 0.9, time window = 10 s, and notch filtered (Butterworth filter order 4 at 50 Hz and 100 Hz). Continuously recorded resting state data were segmented in 4-s length trials. Trials with electrooculography, muscle, and jump artifacts were rejected by means of Fieldtrip package (Oostenveld et al. 2011) (Fig. 1, step 3).
MRI and hippocampal volumes
For each subject, a high-resolution T1-weighted magnetic resonance was acquired at “Hospital Universitario San Carlos” (Madrid) using a General Electric 1.5-T magnetic resonance scanner, with a high-resolution antenna and a homogenization PURE filter (fast spoiled gradient echo sequence, TR/TE/TI = 11.2/4.2/450 ms; flip angle, 12°; 1 mm slice thickness, 256 × 256 matrix; and FOV, 25 cm). To segment the subject’s T1-weighted volume into different regions, Freesurfer software (version 5.1.0) and its specialized tool for automated subcortical segmentation (Fischl et al. 2002) were used. Afterwards, hippocampal volumes were normalized with the overall intracranial volume (ICV) to account for differences in head volume over subjects. Two variables were submitted for statistical analysis: the normalized left hippocampal volumes (LH_ICV) and the normalized right hippocampal volumes (RH_ICV).
Power spectra and statistical analysis
MEG power spectra were computed through Fieldtrip package for all trials, which successfully passed the automatic artifact rejection. A frequency-of-interest range of 0.5 Hz steps from 1 to 30 Hz was employed. In order to obtain the average frequency content of each trial, we applied a multitaper method (mtmfft) with discrete prolate spheroidal sequences (dpss) as windowing function and 1 Hz smoothing. Each trial was visually inspected by an experienced technician blinded to the subjects’ diagnosis by collapsing the power spectrum of all channels (Fig. 1, step 4). Those channels with an aberrant power spectra profile were dismissed. Finally, only MEG recordings with at least 15 survival trials (1 min of brain activity) were submitted for further analyses. The number of survival trials did not differ significantly among groups. Survival trials were averaged across subjects obtaining for each group a 102 channels × 52 frequency steps × “n” subjects matrix (Fig. 1, step 6). For each channel, relative power was calculated as a ratio between power in each 0.5 Hz frequency step and the total power across the 1–30Hz spectrum and was expressed as a percentage (Jelic et al. 2000).
Similarly to previous works (see Fernandez et al. 2006a), we did not use pre-established and conventional frequency bands to analyze power differences among groups. This approach might overcome one of the key problems that emerge when different studies are to be compared: the variability in terms of classification criteria for classical EEG bands. This variability affects low frequencies to a lesser extent; however, the limits between conventional bands in the high-frequency range are difficult to define. Thereby, in order to accomplish a data-driven comparison among groups, we followed a method adapted from Maris and Oostenveld (2007). First, a series of exploratory ANOVA tests were calculated (Fig. 1, step 7) for relative power values in each 0.5-Hz frequency step and sensor. Those comparisons that were found to show significant differences (p < 0.05) were further inspected by means of pairwise t tests. In order to perform such analyses, a series of clusters were built according to a criteria of spatial and frequency adjacency. Thus, each cluster must contain at least five contiguous and significant sensors, and the difference between pairs of groups must remain significant during at least a 2-Hz interval.
Relative power on each cluster of sensors was averaged and submitted to the t test analyses. To control the family-wise error due to multiple comparisons, the test distribution was derived from a permutation test (Ernst 2004). This was accomplished by randomly dividing the participants into two sets, matching the numbers in the original groups. The two-sample t test was then carried out in these two new groups. This procedure was repeated 5,000 times, and the p value from each test was retained in order to obtain a p value distribution. We then identified the fifth percentile of each distribution, and only p values below that threshold were accepted. This strategy was performed for the contrasts a-sd-MCI vs. control, a-md-MCI vs. control, and a-sd-MCI vs. a-md-MCI. Importantly, the criteria utilized to build each cluster (see above) determines the way in which spectral differences between groups will be described in the results section. As above mentioned, results will not be described in terms of conventional frequency bands, but rather in terms of the frequency ranges of those significant clusters that matched the adopted criteria of spatial and frequency adjacency.
Power, hippocampal volumes, and neuropsychology correlation
First, a series of one-way ANOVA tests were performed in order to investigate the distribution of the neuropsychological scores and the hippocampal volumes among groups. In these analyses, the variable “Diagnosis” (a-md-MCI, a-sd-MCI, control) was considered the between groups factor. Then, the relationship among power values, hippocampal volumes, and neuropsychological performance was assessed through Pearson correlation tests. The analyses were performed by correlating the averaged power on each resulting significant cluster of sensors in the t test analyses (see below), and the neuropsychological scores on each test. In order to avoid the multiple comparisons problem, a permutation testing procedure was used (Nichols and Holmes 2001). Five thousand surrogate correlation maps were calculated by randomly distributing the combination of each power value and test score across subjects. The highest absolute value of the Pearson correlation coefficient obtained from each surrogate was retained in order to obtain an empirical null distribution of the statistic. Statistically significant thresholds were obtained from the quantiles of the distribution of these values. For example, the 95th quantile corresponded to a p value of 0.05. This ensures that there is only a 5 % probability that one or more correlation values from the original statistical map would present differences above threshold due to spurious statistical fluctuations.
Results
Description of power spectra
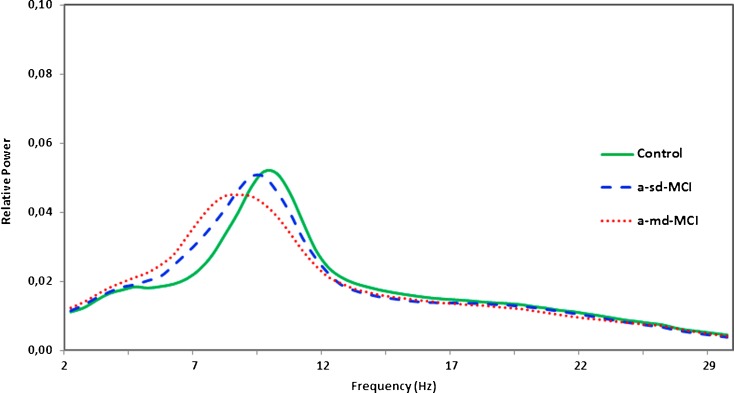
First, only for descriptive purposes, we calculated the averaged relative power in the 1–30 Hz frequency range for each group (see Fig. 2). It can be noticed that a power shift to lower frequencies was observed in MCIs, especially in a-md-MCI patients. Thus, the averaged relative power in a-md-MCI patients showed a frequency peak of about 8.5 Hz, while in a-sd-MCI subjects, the peak appeared at about 9.5 Hz. Healthy controls showed their maximum value at about 10 Hz. Moreover, the profile of the spectral distribution was quite different among groups. Both MCI groups (especially the a-md-MCI group) showed a broader spectral distribution in the 5–12 Hz frequency range, indicating a higher variability and a tendency to lower frequency peaks across subjects. On the contrary, the control group exhibited a narrower spectral distribution, indicating a lower variability and a tendency to frequency peaks that converge within the range of alpha band.
