Abstract
To evaluate the role of ceramide (Cer) in apoptosis signaling, we examined Cer formation induced by CD95, etoposide, or γ-radiation (IR) in relation to caspase activation and mitochondrial changes in Jurkat T cells. The Cer response to all three stimuli was mapped in between caspases sensitive to benzoyloxycarbonyl-VAD-fluoromethylketone (zVAD-fmk) and acetyl-DEVD-aldehyde (DEVD-CHO). Cer production was independent of nuclear fragmentation but associated with the occurrence of other aspects of the apoptotic morphology. Caspase-8 inhibition abrogated Cer formation and apoptosis induced by CD95 but did not affect the response to etoposide or IR, placing CD95-induced Cer formation downstream from caspase-8 and excluding a role for caspase-8 in the DNA damage pathways. CD95 signaling to the mitochondria required caspase-8, whereas cytochrome c release in response to DNA damage was caspase-independent. These results indicate that the caspases required for the Cer response to etoposide and IR reside at or downstream from the mitochondria. Bcl-2 overexpression abrogated the Cer response to etoposide and IR and reduced CD95-induced Cer accumulation. We conclude that the Cer response to DNA damage fully depends on mitochondrion-dependent caspases, whereas the response to CD95 partially relies on these caspases. Our data imply that Cer is not instrumental in the activation of inducer caspases or signaling to the mitochondria. Rather, Cer formation is associated with the execution phase of apoptosis.
Introduction
Elevation of intracellular ceramide (Cer) levels occurs in response to a variety of apoptotic stimuli, including ligation of death receptors and anti-cancer regimens (1). However, the mechanisms that convey activation signals to the enzyme responsible for Cer production are ill defined, and the actual contribution of Cer to the apoptotic response remains poorly understood.
Neutral and acid sphingomyelinases (SMases) have been implicated in the Cer response induced by tumor necrosis factor receptor-1 (TNFR-1) (2), CD95 (3, 4), γ-radiation (IR) (5), and anti-cancer drugs (6), while a role for increased de novo Cer synthesis in anti-cancer, drug-induced Cer formation is controversial (6, 7). Cer formation was shown to be secondary to caspase activation in the CD95 (8, 9), TNFR-1 (10), and Drosophila REAPER pathways (11). However, we have recently shown that CD95-induced Cer production still occurs when caspases and nuclear segmentation sensitive to acetyl-DEVD-aldehyde (DEVD-CHO) are inhibited (12). We therefore consider the possibility that Cer contributes to the effector phase of apoptosis.
Death receptors such as TNFR-1 and CD95 activate death effector domain (DED)–containing caspases via their cytoplasmic tail (13, 14), whereas death receptor–independent stimuli must induce initial caspase processing at other sites. Recent reports have indicated the importance of mitochondria for initiating caspase activity. Apoptotic stimuli can cause the release of mitochondrial cytochrome c (cyt c) into the cytosol by an as yet unknown mechanism (15–17). Studies in vitro (15, 18, 19) and in vivo (20, 21) suggest that cyt c, together with Apaf-1 and caspase-9 (casp-9), mediates the proteolytic activation of casp-3 in a dATP-dependent manner. The apoptosis-inhibitory protein Bcl-2 prevents the release of cyt c (16, 17), most likely by complex formation with Apaf-1 and casp-9 (22, 23). These observations have led to the hypothesis that the mitochondrial membrane, where Bcl-2 resides (24), is a site for activation of inducer caspases that subsequently process and activate executioner caspases, such as casp-3, -6, and -7 (25).
Involvement of the Bcl-2 family is now considered a hallmark for mitochondrial participation in the apoptotic pathway. Bcl-2 and Bcl-xL have a clear cytoprotective effect when apoptosis is induced by ΙR and chemotherapeutic drugs (26–28). In addition, the antagonistic family member Bax facilitates apoptosis induction by DNA-damaging regimens (29). However, the ability of the Bcl-2 family to regulate CD95-induced apoptosis is controversial (27, 29–32). Recently, it was proposed that the amount of activated casp-8 generated at the receptor may vary between cell types and may determine whether a mitochondrion-dependent pathway is used (type II cells) or not (type I cells) (30).
We have investigated how the Cer response relates to mitochondrial changes and caspase activation in Jurkat T cells after apoptosis induction by three distinct stimuli: CD95 ligation, the topoisomerase II inhibitor and anti-cancer drug etoposide (33), and IR. To establish causal relationships, we used peptide-based caspase inhibitors: the casp-8 inhibitory protein FLIPL (FLICE-inhibitory protein; ref. 34) and the mitochondrial regulator Bcl-2 (16, 17). We present evidence that Cer formation depends on inducer caspase activity and cyt c release but is independent of effector caspase activation. This excludes a role for Cer in inducer caspase activation or in conveying the apoptotic signal to the mitochondria.
Methods
Reagents.
