Abstract
The ability to maintain balance deteriorates with increasing age. Anticipatory and compensatory postural adjustments (APAs and CPAs, respectively), both, are known to be affected in the elderly. We examined the effect of aging on the ability of older adults to utilize APAs and its effect on subsequent control of posture (CPAs). Ten elderly individuals were exposed to external predictable and unpredictable perturbations applied to the upper body in the sagittal plane. Body kinematics, electromyographic activity of 13 muscles, and ground reaction forces were analyzed during the anticipatory and compensatory phases of postural control. The elderly were capable of recognizing an upcoming predictable perturbation and activated muscles prior to it. However, the older adults used different muscle strategies and sequence of muscle recruitment than that reported in young adults. Additionally, when the perturbations were unpredictable, no APAs were seen which resulted in large CPAs and greater peak displacements of the center of pressure (COP) and center of mass (COM) following perturbations. As opposed to this, when the perturbations were predictable, APAs were seen in older adults resulting in significantly smaller CPAs. The presence and utilization of APAs in older adults also improved postural stability following the perturbation as seen by significantly smaller COP and COM peak displacements. Using APAs in older adults significantly reduces the need for large CPAs, resulting in greater postural stability following a perturbation. The results provide a foundation for investigating the role of training in improving the interplay between anticipatory and compensatory postural control in older adults.
Keywords: Aging, Postural control, Anticipatory, Compensatory, Stability
Introduction
The ability to maintain balance deteriorates with advancing age since sensory and motor resources required for postural stability and orientation decline with ageing. Age-related proprioceptive and vestibular losses are associated with increased reliance on visual inputs, with vision itself being affected by the process of ageing. Reduction in muscle strength, power, and joint mobility along with impaired sensorimotor integration also contribute to poor postural control in the elderly. These changes affect the ability to resolve sensory conflicts and generate appropriate muscle responses in order to maintain or restore balance (Shaffer and Harrison 2007; Sturnieks et al. 2008). Additionally, older adults are less active and this can lead to a detuning of the balance control system (DiPietro 2001). It is likely that a lack of constant challenge along with systemic changes contribute to the deterioration of balance and mobility in older adults (Frank and Patla 2003). The relation between balance control and independent mobility is important in the elderly where poor postural control is associated with significant mobility losses (Frank and Patla 2003), physical inactivity (Merom et al. 2012), and an increase in the fear of falling (Skelton and Beyer 2003).
It is known that anticipatory and compensatory postural strategies are the two main mechanisms used by the central nervous system (CNS) in order to deal with body perturbations that may either be internally generated (e.g., self-initiated movements) or externally generated (e.g., being pushed at shoulder level while walking). A disturbance to balance that can be predicted, elicits changes in background activity of muscles that cause a rearrangement of body segments prior to the moment of perturbation in a feedforward manner such that the impact of the upcoming perturbation is minimized (Belen’kii et al. 1967; Bouisset and Zattara 1987; Massion 1992; Aruin and Latash 1995). These adjustments, known as anticipatory postural adjustments (APAs), are based on anticipation or prior experience and help in dealing with the potential effects of an expected perturbation. However, when the disturbance to balance is unpredictable, postural muscles are activated after the moment of perturbation with the aim of restoring stability (Nashner and Cordo 1981). These changes, known as compensatory postural adjustments (CPAs), are triggered by sensory feedback signals and help in dealing with the actual effects of a perturbation (Park et al. 2004; Alexandrov et al. 2005). While APAs are observed only in the case of predictable perturbations, CPAs are seen during both, predictable (following APAs) and unpredictable (CPAs) perturbations.
Age-related changes to postural control are reflected in the ability to maintain or re-establish balance when perturbed. For instance, older adults show increased spontaneous sway while standing, especially when two or more sensory inputs are eliminated or distorted (Shaffer and Harrison 2007; Sturnieks et al. 2008). Following unexpected perturbations, there is a delay in the onset latencies of postural muscles and more time is needed to stabilize the center of pressure (COP) position (Woollacott and Shumway-Cook 1990; Lin et al. 2004). In contrast to young individuals, the elderly tend to use more of a hip strategy and show patterns of increased co-activation of agonist–antagonist muscles (Manchester et al. 1989; Woollacott and Shumway-Cook 1990; Lin et al. 2004). Older adults tend to have difficulties in sensing the onset of perturbations as well (an increase in the threshold of perturbation recognition) and therefore, a limited response capacity (Woollacott and Shumway-Cook 1990). Balancing reactions such as rapid stepping or reaching movements that are critical for preventing falls are also impaired in healthy older adults (Maki and McIlroy 2006). Thus, compensatory postural adjustments which are crucial to balance recovery are significantly affected by the ageing process. At the same time, anticipatory mechanisms of postural control that play a vital role in preparing the body for an upcoming perturbation are also impaired in healthy older adults. Thus, it has been shown that the elderly have delayed onset (closer to or after the moment of impact) and reduced magnitude of muscle activation prior to self-initiated and externally induced predictable perturbations (Man’kovskii et al. 1980; Inglin and Woollacott 1988; Rogers et al. 1992; Woollacott and Manchester 1993; Kanekar and Aruin 2014). Additionally, the pattern of muscle recruitment shifts from a classic distal to proximal sequence to more of a hip strategy or involves a selective or increased activation of certain muscles (Inglin and Woollacott 1988; Bleuse et al. 2006). Delays in the anticipatory vertical torque (Tz) and COP latency have also been reported in the elderly during performance of an arm raising task (Bleuse et al. 2006). Moreover, prior to a predictable external perturbation, delayed and reduced anticipatory muscle activity in older adults is also associated with smaller anticipatory COP displacements and directionally impaired anticipatory movement of the center of mass (COM) (Kanekar and Aruin 2014). While APAs are delayed and reduced in magnitude in older adults, nevertheless, the ability to recruit these muscles anticipatorily is largely preserved with aging (Rogers et al. 1992; Garland et al. 1997; Bleuse et al. 2006; Claudino et al. 2013).
