Abstract
The role of macrophages and their interactions with T cells during aging is not well understood. We determined if activating elderly-derived macrophages could rescue age-related and tumor-induced T cell dysfunction. Healthy elderly (18–24 months) Balb/c contained significantly more splenic IL-10-secreting M2-macrophages and myeloid-derived suppressor cells than young (6–8 weeks) mice. Exposure to syngeneic mesothelioma or lung carcinoma-conditioned media polarized peritoneal macrophages into suppressive M2-macrophages regardless of age. Tumor-exposed, elderly, but not young-derived, macrophages produced high levels of IL-4 and could not induce T cell IFN-γ production. We attempted to rescue tumor-exposed macrophages with LPS/IFN-γ (M1 stimulus) or IL-2/agonist anti-CD40 antibody. Tumor-exposed, M1-stimulated macrophages retained high CD40 expression, yet TNF-α and IFN-γ production were diminished relative to non-tumor-exposed, M1-stimulated controls. These macrophages induced young and elderly-derived T cell proliferation however, T cells did not secrete IFN-γ. In contrast, tumor-exposed, IL-2/CD40-stimulated macrophages rescued elderly-derived T cell IFN-γ production, suggesting that IL-2/CD40-activated macrophages could rescue T cell immunity in aging hosts.
Keywords: Innate immunity, Elderly, Immune restoration, Cancer immunotherapy, Macrophage, T cell
Introduction
Recent studies showed that components of immune function decline with age (Fulop et al. 2010). In particular, T cell function is increasingly compromised with advancing age (Haynes and Maue 2009) which may account for the increased susceptibility to infection and cancer in the elderly (Fulop et al. 2010). Similarly, tumor cells sabotage T cell function in young hosts using multiple mechanisms including secreting factors such as transforming growth factor-beta (TGF-β) that induce and/or recruit anti-inflammatory CD4+ Th2 cells and CD4+CD25+Foxp3+ regulatory T cells (Treg). Both Th2 and Treg cells interfere with CD8+ cytotoxic T lymphocyte (CTL) function (Chappert et al. 2010) and Tregs increase in healthy, cancer-free, elderly hosts (Gregg et al. 2005). However, it is Th1 cells that promote cell-mediated immunity, in particular CD8+ T cell function and secrete IFN-γ, (Chamoto et al. 2003) a cytokine with potent anti-infection and anti-cancer effects. TGF-β and other tumor-derived factors may also directly impair CD8+ CTL function (Kapp et al. 2007), a key cell that mediates tumor regression. Taken together, the data suggest that T cells in cancer-bearing elderly hosts are likely to be severely compromised due to age-related and tumor-induced effects. Thus, it is possible that even standard chemotherapy may not be effective in the elderly as there is increasing evidence that some chemotherapy requires functioning T cells (Jackaman et al. 2012b; Nowak et al. 2003; Zitvogel et al. 2008). Furthermore, immunotherapy which deliberately targets tumor-specific CD8+ T cells may also not be as effective in elderly hosts.
Currently, long-term eradication of many cancers including mesothelioma and lung cancer in humans is not possible using chemotherapy and surgery. Immunotherapy has shown promise in both cancers particularly mesothelioma (Bundell et al. 2006; Jackaman et al. 2003; Jackaman et al. 2009; Jackaman et al. 2012b; Jackaman et al. 2008; Jackaman and Nelson 2010, Jackaman and Nelson 2012; van Bruggen et al. 2005); however, most preclinical analysis are performed in young adult mice and not representative of human cancers, such as mesothelioma, that usually occur in aging populations (Bianchi and Bianchi 2007). Furthermore, most immunotherapeutic strategies focus on T cell activation, yet T cell number and function decline with age meaning that other cells may have to be targeted (Haynes and Maue 2009).
Unlike T cell immunity, innate immunity may be better preserved in the elderly (Fulop et al. 2010). Use of a mouse breast cancer model showed that effective anti-cancer immunity in young animals was mediated by T cells, in contrast responses in old animals relied on innate immunity (Provinciali et al. 2000). Macrophages play a key role in innate immunity. They represent a numerically abundant tissue-resident cell that is highly sensitive to its microenvironment. Furthermore, many solid tumors, including mesothelioma, contain large numbers of macrophages (Burt et al. 2011; Jackaman et al. 2009). Thus, macrophages are strategically placed to function as sensors for, and eliminators of, tumor cells. However, they may also promote tumor growth. There is still controversy in regard to the effect of aging on macrophage function in healthy hosts, and even less is known about their role in tumor-bearing hosts, and there are few studies addressing the effect tumor-derived factors have on macrophage-T cell interactions in aging hosts.