Fig. 2.
Average relative power spectra for all channels in the control group (green line), the a-sd-MCI group (blue dashed line), and the a-md-MCI group (red dotted line). Spectra are represented in the “x” axis from 1 to 30 Hz frequency band and relative power values in the “y” axis. It is very important to point out for the reader that, in all figures, the red color represents the a-md-MCI group, the blue color represents the a-sd-MCI group and the green color represents the control group
Differences in relative power among groups
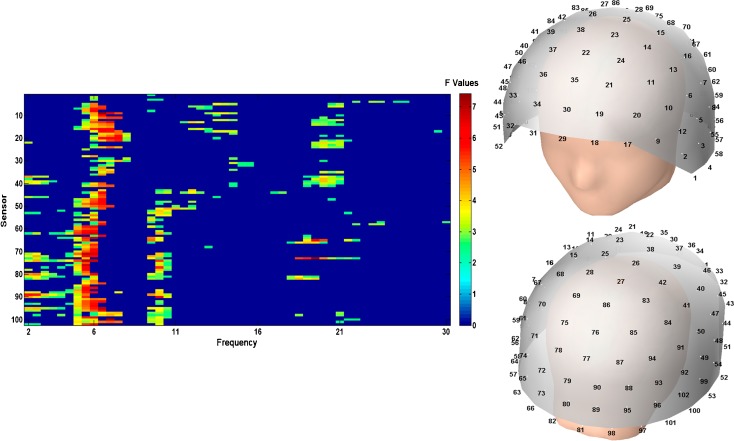
As previously mentioned, a series of exploratory ANOVA tests were calculated for each 0.5 Hz frequency step and sensor. The results of those ANOVAs are displayed in Fig. 3. Overall, significant differences seem to converge within three frequency ranges: (1) a low-frequency range that includes frequencies between 2 and 8 Hz, (2) a range that includes frequencies between 9 and 12 Hz, and (3) a high-frequency range that includes frequencies between 16 and 23 Hz. These preliminary results might give us a hint about the frequency distribution of the significant clusters in the pairwise comparison.
Fig. 3.
Significant F values corresponding to the exploratory ANOVA tests for each sensor and frequency step (left side). MEG helmet layout showing the distribution of sensors (right side)
In order to compare our results with previous MEG and EEG literature, significant clusters were referred in the range of the classical frequency bands (delta, theta…).
Differences within the delta band range
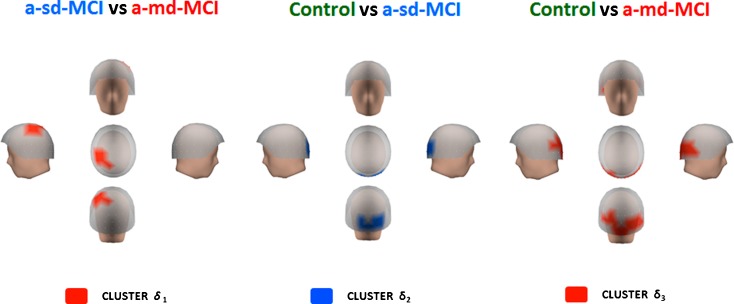
The a-md-MCI group showed a significant increase in activity within the delta range as compared with a-sd-MCIs and healthy controls (see Fig. 4). Differences between a-md-MCIs and a-sd-MCIs (t = −3.331; p < 0.001) appeared within a frequency range of 2–4 Hz in a cluster of sensors located in left centro-parietal regions (henceforth called cluster δ1). The significant differences between a-sd-MCIs and controls (t = −2.280; p < 0.05) emerged from cluster of sensors also within the 2–4 Hz frequency range (cluster δ2) located in occipital regions. Finally, significant differences between a-md-MCIs and controls (t = −3.123; p < 0.001) emerged within the same 2–4 Hz frequency range in a cluster (δ3) with a broader occipito-temporal distribution as compared with cluster δ2. Importantly (see below), cluster δ3 contains all sensors included in cluster δ2.
Fig. 4.
Differences within the delta band range. Red color indicates that the a-md-MCI group had more relative power than a-sd-MCI in left centro-parietal regions (cluster δ 1) and in occipito-temporal areas in comparison with the control group (cluster δ 3). Blue color indicates that the a-sd-MCI had more relative power in posterior regions than the control group (cluster δ 2)
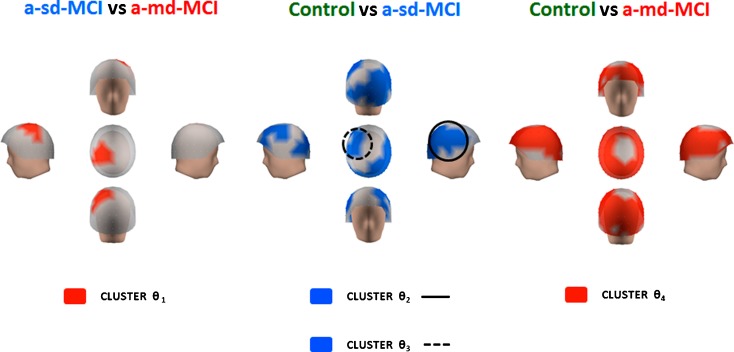
Differences within the theta band range
Very similar to previous results, power within the theta range was significantly higher in the a-md-MCI group. The a-md-MCI group showed increased relative power (t = −2.007; p < 0.05) as compared with a-sd-MCIs within a 5–7 Hz frequency range in a cluster of sensors (cluster θ1) located in left centro-parietal regions (see Fig. 5). The a-sd-MCI group showed increased power within the typical 4–8 Hz range of theta band in two clusters of sensors (clusters θ2 and θ3) when compared with the control group. Cluster θ2 (t = −2.939; p < 0.005) extended over most of the right lateral fronto-temporo-parieto-occipital region, while cluster θ3 (t = −2.231; p < 0.05) had a left fronto-central location. When a-md-MCIs and controls were compared, the a-md-MCIs exhibited the same pattern of increased relative power within the 4–8 Hz range (t = −4.107; p < 0.0001), but in this case, such differences emerged from a cluster that basically included all sensors excepting those located around the vertex (cluster θ4). Consequently, cluster θ4 encompassed sensors and frequency ranges in clusters θ1, θ2, and θ3 (see below).