The role of APA utilization in subsequent control of posture has been studied in healthy young adults using a pendulum-impact paradigm that allows triggering predictable and unpredictable external perturbations of the same magnitude while the level of generation of anticipatory postural adjustments is manipulated (Santos et al. 2010a, b). It was found that an unavailability of APAs resulted in huge compensatory muscle activation and greater center of mass–center of pressure (COM-COP) displacements whereas utilization of robust APAs was associated with significantly smaller compensatory muscle activation and COM-COP displacements. As such, these findings highlight the importance of APAs in control of posture, and point out the existence of a relationship between anticipatory and compensatory components of postural control.
While such an association is established in healthy young adults, little is known about how aging affects the interaction between these two mechanisms of postural control. Given that one of the functional goals of both APAs and CPAs is to minimize and restore the body’s COM displacement after a perturbation and the fact that both these control mechanisms are affected in older adults, comprehending the effect of aging on the interplay between these two mechanisms of control is vital. While an impairment of anticipatory postural control in the older adults (delayed and reduced APAs) has been demonstrated, it is not specifically known how these changes influence the functional interaction of APAs and CPAs in controlling the body’s COM displacement. The outcome of a recent study on postural control in the elderly responding to external perturbations induced in the lateral plane revealed that there is no difference in the APAs between the young and elderly subjects. At the same time, it was shown that when the perturbations were predictable, the young and the elderly demonstrated anticipatory muscle activity that was followed by decreased CPAs as opposed to the unpredictable perturbations wherein an absence of APAs resulted in large CPAs (Claudino et al. 2013). However, the COP displacement following the disturbance was similar between the predictable and unpredictable conditions in the older adults, indicating greater postural instability even in the presence of anticipatory muscle activity. Thus, the findings of this study indicate that while dealing with perturbations in the lateral plane older adults may have difficulties in utilizing APAs. On the other hand, it is important to note that obtaining the timing of anticipatory activation of postural muscles provides valuable information for understanding the changes in APAs with aging. However, the onsets of anticipatory muscle activity were not reported in the above study, thereby, making it difficult to determine the precise role of anticipatory and compensatory postural mechanisms in balance control of the elderly. As such, it is important to investigate whether older adults are able to effectively utilize preparatory muscle activity to improve postural stability after a perturbation has occurred. This issue is critical because inability of the older adults to optimally generate postural adjustments prior to an upcoming balance threat as well as possible impairment of the interaction between the two mechanisms of postural control in the elderly (the CNS of an elderly individual may have difficulties in accurately assessing the effect of involvement of APAs in control of posture) may put additional demands on their postural control system, thereby, placing them at a greater risk for losing balance.
This study was therefore focused on examining the interaction between anticipatory and compensatory mechanisms of balance control in healthy older adults exposed to external perturbations induced in the sagittal plane. We hypothesized that: (1) during an unpredictable perturbation (no vision condition), APAs will be absent; only large CPAs will be observed. As a result, large peak COP and COM displacements will occur and (2) during a predictable perturbation (full vision condition), APAs will be present. As a result, smaller CPAs and smaller peak COP and COM displacements will be seen.
Methods
Subjects
A group of ten healthy aging adults (six males and four females, mean age 69.9 ± 4.04 years; mean body mass 76.42 ± 17.39 kg, and mean height 1.70 ± 0.13 m) without any neurological or musculoskeletal disorders participated in the study. All the subjects signed an informed consent approved by the university’s Institutional Review Board.
Experimental set-up and procedure
The subjects were instructed to stand barefoot with their feet shoulder width apart on a force platform. They were exposed to external perturbations in the sagittal plane induced by a pendulum with an additional load (3 % of the subjects’ body weight) attached to it. The subjects were required to receive each pendulum impact with their hands, while their arms, wrists, and fingers were extended at the shoulder level, and to maintain their balance after the perturbation (for more detail see (Santos et al. 2010a)). There were two experimental conditions: (1) external perturbations applied when eyes were open, thus, predictable in their timing; “predictable perturbations” and (2) external perturbations applied when eyes were closed; “unpredictable perturbations”. Two to three practice trials were given prior to each experimental condition. No advance warning of the impending perturbation was provided. The subjects wore wireless headphones and listened to music in both the experimental conditions, to prevent them from obtaining any auditory information about the moment of the pendulum release. For safety purposes in all the experiments, the subjects wore a harness (NeuroCom, USA) with two straps attached to the ceiling. Ten trials, each 5 s in duration, were collected for each experimental condition and the order of experimental conditions (predictable and unpredictable) was randomized.
Instrumentation
Electromyographic (EMG) activity of muscles was recorded with bipolar disposable surface electrodes (Red Dot 3 M) from 13 right lower limb and trunk muscles. After the skin area was cleaned with alcohol preps, electrodes were attached to the muscle belly of soleus (SOL), lateral gastrocnemius (GASL), medial gastrocnemius (GASM), tibialis anterior (TA), rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF), semitendinosus (ST), gluteus medius (GMED), external oblique (EO), rectus abdominis (RA), and erector spinae longus (ESL). The distance between two electrodes in a pair was 20 mm. A ground electrode was attached to the anterior aspect of the leg over the tibial bone. EMG signals were collected, filtered, and amplified (10–500 Hz, gain 2,000) using an EMG system (Myopac, RUN Technologies, USA). A force platform (model OR-5, AMTI, USA) was used to record the ground reaction forces and moments of forces. The signal obtained from an accelerometer (Model 208CO3, PCB Piezotronics, Inc, USA) attached to the distal end of the pendulum was used to identify the moment of the perturbation.