Macrophages can be divided into pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages (Mantovani et al. 2004) and can switch phenotypes based on the stimuli present (Arnold et al. 2007). M1 macrophages secrete pro-inflammatory cytokines including tumor necrosis factor (TNF-α) and exert anti-tumoricidal activity (Mantovani et al. 2004). M2 macrophages secrete anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β which promote angiogenesis, repair, and tumor growth (Deonarine et al. 2007). Myeloid-derived suppressor cells (MDSCs) are macrophage-like immature cells that are often increased in cancer and have similarities to M2 macrophages (Sinha et al. 2007). Aging has been associated with the emergence of M2-like macrophages within the eye (Ly et al. 2010) which coincides with impaired macrophage responses to M1 stimuli (Mahbub et al. 2012). In contrast, aging has also been associated with enhanced M1 macrophage function through the production of reactive oxygen species (Smallwood et al. 2011). However, in agreement with the former study, we recently showed that lymphoid organs of geriatric 27-month-old C57BL/6J mice contain significantly more suppressive, IL-10-secreting, M2 macrophages and MDSCs than young adult mice (Jackaman et al. 2013): these M2 macrophages and MDSCs are likely to more readily support tumor growth than their younger counterparts by sabotaging T cell function.
Recent studies using young adult mice showed that targeting tumor-associated M2 macrophages such that they skew back to the M1 phenotype may be an effective anti-cancer treatment strategy (Fridlender et al. 2011; Mantovani et al. 2004). Whether this will be an effective treatment in the elderly is yet to be determined. Promising data has shown that macrophage activation can be effective in generating tumor regression in old, but not young mice (Leibovici et al. 2009). Furthermore, we have recently shown that, if appropriately activated, macrophages from elderly hosts can adopt a pro-inflammatory M1 phenotype and restore T cell IFN-γ production (Jackaman et al. 2013). We have also shown that exposure to tumor-derived factors polarized geriatric and young-derived macrophages toward the M2-phenotype; however, only geriatric-derived macrophages produced IL-4 (Jackaman et al. 2013). These data imply increased immunosuppression in elderly hosts with cancer, i.e., a dual dilemma of age-related and tumor-induced macrophage and T cell dysfunction.
We did not attempt to rescue tumor-exposed macrophages in our previous studies, nor did we address genetic differences between strains. Here, we extend those studies to include Balb/c mice. Spleens, lymph nodes, bone marrow, and peritoneal macrophage subpopulations from healthy young adult (6–8 weeks old) versus healthy elderly (18 months) Balb/c or C57BL/6J mice were analyzed. We mimicked conditions that may occur in mesothelioma or lung carcinoma-bearing hosts by exposing macrophages from young and elderly mice to syngeneic murine mesothelioma and Lewis lung tumor cell-conditioned media and examined changes to phenotype and cytokine secretion. The consequences of macrophage-T cell interactions were examined, focussing on the capacity of tumor-exposed elderly versus young-derived macrophages to induce proliferative and functional (IFN-γ secretion) responses by T cells from young versus elderly hosts. Finally, we attempted to rescue tumor-exposed, elderly-derived macrophages using classical M1 stimulus (LPS/IFN-γ) as well as IL-2/agonist anti-CD40 antibody (Ab) immunotherapy (Jackaman et al. 2008).
Materials and methods
Mice
Balb/c or C57BL/6J mice aged 6–8 weeks (young) and 17–18 months (elderly) were obtained from Animal Resources Centre (ARC, Murdoch, Western Australia) and maintained under specific pathogen-free conditions at Curtin University. Balb/c mice are more difficult to manage during aging than C57BL/6J mice; therefore, we used 17–18-month-old mice that were still healthy (Yuan et al. 2009); after this age, there is a rapid decline in survival. The Jackson laboratory defines 18- to 24-month-old mice as old or elderly and equivalent to 56- to 69-year-old humans and refers to a period when senescent changes can be detected in many biomarkers in all animals (Yuan et al. 2009). This is also an age where sarcopenia (age-related loss of muscle mass) becomes evident and is similar to the sarcopenia seen in 70-year-old humans (Shavlakadze et al. 2010). All experiments were performed according to the Australian Code of Practice for the care and use of animals for scientific purposes as per Curtin University Animal Ethics Committee (AEC#2012_21).
Tumor cell lines and tumor cell-conditioned media (supernatant)
AB1 and AE17 are malignant mesothelioma cell lines derived from the peritoneal cavity of either Balb/c mice or C57BL/6J mice injected with asbestos fibers respectively and have been previously described (Davis et al. 1992; Jackaman et al. 2003). The murine Lewis lung (LL/2) cell line was obtained from the ATCC (ATCC CRL-1640; Maryland, USA). The tumor cell lines were maintained in complete medium, consisting of RPMI 1640 (Invitrogen, CA, USA) supplemented with 10 % fetal calf serum (FCS; ThermoScientific, Victoria, Australia), 50 mg/L gentamicin (Pharmacia and Upjohn, Western Australia, Australia), 60 mg/L benzylpenicillin (CSL Ltd, PA, USA), 2 mM L glutamax (Invitrogen), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, MO, USA). Cells were cultured at 37 °C in a 5 % CO2 atmosphere. For the generation of tumor cell-conditioned media, cells were cultured in a serum-free medium (Invitrogen). After 48–72 h, conditioned media from tumor cell cultures were centrifuged and cell-free supernatants collected and stored at −80 °C until use.