L-[3-14C]serine (54 mCi/mmol) and an enhanced chemiluminescence kit were purchased from Amersham Pharmacia Biotech(Roosendahl, the Netherlands); the etoposide from Sigma Chemical Co. (St. Louis, Missouri, USA); and DEVD-CHO, benz-oyloxycarbonyl-VAD-fluoromethylketone (zVAD-fmk), and benzoyloxycarbonyl-LEHD-fluoromethylketone (zLEHD-fmk) were from Calbiochem-Novabiochem Corp. (San Diego, California, USA). Mouse anti–human CD95 monoclonal antibody (mAb) 7C11 was obtained from Immunotech (Marseille, France); anti–cyt c mAb 7H8.2C12 was purchased from PharMingen (San Diego, California, USA); and anti-actin mAb C4 from Boehringer Mannheim (Almere, the Netherlands). Horseradish peroxidase–conjugated rabbit anti–mouse Ig was purchased from DAKO A/S (Glostrup, Denmark). DiOC6(3) was obtained from Molecular Probes Inc. (Eugene, Oregon, USA).
Cells.
The J16 clone was derived by limiting dilution from the human T-acute lymphoblastic leukemia cell line Jurkat (12). The Jurkat cell line JFL2, stably transfected with FLIPL cDNA (34), was kindly provided by J. Tschopp (Institute of Biochemistry, University of Lausanne, Epalinges, Switzerland). Jurkat cells stably transfected with the human bcl-2 cDNA and empty vector control cells were described previously (31).
Cell culture and stimulation.
Cells were cultured as described previously (12). The bcl-2– and empty vector–transfected Jurkat cells received additional neomycin sulphate (G418) at 200 μg/ml. Before stimulation, cells were incubated overnight in synthetic Yssel’s medium (35) and resuspended in Yssel’s medium at 106 per ml in 96-well culture plates for apoptosis assays, or at 5–10 × 106 per ml in 24-well culture plates for caspase and cyt c assays. Cells were stimulated with medium, etoposide, or anti-CD95 mAb, or irradiated using a 137Cs source (2 × 415 Ci; Von Gahlen Nederland BV, Didam, the Netherlands), and incubated for various time periods at 37°C, 5% CO2.
Apoptosis and viability assays.
To measure nuclear fragmentation, cells were lysed in 0.1% sodium citrate, 0.1% Triton X-100, and 50 μg/ml propidium iodide (36). Fluorescence intensity of propidium iodide–stained DNA was determined in 5,000 cells on a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, California, USA), and data were analyzed using Lysys software (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). Fragmented, apoptotic nuclei are recognized by their subdiploid DNA content. To analyze loss of mitochondrial transmembrane potential (Δψm), cells (1–2 × 105) were incubated with DiOC6 (3) at 25 ng/ml in PBS for 10 min at 37°C in the dark followed by FACScan analysis (37). Cell viability was assessed by determining the ability of cells to exclude propidium iodide (10 μg/ml).
Preparation of cytosolic extracts and immunoblot analysis for cyt c.
Cells (5 × 106 per sample) were washed twice with ice-cold PBS and resuspended in 100 μl of extraction buffer (50 mM PIPES-KOH [pH 7.4], 220 mM mannitol, 68 mM sucrose, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, and protease inhibitors) and allowed to swell on ice for 30 min. Cells were homogenized by passing the suspension through a 25-gauge needle (10 strokes). Homogenates were centrifuged in a Beckman Airfuge (Beckman Instruments Nederland BV, Mijdrecht, the Netherlands) at 100,000 g for 15 min at 4°C, and supernatants were harvested and stored at –70°C until analysis by gel electrophoresis. Cytosolic protein (10 μg), as determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Munich, Germany), was loaded onto a 12% SDS–polyacrylamide gel (equivalents of ∼106 cells per lane). Proteins were transferred to nitrocellulose sheets (Schleicher and Schuell, Dassel, Germany). Blots were blocked with 5% (wt/vol) nonfat dry milk in PBS, 0.05% Tween-20, and probed with anti–cyt c mAb (1:2,000) and anti-actin mAb (1:3,000) in PBS, 0.05% Tween-20, 1% nonfat dry milk, followed by a 1:7,500 dilution of horseradish peroxidase–conjugated rabbit anti–mouse Ig, and developed by ECL.
Cer quantification.
Cellular Cer levels were determined essentially as described previously (12, 38). Cells (106 per ml) were metabolically labeled with [14C]serine (0.2 μCi/ml) in Yssel’s medium for 20–48 h, washed once with Yssel’s medium, and resuspended at 5 × 106 per ml in 24-well plates. After cell stimulation, total lipids were extracted with chloroform/methanol (1:2 vol/vol), and phases were separated using 20 mM HAc. Extracts were spotted on Silica Gel 60 TLC plates (Merck-Vel Nederland BV, Amsterdam, the Netherlands) and developed as described previously (12, 38). Radioactive lipids were made visible and quantitated using a Fujix BAS 2000 TR PhosphorImager (Fuji Photo Film Co., Tokyo, Japan) and identified using external lipid standards. Cer was expressed relative to total radioactivity in phosphatidylserine and phosphatidylethanolamine, both of which remained unaltered upon stimulation.