Three-dimensional kinematic data was collected using a six-camera VICON 612 system (Oxford Metrics, UK). Retroreflective markers were placed over anatomical landmarks bilaterally according to the Plug-In-Gait (PIG) model (Oxford Metrics), which includes: second metatarsal head, calcaneus, lateral malleolus, lateral epicondyle of the femur, a marker on the lateral border of the leg (between the lateral malleolus and femoral epicondyle markers), anterior/posterior superior iliac spines, a marker on the lateral border of the thigh (between the femoral epicondyle and anterior superior iliac spines), second metacarpal, lateral epicondyle of the humerus, acromio-clavicular joint, and a marker on the lateral border of the arm (between the humeral epicondyle and the acromio-clavicular joint markers). Also, subjects wore head and wrists bands with four and two markers attached on them, respectively. Finally, five additional markers were attached over the following landmarks: seventh cervical vertebra, tenth thoracic vertebra, inferior angle of the right scapula, between the two sternoclavicular joints, and xiphoid process of the sternum bone.
EMG, forces, moments of force, and accelerometer signals were acquired at 1,000 Hz and kinematic data were acquired at 100 Hz by means of the VICON 612 data station.
Data processing
MATLAB (MathWorks, Natick, MA, USA) programs were used for off-line data analysis. EMG signals were rectified and filtered with a 100 Hz low-pass, second order, zero-lag Butterworth filter, while the ground reaction forces, moments, and COM were filtered with a 40-Hz low-pass, second order, zero-lag Butterworth filter. The ‘time-zero’ (T0 = 0, moment of pendulum impact) was calculated from the accelerometer signal as a point in time at which the signal exceeded 5 % of the maximum acceleration. This value was confirmed by visual inspection by an experienced researcher. Data in the range from −600 ms (before T0) to +1,000 ms (after T0) were selected for further analysis. Individual trials were aligned according to T0 and this was used as a common reference point for all the signals.
The beginning of activation or inhibition of each muscle (muscle onset or muscle latency) in each trial was detected in a time window from −250 to +250 ms in relation to T0 by a combination of computer algorithm and visual inspection of the trials. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean ± 2 SD of its baseline value, measured from −500 to −400 ms. The onset latencies for each muscle were then averaged across the trials within each condition for each subject.
Integrals of anticipatory and compensatory EMG activity were derived using average trials for each subject. Integrals of the EMG activities (IntEMGi) were calculated for four different epochs, each of 150 ms duration in relation to T0. The time windows for the four epochs were: (1) from −250 to −100 ms (anticipatory activity, APA1); (2) −100 ms to +50 ms (anticipatory activity, APA2); (3) +50 ms to +200 ms (compensatory reactions, CPA1); and (4) + 200 ms to +350 ms (late compensatory reactions, CPA2). The IntEMGi for each of the four epochs was further corrected by the EMG integral of the baseline activity from −600 to −450 ms in relation to T0. The integrals of EMG activity were then normalized by the peak muscle activity across all conditions within an experiment for each muscle for each subject. As a result of the normalization, all the normalized integrals (IEMGNORM) are within the range from +1 to −1, with the positive values indicating an activation of the muscle and negative values indicating a decrease in the background activity (inhibition).
Displacements of the center of pressure in the anterior–posterior direction were calculated based on previous literature (Winter et al. 1996; Santos et al. 2010a).
Vicon and BodyBuilder 3D modeling software were used for initial processing of the kinematic data. The PIG model consisted of 15 body segments, including pelvis, femur (2), tibia (2), feet (2), humerus (2), radius (2), hands (2), thorax, and head. Body mass and height, seven anthropometrical measures such as leg length, knee, ankle, elbow, and wrist width and shoulder offset and hand thickness for each subject were entered in the PIG model. These measures together with the kinematic data were used to calculate body’s COM position. Customized MATLAB programs were thereafter used for further analysis. Aligned trials were averaged for each subject. The displacements of COP and COM at T0 which is anticipatory in nature and the peak displacement (maximum displacement after T0) that is compensatory in nature were calculated. The COP and COM displacements in the anterior–posterior direction (Y-axis according to our experimental set up) will be reported since the induced perturbations were symmetrical in nature, and were therefore associated with only negligible displacements in the medial-lateral direction. It is important to note that larger peak displacements following the perturbation indicate greater postural instability. The times at which the peak displacements occurred were also calculated for each of the above variables. These measures indicated the individuals’ balance control ability after the perturbations.
