Abstract
Aortic stiffness, assessed by carotid-to-femoral pulse wave velocity (PWV), often fails to predict cardiovascular (CV) risk and mortality in the very elderly. This may be due to the non-linear association between PWV and compliance or to blood pressure decrease in the frailest subjects. Total arterial compliance (CT) is the most relevant arterial property regarding CV function, compared to local or regional arterial stiffness. A new method for CT estimation, based on PWV, was recently proposed. We aimed to investigate the value of CT to predict all-cause mortality at the elderly. PWV was estimated in 279 elderly subjects (85.5 ± 7.0 years) who were followed up for a mean period of 12.8 ± 6.3 months. CT was estimated by the formula CT = k × PWV−2; coefficient k is body-size dependent based on previous in silico simulations. Herein, k was adjusted for body mass index (BMI) with a 10 % change in BMI corresponding to almost 11 % change in k. For a reference BMI = 26.2 kg/m2, k = 37. Survivors (n = 185) and non-survivors (n = 94) had similar PWV (14.2 ± 3.6 versus 14.9 ± 3.8 m/s, respectively; p = 0.139). In contrast, non-survivors had significantly lower CT than survivors (0.198 ± 0.128 versus 0.221 ± 0.1 mL/mmHg; p = 0.018). CT was a significant predictor of mortality (p = 0.022, odds ratio = 0.326), while PWV was not (p = 0.202), even after adjustment for gender, mean pressure and heart rate. Age was an independent determinant of CT (p = 0.016), but not of PWV. CT, estimated by a novel method, can predict all-cause mortality in the elderly. CT may be more sensitive arterial biomarker than PWV regarding CV risk assessment.
Keywords: Arterial stiffness, Pulse wave velocity, Distensibility, Elasticity, Aorta, Cardiovascular risk
Introduction
In our days, population ageing is an unprecedented phenomenon. In almost every country, especially the industrialized ones, the proportion of people over 60 years is growing faster than any other age group, as a result of both longer life expectancy and declining birth rates. Several parameters have been identified as risk factors for mortality in frail elderly populations. Many great discoveries regarding arterial macro- and microcirculation from the antiquity (Karamanou and Androutsos 2010; Androutsos et al. 2012) as well as recent knowledge gained from prospective clinical studies have led to the appreciation that arterial ageing and impairment of physiological mechanical properties of arterial walls (i.e. stiffening) are indisputably predisposing factors for increased cardiovascular (CV) risk and mortality rates. However, in elderly and other populations, there is a controversy regarding the prognostic value of arterial stiffness as assessed by existing methodologies (Megnien et al. 1998; Meaume et al. 2001; Protogerou et al. 2007; Verwoert et al. 2012).
Compliance is a main biomechanical property of the arterial walls with established hemodynamic and pathophysiological relevance. Arterial compliance is the ability of the arterial wall to distend and increase volume with increasing transmural pressure. Arterial compliance as a property is the inverse of arterial stiffness. The classic definition of arterial compliance (C) is the change in arterial blood volume (ΔV) due to a given change in arterial blood pressure (ΔP); thus, C = ΔV / ΔP (Spencer and Dennison 1963). When the left ventricle (LV) ejects into a compliant system, a slower rise in systolic pressure for a given stroke volume causes a lower wall stress and a lower oxygen consumption (McVeigh et al. 2007); namely, LV performance is maintained at lower energetic cost (Kolh et al. 2000). Furthermore, the stored blood volume during cardiac systole, by the distensible arterial walls, contributes to the maintenance of blood flow during cardiac diastole. On the other hand, a decrease in total arterial compliance (CT) can cause an enhancement in pressure and flow wave amplitude and increase LV load (Kelly et al. 1992; Papaioannou et al. 2003; Mottram et al. 2005). Coronary perfusion may be also affected due the decline in diastolic pressure. In parallel, CT reduction results to an increase in pressure wave speed, and consequently, the return of reflected waves at central aorta occurs during early systole, leading to an augmentation of peak systolic pressure and pulse pressure.
Several methods and techniques have been proposed for the non-invasive estimation of compliance of (1) a specific arterial location which is mainly assessed by ultrasound-based techniques (Stefanadis et al. 1990; Li and Khir 2011), (2) a whole arterial segment or region which is most commonly assessed by carotid-to-femoral pulse wave velocity (Van Bortel et al. 2012) and (3) the entire arterial tree (Stergiopulos et al. 1995). In a theoretical and physiological basis, the most relevant arterial property, in respect to cardiac function and ventriculo-arterial coupling, is CT rather than the stiffness of a single arterial location or segment, since CT is a main parameter that influences aortic input impedance (Westerhof et al. 2010). Most methods of CT estimation require both pressure and flow wave recording at the aorta (Stergiopulos et al. 1995, 1999; Segers et al. 1999). Consequently, these techniques are complex in their use with limited applicability in clinical research.