Results
Etoposide and IR induce Cer formation and apoptosis in Jurkat cells.
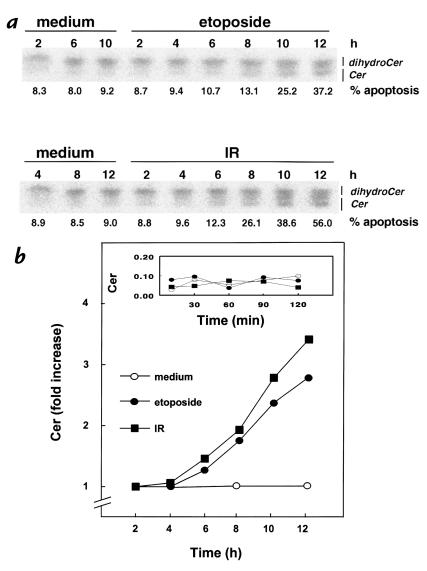
We demonstrated previously that CD95 ligation with a specific mAb induces Cer formation in Jurkat cells (J16), with kinetics closely paralleling nuclear fragmentation (12). Figure 1a shows that apoptosis induction by etoposide or IR is also accompanied by elevation of intracellular Cer levels, measured after metabolic labeling of cells with [14C]serine. The first significant increase in Cer levels could be observed after several hours (Figure 1b), shortly before nuclear changes became apparent. Note that no significant increase in Cer levels was observed up to two hours after exposure to etoposide or IR (Figure 1b, inset). The temporal relationship between Cer formation and the onset of nuclear apoptosis after these treatments resembles that observed earlier for CD95 stimulation (12).
Figure 1.
Etoposide and IR induce Cer formation and apoptosis. Jurkat T cells (J16) labeled until equilibrium with [14C]serine were exposed to etoposide (10 μg/ml) or IR (30 Gy), or left untreated (medium). At the indicated times, Cer levels and apoptosis were determined. (a) TLC separation of Cer from dihydroceramide. Apoptosis is expressed as percentage of nuclei with subdiploid DNA content. (b) Time course of changes in Cer levels induced by etoposide or IR. Cer data are expressed as -fold increase relative to control and are representative of three experiments. The inset shows Cer levels (expressed relative to total radioactivity in phosphatidylserine and phosphatidylethanolamine) at shorter time points. Cer, ceramide; IR, γ-radiation.
Cer formation induced by CD95, etoposide, and IR requires caspase activation.
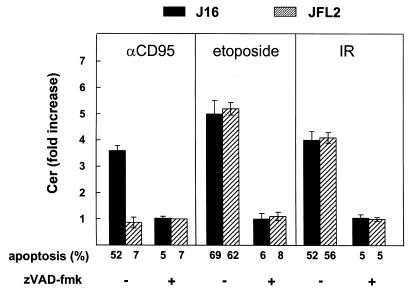
To evaluate whether caspase activation is required for Cer generation induced by CD95, etoposide, and IR, we made use of the synthetic peptide zVAD-fmk, an irreversible broad-specificity caspase inhibitor (25). In Jurkat cells (J16), stimulation with anti-CD95 mAb for six hours resulted in a 3.6-fold increase in Cer levels (Figure 2, filled bars), which was blocked in the presence of zVAD-fmk, in agreement with published data (8, 9). In response to etoposide or IR, cells showed an even higher elevation of Cer levels (Figure 2), which was also abrogated by zVAD-fmk. Thus, caspase activity is not only required for Cer formation upon CD95 ligation but also for Cer generation in response to DNA-damaging treatments.
Figure 2.
Role of caspases in CD95-, etoposide-, and IR-induced Cer accumulation and apoptosis. Control Jurkat cells (J16; filled bars) or Jurkat cells stably transfected with FLIPL cDNA (JFL2; hatched bars) (34) were treated with anti-CD95 mAb (200 ng/ml) for 6 h, or etoposide (10 μg/ml) or IR (30 Gy) for 16 h. Where indicated (+), cells were pretreated (2 h) and further incubated with 50 μM zVAD-fmk. Cer levels were quantitated and expressed as -fold increase relative to time-matched medium controls. Apoptosis sensitivity of J16 and JFL2 was determined in parallel and expressed as percentage hypoploid cells. Cer data are representative of two independent experiments and show mean ± SD from duplicate samples in one experiment. FLIPL, FLICE-inhibitory protein; mAb, monoclonal antibody; zVAD-fmk, benzoyloxycarbonyl-VAD-fluoromethylketone.
Cer formation is independent of effector caspases.