Statistical analysis
Statistical analysis was performed in SPSS 17 for Windows XP (SPSS Inc., Chicago, USA). Means with standard errors are reported. For IEMGNORM, separate 2 × 4 repeated measures ANOVAs were performed for each muscle. There were two within-subject factors: condition (two levels: predictable and unpredictable) and epoch (four levels: APA1, APA2, CPA1, and CPA2). When condition × epoch interactions was significant, paired t tests with Bonferroni’s correction were used for post hoc comparisons. A paired t test was used for comparison of latencies of individual muscles between the two experimental conditions (predictable and unpredictable). Paired t tests were used for comparing displacements of COP and COM at T0 and at the peak after T0 between the two experimental conditions (predictable and unpredictable). Paired t tests were also used to compare the time of peak displacements of COP and COM between the two experimental conditions (predictable and unpredictable). Statistical significance was set at alpha <0.05 for all tests except for the post hoc comparisons performed for IEMGNORM. When post hoc comparisons were performed for investigating the differences in IEMGNORM between the two conditions across each epoch, Bonferroni’s correction was applied such that alpha <0.012 was considered statistically significant. When post hoc comparisons were performed for investigating the differences in IEMGNORM between the four epochs within a given condition, Bonferroni’s correction was applied such that alpha <0.008 was considered statistically significant.
Results
Onset of muscle activity (muscle latency)
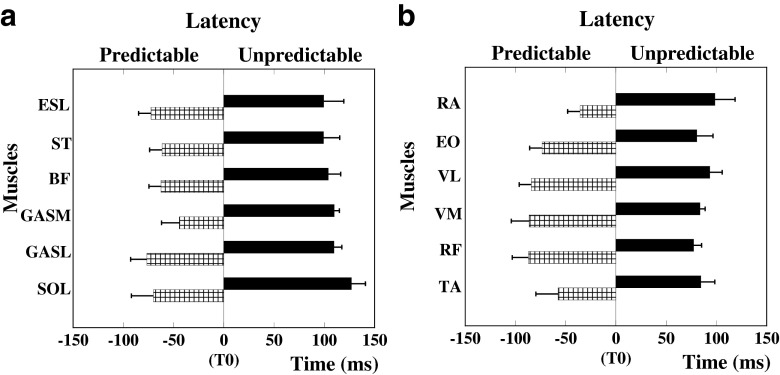
In conditions with the predictable perturbations, onset of EMG activity was seen before the pendulum impact in all the studied muscles (Fig. 1). The sequence of muscle activation in the predictable conditions was from distal to proximal for the dorsal muscles (Fig. 1a); however, a clear pattern was not seen for the ventral muscles where the knee extensors were activated before the distal and proximal muscles (Fig. 1b). In the dorsal muscles, GASL and SOL were the first muscles to be inhibited at −76.5 ± 16.14 ms and −69.95 ± 22.02 ms before the perturbation, respectively. This was followed by inhibition in the BF (−62.45 ± 12.02 ms) and ST (−61.45 ± 12.38 ms) muscles. The activity in the GASM and ESL muscles did not follow the sequence of activation; their inhibition occurred at −44.12 ± 17.80 ms and at −72.36 ± 12.36 ms, respectively. In the ventral muscles, RF, VM, and VL were the first muscles to be activated at −86.98 ± 12.50, −86.33 ± 28.73, and −84.16 ± 28.22 ms before the perturbation, respectively. This was followed by anticipatory activation in the EO (−73.33 ± 11.67 ms), TA (−57.58 ± 16.53 ms), and RA (−35.69 ± 9.64 ms) muscles.
Fig. 1.
Muscle activity onsets for dorsal (a) and ventral (b) muscles. Note that for predictable perturbations the onset of muscle activity occurred prior to the perturbation (T 0). In case of unpredictable perturbations, the muscle onsets occurred after the perturbation. Dorsal muscles: SOL (soleus), GASL (lateral gastrocnemius), GASM (medial gastrocnemius), BF (biceps femoris), ST (semitendinosus), and ESL (erector spinae longus). Ventral muscles: TA (tibilais anterior), RF (rectus femoris), VM (vastus medialis), VL (vastus lateralis), EO (external oblique), and RA (rectus abdominis). Differences in latencies between predictable and unpredictable conditions are significant for all muscles shown, p < 0.01
None of the 13 studied muscles showed changes in their background activity before the pendulum impact during the series with the unpredictable perturbations; instead, all the muscles became active after the perturbation impact. The sequence of activation of muscles was from proximal to distal in the dorsal muscles; however a distinct pattern was not seen in the ventral muscles. In the dorsal muscles, ESL and ST were the first muscles to show compensatory activity with onsets at 99.33 ± 20.01 and 99.29 ± 16.05 ms after the perturbation, respectively. This was followed by onsets in BF (103.97 ± 12.35 ms), GASL (109.61 ± 7.88 ms), GASM (109.84 ± 5.22 ms), and SOL (126.98 ± 13.93 ms) muscles. In the ventral muscles, RF was the first to get activated at 77.37 ± 3.34 ms, followed by EO at 80.88 ± 2.75 ms, VM at 83.63 ± 0.31 ms, TA at 84.51 ± 5.05 ms, VL at 93.37 ± 6.31 ms, RA at 98.60 ± 9.88 ms, and GMED at 112.38 ± 9.44 ms after the perturbation. The differences in the onsets of muscles between predictable and unpredictable conditions were statistically significant for all muscles, except GMED (p < 0.01).