Thus, up to now, measurement of carotid-to-femoral pulse wave velocity (PWV) has prevailed in clinical practice as the most broadly applied method for the assessment of arterial stiffness. A great body of evidence and data indicates that aortic stiffness is an independent predictor of CV risk and mortality in several populations (Laurent et al. 2001; Blacher et al. 2002; Mattace-Raso et al. 2006; Mitchell et al. 2010). Nevertheless, other studies failed to prove the prognostic value of PWV in elderly populations or subjects with increased arterial stiffness (Megnien et al. 1998; Protogerou et al. 2007; Verwoert et al. 2012). The rationale behind the limited prognostic value of PWV in the aforementioned studies may be due to (1) the non-linear association between PWV and compliance as previously suggested (Vardoulis et al. 2012), (2) the non-linear association between PWV and age (Taviani et al. 2011) or (3) decrease of blood pressure (BP) in the frailest subjects and BP dependence of PWV.
Recently, a new method for CT assessment was described (Vardoulis et al. 2012), based on the Bramwell-Hill theory (Bramwell and Hill 1922). This method allows the estimation of CT only from aortic PWV with a correction based primarily on body size. The aim of the present study was to investigate the ability of CT (estimated by this new method) to predict all-cause mortality in elderly people. Our hypothesis was that although, as previously published (Protogerou et al. 2007), aortic PWV fails to predict mortality in the elderly, CT may have a predictive ability to detect individuals at increased risk.
Methods
Study cohort
A total of 331 consecutive patients examined at the geriatric departments of Charles Foix and Emile Roux Hospitals, Ile de France from May 2000 to November 2001 were initially enrolled in the “PRonostic cardiovasculaire Optimisation Therapeutique En GERiatric Study” (PROTEGER study) (Protogerou et al. 2007; Zhang et al. 2010a, b). The inclusion criteria were as follows: age above 70 years old; history of CV disease including coronary heart disease, hypertension, cerebrovascular disease or any other CV events of the upper or lower limbs, abdominal or thoracic aorta or renal arteries; Mini Mental Status Examination greater than 15 of 30; absence of fatal disease with life expectancy up to 1 month; and willingness to give a written informed consent to participate in this study. Patients with cachexia (body mass index <17 kg/m2) and/or evolutive cancer and/or advanced renal failure (plasma creatinine >250 μmol/L) were excluded from the study.
The study protocol was approved by the Committee for the Protection of Human Subjects in Biomedical Research of Saint Germain Hospital (Ile de France). Written informed consent was obtained from all participants. Further details regarding the study population and protocol have been described previously (Protogerou et al. 2007; Zhang et al. 2010a, b, 2012), and only parameters relevant to the present analysis are presented here.
Assessment of aortic stiffness and wave reflections
All measurements were performed in the morning with each subject being at the supine position after 15 min of rest. Brachial BP was measured by the semi-automatic oscillometric device Dynamap (Kontron). Aortic hemodynamics was also estimated by the use of generalized transfer function and applanation tonometry of the radial artery (SphygmoCor, AtCor, Sydney, Australia). Wave reflections were assessed by using augmentation index (AIx) which was calculated by pulse wave analysis as previously described (Vlachopoulos and O'Rourke 2000).
Aortic PWV was estimated using the Complior apparatus (Alam Medical, Paris, France). The distance covered by the pulse wave was measured directly from the carotid to the femoral artery, and the transit time was estimated by a previously described foot-to-foot method (Asmar et al. 1995). The superficial distance covered by the pulse wave was measured directly from the carotid to the femoral artery. It should be note that this method for distance assessment may overestimate PWV by approximately 2 m/s (Van Bortel 2006).