Fig. 5.
Differences within the theta band range. Red color indicates that the a-md-MCI group exhibited more relative power in left centro-parietal regions than a-sd-MCI group (cluster θ 1) and in practically the whole head when compared with control group (cluster θ 4). The a-sd-MCI exhibited a significant power increase compared with the control group in two clusters of sensors, represented in blue color, which involved the right lateral fronto-temporo-parieto-occipital region (cluster θ 2) and the left fronto-central location (cluster θ 3)
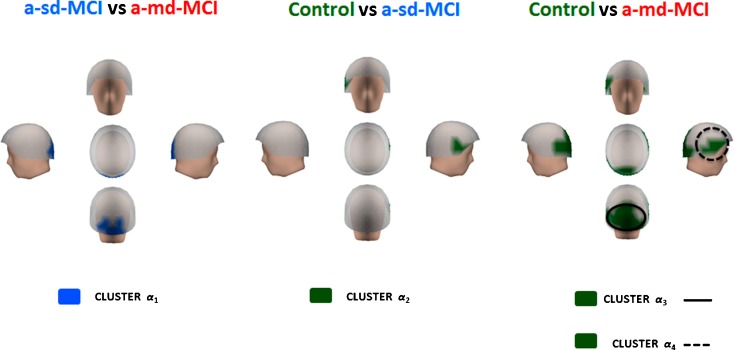
Differences within the alpha band range
The a-md-MCI group (see Fig. 6) showed reduced relative power values as compared to a-sd-MCIs in a small occipital cluster of sensors (cluster α1) within a frequency range of 9–11 Hz (t = 2.457; p < 0.01). The a-sd-MCI group exhibited reduced relative power as compared to controls (t = 2.279; p < 0.05) within a 10–12 Hz frequency range in a small right fronto-temporal cluster of sensors (cluster α2). Finally, a-md-MCIs showed significantly lower relative power values within an 8–12 Hz frequency range in two different clusters of sensors. Cluster α3 (t = 2.752; p < 0.001) extended bilaterally over the occipital region and contained all sensors in cluster α1 (see below). Cluster α4 (t = 2.703; p < 0.001) extended over the right lateral fronto-temporal region and contained all sensors in cluster α2.
Fig. 6.
Differences within the alpha band range. The cluster in blue represents an increase in relative power within the a-sd-MCI group in occipital regions (cluster α 1), as compared with the a-md-MCI group. The control group showed more relative power in right fronto-temporal areas (cluster α 2), in bilateral occipital regions (cluster α 3), and in right lateral fronto-temporal regions (cluster α 4) than both MCI groups. These clusters are represented in green color
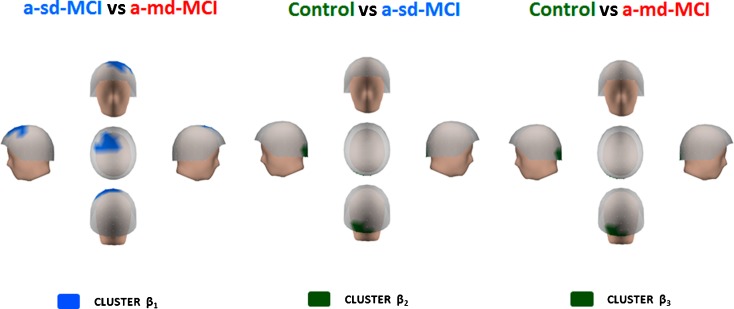
Differences within the beta band range
As previously described for the alpha range, relative power within the beta range was significantly decreased in the MCI groups (see Fig. 7). Of note, a-md-MCIs showed reduced relative power as compared with a-sd-MCIs (t = 2.262; p < 0.05) in a fronto-central cluster with left predominance (cluster β1) within a frequency range of 20–22 Hz. The a-sd-MCI group exhibited lower power values than controls within a broader frequency range of 16–23 Hz (t = 2.654; p < 0.001) in a small cluster of occipital sensors (cluster β2). Similarly, a-md-MCIs showed reduced power values as compared with controls (t = 2.126; p < 0.05) in the same 16–23 frequency range of 16–23 Hz and basically within the same occipital cluster of sensors observed in the controls vs. a-sd-MCI comparison (cluster β3).
Fig. 7.
Differences within the beta band range. Blue color indicates that the a-sd-MCI group exhibited increased relative power in a fronto-central cluster (cluster β 1) as compared with a-md-MCI. In the control group, there is an increased of relative power, indicated in green color, in occipital areas (clusters β 2 and β 3) when compared with a-sd and a-md-MCI groups
Power, hippocampal volumes, and neuropsychology correlations
As previously described, a series of exploratory one-way ANOVAs were performed to assess the differences in terms of neuropsychological tests performance and hippocampal volumes among groups. Firstly, the ANOVA revealed a significant effect of “Diagnosis” on LH_ICV and RH_ICV (p < 0.0001) (see Table 1). Post hoc comparisons with Bonferroni correction showed significant differences for LH_ICV and RH_ICV between controls and MCIs (p < 0.001), with larger hippocampal volumes within the healthy group. On the other hand, a-md-MCIs exhibited smaller volumes than a-sd-MCIs, but the comparison failed to reach the level of statistical significance (p > 0.05). A significant effect of “Diagnosis” was also found for all neuropsychological tests, with the exception of clock drawing test, VOSP, and TMTA accuracy. This initial finding was further explored by means of post hoc pair-wise comparison with Bonferroni correction. MMSE scores were significantly higher in control subjects as compared with both clinical groups (p < 0.001), but in addition, a-sd-MCI’s MMSE scores were significantly higher as compared to those of a-md-MCIs (p < 0.01; see Table 1). Direct digit span scores were significantly higher in controls, as compared with both clinical groups (p < 0.05), but no significant differences emerged from the comparison of MCI subtypes. Identical results were obtained in the analysis of inverse digit span, and immediate and delayed recall. Phonemic fluency scores were also significantly higher in controls when compared with both clinical groups (p < 0.05), and a-sd-MCIs showed significantly increased fluency values than a-md-MCIs (p < 0.05). Controls also exhibited increased semantic fluency values (p < 0.05), but no significant differences were found between MCI subtypes. An identical pattern was found in the comparison of TMTA time and TMTB accuracy and time scores. In both cases, a-sd-MCI showed higher scores as compared to a-md-MCIs, but the effect did not reach the significance level. Ideomotor Praxis values were also significantly higher in controls (p < 0.05). Finally, BNT scores presented a slightly different behavior. First, no significant differences emerged from the comparison of controls and a-sd-MCI patients. However, controls showed higher scores than a-md-MCIs (p < 0.01), and a-md-MCIs exhibited significantly higher scores than a-sd-MCIs (p < 0.05). According to these results, it seems that both MCI groups are similarly impaired in memory performance, but a-md-MCIs tend to show a poorer performance in language tasks, especially in the BNT. Additionally, a-md-MCIs presented the lowest MMSE scores, indicating a significantly more impaired general cognitive status.