Integrated electromyographic activity
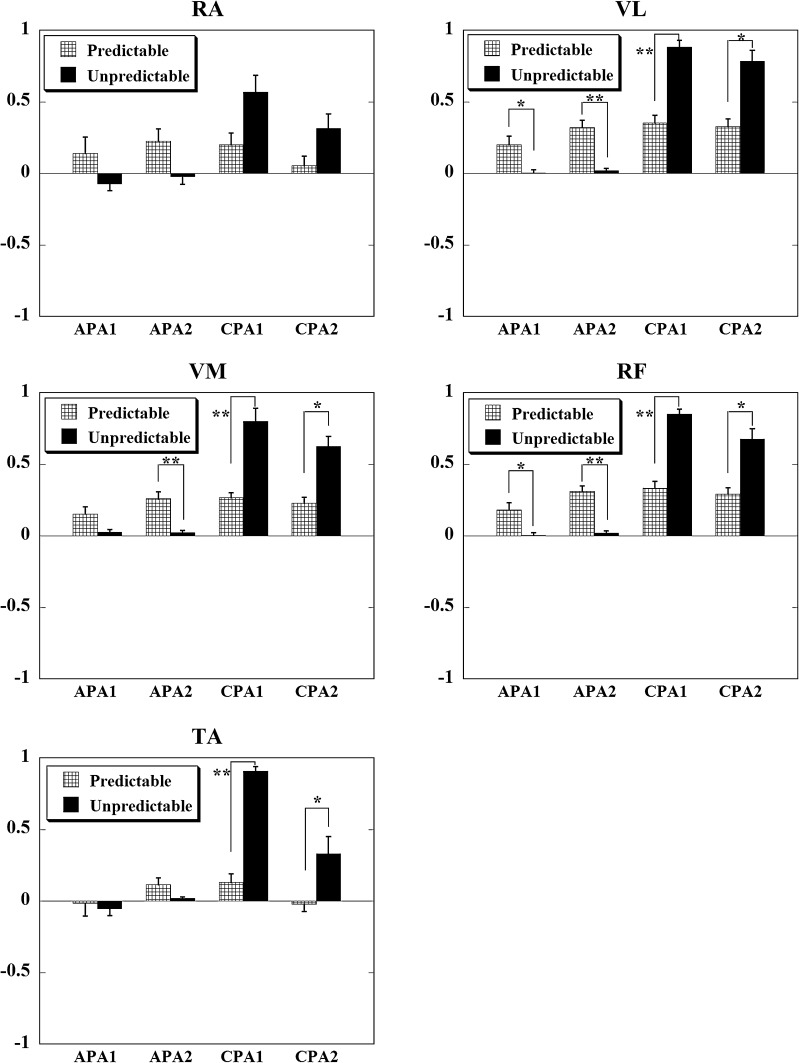
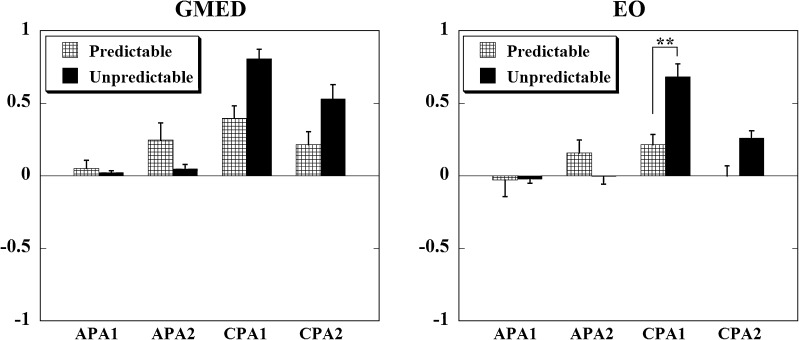
Integrals of EMG activity (IEMGNORM) of the ventral muscles during predictable and unpredictable perturbations are shown in Fig. 2 while the IEMGNORM for the lateral muscles are shown in Fig. 3. A significant main effect for condition × epoch interaction was seen for the IEMGNORM in seven muscles, namely: TA (p < 0.001), RF (p < 0.001), VM (p < 0.001), VL (p < 0.001), RA (p < 0.01), EO (p < 0.001), and GMED (p < 0.001). In the series with unpredictable perturbations, IEMGNORM observed prior to the perturbation onset during both, the APA1 and APA2 epochs, were negligible for all the muscles. Instead, large IEMGNORM could be seen after the pendulum impact. On the other hand, in series with predictable perturbations, larger IEMGNORM were observed prior to the perturbation onset during both, the APA1 and APA2 epochs, in most of the muscles. At the same time, smaller IEMGNORM were seen after the pendulum impact. Similar patterns of muscle activity were seen for the GASM, BF, and ST muscles, however, the differences between conditions were not significant.
Fig. 2.
Normalized integrated EMG activities in older adults during the four epochs (APA1, APA2, CPA1, and CPA2) are shown for the ventral muscles: RA (rectus abdominis), VL (vastus lateralis), VM (vastus medialis), RF (rectus femoris), and TA (tibialis anterior). Positive values indicate an activation of the muscle, while negative values indicate a muscle inhibition. *p < 0.01 and **p < 0.001
Fig. 3.
Normalized integrated EMG activities in older adults during the four epochs (APA1, APA2, CPA1, and CPA2) are shown for the lateral muscles: GMED (gluteus medius) and EO (external oblique). Positive values indicate an activation of the muscle, while negative values indicate a muscle inhibition. **p < 0.001
Post hoc analysis revealed significant differences for five muscles. The IEMGNORM during the APA1 epoch for the unpredictable condition was significantly smaller than the predictable condition for RF (p < 0.01) and VL (p = 0.01) muscles, whereas during the APA2 epoch, the same pattern (smaller muscle activity for the unpredictable than the predictable condition) was significant in RF (p < 0.001), VM (p < 0.001), and VL (p < 0.001) muscles. For the RA muscle, the difference in the anticipatory muscle activity between the two conditions was close to significance for the APA2 epoch (p = 0.015). Consequently, the IEMGNORM during the CPA1 epoch for the unpredictable condition was significantly larger than for the predictable condition for TA (p < 0.001), RF (p < 0.001), VM (p < 0.001), VL (p < 0.001), and EO (p < 0.001) muscles, whereas during the CPA2 epoch, the same pattern was significant in TA (p < 0.01), RF (p < 0.01), VM (p < 0.01), and VL (p < 0.01) muscles. For the RA and GMED muscles, the difference in the compensatory muscle activity between the two conditions was close to significance for the CPA1 epoch (p = 0.017 for both the muscles).