Estimation of total arterial compliance
The following mathematical equation relating CT with aortic PWV was recently proposed, by using a theoretical approach based on the Bramwell-Hill theory (Bramwell and Hill 1922):
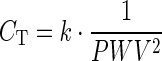 |
where k is a coefficient that accounts for the contribution of local geometry and wave speed of each arterial segment and it was determined in silico (k = 36.7 with 95 % confidence interval 36.2–37.2) (Vardoulis et al. 2012). Coefficient k was found to be primarily body-size-dependent. A good agreement regarding the proportionality of k values to the volume of the arterial tree was reported (Vardoulis et al. 2012); namely, an increase of arterial volume by 10 % (from a reference value of arterial volume) resulted in an 11.8 % increase in k value, while a 10 % volume reduction resulted in a 10.8 % decrease of k. Specifically, k values were 32.8, 36.9 and 41.2 for 10 % decrease, baseline and +10 % increase in arterial volume, respectively. For a reference arterial model that has been previously validated against human data (Reymond et al. 2009, 2011), baseline body mass index (BMI) was 26.23 kg/m2. For each subject, an individualized k value was calculated based on the above-mentioned correspondence between percent changes in size and k coefficient.
Coefficient k is principally dependent on geometry and, specifically, on the weighted sum of all arterial segment volumes (Vardoulis et al. 2012). We have shown in silico that there is a good agreement regarding the proportionality of the k values to the volume of the arterial tree (Vardoulis et al. 2012). Hence, we can assume that coefficient k is principally dependent upon body size as estimated by BMI, but of course, this needs to be verified by further studies either in silico or with in vivo measurements.
Statistics
Distribution of continuous variables was assessed for normality by using the Kolmogorov-Smirnov test. Variables that were not normally distributed were transformed when needed (i.e. logarithmic transformation). The bivariate association between continuous variables was examined by the Pearson correlation coefficient. Multiple stepwise regression analysis was performed to assess the independent determinants of AIx, PWV and CT. Differences in arterial biomarkers between survivors and non-survivors were evaluated by Student’s t test for independent samples. Cox regression analysis was performed to examine whether AIx, PWV and CT predict all-cause mortality. Stepwise models were also constructed to assess confounding effects of other parameters. Receiver-operator (ROC) curves analysis was used to determine the predictive value of AIx, PWV and CT. Paired comparisons of the areas under the ROC curves were also performed to examine whether CT is superior than PWV and AIx in mortality prediction. Statistical significance was accepted for p values lower than 0.05. All comparison tests were two-sided. Statistical analysis was performed by SPSS (SPSS Inc., IL, USA) and Stata (StataCorp LP, TX, USA).
Results
Data from a total of 279 elderly subjects were finally analyzed (subjects with missing data of either PWV, BMI or mortality were excluded). All subjects were followed up for an average period of 12.8 ± 6.3 months. A 33.7 % all-cause mortality was observed (94 subjects died during the 28 months of follow-up). Descriptive and hemodynamic characteristics of the study population are reported in Table 1.
Table 1.
Descriptive characteristics of the examined elderly population
| Cardiovascular risk factors | |
| Age (years) | 85.4 ± 7.0 |
| Gender (%, males) | 25.8 |
| Body mass index (kg/m2) | 26.9 ± 5.3 |
| Smoking (%) | 21.2 |
| Hypertension (%) | 76 |
| Diabetes (%) | 20.1 |
| Dyslipidemia (%) | 17.3 |
| Biochemical parameters | |
| Glucose (mmol/L) | 5.8 ± 2.2 |
| Total cholesterol (mmol/L) | 5.3 ± 1.2 |
| HDL (mmol/L) | 1.1 ± 0.3 |
| LDL (mmol/L) | 3.4 ± 1.0 |
| Triglycerides (mmol/L) | 1.6 ± 0.9 |
| Hemodynamic and vascular parameters | |
| Systolic BP (mmHg) | 132.5 ± 19.5 |
| Diastolic BP (mmHg) | 65.8 ± 12.1 |
| Mean BP (mmHg) | 91.3 ± 15.4 |
| Heart rate (b.p.m.) | 71.3 ± 12.5 |
| Augmentation index (%) | 27.8 ± 13.5 |
| PWV (m/s) | 14.4 ± 3.7 |
Data are mean ± standard deviation or percentages
BP blood pressure, HDL high-density lipoprotein, LDL low-density lipoprotein, PWV carotid-to-femoral pulse wave velocity
Three widely used surrogates of arterial biomechanical properties were assessed: wave reflections (AIx), aortic stiffness (carotid-to-femoral PWV) and the newly proposed index of total arterial compliance (CT). Parameters which are typically associated with the aforementioned biomarkers—such as age, gender, mean BP, heart rate and CV risk factors—were analyzed by linear regression analysis. AIx was significantly related to mean BP and heart rate, but not with age; PWV was correlated with mean BP and not with age; and finally, CT was significantly related to age and gender but not with mean BP (Table 2).