Once this exploratory analysis was carried out, and with the aim of investigating the relationship between neurophysiological activity, hippocampal atrophy and cognitive performance, averaged power values in the significant clusters were correlated with hippocampal volumes and neuropsychological test scores in the whole sample (a-md-MCIs + a-sd-MCIs + controls). In order to avoid redundant information, and considering the high degree of ovelapping among clusters, we decided to submit for correlation analyses only those clusters that matched the following criteria: (1) clusters without spatial or spectral coincidence with other clusters (i.e., clusters δ1 and β1), and (2) broader clusters that encompassed the sensors and spectral ranges of smaller clusters (i.e., δ3, θ4, α3, α4, and β3). Pearson’s “r” and “p” values of all significant correlations are displayed in Table 2. Within the delta band range, cluster δ1 was inversely correlated with MMSE, inverse digit span, and TMTA accuracy, indicating that higher scores in this cluster are associated with a lower cognitive status. Cluster δ1 was positively correlated with TMTA time. Cluster δ3 was inversely correlated with MMSE, inverse digit span, immediate and delayed recall, phonemic fluency, and TMTB accuracy. Mirroring cluster δ1, cluster δ3 was also directly correlated with TMTA time.
Table 2.
Pearson correlation analyses of averaged power values in the significant clusters with neuropsychological test scores and hippocampal volumes in the whole sample
| Cluster | δ 1 | δ 3 | θ 4 | α 3 | α 4 | β 1 | β 3 |
|---|---|---|---|---|---|---|---|
| MMSE |
p = 0.01115 r = −0.2528 |
p = 0.01201 r = −0.25033 |
p = 0.00032 r = −0.35269 |
n.s. |
p = 0.00125 r = 0.31824 |
p = 0.03455 r = 0.21162 |
n.s. |
| Inverse Digit Span |
p = 0.03296 r = −0.2114 |
p = 0.00078 r = −0.32741 |
p = 0.00700 r = −0.26550 |
p = 0.02979 r = 0.21526 |
p = 0.02038 r = 0.22940 |
n.s. | n.s. |
| Immediate Recall | n.s. |
p = 0.00003 r = −0.39799 |
p = 0.000001 r = −0.45913 |
p = 0.00992 r = 0.25424 |
p = 0.00041 r = 0.34337 |
n.s. |
p = 0.00331 r = 0.28818 |
| Delayed Recall | n.s. |
p = 0.00118 r = −0.32295 |
p = 0.000001 r = −0.49822 |
p = 0.01768 r = 0.23922 |
p = 0.00037 r = 0.35267 |
n.s. |
p = 0.00083 r = 0.33234 |
| TMT A (acc.) |
p = 0.00417 r = −0.2827 |
n.s. |
p = 0.03475 r = −0.21034 |
n.s. | n.s. | n.s. | n.s. |
| TMT A (time) |
p = 0.03870 r = 0.2061 |
p = 0.01142 r = 0.25080 |
n.s. | n.s. | n.s. | n.s. | n.s. |
| TMT B (acc.) | n.s. |
p = 0.02988 r = −0.22656 |
p = 0.00125 r = −0.33153 |
n.s. | n.s. |
p = 0.01580 r = 0.25102 |
n.s. |
| TMT B (time) | n.s. | n.s. | n.s. |
p = 0.01086 r = −0.26303 |
n.s. | n.s. | n.s. |
| BNT | n.s. | n.s. |
p = 0.01214 r = −0.24996 |
n.s. | n.s. |
p = 0.04702 r = 0.19912 |
n.s. |
| Phonemic Fluency | n.s. |
p = 0.00078 r = −0.32889 |
p = 0.02630 r = −0.22109 |
p = 0.02740 r = 0.21953 |
n.s. | n.s. | n.s. |
| LH_ICV | n.s. | n.s. |
p = 0.0032 r = −0.3196 |
n.s. |
p = 0.0022 r = 0.2501 |
n.s. |
p = 0.0235 r = 0.2485 |
| RH_ICV | n.s. | n.s. |
p = 0.0022 r = −0.3309 |
n.s. |
p = 0.0533 r = 0.2129 |
n.s. |
p = 0.0003 r = 0.3882 |
The table shows the “p” and “r” (correlation index) values that were significant (p < 0.05)
Cluster θ4 was inversely correlated with both hippocampus and several tests: MMSE, inverse digit span, immediate recall, delayed recall, rule shift cards, phonemic fluency, TMTA and TMTB accuracy, and BNT. On the other hand, cluster θ4 was directly correlated with TMTA and TMTB time. The pattern of correlations within the low-frequency domain clearly suggests that the higher activity within delta and theta ranges, the poorer cognitive performance in several domains.
Cluster α3 was directly correlated with immediate and delayed recall, inverse digit span, and phonemic fluency, indicating that higher posterior activity within the alpha range is associated with a better cognitive performance. Cluster α3 was inversely correlated with TMTB time. Supporting this idea, cluster α4 was also positively correlated with both hippocampus, MMSE, inverse digit span, and immediate and delayed recall.
Finally, within the beta range, cluster β1 was directly correlated with MMSE, BNT, and TMTB accuracy. Cluster β3 was positively correlated with both hippocampal volumes, immediate and delayed recall. This correlation pattern suggests that an enhanced activity within the high-frequency range is associated with a lesser hippocampal atrophy and a better cognitive performance.