Post hoc analysis comparing the four epochs within the unpredictable condition showed that the IEMGNORM during both, the APA1 and APA2 epochs, was significantly smaller (negligible) than that during both, the CPA1 and CPA2 epochs, for RF, VM, VL, RA, EO, and GMED muscles (p < 0.001 for all comparisons) and significantly smaller than only the CPA1 epoch for TA muscle (p < 0.001). Also, for the unpredictable condition, the IEMGNORM during the CPA1 epoch was significantly larger than that during the CPA2 epoch for TA (p < 0.001) and EO (p = 0.001) muscles.
Post hoc analysis comparing the four epochs within the predictable condition showed that, the IEMGNORM during the APA2 epoch was significantly larger than that during the CPA2 epoch for the EO muscle (p = 0.005) and the muscle activity for GMED during the APA1 epoch was smaller than the CPA1 epoch (p = 0.009, close to significance). Also, for the predictable condition, the IEMGNORM during the CPA1 epoch was significantly larger than that during the CPA2 epoch for TA and RA muscles (p = 0.001 for both the muscles).
Displacements of center of pressure and center of mass
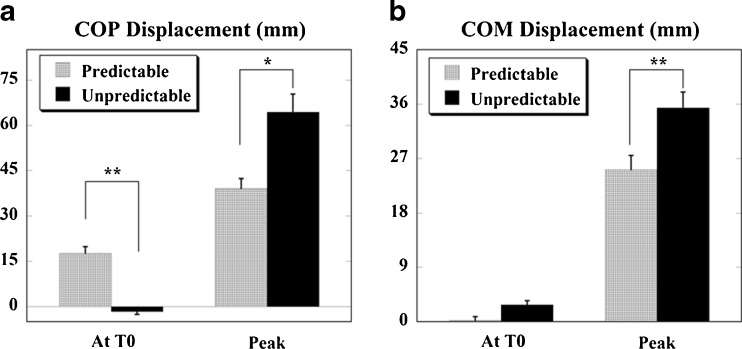
Displacements of COP and COM at T0 and the peak displacements after T0 for the predictable and unpredictable conditions are shown in Fig. 4. It can be seen in Fig. 4a that there was minimal anticipatory COP displacement (at T0) during unpredictable condition (−1.62 ± 0.00 mm) as compared to the predictable condition (17.62 ± 2.21 mm); the difference was statistically significant (p < 0.001). At the same time, the peak displacement was larger (almost 1.5 times greater) for the unpredictable perturbation (64.36 ± 5.97 mm) than for the predictable perturbation (39.12 ± 3.22 mm); the difference was also statistically significant (p = 0.019).
Fig. 4.
Displacements of COP (a) and COM (b) at T 0 (anticipatory) and peak displacements (after T 0, compensatory) for predictable and unpredictable perturbations are shown. Positive values indicate displacement in the posterior/backward direction and negative values indicate displacement in the anterior/forward direction. **p < 0.001 and *p < 0.05
The anticipatory COM displacement (Fig. 4b) for unpredictable condition (2.82 ± 0.65 mm) was higher than that for predictable condition (0.23 ± 0.60 mm); the difference was close to significance (p = 0.05). The peak COM displacement was significantly larger for the unpredictable condition (35.41 ± 2.61 mm) as compared to the predictable condition (25.13 ± 2.36 mm); the difference was statistically significant (p < 0.001). During the predictable condition, the COP moved in the posterior direction (i.e., in the direction of perturbation) prior to the perturbation and continued moving in the same direction after the perturbation. However, the COM moved backwards (i.e., in the direction of perturbation) prior to the perturbation (as opposed to movement in the forward direction, in preparation for the impact in young adults that we described previously—(Santos et al. 2010b)) and continued moving in the same direction after the impact. During the unpredictable condition, the COP moved slightly forwards (i.e., opposite to the direction of perturbation) prior to the impact and then moved backwards (i.e., in the direction of perturbation) after the impact, whereas the COM moved backwards prior to the impact and continued moving in the same direction after the impact (Fig. 4b).
The COP was the first to reach its peak after the impact for both, unpredictable and predictable perturbations. The time of the peak COP displacement for unpredictable perturbation was 345.14 ± 36.85 ms and for predictable perturbation it was 313.14 ± 48.54 ms after T0. The COM reached its peak after the COP; its times were 510 ± 4.26 ms for unpredictable perturbation and 564.3 ± 3.30 ms after T0 for predictable perturbation. The time when the COP and COM variables reached their peaks was not significantly different between the two conditions.
Discussion
Age-related changes in postural control mechanisms have been consistently documented (Woollacott and Shumway-Cook 1990; Rogers et al. 1992; Woollacott and Manchester 1993; Frank and Patla 2003; Lin et al. 2004; Bleuse et al. 2006; Horak 2006; Shaffer and Harrison 2007; Sturnieks et al. 2008; Claudino et al. 2013). However, anticipatory and compensatory postural control strategies used by the elderly have largely been investigated independently, as such the interaction between these mechanisms is not clearly understood. This study was focused on investigating the effect of aging on the utilization of anticipatory postural control strategies in preserving postural stability following a perturbation. It was hypothesized that older adults would exhibit an interaction of APAs and CPAs where a lack of APAs would be associated with larger CPAs and larger peak displacements of COP and COM following a perturbation. Likewise, the presence of APAs would result in lower magnitude of CPAs and smaller peak COP and COM displacements following the perturbation. Overall, the study hypotheses were supported such that the ability to utilize anticipatory postural adjustments in compensatory control of posture is retained in the elderly.