Table 2.
Linear regression analysis: dependent variables were aortic pulse wave velocity (PWV), aortic augmentation index (AIx) and total arterial compliance (C T) and independent variables were demographic and hemodynamic parameters as well as risk factors which are known to affect arterial biomechanical properties
| AIx (%) | PWV (m/s) | C T (mL/mmHg) | |
|---|---|---|---|
| Age (years) | −0.017 (0.788) | 0.015 (0.795) | −0.116 (0.068) * |
| Gender (females) | 0.098 (0.111) * | −0.104 (0.080) | 0.095 (0.138) * |
| Mean BP (mmHg) | 0.319 (<0.001) ** | 0.168 (0.005) ** | −0.105 (0.099) |
| Heart rate (b.p.m.) | −0.311 (<0.001) ** | 0.060 (0.327) | −0.028 (0.665) |
| Smoking (yes) | −0.148 (0.244) | −0.010 (0.933) | −0.004 (0.974) |
| Diabetes (yes) | −0.137 (0.026) | 0.096 (0.105) | −0.011 (0.869) |
| Dyslipidemia (yes) | 0.034 (0.557) | −0.061 (0.304) | 0.110 (0.084) |
Values correspond to beta coefficients (p values)
*p < 0.05; **p < 0.01 (indicate the independent determinants of the dependent variable in a stepwise multiple regression model)
In multivariate regression analysis, AIx was independently related to gender (AIx was higher in females than in males, p = 0.002), mean BP (p < 0.001) and heart rate (p < 0.001). The stronger independent determinant of PWV was mean BP (p = 0.007). Finally, CT was independently related to age (p = 0.016) and gender (females had higher total arterial compliance than males, p = 0.034).
AIx and PWV did not differ significantly between survivors and non-survivors (Table 3). On the contrary, non-survivors had significantly lower values of CT compared to survivors (p = 0.018, Table 3). BMI of survivors and non-survivors was 24.2 ± 5.0 versus 23.3 ± 4.7 kg/m2, respectively (p = 0.167).
Table 3.
Comparison of aortic pulse wave velocity (PWV), aortic augmentation index (AIx) and total arterial compliance (C T) between survivors and non-survivors
| Survivors | Non-survivors | p value | |
|---|---|---|---|
| AIx (%) | 142.9 ± 24.3 | 143.2 ± 20.4 | 0.912 |
| PWV (m/s) | 14.2 ± 3.6 | 14.9 ± 3.8 | 0.139 |
| C T (mL/mmHg) | 0.221 ± 0.1 | 0.198 ± 0.128 | 0.018 |
Values are presented as mean ± standard deviation (number of valid cases)
The ability of AIx, PWV and CT to predict all-cause mortality was first assessed by univariate Cox regression analysis. Among these three parameters, only CT had a significant predictive value (odds ratio 0.326, p = 0.022; Table 4).
Table 4.
Univariate Cox regression analysis of aortic pulse wave velocity (PWV), aortic augmentation index (AIx) and total arterial compliance (C T), for the prediction of mortality at the elderly
| Odds ratio | 95 % confidence intervals | p value | |
|---|---|---|---|
| AIx (%) | 0.998 | 0.990–1.007 | 0.686 |
| PWV (m/s) | 3.566 | 0.506–25.14 | 0.202 |
| C T (mL/mmHg) | 0.326 | 0.125–0.853 | 0.022 |
Total arterial compliance predicted significantly all-cause mortality, in this elderly population, even after adjustment for the effect of gender, mean BP and heart rate (Table 5). The statistical significance was marginally lost (p = 0.078) after adjustment for age. When all confounders (gender, mean BP, heart rate and age) were included in a single multivariate Cox regression model, the independent predictive value of CT was observed at a significance level 0.097 (Table 5).
Table 5.