Lastly, in order to explore the relationship between hippocampal volumes and neuropsychological scores, both measures were correlated. LH_ICV was positively correlated with MMSE (p = 0.0040; r = 0.3832), immediate recall (p = 1.8 × 10−6; r = 0.4992), delayed recall (p = 6.2 × 10−10; r = 0.5941), VOSP (p = 0.0212; r = 0.2558), and TMT B (accuracy) (p = 0.0430; r = 0.2328) and negatively with TMT B (time) (p = 0.032; r = −0.2446). Similarly, RH_ICV was directly correlated with MMSE (p = 0.0018; r = 0.3413), immediate recall (p = 0.8 × 10−5, r = 0.4709); delayed recall (p = 3.93 × 10−7; r = 0.5314), rule shift cards (p = 0.0422; r = 0.2292), TMT B (accuracy) (p = 0.0164; r = 0.2745), and inversely correlated with TMT B (time) (p = 0.0064; r = −0.3081). These findings confirm the relationship among the hippocampal atrophy, the neurophysiological changes, and the global deficit observed in different cognitive domains, such as memory, perception, or executive functioning.
Discussion
We are aware that the clusterization of the frequency bands done in this study rendered difficult the comparison of our findings with the previously published literature. For this reason, we decided to compare the current findings with the classical frequency band closer to the frequency cluster found in this study.
The results of the present study basically support previous reports about EEG/MEG spectral analysis in MCI and add new information on the spectral profiles of MCI subtypes that was not previously addressed. As it might be expected, MCI patients (especially the a-md-MCI group) showed a generalized power increase within the theta range accompanied by a power decrease within the alpha and beta ranges in the posterior regions of the brain. More importantly, a-md-MCIs exhibited increased power within the delta and theta ranges as compared with a-sd-MCIs. This tendency (see Fig. 2) is a new example of the “shift to the left” in the spectral profile observed in AD and MCI patients that correlates with the degree of cognitive impairment (Fernandez et al. 2006b; Rodriguez et al. 1999).
From the last years, there is evidence of hyperactivation (Dickerson et al. 2005; Maestú et al. 2008) or hypersynchronization (Bajo et al. 2010) of different regions of the brain in patients with amnestic MCI in comparison to healthy controls but as well to AD patients. This increase is an indication of compensatory activity due to the loss of efficiency of the memory networks that leads to the idea of a nonclear linear neurophysiological process between healthy aging and dementia. In addition, there are some biochemical (Lavenex and Amaral 2000) or resting state functional studies (Osipova et al. 2006) that suggest that the differences between controls and MCI patients seem not to be so linear as our present results. Nonetheless, overall our findings seem to confirm the hypothesis of a spectral pattern more proximate to the typical AD profile, mainly in a-md-MCIs. Previous EEG (Babiloni et al. 2004; Dauwels et al. 2011; Huang et al. 2000; Jeong 2004) and MEG studies (Berendse et al. 2000; Fernandez et al. 2002, 2003, 2006b) of AD found an increased power in the low-frequency bands (delta and theta), accompanied by a decreased power in the high-frequency range (alpha, beta, and gamma). Such pattern of spectral changes is a consistent finding that correlates with cognitive performance and functional status (Fernandez et al. 2002; Prichep et al. 1994; van Deursen et al. 2008). More specifically, in a previous study of our group (Fernandez et al. 2006a), AD patients were characterized by a power increase within the 2–4-Hz frequency range, accompanied by a power decrease within the 16–28 Hz frequency range that basically parallels the spectral changes observed in the present investigation of MCI patients.
The sequence of spectral changes in AD is assumed to start with an increase in theta and a decrease in beta activity, which are followed by a decrease in alpha activity. Delta power is usually believed to increase only in more severe stages of the disease (Jeong 2004). However, several studies also reported an increase in delta power in MCI patients when compared with healthy aged controls (see, for example, Babiloni et al. 2006a, b, 2010; Rossini et al. 2008). Moreover, Fernandez et al. (2006c) demonstrated than MCI patients with elevated delta activity in posterior parietal regions had a significantly elevated risk of conversion to AD. This line of evidence indicates that very similar patterns of neurophysiological activity can be found in MCI patients and AD patients, supporting the idea of some degree of overlapping (Fernandez et al. 2006b). An increase in low-frequency activity is not the only shared characteristic. MCI and AD share neuropathological and functional features, including: tau and ApoE abnormal proteins (Mufson et al. 1999), reduced hippocampal and temporal lobe volumes (Jack et al. 1999; Martin et al. 2010), temporo-parieto-occipital hypometabolism–hypoperfusion in PET or SPECT scans (Nestor et al. 2004), and cholinergic dysfunction (Haense et al. 2012).
The “proximity” of a-md-MCIs’ spectral profiles to the typical AD pattern, together with the significantly lower MMSE, phonemic fluency, and language scores within this group (see Table 1), may be interpreted as a sign of greater deterioration in these patients. This affirmation is supported by the correlations between neurophysiological, structural, and neuropsychological data. The a-md-MCI group showed the highest levels of activity within delta and theta ranges, and such activity was inversely correlated with MMSE scores and hippocampal volumes (in this case, only theta range power). In addition, activity within delta and theta ranges was inversely correlated with the performance on tests of memory, language, attention, and executive functions such as immediate and delayed recall, inverse digit span, TMTA and TMTB, BNT, etc. The correlation pattern of clusters within alpha and beta ranges was also illustrative. The a-md-MCI group exhibited the lowest power within the alpha and beta ranges, especially in posterior sites, compared with the control group. Relative power values within the alpha range were directly correlated with hippocampal volumes and scores of immediate and delayed recall, inverse digit span, etc. Similarly, an increased activity within the beta range was positively correlated not only with hippocampal volumes and global cognitive status (i.e., MMSE) but also with the performance on tests of memory, language, and executive functioning. Overall, a-md-MCIs exhibited the highest power values in the low-frequency range, the lowest power values in the high-frequency range, the poorer cognitive performance, and the smaller hippocampal volumes.