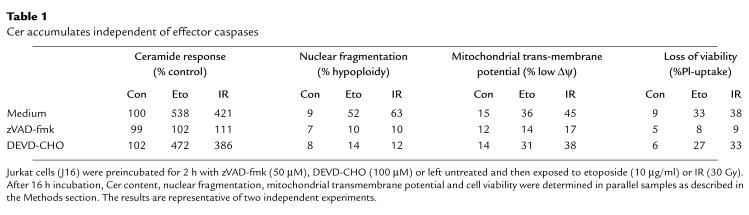
We established previously that a large proportion of the Cer response to CD95 ligation remains unaffected upon caspase inhibition by the reversible inhibitor peptide aldehyde DEVD-CHO (12). Since DEVD-CHO preferentially inhibits casp-3 and related effector caspases (23), this indicated that Cer accumulation is independent of effector caspase activation. Here, we investigated how etoposide- and IR-induced Cer production relate to effector caspases. In marked contrast to the general caspase inhibitor zVAD-fmk, DEVD-CHO only minimally affected Cer accumulation induced by etoposide or IR, whereas both inhibitors efficiently prevented nuclear segmentation (Table 1). However, whereas mitochondrial depolarization (Δψm) (37) upon exposure to etoposide and IR was completely prevented by zVAD-fmk, DEVD-CHO had almost no protective effect (Table 1). In addition, in contrast to zVAD-fmk, DEVD-CHO did not protect against the loss of membrane integrity, measured by propidium iodide uptake (Table 1).
Table 1.
Cer accumulates independent of effector caspases
These results indicate that Cer formation occurs downstream from inducer caspases yet is independent of DEVD-CHO–sensitive effector caspases. Because DEVD-CHO inhibited nuclear segmentation, but not Δψm collapse and membrane permeability, we conclude that Cer formation is associated with the occurrence of certain, but not all, aspects of the apoptotic morphology.
Role of casp-8 in Cer formation and apoptosis.
To identify the caspase(s) upstream of Cer formation, we first examined the requirement for casp-8 activation in CD95-, etoposide- and IR-induced Cer formation and apoptosis using Jurkat cells stably transfected with FLIPL (JFL2; ref. 34). FLIPL is structurally similar to casp-8: it has two DEDs and a caspase-like domain but lacks the active-site cysteine that renders it catalytically inactive. Both viral and cellular FLIPs interact via their DED with homologous domains in FADD and casp-8 (potentially also in casp-10) and therewith inhibit apoptosis induced by CD95 and other death receptors that signal via DED-containing caspases (34, 39–41). CD95-induced Cer accumulation was completely blocked in the FLIPL transfectant JFL2 (Figure 2, hatched bars). In contrast, Cer production in response to etoposide or IR was unaffected by FLIPL (Figure 2). zVAD-fmk prevented DNA damage–induced Cer accumulation in JFL2 cells, just like it did in control cells. CD95-induced apoptosis was completely blocked in JFL2, as shown previously (34), whereas treatment of these cells with etoposide or IR resulted in comparable levels of apoptosis as observed in Jurkat control cells (Figure 2). Thus, casp-8 activity is required for CD95-induced Cer accumulation and apoptosis but not for Cer formation and apoptosis induced by etoposide or IR.
Role of caspases in signaling to the mitochondria.
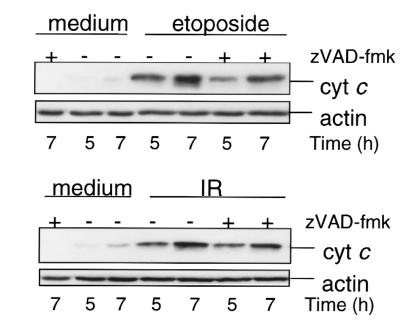
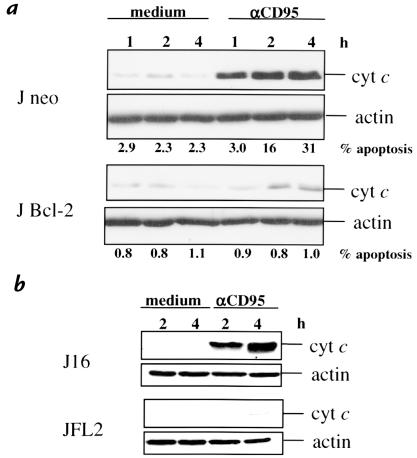
We investigated the participation of the mitochondria in CD95- and DNA damage–induced apoptosis by the analysis of cyt c release into the cytoplasm. Cytosolic, mitochondria-free extracts of cells treated with etoposide or IR showed a progressive release of cyt c (Figure 3) consistent with other reports (42, 43); this was evident within five hours. CD95 ligation also induced a rise in cytoplasmic cyt c levels, clearly preceding the onset of nuclear apoptosis (Figure 4), testifying to the common involvement of mitochondria in the response to etoposide treatment, IR, and CD95. Cyt c release and apoptosis were inhibited in Bcl-2–overexpressing cells (J Bcl-2), corroborating mitochondrial involvement in CD95-induced Jurkat cell death (Figure 4a).
Figure 3.
Etoposide and IR induce cyt c release in a caspase-independent manner. Jurkat cells (J16) were treated with zVAD-fmk (2 h, 50 μM) or left untreated and then induced to undergo apoptosis by etoposide (10 μg/ml) or IR (30 Gy). Cytosolic extracts were prepared at the indicated times and separated by 12% SDS-PAGE. Cyt c and actin content were evaluated by immunoblot analysis. Cyt c, cytochrome c.
Figure 4.