Multivariate Cox regression analysis of aortic pulse wave velocity (PWV), aortic augmentation index (AIx) and total arterial compliance (C T), for the prediction of mortality at the elderly
| Model | Covariates | Dependent variable | ||
|---|---|---|---|---|
| AIx | PWV | C T | ||
| Model-1 | Gender | 0.99 (p = 0.814) | 3.25 (p = 0.241) | 0.33 (p = 0.023) |
| Model-2 | Mean BP | 0.99 (p = 0.767) | 3.75 (p = 0.19) | 0.32 (p = 0.019) |
| Model-3 | Heart rate | 0.99 (p = 0.736) | 3.55 (p = 0.212) | 0.32 (p = 0.022) |
| Model-4 | Age | 0.99 (p = 0.517) | 2.86 (p = 0.29) | 0.41 (p = 0.078) |
| Model-5 | Gender, mean BP | 1.00 (p = 0.952) | 2.13 (p = 0.472) | 0.43 (p = 0.097) |
| Heart rate and age | ||||
Odds ratio (p value)
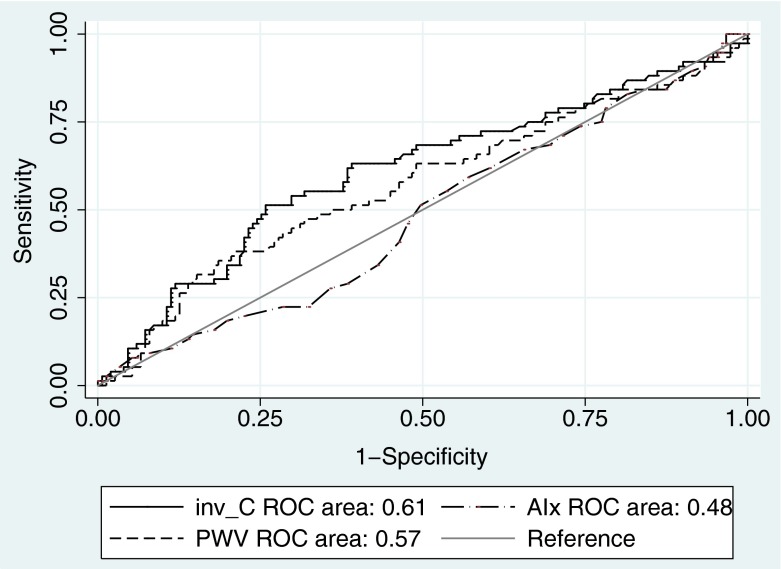
Receiver operator curve analysis (Fig. 1) showed that the area under the curve for CT was 0.61, for PWV 0.569 and for AIx 0.483. The area under the curve of CT was significantly higher than both PWV and AIx areas (p = 0.011 and p = 0.032, respectively).
Fig. 1.
Receiver-operator-curve analysis of aortic pulse wave velocity (PWV) and total arterial compliance (C T) for the prediction of all-cause mortality. In order to compare directly the areas under the two curves, C T was inversed so that high values of 1/C T, AIx and PWV have predictive value at the same direction
Discussion
This is the first study demonstrating the significant value of total arterial compliance to predict all-cause mortality in an elderly population, when at the same time the typical measurement of carotid-to-femoral PWV and pressure wave reflections did not. A recently proposed method of CT estimation (Vardoulis et al. 2012), using a modified approach of Bramwell-Hill theory, was applied on a previously investigated cohort of elderly people (Protogerou et al. 2007; Zhang et al. 2010a, b). It was revealed that CT, assessed by this technique, is a significant predictor of all-cause mortality, independently from gender, mean blood pressure and heart rate.
Currently, the “gold standard” method for the assessment of arterial stiffness is measurement of the transmission velocity of a pressure wave between two arterial sites which is performed non-invasively, easily and reproducibly by the existing technologies. In contrast, traditional methods for CT estimation are too complex since they require both blood pressure and velocity/flow recordings at the aorta. Most commonly, PWV is measured between carotid and femoral artery, providing a surrogate of aortic stiffness. Emerging evidence exists supporting that aortic PWV is an independent predictor of CV risk and mortality (Laurent et al. 2001; Blacher et al. 2002; Cruickshank et al. 2002). The inclusion of PWV in clinical practice, though, is still a conflicting issue. PWV measurement is proposed by the guidelines for the management of arterial hypertension of the European Society of Hypertension/European Society of Cardiology as a tool for assessment of subclinical target organ damage (Mancia et al. 2007). On the contrary, the guidelines for assessment of CV in asymptomatic adults, which were published in 2010 by the American College of Cardiology Foundation and the American Heart Association, raised several concerns which restrict, at the moment, the recommendation of arterial stiffness measurement for clinical research (Greenland et al. 2010).