The correlation between spectral profiles and cognitive performance was previously assessed in several studies. For example, in their classic study, Jelic et al. (1996) reported negative correlations between relative theta power, visuospatial function, memory and attention. More recent investigations showed very similar tendencies. Van der Hiele et al. (2007) found negative correlations between theta power and tests of global domain. In addition, negative correlations with tests such as the TMTB or semantic fluency were described by the authors. Babiloni et al. (2010) described negative correlations between delta power, Stroop and digit-symbol tests, while alpha 1 power was directly correlated with these neuropsychological measures. Although these investigations assessed the correlation between spectral and neuropsychological data, the importance of the regional distribution was not explicitly explored. It is noteworthy that the most significant difference between a-md-MCIs (i.e., patients with the poorest cognitive performance and lowest mental status) and a-sd-MCI patients appeared in fronto-central region (cluster δ1). The implication of more anterior sites is associated with a posterior-to-anterior tendency of neurophysiological abnormalities, such as focal delta activity, observed with the progress of the disease (Fouquet et al. 2009; Osipova et al. 2005).
As in some previous studies (Whitwell et al. 2007; He et al. 2009; Zhang et al. 2012), where a-MCI subtypes were compared, we failed to find significant statistical differences in hippocampal volumes. However, in a follow-up study, Tabert et al. (2006) found that 38 out of 39 patients that converted to AD had a baseline diagnosis of a-md-MCI, while none of them had a baseline diagnosis of a-sd-MCI. Diniz et al. (2009) found that those MCI who progress to dementia showed a worse global cognitive performance and multiple cognitive deficits at baseline than those MCI who remained stable. Similarly, Han et al. (2012) reported that the rate of progression to dementia in a-sd-MCI patients was very similar to the rate of reversion to the normal cognition, while in the a-md-MCI group, the rate of conversion to dementia was significantly higher when compared to the rate of reversion. Interestingly, the authors interpreted these findings as a sign of broader cerebral “degeneration” in the a-md-MCI group. The findings of Brodaty et al. (2012) mirrored those reported by Han and coworkers.
The relationship between elevated risk of conversion and presence of neuropathological signs has been established in a-md-MCI patients. For instance, Caffarra et al. (2008) accomplished a follow-up study where a-sd-MCI, a-md-MCIs, and “dysexecutive MCI” patients underwent SPECT evaluation at baseline. Both amnestic groups showed significant hypoperfusion in the left hippocampus and parahippocampal gyrus, but a-md-MCIs showed an additional hypoperfusion in left posterior cingulated gyrus. Notably, the 55 % of a-md-MCIs progressed to AD in a maximum follow-up period of 24 months, while none of the a-sd-MCI patients progressed to dementia. These results might suggest that, contrary to the classical understanding of a-MCI as a risk factor for dementia, the a-sd-MCI subtype is in fact a “benign” form of MCI. This challenging idea was posed by Nordlund et al. (2010) in an excellent investigation. The authors evaluated the 2-year outcome in a sample composed of a-sd-MCIs, a-md-MCIs, na-sd-MCIs, and na-md-MCIs. In addition, cerebrospinal fluid (CSF) samples were collected to estimate tau levels at baseline. Paralleling previous studies, only multidomain cases progressed to dementia, but more specifically 18 out of 21 patients that progressed to dementia had a baseline diagnosis of a-md-MCI. In fact, elevated tau levels in CSF and a diagnosis of a-md-MCI were the best predictors of progression to dementia. The results of Norlund et al. further confirm the findings of Wolk et al. (2009) of elevated levels of AD pathology in a-md-MCIs, in this case represented by a significantly higher number of Pittsburgh Compound B-positive cases that exhibited an increased risk of conversion to AD.
At this point, three lines of evidence converge: (1) a-md-MCI and AD biological markers seem to overlap in a significant percentage of cases; (2) several studies support the notion of a more severe brain deterioration with a more frequent presence of AD-pathology and an elevated risk of conversion to AD in a-md-MCI subtype; and (3) our results indicate that a-md-MCIs exhibit a spectral profile very similar to that observed in AD, which is associated with a more severe cognitive deterioration. Taken together, these lines of evidence lead us to discuss the notion of a-md-MCI as an independent clinical condition. Recently, in a key position article, Dubois et al. (2010) revised the definition of AD and some related disorders. According to the new perspective proposed by the authors, some patients previously described as having MCI should be actually considered as suffering from “prodromal” AD. This is to say that they already have AD. The category of prodromal AD encompasses all patients previously considered as having MCI who show positive biomarker evidence of AD pathology in their brains. The concept of MCI only remains to define those patients that do not show biomarker evidence of AD pathology.
According to previous literature and our own results, a-md-MCIs might be more likely to fulfill the criteria of prodromal AD proposed by Dubois et al. (2010). Nevertheless, the more important question addressed by our investigation is the potential role of neurophysiological techniques (EEG or MEG) as markers for AD pathology. In a very recent study of our group (Fernandez et al. 2013), it was demonstrated that delta current densities in posterior parietal, occipital, prerolandic, and precuneus cortices distinguished among MCI patients, AD patients with different severity scores, and controls. More importantly, an increase in delta activity in posterior regions such as the right posterior parietal cortex and the precuneus indexed the transition from MCI to mild and from mild to more severe dementia. Considering the close relationship between cholinergic inputs and neurophysiological activity, we proposed that MEG spectral mapping might be a serious candidate for a “neural degeneration” marker of AD reflecting dysfunctional synaptic transmission. The new results presented here might support the role of MEG spectral analysis in the investigation within the healthy aging–AD continuum, particularly as an objective marker of disease progression associated with cognitive deterioration. Notwithstanding, the confirmation of such role should be attained by correlating MEG spectral data with CSF or imaging markers of AD. In addition, the spectral information should be evaluated in individuals with elevated risk of developing AD such as the APOE4 carriers. According to our results, we hypothesize that patients with a diagnosis of a-md-MCI showing an exaggerated activity within the delta and theta ranges are cases of prodromal AD (according to the definition of Dubois et al.) with an elevated risk of rapid progression to a fully declared dementia. If this strong hypothesis is confirmed, MEG spectral analysis might serve as a quantitative measure to define the neurophysiological characteristics of prodromal AD.
Acknowledgments
This study was supported by two projects, PSI2009-14415-C03-01 and PSI2012-38375-C03-01, from the Spanish Ministry of Science and Economy, and a predoctoral fellowship from the Ministry of Education (FPU AP-2008- 00175), a predoctoral fellowship from the Spanish Ministry of Science and Innovation (BES-2010-036469), a PICATA predoctoral fellowship of the Moncloa Campus of International Excellence (UCM-UPM), and a predoctoral fellow from the Basque Government.