CD95 induces cyt c release in a Bcl-2– and FLIPL-inhibitable manner. Cytosolic extracts (10 μg of protein) of J neo and J Bcl-2 (a), and J16 and JFL2 (b), prepared after the indicated times of treatment with anti-CD95 mAb (200 ng/ml), were separated by SDS-PAGE, and cyt c and actin content were evaluated by immunoblotting.
Although the involvement of mitochondria in DNA damage–induced apoptosis is well established, the mechanism by which these stimuli signal to the mitochondria remains elusive. To determine whether etoposide and IR require caspase activation to signal to the mitochondria, cyt c release was assessed in the presence or absence of the pan-caspase inhibitor zVAD-fmk. Figure 3 shows that incubation with zVAD-fmk still allowed cytosolic accumulation of cyt c in response to etoposide or IR, whereas zVAD-fmk effectively suppressed apoptosis (Table 1). This indicates that caspase activity is not required for the mitochondrial release of cyt c in response to etoposide or IR. Importantly, it implies that zVAD-fmk inhibits DNA damage–induced Cer accumulation and apoptosis (Figure 2 and Table 1) by targeting caspase(s) at, or downstream from, the mitochondria. By contrast, CD95-induced cyt c release was inhibited by FLIPL (Figure 4b), indicating that CD95 requires casp-8 to signal to the mitochondria.
Effect of Bcl-2 overexpression on Cer accumulation.
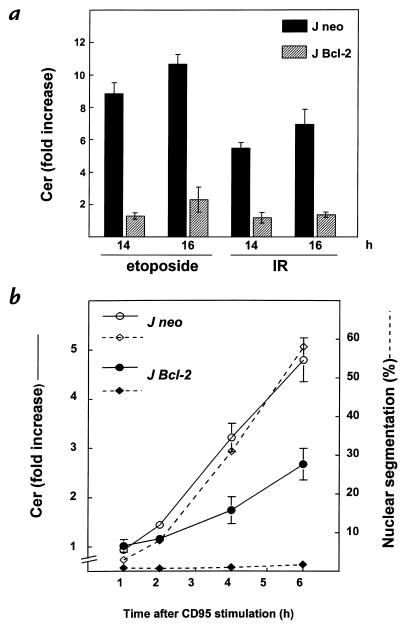
To establish possible involvement of mitochondria in the Cer response induced by CD95 cross-linking or DNA damage, we examined the effect of Bcl-2 overexpression on the Cer response. Etoposide and IR evoked a dramatic Cer response in control J neo cells, whereas Cer levels in Bcl-2–transfected cells remained at basal values or were only marginally elevated (Figure 5a). Apparently, Cer formation induced by etoposide and IR is dependent on the mitochondria. The caspases that were shown to act upstream of Cer formation by these stimuli reside at, or downstream from, the mitochondria. In the J Bcl-2 transfectant, CD95-induced apoptosis was decreased by ∼50% compared with the response in control J neo cells (Figure 5b), whereas nuclear segmentation was completely inhibited (Figure 4a). Apparently, CD95 ligation, in contrast to etoposide and IR, can still induce significant Cer formation under conditions where mitochondrial participation is blocked.
Figure 5.
Bcl-2 abrogates Cer formation induced by etoposide or IR and reduces CD95-induced Cer formation. (a) Jurkat cells transfected with vector (J neo; filled bars) or with human bcl-2 cDNA (J Bcl-2; hatched bars) labeled with [14C]serine were exposed to etoposide (10 μg/ml) or IR (30 Gy). Cer levels were determined after 14 and 18 h and expressed as -fold increase (means ± SD) relative to Cer levels in time-matched untreated cells. (b) J Neo or J Bcl-2 cells labeled with [14C]serine were treated with anti-CD95 mAb (200 ng/ml). Cer formation and nuclear segmentation were determined in the same set of samples at the indicated times. Solid lines show Cer expressed as -fold increase relative to control cells and represent three independent experiments. Dotted lines show the percentage of nuclear segmentation.
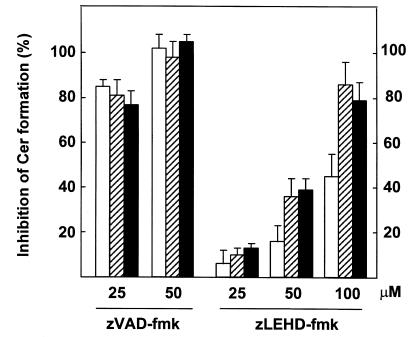
Studies in vitro and in vivo suggest that casp-9 is at the apex of the apoptotic protease cascade triggered by cytosolic cyt c (18, 20, 21). Thus, casp-9 is likely to be the most upstream zVAD-fmk target in case of DNA damage–induced Cer formation and apoptosis. We therefore tested the effect of casp-9 inhibition on Cer production, using the commercially available inhibitory peptide zLEHD-fmk, designed to specifically inhibit casp-9 based on its strong preference for the tetrapeptide substrate LEHD-AMC (25). zLEHD-fmk significantly reduced etoposide- and IR-induced Cer formation at 50 μM and achieved nearly complete inhibition at 100 μM, whereas the Cer response to CD95 was only partially sensitive to zLEHD-fmk (Figure 6). In contrast, zVAD-fmk appeared to be a more potent and general inhibitor, blocking both DNA damage–induced and CD95-induced Cer formation completely at 50 μM (Figure 6). These results suggest that the inducer casp-9 is upstream of etoposide- and radiation-induced Cer accumulation, whereas CD95-induced Cer formation only partially depends on casp-9 activation, which is consistent with our results obtained using Bcl-2 transfectants (Figure 5b).