In the present study, all muscles in the older adults showed anticipatory muscle activity when the perturbations were predictable. These results suggest that aging itself does not seem to affect anticipatory recruitment of postural muscles. This finding is in line with previous literature (Rogers et al. 1992; Garland et al. 1997; Bleuse et al. 2006; Claudino et al. 2013), in fact, one study demonstrated that the elderly recruit their lower limb and trunk muscles prior to a focal movement as frequently as (96–100 % of trials) the young subjects (Rogers et al. 1992). Thus, it appears that deficits in postural control with aging may not be due to an absence of APAs per se, which have otherwise been found to be sometimes absent in individuals with neurological conditions associated with aging such as Parkinson’s disease (Bazalgette et al. 1987). Moreover, the present study also illustrated the ability of older adults to effectively utilize APAs for postural control, when the APAs are available. Thus when the perturbation was predictable, muscles were recruited prior to the perturbation, as opposed to being activated after the impact when the perturbation was unpredictable. Also, a distal to proximal sequence of anticipatory recruitment was maintained for the dorsal muscles in the older adults during the predictable perturbation. This pattern of recruitment is similar to that observed in healthy young adults during similar external perturbations (Santos et al. 2010a). The ventral muscles, however, depicted a different strategy wherein the knee extensor muscles were activated prior to the hip and leg muscles. Such differences in anticipatory strategies have also been reported in the literature on self-generated perturbations, suggesting that a lack of stability induced by delayed postural preparation in the elderly is compensated for by different muscle strategies (Inglin and Woollacott 1988). On the other hand, in the present study, when the perturbation was unpredictable, the dorsal muscles in the older adults were recruited after the impact in a proximal to distal sequence, similar to the pattern seen in young adults (Santos et al. 2010a). However, the ventral muscles did not show a clear sequence of activation in older adults as opposed to the same proximal to distal sequence seen in young adults (Santos et al. 2010a).
The interplay between the anticipatory and compensatory postural control mechanisms (in terms of magnitude of muscle activity) was also maintained in older adults and the relationship was similar to the one we observed in young adults (Santos et al. 2010a). For older adults in the series with unpredictable perturbations, IEMGNORM observed prior to the perturbation onset during both, the APA1 and APA2 epochs, were negligible for all the muscles. Instead, large IEMGNORM could be seen after the pendulum impact, especially during the first compensatory phase (CPA1 epoch) when compared with the second compensatory phase (CPA2 epoch). On the other hand, in series with predictable perturbations, larger IEMGNORM were observed prior to the perturbation onset during both, the APA1 and APA2 epochs (greater for APA2 epoch), in most of the muscles. At the same time, smaller IEMGNORM were seen after the pendulum impact. Similar patterns of muscle activity have been reported in the elderly subjects exposed to external perturbations in the lateral plane (Claudino et al. 2013).
In the present study, a reciprocal pattern of activation was seen before and after T0 during the predictable perturbations. Within this pattern, dorsal muscles were generally inhibited whereas ventral and lateral muscles were activated. During unpredictable perturbations a co-activation pattern was seen during the CPA1 epoch between the thigh and the trunk muscle pairs, whereas during the CPA2 epoch a reciprocal pattern was seen (dorsal muscles inhibited and ventral and lateral muscles remained activated). The distal leg muscle pair showed more of a reciprocal strategy during the CPA1 and CPA2 epochs (SOL, GASM were inhibited and GASL and TA were activated). Thus, when the perturbations were unpredictable, the older adults chose to increase the stiffness of the upper body while a reciprocal strategy was used to control the ankle joint during the CPA1 epoch. During the CPA2 epoch, a reciprocal pattern was used across all the joints. Thus, it seems that in order to maintain the vertical head–trunk orientation during the unpredictable perturbation, older adults initially employed a more conservative strategy of increasing the trunk stiffness. Similar strategies of increasing joint stiffness with co-activation of trunk and thigh muscles in response to a perturbation have been previously reported in healthy young and old individuals (Allum et al. 1989; Berger et al. 1992; Nagai et al. 2011) and in individuals with Parkinson’s disease (Dimitrova et al. 2004).
Larger compensatory reactions were seen during the unpredictable perturbations because these conditions were not associated with any anticipatory activity. However, when the perturbations were predictable, the subjects generated strong APAs which resulted in significantly smaller compensatory reactions. Changes in EMG activity were also associated with differences in COP and COM displacements between the two conditions. Thus, when anticipatory EMG activity was present (predictable perturbation); larger COP displacements were seen in preparation to the perturbation, but COM movement was minimal. This was associated with significantly smaller peak COP and COM displacements after the perturbation. On the contrary, in the absence of anticipatory EMG activity (unpredictable perturbation), significantly larger peak COP and COM displacements were seen following the perturbation, indicating greater postural instability. These findings highlight the critical role played by anticipatory postural mechanisms in dealing with balance perturbations in the elderly. The important role of APAs in preserving stability was also confirmed in a recent study on unilateral platform perturbations in the elderly. It was demonstrated that an inability to produce APAs relates to an increased likelihood of falls in the elderly, whereas elderly subjects with APAs show no differences in stability as compared to young adults (Hyodo et al. 2012). Thus, in the present study the observed changes in the electrical activity of muscles, COP, and COM displacements suggest that in spite of aging, the CNS of the older adults is capable of assessing the effect of involvement of APAs and generates or scales down CPAs accordingly to improve postural stability.