Although PWV can predict CV risk and mortality in various populations, it has limited predictive value in situations where arterial stiffness is increased, as previously discussed (Vardoulis et al. 2012). In an analysis which was performed within the framework of the Rotterdam Study, it was investigated whether aortic stiffness (assessed by PWV) improves the prediction of coronary heart disease; 2,849 elderly subjects with mean age of 71.5 years and mean PWV of 13.3 m/s were examined, and it was shown that there is a low additional value of aortic stiffness in the clinical management of coronary heart disease (Verwoert et al. 2012). In another clinical study by Megnien et al. (1998), aortic PWV and distensibility did not predict coronary and extracoronary atherosclerosis in asymptomatic men at risk for CV disease. In the same direction, Matsusima et al. found that brachial-ankle PWV was not an independent predictor of coronary artery disease severity in a population of 205 patients (mean age 65 years) with increased PWV levels (16.4 ± 3.6 m/s) who underwent coronary angiography (Matsushima et al. 2004). Another study on the prediction of mortality by PWV at the elderly showed that aortic PWV was an independent predictor of cardiovascular and not all-cause mortality in a population of 141 patients older than 70 years (Meaume et al. 2001). However, only PWV values higher than 17.7 m/s could predict CV mortality in that study (Meaume et al. 2001).
The aforementioned studies have revealed a limitation of PWV to predict CV risk and mortality in the elderly or in subjects with increased arterial stiffness. A possible explanation for this may be due to the non-linear relationship between CT and PWV. Another potential explanation is the fact that BP is decreased in the frailest subjects while at the same time PWV is BP dependent and thus PWV’s predictive value may be confounded by BP levels.
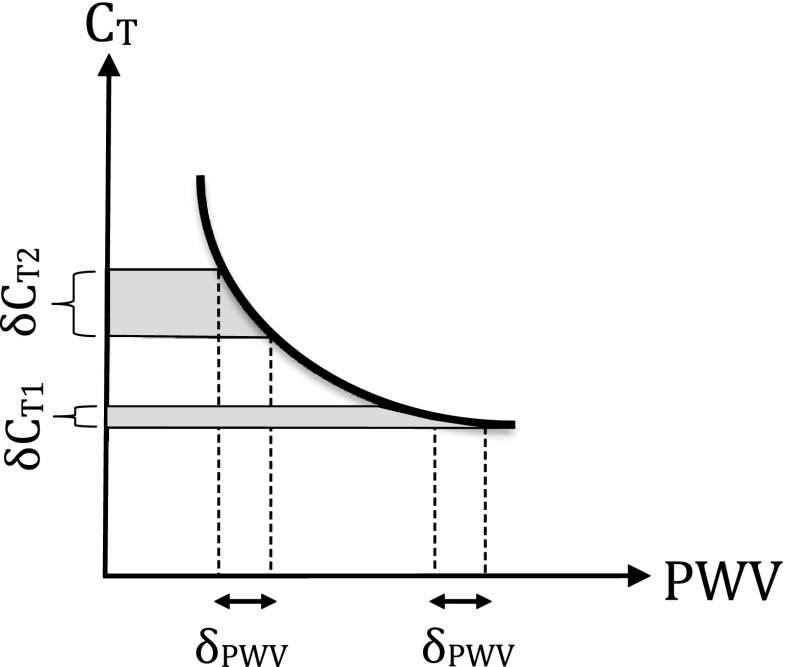
In this study, CT was found to predict all-cause mortality while PWV did not. The two aforementioned mechanisms can explain this finding. First, our approach for CT estimation takes into consideration the non-linear relationship between compliance and PWV. Thus, small differences in PWV among subjects correspond to greater differences in CT values (Fig. 2), rendering the latter parameter a more sensitive index. Secondly, although we verified the BP dependence of PWV in this population too, we also found that CT was not related significantly with mean BP. Therefore, mean BP likely confounds the predictive value of PWV, but it does not affect that much the respective predictive value of CT, in the examined elderly population. The latter finding was further supported by Cox regression analysis with both CT and mean BP as independent covariates in a multivariate model for the prediction of all-cause mortality.
Fig. 2.
Nonlinear relation between total arterial compliance (C T) and pulse wave velocity (PWV)
Another observation that might likely explain the superior prognostic value of CT compared to PWV was that CT better reflects the age-related effects on arterial properties; specifically, it was found that CT is significantly related to age, while PWV is not.