Footnotes
M. E. López and P. Cuesta contributed equally to this work.
Contributor Information
M. E. López, Phone: +34-91-3367307, Phone: +34-91-3364632, FAX: +34-91-3366828, Email: meugenia.lopez@ctb.upm.es
P. Cuesta, Phone: +34-91-3364632, Email: pablo.cuesta@ctb.upm.es
P. Garcés, Phone: +34-91-3364632, Email: pilar.garces@ctb.upm.es
P. N. Castellanos, Phone: +34-91-3364632, Email: nazareth.castellanos@ctb.upm.es
S. Aurtenetxe, Phone: +34-91-3364632, Email: sara.aurtenetxe@ctb.upm.es
R. Bajo, Phone: +34-628909785, Email: ricardo.bajo@ctb.upm.es
A. Marcos, Email: amarcosdolado@gmail.com
M. L. Delgado, Email: marisadelgadolosada@yahoo.es
P. Montejo, Email: montejop@madrid.es
J. L. López-Pantoja, Email: jlopezdos@wanadoo.es
F. Maestú, Phone: +34-91-3364632, Email: fernando.maestu@ctb.upm.es
A. Fernandez, Email: aferlucas@med.ucm.es
References
- Agrell B, Dehlin O. The clock-drawing test. Age Ageing. 1998;27:399–403. doi: 10.1093/ageing/27.3.399. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatry. 1997;9(1):167–171. doi: 10.1017/S1041610297004869. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Cerboneschi D, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual-Marqui RD, Rodriguez G, Romani GL, Salinari S, Tecchio F, Vitali P, Zanetti O, Zappasodi F, Rossini PM. Mapping distributed sources of cortical rhythms in mild Alzheimer's disease. A multicentric EEG study. Neuroimage. 2004;22(1):57–67. doi: 10.1016/j.neuroimage.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Benussi L, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Ghidoni R, Miniussi C, Rodriguez G, Romani GL, Squitti R, Ventriglia MC, Rossini PM. Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: a multicentric electroencephalogram study. Ann Neurol. 2006;59:323–334. doi: 10.1002/ana.20724. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin Neurophysiol. 2006;117:252–268. doi: 10.1016/j.clinph.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni G, Steriade M, Bresciani L, Binetti G, Del Percio C, Geroldi C, Miniussi C, Nobili F, Rodriguez G, Zappasodi F, Carfagna T, Rossini PM. Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer's disease. Clin Neurophysiol. 2006;117(5):1113–1129. doi: 10.1016/j.clinph.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni GB, Pievani M, Vecchio F, Lizio R, Buttiglione M, Geroldi C, Fracassi C, Eusebi F, Ferri R, Rossini PM. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. Neuroimage. 2009;44:123–135. doi: 10.1016/j.neuroimage.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Visser PJ, Frisoni G, De Deyn PP, Bresciani L, Jelic V, Nagels G, Rodriguez G, Rossini PM, Vecchio F, Colombo D, Verhey F, Wahlund LO, Nobili F. Cortical sources of resting EEG rhythms in mild cognitive impairment and subjective memory complaint. Neurobiol Agin. 2010;31:1787–1798. doi: 10.1016/j.neurobiolaging.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Bajo R, Maestú F, Nevado A, Sancho M, Gutiérrez R, Campo P, Castellanos NP, Gil P, Moratti S, Pereda E, Del-Pozo F. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J Alzheimers Dis. 2010;22(1):183–193. doi: 10.3233/JAD-2010-100177. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K (1989) Multilingual aplasia examination, 2nd edn. Department of Neurology and Psychology, The University of Iowa, Iowa City
- Berendse HW, Verbunt JP, Scheltens P, van Dijk BW, Jonkman EJ. Magnetoencephalographic analysis of cortical activity in Alzheimer's disease: a pilot study. Clin Neurophysiol. 2000;111:604–612. doi: 10.1016/S1388-2457(99)00309-0. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Heffernan M, Kochan NA, Draper B, Trollor JN, Reppermund S, Slavin MJ, Sachdev PS. Mild cognitive impairment in a community sample: The Sydney Memory and Ageing Study. Alzheimers Dement. 2012;9(3):310–317. doi: 10.1016/j.jalz.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Caffarra P, Ghetti C, Concari L, Venneri A. Differential patterns of hypoperfusion in subtypes of mild cognitive impairment. Open Neuroimag J. 2008;2:20–28. doi: 10.2174/1874440000802010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Srinivasan K, Ramasubba Reddy M, Musha, T, Vialatte FB, Latchoumane C, Jeong J, Cichocki A (2011) Slowing and loss of complexity in Alzheimer's EEG: two sides of the same coin? Int J Alzheimers Dis 539621 [DOI] [PMC free article] [PubMed]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Nunes PV, Yassuda MS, Forlenza OV. Diagnosis of mild cognitive impairment revisited after one year. Preliminary results of a prospective study. Dement Geriatr Cogn Disord. 2009;27(3):224–231. doi: 10.1159/000203346. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Cummings JL, De Kosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, de Souza LC, Stern J, Visser PJ, Scheltens P. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Stat Sci. 2004;19(4):676–685. doi: 10.1214/088342304000000396. [DOI] [Google Scholar]
- Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Maestu F, Amo C, Gil P, Fehr T, Wienbruch C, Rockstroh B, Elbert T, Ortiz T. Focal temporoparietal slow activity in Alzheimer's disease revealed by magnetoencephalography. Biol Psychiatry. 2002;52:764–770. doi: 10.1016/S0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Arrazola J, Maestu F, Amo C, Gil-Gregorio P, Wienbruch C, Ortiz T. Correlations of hippocampal atrophy and focal low-frequency magnetic activity in Alzheimer disease: volumetric MR imaging-magnetoencephalographic study. AJNR Am J Neuroradiol. 2003;24:481–487. [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Hornero R, Mayo A, Poza J, Maestu F, Ortiz T. Quantitative electroencephalography of spontaneous brain activity in Alzheimer disease: an exhaustive frequency analysis. Alzheimer Dis Assoc Disord. 2006;20:153–159. doi: 10.1097/00002093-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Hornero R, Mayo A, Poza J, Gil-Gregorio P, Ortiz T. MEG spectral profile in Alzheimer's disease and mild cognitive impairment. Clin Neurophysiol. 2006;117:306–314. doi: 10.1016/j.clinph.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Turrero A, Zuluaga P, Gil P, Maestu F, Campo P, Ortiz T. Magnetoencephalographic parietal delta dipole density in mild cognitive impairment: preliminary results of a method to estimate the risk of developing Alzheimer disease. Arch Neurol. 2006;63:427–430. doi: 10.1001/archneur.63.3.427. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Turrero A, Zuluaga P, Gil-Gregorio P, del Pozo F, Maestu F, Moratti S. MEG delta mapping along the healthy aging-Alzheimer’s disease continuum: diagnostic implications. J Alzh Dis. 2013;35(3):495–507. doi: 10.3233/JAD-121912. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, Viader F, Baron JC, Eustache F, Chetelat G. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132:2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris S, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Grunwald M, Busse F, Hensel A, Riedel-Heller S, Kruggel F, Arendt T, Wolf H, Gertz HJ. Theta-power differences in patients with mild cognitive impairment under rest condition and during haptic tasks. Alzheimer Dis Assoc Disord. 2002;16:40–48. doi: 10.1097/00002093-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Haense C, Kalbe E, Herholz K, Hohmann C, Neumaier B, Krais R, Heiss WD. Cholinergic system function and cognition in mild cognitive impairment. Neurobiol Aging. 2012;33:867–877. doi: 10.1016/j.neurobiolaging.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Han JW, Kim TH, Lee SB, Park JH, Lee JJ, Huh Y, Park JE, Jhoo JH, Lee DY, Kim KW. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement. 2012;8:553–559. doi: 10.1016/j.jalz.2011.08.007. [DOI] [PubMed] [Google Scholar]