Figure 6.
Differential effects of zVAD-fmk and zLEHD-fmk on Cer production induced by CD95 ligation or DNA damage. Jurkat cells (J16) labeled with [14C]serine were left untreated or preincubated for 2 h in the presence of zVAD-fmk or zLEHD-fmk at the indicated concentrations before addition of anti-CD95 mAb (200 ng/ml), etoposide (10 μg/ml), or exposure to IR (30 Gy). Quantitation of cellular Cer was performed after 5 h (anti-CD95; open bars) or 15 h (Eto/IR; hatched bars and filled bars, respectively) of further incubation. Results are expressed as percentage inhibition (means ± SD) of the Cer response observed in the absence of inhibitor peptide. Shown are data from a representative experiment containing duplicate samples performed two times and showing similar results. zLEHD-fmk, benzoyloxycarbonyl-LEHD-fluoromethylketone.
Discussion
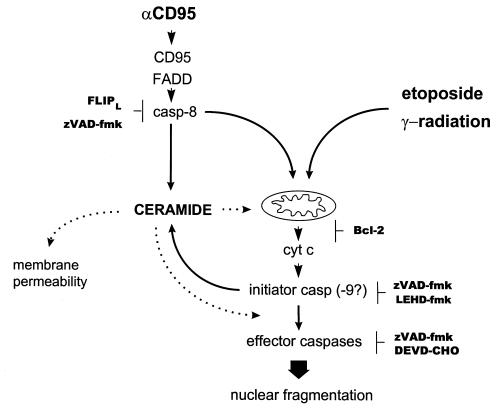
To evaluate the role of endogenous Cer in apoptosis induction, we investigated the molecular ordering of caspase activation, mitochondrial events, and Cer accumulation induced by etoposide, IR, or CD95 ligation in Jurkat T cells. According to our results, we propose the following sequence of events (Figure 7).
Figure 7.
Mapping the Cer response in relation to caspase activation and mitochondrial changes in the CD95, etoposide, and IR pathways in Jurkat cells. Cer is placed in between initiator and executioner caspases in all cases, because its accumulation is inhibited by zVAD-fmk but not by DEVD-CHO. CD95 requires casp-8, whereas DNA damage–induced Cer formation does not involve casp-8 or death receptor signaling. In the case of etoposide or IR, the most proximal zVAD-fmk target resides at, or downstream from, the mitochondria, since zVAD-fmk does not block cyt c release. Bcl-2 overexpression abrogates Cer formation in response to etoposide or IR but only partially interferes with the CD95-induced Cer response. Possible roles of Cer are indicated by dotted lines. DEVD-CHO, acetyl-DEVD-aldehyde.
In all cases, Cer formation is dependent on inducer caspase activation but independent of DEVD-CHO–sensitive effector caspases. Our data support earlier evidence that caspases control Cer formation in response to CD95 (8, 9), TNF-α (10), or REAPER (11). In addition, we provide direct information on the identity of the responsible caspases. Using FLIPL-transfected Jurkat cells, we demonstrate that casp-8 activation is a crucial event for CD95-induced Cer formation (Figure 2). In addition, our findings strongly suggest that it is the casp-8 pathway that couples to Cer formation, rather than the pathway initiated by the adaptor Daxx, which can also interact with the CD95 death domain and which activates JNK/SAPK (44).
Cer formation and apoptosis induction upon etoposide treatment or IR does not rely on casp-8 or any other pathway dependent on DED-containing caspases, since it was not inhibited by FLIPL (Figure 2). Because FLIPL inhibits signaling of CD95, TNF receptor 1, TRAIL receptor, and TRAMP (34), our data support the contention that DNA damage–induced apoptosis does not involve death receptor signaling (27, 45–47). This is in direct conflict with the reported finding that doxorubicin- and IR-induced Cer production are dependent on the CD95 system in Jurkat cells (48).
To our knowledge, our study is the first to document caspase requirement upstream of DNA damage–induced Cer generation based on the complete abrogation of Cer accumulation by the pan-caspase inhibitor zVAD-fmk. We realize that the interpretation of studies as presented here using peptide-based inhibitors is compromised by an incomplete understanding of their selectivities. The fluoromethylketone moiety of zVAD-fmk is known to irreversibly inactivate caspases by forming a thiomethyl-ketone adduct with the active-site cysteine (49), and it similarly modifies other sulfhydryl-dependent enzymes. We have ruled out a direct inhibition of SMase by zVAD-fmk, independent of its effect on caspases, because pretreatment with zVAD-fmk (2 hours, 50 μM) of either intact cells, cell lysates, or Bacillus cereus SMase did not affect in vitro SMase activity assayed at pH 5.0 and 7.4 (data not shown).