From a pathophysiological point of view, there is no doubt that CT is more relevant than regional or local arterial compliance (or their surrogate PWV), in terms of modulation of cardiac load, LV function, arterio-ventricular coupling and CV risk. The previously proposed method for CT estimation (Vardoulis et al. 2012) can be widely and even retrospectively used, providing a more close approximation of global systemic arterial compliance. Of course, this method has strengths and weaknesses that should be acknowledged. Its advantages rely on the use of a broad, easy and simple method that is clinically available for the assessment of aortic stiffness (namely carotid-to-femoral PWV) combined with a simple adjustment for body size (i.e. BMI). The accuracy and precision of this method were found to be quite high in a previous validation study (Vardoulis et al. 2012). The fact that BMI does not always represent the arterial size, which is directly involved in the estimation of k coefficient, is considered as a weakness of this technique. Another limitation of the study is the small sample size which bounded the significance level of the predictive value of CT (p value = 0.097) when more covariates were entered in the multiple Cox regression model. It is estimated that a larger population (~350 subjects) would be enough to provide a p value lower than 0.05 for the independent prediction of all-cause mortality by CT.
Finally, it should be mentioned that a previous relevant study of elderly women showed that systemic arterial compliance was not a significant predictor of cardiovascular events (Dart et al. 2006). The findings of the present study are not in line with those of that study which is mostly a result of major methodological differences between the two cohorts. In the study of Dart et al., only females were examined, while in this study, both genders were included. Furthermore, CT was estimated by a much more complex technique (which was based on both aortic flow and pressure waves recording) than the simple one used in this study. Finally, Dart et al. examined the ability of CT to predict “cardiovascular disease-free survival” and not total mortality per se. Since PWV was not measured in that study (Dart et al. 2006), direct comparison of the results of that cohort with the present one is not possible.
Conclusions
Population ageing challenges health care systems to maximize the effectiveness of diagnostic and prognostic strategies in these subjects, aiming to the better understanding and management of their risk for CV complications and death. In this direction, a small step towards a more accurate and timely assessment of CV risk and prediction of all-cause mortality in elderly populations may be achieved by the evaluation of total arterial compliance. This can be achieved by using a simple and non-invasive technique principally based on PWV and a modified Bramwell-Hill approach. The opinion of Sir William Osler (Canadian Physician, 1849–1919), one of the four founding professors of Johns Hopkins Hospital, best reflects the critical role of arterial properties on longevity: “Longevity is a vascular question, which has been well expressed in the axiom that man is only as old as his arteries”. The age of the arterial tree is definitely reflected by its global compliance which remains a challenging property from methodological and clinical perspective.
References
- Androutsos G, Karamanou M, Stefanadis C. William Harvey (1578-1657): discoverer of blood circulation. Hell J Cardiol. 2012;53:6–9. [PubMed] [Google Scholar]
- Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.HYP.26.3.485. [DOI] [PubMed] [Google Scholar]
- Blacher J, Safar ME, Pannier B, et al. Prognostic significance of arterial stiffness measurements in end-stage renal disease patients. Curr Opin Nephrol Hypertens. 2002;11:629–634. doi: 10.1097/00041552-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond B. 1922;93:298–306. doi: 10.1098/rspb.1922.0022. [DOI] [Google Scholar]
- Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.CIR.0000033824.02722.F7. [DOI] [PubMed] [Google Scholar]
- Dart AM, Gatzka CD, Kingwell BA, et al. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- Karamanou M, Androutsos G. Completing the puzzle of blood circulation: the discovery of capillaries. Ital J Anat Embryol. 2010;115:175–179. [PubMed] [Google Scholar]
- Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71:490–502. doi: 10.1161/01.RES.71.3.490. [DOI] [PubMed] [Google Scholar]