Conclusion
Anticipatory postural adjustments are delayed and reduced in magnitude with aging. Nonetheless, while APAs are impaired in older adults, the ability to recruit muscles anticipatorily is largely preserved. Additionally, the use of APAs in older adults significantly reduces the need for large CPAs resulting in greater postural stability following a perturbation. Therefore, the age-related decline in control of posture is not due to an inability to utilize anticipatory postural adjustments, per se. The CNS of the older adults is capable of assessing the involvement of APAs, when available, and is also able to effectively employ them in enhancing postural stability. Therefore, improvements in anticipatory postural strategies with training can be a potential focus of balance rehabilitation in people with postural instability due to ageing or neurological disorders. The outcomes of the current study provide important information for the future investigations of the role of anticipatory and compensatory mechanisms of postural control during maintenance of posture in more destabilizing dynamic conditions.
Acknowledgments
This work was supported in part by the National Institutes of Health grant #HD064838. We also thank Vennila Krishnan for help in data collection and Xiaoyan Li for assistance in data processing.
References
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH, Honegger F, Pfaltz CR. The role of stretch and vestibulo-spinal reflexes in the generation of human equilibrating reactions. Prog Brain Res. 1989;80:399–409. doi: 10.1016/S0079-6123(08)62236-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Bazalgette D, Zattara M, Bathien N, Bouisset S, Rondot P. Postural adjustments associated with rapid voluntary arm movements in patients with Parkinson’s disease. Adv Neurol. 1987;45:371–374. [PubMed] [Google Scholar]
- Belen’kii VE, Gurfinkel VS, Pal’tsev EI. Control elements of voluntary movements. Biofizika. 1967;12:135–141. [PubMed] [Google Scholar]
- Berger W, Trippel M, Discher M, Dietz V. Influence of subjects’ height on the stabilization of posture. Acta Otolaryngol. 1992;112:22–30. doi: 10.3109/00016489209100778. [DOI] [PubMed] [Google Scholar]
- Bleuse S, Cassim F, Blatt JL, Labyt E, Derambure P, Guieu JD, Defebvre L. Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture. 2006;24:203–210. doi: 10.1016/j.gaitpost.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech. 1987;20:735–742. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Claudino R, dos Santos EC, Santos MJ. Compensatory but not anticipatory adjustments are altered in older adults during lateral postural perturbations. Clin Neurophysiol. 2013;124:1628–1637. doi: 10.1016/j.clinph.2013.02.111. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol. 2004;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):13–22. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- Frank JS, Patla AE. Balance and mobility challenges in older adults: implications for preserving community mobility. Am J Prev Med. 2003;25:157–163. doi: 10.1016/S0749-3797(03)00179-X. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Arch Phys Med Rehabil. 1997;78:1072–1077. doi: 10.1016/S0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- Hyodo M, Saito M, Ushiba J, Tomita Y, Minami M, Masakado Y. Anticipatory postural adjustments contribute to age-related changes in compensatory steps associated with unilateral perturbations. Gait Posture. 2012;36:625–630. doi: 10.1016/j.gaitpost.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. J Gerontol. 1988;43:M105–M113. doi: 10.1093/geronj/43.4.M105. [DOI] [PubMed] [Google Scholar]
- Kanekar N, Aruin AS (2014) The effect of aging on anticipatory postural control. Exp Brain Res. doi:10.1007/s00221-014-3822-3 [DOI] [PMC free article] [PubMed]
- Lin SI, Woollacott MH, Jensen JL. Postural response in older adults with different levels of functional balance capacity. Aging Clin Exp Res. 2004;16:369–374. doi: 10.1007/BF03324566. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing. 2006;35(Suppl 2):ii12–ii18. doi: 10.1093/ageing/afl078. [DOI] [PubMed] [Google Scholar]
- Man’kovskii NB, Mints AY, Lysenyuk VP. Regulation of the preparatory period for complex voluntary movement in old and extreme old age. Hum Physiol (Moscow) 1980;6:46–50. [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–M127. doi: 10.1093/geronj/44.5.M118. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-C. [DOI] [PubMed] [Google Scholar]
- Merom D, Pye V, Macniven R, van der Ploeg H, Milat A, Sherrington C, Lord S, Bauman A. Prevalence and correlates of participation in fall prevention exercise/physical activity by older adults. Prev Med. 2012;55:613–617. doi: 10.1016/j.ypmed.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Nagai K, Yamada M, Uemura K, Yamada Y, Ichihashi N, Tsuboyama T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch Gerontol Geriatr. 2011;53:338–343. doi: 10.1016/j.archger.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Cordo PJ. Relation of automatic postural responses and reaction-time voluntary movements of human leg muscles. Exp Brain Res. 1981;43:395–405. doi: 10.1007/BF00238382. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. J Gerontol. 1992;47:M159–M165. doi: 10.1093/geronj/47.5.M159. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010;20:388–397. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87:193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Beyer N. Exercise and injury prevention in older people. Scand J Med Sci Sports. 2003;13:77–85. doi: 10.1034/j.1600-0838.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467–478. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J Gerontol. 1993;48:M64–M70. doi: 10.1093/geronj/48.2.M64. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A. Changes in posture control across the life span—a systems approach. Phys Ther. 1990;70:799–807. doi: 10.1093/ptj/70.12.799. [DOI] [PubMed] [Google Scholar]