Cyt c release induced by DNA damage still occurred in the presence of zVAD-fmk (Figure 3), indicating that these stimuli signal to the mitochondria in a caspase-independent manner. Thus, in case of DNA damage, zVAD-fmk prevents Cer accumulation and apoptosis by targeting a caspase residing at, or downstream from, the mitochondria. The inhibitory effect of zLEHD-fmk supports a role for casp-9 as inducer caspase required for DNA damage–induced Cer accumulation (Figure 6).
Overexpression of Bcl-2 completely blocked the Cer response to etoposide and IR (Figure 5a), confirming that upon DNA damage, Cer accumulates downstream from the mitochondria and cyt c release, probably upon activation of a caspase cascade initiated at the mitochondrial membrane. In the case of CD95, the Cer response is in part independent of a mitochondrial contribution because it is only partially inhibited by Bcl-2 (Figure 5b). This pool of Cer is probably generated in parallel rather than upstream of the mitochondrial changes, because it is preceded by cyt c release (Figures 4a and 5b).
The inhibitory effect of Bcl-2 overexpression, together with the observation that cyt c release precedes Cer formation upon all stimuli, argues against a role for Cer in signaling to the mitochondria. This is in agreement with reported inhibition of Cer formation by Bcl-2 during hypoxic cell death (50). However, in some studies, Bcl-2 or Bcl-xL overexpression did not affect Cer accumulation in response to certain anti-cancer drugs (51, 52) or receptor agonists (10, 53), whereas Bcl-xL, but not Bcl-2, interferes with TNF-α– and camptothecin-induced Cer formation (54). At present, we have no explanation for these discrepancies.
Short-chain, cell-permeable Cer analogues (e.g., C2-Cer) are commonly used and assumed to mimic the action of natural endogenous Cer species. However, whereas endogenous Cer was shown to accumulate downstream from the mitochondria, synthetic Cer analogues seem to act upstream: they induce cyt c release in Jurkat cells (data not shown) and HL60 cells (42), whereas apoptosis induction is inhibited by Bcl-2 or Bcl-xL overexpression (50–55). This demonstrates that data obtained with short-chain Cer analogues should be interpreted with caution.
Whether Cer plays a role in apoptosis signaling should be established by experiments in which the Cer response is selectively abrogated and effects on apoptosis-induction are examined. Using aSMase-deficient (aSMase=acid SMase) Niemann-Pick lymphoblasts and their aSMase-reconstituted counterparts, we previously ruled out a role for aSMase in the CD95-induced Cer response of human B-lymphoblastoid cells (38). We found that etoposide and IR also induced Cer formation, caspase processing, and apoptosis with similar efficiency in the presence or absence of aSMase in these cells (Tepper et al, unpublished results); these results are in conflict with studies suggesting a requirement for aSMase (5, 48). Because we also excluded a role for de novo Cer biosynthesis, using the inhibitor Fumonisin B1 (Tepper, A.D., manuscript in preparation), our present data are in agreement with a role for neutral SMase (nSMase) in the generation of the CD95-, etoposide-, and IR-induced Cer response. A purported nSMase cDNA has recently been cloned (56), but the involvement of this molecular species in the Cer response to apoptotic stimuli has not yet been fully addressed.
We consider the possibility that Cer accumulation affects the structure of cell membranes. Enzymatic generation of Cer from SM induces microdomain formation in large unilamellar vesicles (57), supporting earlier reports that Cer induces membrane destabilization and leakage of aqueous solutes from vesicles (58). In vivo, Cer may accumulate to high local concentrations in the plasma membrane, sufficient to cause defects in the permeability barrier. Our observation that both Cer production and loss of membrane integrity occur in the absence of effector caspase activity would support such a causal relationship.
In conclusion, our data argue against a primary function of Cer in inducer caspase activation or signaling to the mitochondria. Rather, Cer formation is associated with the effector phase of apoptosis. It might provide an amplifying signal to mitochondria in a feedback loop of caspase activation (59). Alternatively, Cer may participate in propagation of the death signal, for instance by facilitating the activation of effector caspases or the induction of membrane-related (morphological) changes. Yet another possibility is that Cer accumulation has no role in effecting the ultimate apoptotic phenotype. Future availability of the proper genetic tools will permit adequate evaluation of these possibilities.
Acknowledgments
The authors thank Jürg Tschopp and Marcus Peter for kindly providing reagents. This work was supported by a grant from the Dutch Cancer Foundation (KWF).