- He J, Farias S, Martinez O, Reed B, Mungas D, Decarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66(11):1393–1399. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V (2000) Discrimination of Alzheimer's disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol 111:1961–1967 [DOI] [PubMed]
- Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/WNL.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO. Quantitative electroencephalography power and coherence in Alzheimer's disease and mild cognitive impairment. Dementia. 1996;7:314–323. doi: 10.1159/000106897. [DOI] [PubMed] [Google Scholar]
- Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer's disease. Neurobiol Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry. 2005;77(4):429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S (1983) The Boston Naming Test Philadelphia: Lea and Febiger
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.WNL.0000034176.07159.F8. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brodie EM. Assessment of older people: self maintaining and instrumental activity of daily living. J Gerontol. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Lobo A, Ezquerra J, Gomez BF, Sala JM, Seva DA. Cognitive mini-test (a simple practical test to detect intellectual changes in medical patients) Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1979;7:189–202. [PubMed] [Google Scholar]
- Maestú F, Campo P, Del Río D, Moratti S, Gil-Gregorio P, Fernández A, Capilla A, Ortiz T. Increased biomagnetic activity in the ventral pathway in mild cognitive impairment. Clin Neurophysiol. 2008;119(6):1320–1327. doi: 10.1016/j.clinph.2008.01.105. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010;31:1099–1106. doi: 10.1016/j.neurobiolaging.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhan G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Mufson JC, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neuro. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nat Med. 2004;10(Suppl):S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for human neuroimaging: a primer with examples. Hum Brain Mapping. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81:541–546. doi: 10.1136/jnnp.2008.171066. [DOI] [PubMed] [Google Scholar]
- Norris G, Tate RL. The behavioural assessment of the dysexecutive syndrome (BADS): ecological, concurrent and construct validity. Neuropsychological Rehabilitation. 2000;10:33–45. doi: 10.1080/096020100389282. [DOI] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J‐M (2011) FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. doi:10.1155/2011/156869 [DOI] [PMC free article] [PubMed]
- Osipova D, Ahveninen J, Jensen O, Ylikoski A, Pekkonen E. Altered generation of spontaneous oscillations in Alzheimer's disease. Neuroimage. 2005;27:835–841. doi: 10.1016/j.neuroimage.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Osipova D, Rantanen K, Ahveninen J, Ylikoski R, Häppölä O, Strandberg T, Pekkonen E. Source estimation of spontaneous MEG oscillations in mild cognitive impairment. Neurosci Lett. 2006;405(1–2):57–61. doi: 10.1016/j.neulet.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Peña-Casanova J (1990) Programa Integrado de Exploración Neuropsicológica- Test Barcelona Protocolo Masson, SA, Barcelona
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: where are we? Alzheimer Dis Assoc Disord. 2005;19:166–169. doi: 10.1097/01.wad.0000179417.95584.90. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, Cancro R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging. 1994;15:85–90. doi: 10.1016/0197-4580(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, Torossian C, Reisberg B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006;27:471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de León MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatr. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Rodriguez G, Copello F, Vitali P, Perego G, Nobili F. EEG spectral profile to stage Alzheimer's disease. Clin Neurophysiol. 1999;110:1831–1837. doi: 10.1016/S1388-2457(99)00123-6. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Buscema M, Capriotti M, Grossi E, Rodriguez G, Del Percio C, Babiloni C. Is it possible to automatically distinguish resting EEG data of normal elderly vs mild cognitive impairment subjects with high degree of accuracy? Clin Neurophysiol. 2008;119:1534–1545. doi: 10.1016/j.clinph.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Shah Y, Tangalos EG, Petersen RC. Mild cognitive impairment. When is it a precursor to Alzheimer's disease? Geriatrics. 2000;55(9):62, 65–8. [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M. Presentation of electromagnetic multichannel data: the signal space separation method. J Appl Phys. 2005;97:124905. doi: 10.1063/1.1935742. [DOI] [Google Scholar]
- van der Hiele K, Vein AA, Reijntjes RH, Westendorp RG, Bollen EL, van Buchem MA, van Dijk JG, Middelkoop HA. EEG correlates in the spectrum of cognitive decline. Clin Neurophysiol. 2007;118:1931–1939. doi: 10.1016/j.clinph.2007.05.070. [DOI] [PubMed] [Google Scholar]
- van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH, Riedel WJ. Increased EEG gamma band activity in Alzheimer's disease and mild cognitive impairment. J Neural Transm. 2008;115:1301–1311. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery bury St Edmunds. UK: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—revised (manual) San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64(8):1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sachdev PS, Wen W, Kochan NA, Crawford JD, Brodaty H, Slavin MJ, Reppermund S, Draper B, Zhu W, Kang K, Trollor JN. Gray matter atrophy patterns of mild cognitive impairment subtypes. J Neurol Sci. 2012;315:26–32. doi: 10.1016/j.jns.2011.12.011. [DOI] [PubMed] [Google Scholar]