- Kolh P, D'Orio V, Lambermont B, et al. Increased aortic compliance maintains left ventricular performance at lower energetic cost. Eur J Cardiothorac Surg. 2000;17:272–278. doi: 10.1016/S1010-7940(00)00341-9. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Li Y, Khir AW. Experimental validation of non-invasive and fluid density independent methods for the determination of local wave speed and arrival time of reflected wave. J Biomech. 2011;44:1393–1399. doi: 10.1016/j.jbiomech.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- Matsushima Y, Kawano H, Koide Y, et al. Relationship of carotid intima-media thickness, pulse wave velocity, and ankle brachial index to the severity of coronary artery atherosclerosis. Clin Cardiol. 2004;27:629–634. doi: 10.1002/clc.4960271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Bank AJ, Cohn JN. Arterial compliance. In: Willerson JT, Cohn JN, Wellens HJJ, Holmes DR, editors. Cardiovascular medicine. London: Springer-Verlag; 2007. pp. 1811–1831. [Google Scholar]
- Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- Megnien JL, Simon A, Denarie N, et al. Aortic stiffening does not predict coronary and extracoronary atherosclerosis in asymptomatic men at risk for cardiovascular disease. Am J Hypertens. 1998;11:293–301. doi: 10.1016/S0895-7061(97)00477-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram PM, Haluska BA, Leano R, et al. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou TG, Mathioulakis DS, Tsangaris SG. Simulation of systolic and diastolic left ventricular dysfunction in a mock circulation: the effect of arterial compliance. J Med Eng Technol. 2003;27:85–89. doi: 10.1080/0309190021000043701. [DOI] [PubMed] [Google Scholar]
- Protogerou AD, Safar ME, Iaria P, et al. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension. 2007;50:172–180. doi: 10.1161/HYPERTENSIONAHA.107.089797. [DOI] [PubMed] [Google Scholar]
- Reymond P, Bohraus Y, Perren F, et al. Validation of a patient-specific one-dimensional model of the systemic arterial tree. Am J Physiol Heart Circ Physiol. 2011;301:H1173–H1182. doi: 10.1152/ajpheart.00821.2010. [DOI] [PubMed] [Google Scholar]
- Reymond P, Merenda F, Perren F, et al. Validation of a one-dimensional model of the systemic arterial tree. Am J Physiol Heart Circ Physiol. 2009;297:H208–H222. doi: 10.1152/ajpheart.00037.2009. [DOI] [PubMed] [Google Scholar]
- Segers P, Verdonck P, Deryck Y, et al. Pulse pressure method and the area method for the estimation of total arterial compliance in dogs: sensitivity to wave reflection intensity. Ann Biomed Eng. 1999;27:480–485. doi: 10.1114/1.192. [DOI] [PubMed] [Google Scholar]
- Spencer MP, Dennison AB. Pulsatile blood flow in the vascular system. In: Hamilton WF, editor. Handbook of physiology. Washington, DC: American Physiology Society; 1963. p. 842. [Google Scholar]
- Stefanadis C, Stratos C, Boudoulas H, et al. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–996. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- Stergiopulos N, Meister JJ, Westerhof N. Evaluation of methods for estimation of total arterial compliance. Am J Physiol. 1995;268:H1540–H1548. doi: 10.1152/ajpheart.1995.268.4.H1540. [DOI] [PubMed] [Google Scholar]
- Stergiopulos N, Segers P, Westerhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. Am J Physiol. 1999;276:H424–H428. doi: 10.1152/ajpheart.1999.276.2.H424. [DOI] [PubMed] [Google Scholar]
- Taviani V, Hickson SS, Hardy CJ, et al. Age-related changes of regional pulse wave velocity in the descending aorta using Fourier velocity encoded M-Mode. Magn Reson Med. 2011;65:261–268. doi: 10.1002/mrm.22590. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM. Is arterial stiffness ready for daily clinical practice? J Hypertens. 2006;24:281–283. doi: 10.1097/01.hjh.0000199805.03058.78. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- Vardoulis O, Papaioannou TG, Stergiopulos N. On the estimation of total arterial compliance from aortic pulse wave velocity. Ann Biomed Eng. 2012;40:2619–2626. doi: 10.1007/s10439-012-0600-x. [DOI] [PubMed] [Google Scholar]
- Verwoert GC, Elias-Smale SE, Rizopoulos D, et al. Does aortic stiffness improve the prediction of coronary heart disease in elderly? The Rotterdam Study. J Hum Hypertens. 2012;26:28–34. doi: 10.1038/jhh.2010.124. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, O'Rourke M. Genesis of the normal and abnormal arterial pulse. Curr Probl Cardiol. 2000;25:303–367. doi: 10.1067/mcd.2000.104057. [DOI] [PubMed] [Google Scholar]
- Westerhof N, Stergiopulos N, Noble MIM. Snapshots of hemodynamics: an aid for clinical research and graduate education. New York: Springer; 2010. [Google Scholar]
- Zhang Y, Agnoletti D, Iaria P, et al. Gender difference in cardiovascular risk factors in the elderly with cardiovascular disease in the last stage of lifespan: the PROTEGER study. Int J Cardiol. 2012;155:144–148. doi: 10.1016/j.ijcard.2011.09.073. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Safar ME, Iaria P, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in the elderly: the PROTEGER Study. Am Heart J. 2010;160:471–478. doi: 10.1016/j.ahj.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Safar ME, Iaria P, et al. Cardiac and arterial calcifications and all-cause mortality in the elderly: the PROTEGER Study. Atherosclerosis. 2010;213:622–626. doi: 10.1016/j.atherosclerosis.2010.09.020. [DOI] [PubMed] [Google Scholar]