References
- 1.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 2.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 3.Cifone MG, et al. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepper CG, et al. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santana P, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 6.Jaffrezou J-P, et al. Daunorubicin induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- 7.Bose R, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 8.Sillence D, Allan D. Evidence against an early signalling role for ceramide in Fas-mediated apoptosis. Biochem J. 1997;324:29–32. doi: 10.1042/bj3240029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genestier L, et al. Caspase-dependent ceramide production in Fas- and HLA class I-mediated peripheral T cell apoptosis. J Biol Chem. 1998;273:5060–5066. doi: 10.1074/jbc.273.9.5060. [DOI] [PubMed] [Google Scholar]
- 10.Dbaibo GS, et al. Cytokine response modifier A (crmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-α: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J Exp Med. 1997;3:481–490. doi: 10.1084/jem.185.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 12.Tepper AD, Boesen-de Cock JGR, de Vries E, Borst J, van Blitterswijk WJ. CD95/Fas-induced ceramide formation proceeds with slow kinetics and is not blocked by caspase-3/CPP32 inhibition. J Biol Chem. 1997;272:24308–24312. doi: 10.1074/jbc.272.39.24308. [DOI] [PubMed] [Google Scholar]
- 13.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medema JP, et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of the apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 17.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 18.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 19.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 20.Hakem R, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 21.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Wallen HD, Nunez G. Interaction and regulation of the Caenorhabditis elegans death protease CED-3 by CED-4 and CED-9. J Biol Chem. 1997;272:21449–21454. doi: 10.1074/jbc.272.34.21449. [DOI] [PubMed] [Google Scholar]
- 23.Chinnayian AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 24.De Jong D, et al. Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994;54:256–260. [PubMed] [Google Scholar]
- 25.Thornberry NA, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 27.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao DT, et al. Bcl-xL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brady HJM, Salomons GS, Bobeldijk RC, Berns AJM. T cells from baxα transgenic mice show accelerated apoptosis in response to stimuli but do not show restored DNA damage-induced cell death in the absence of p53. EMBO J. 1996;15:1221–1230. [PMC free article] [PubMed] [Google Scholar]
- 30.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong RC, et al. Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 32.Jaatela M, Benedict M, Tewari M, Shayman JA, Dixit VM. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 33.Chen GL, et al. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984;259:13560–13566. [PubMed] [Google Scholar]
- 34.Irmler M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 35.Yssel H, de Vries JE, Koken M, van Blitterswijk WJ, Spits H. Serum-free medium for the generation and the propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 36.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi CA. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 37.Zamzami N, et al. Mitochondrial control of apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesen-de Cock JGR, Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. CD95 (Fas/APO-1) induces ceramide formation and apoptosis in the absence of a functional acid sphingomyelinase. J Biol Chem. 1998;273:7560–7565. doi: 10.1074/jbc.273.13.7560. [DOI] [PubMed] [Google Scholar]
- 39.Thome M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasula SM, et al. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 41.Goltsev YV, et al. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 42.Amarante-Mendes GP, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- 43.Chauhan D, et al. Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boesen-de Cock JGR, de Vries E, Williams GT, Borst J. The anti-cancer drug etoposide can induce caspase-8 processing and apoptosis in the absence of CD95 receptor-ligand interaction. Apoptosis. 1998;3:17–25. doi: 10.1023/a:1009603001888. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka T, et al. FLIP prevents apoptosis induced by death receptors, but not by perforin/granzyme B, chemotherapeutic drugs, and γ-irradiation. J Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- 47.McGahon AJ, Costa Pereira AP, Daly L, Cotter TG. Chemotherapeutic drug-induced apoptosis in human leukaemic cells is independent of the Fas (APO-1/CD95) receptor/ligand system. Br J Haematol. 1998;101:539–547. doi: 10.1046/j.1365-2141.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 48.Herr I, Wilhelm D, Bohler T, Angel P, Debatin K-M. Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornberry NA, et al. Inactivation of interleukin-1β converting enzyme by peptide (acyloxy)methyl ketones. Biochemistry. 1994;33:3934–3940. doi: 10.1021/bi00179a020. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura S, et al. Ceramide formation leads to caspase-3 activation during hypoxic PC12 cell death. Inhibitory effects of Bcl-2 on ceramide formation and caspase-3 activation. J Biol Chem. 1998;273:6921–6927. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, et al. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allouche M, et al. Influence of Bcl-2 overexpression on the ceramide pathway in daunorubicin-induced apoptosis of leukemic cells. Oncogene. 1997;14:1837–1845. doi: 10.1038/sj.onc.1201023. [DOI] [PubMed] [Google Scholar]
- 53.Wiesner DA, Kilkus JP, Gottschalk AR, Quintas J, Dawson G. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by Bcl-xL. J Biol Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- 54.El-Assaad W, El-Sabban M, Awarja C, Abboushi N, Dbaibo G. Distinct sites of action of Bcl-2 and Bcl-XL in the ceramide pathway of apoptosis. Biochem J. 1998;336:735–741. doi: 10.1042/bj3360735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smyth MJ, et al. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem J. 1996;316:25–28. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci USA. 1998;95:3638–3643. doi: 10.1073/pnas.95.7.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holopainen JM, Subramanian M, Kinnunen PKJ. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Arguello MB, Goni FM, Alonso A. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem. 1996;271:26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- 59.Green D, Kroemer G. The central executioners in apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]