Collection and in vitro stimulation of peritoneal macrophages
Cells obtained from the peritoneal cavity of mice by washing with ice-cold PBS were incubated for 2–4 h at 37 °C, after which non-adherent cells were removed; the remaining adherent population was >95 % F4/80+ macrophages (Jackaman and Nelson 2010). Where specified, macrophages were cultured overnight with 50 % AB1 mesothelioma-conditioned media, 50 % AE17 mesothelioma-conditioned media, 50 % LL-conditioned media, M2 stimuli (20 ng/ml IL-4; Shenandoah Biotechnology, PA, USA), M1 stimuli (20 ng/ml IFN-γ, Shenandoah Biotechnology; and 1 μg/ml LPS, Sigma-Aldrich) or IL-2/anti-CD40 Ab (10 μg/ml of each, IL-2 obtained from Cetus Corporation, CA, USA; anti-CD40 Ab, or FGK45 obtained from AbSolutions, Western Australia).
Flow cytometry
Spleen, bone marrow, and lymph node samples for intracellular staining were collected in PBS/2 % FCS solution containing Brefeldin A (Biolegend). Tissue samples were disaggregated into single-cell suspensions by gentle dispersion between two frosted glass slides and stained for flow cytometric analysis. The following anti-mouse primary antibodies were incubated for 1 h at 4 °C in the dark: anti-CD11b-PE-Cy7 (Biolegend, CA, USA), anti-F4/80-APC-Cy7 (Biolegend), anti-Ly6C biotin (Biolegend), anti-Ly6G-PerCP-Cy5.5 (Biolegend), anti-CD40-PE and rabbit anti-CX3CR1 (Abcam, MA, USA). Following three washes in PBS/2 % FCS, secondary antibody Alexafluor® 488-conjugated anti-rabbit antibody (Biolegend) and streptavidin-V500 (Becton Dickinson) was incubated for 30 min at 4 °C in the dark. For intracellular staining, cells were fixed in 1 % paraformaldehyde on ice for 15 min, followed by permeabilization with PBS/2 % FCS solution containing 0.1 % saponin. Cells were then stained with anti-TNF-α-APC and anti-IL10-Brilliant Violet 421. Cells were washed and resuspended in PBS/2 % FCS for analysis on a FACSCanto II using FACSDiva software (Becton Dickinson) or FlowJo software (TreeStar, OR, USA).
Allogeneic mixed lymphocyte reaction (MLR) using CFSE-labelled T cells
Cross-over MLR assays were used to determine whether any defect seen was at the macrophage or T cell level. Macrophages from young or elderly Balb/c mice were co-cultured with allogeneic T cells from C57BL/6J mice. Alternatively, macrophages from young C57BL/6J mice were co-cultured with allogeneic T cells from young or elderly Balb/c mice. Prior to addition of T cells, all stimuli were removed and macrophages were washed with PBS. Splenocytes collected from either Balb/c mice or C57BL/6J mice were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), a fluorescent dye that binds to cell membranes (Lyons and Parish 1994). Balb/c splenocytes were used as allogeneic responder T cells for C57BL/6J macrophages, and C57BL/6J splenocytes were used as allogeneic responder T cells for Balb/c macrophages. The cells were washed in PBS, resuspended at 2 × 107 cells/ml in RPMI containing 3.5 μM CFSE, incubated at room temperature for 10 min, then washed three times with RPMI. Labelled splenocytes were cultured for 2–4 h to remove adherent cells. Non-adherent cells (consisting of 39.3 ± 1.3 % T cells) were co-cultured for 3–5 days with varying macrophage: T cell ratios (1:2 to 1:200). Splenocytes cultured with 1 μg/mL Concanavalin A, (ConA; Sigma-Aldrich) were used as a positive control. After 5 days, cells were stained with anti-mouse antibodies: anti-CD4-APC-Cy7, anti-CD8-PerCP-Cy5.5, and anti-CD44-APC (Beckton Dickinson). As T cells proliferate, CFSE segregates equally between each daughter population, and in flow cytometric analysis, each round of proliferation is seen as sequential halving of CFSE staining intensity (Lyons and Parish 1994). The percentage of T cell proliferation was calculated based on loss of staining intensity of the parent peak.
Cytokine bead array (CBA)
Concentrations of the cytokines TNF-α, IL-4, and IFN-γ in stimulated macrophage supernatants and MLR supernatants (macrophage:T cell ratio 1:2) were measured using the mouse Th1/Th2 CBA (BD Biosciences). The data shown for IFN-γ in the MLR supernatants was calculated by subtracting IFN-γ secreted by macrophage only controls, to exclude macrophage-derived cytokines and reveal T cell-derived cytokines. Analysis was performed on a FACSCanto II (BD) using FACSDiva software (BD).
Data analysis
Statistical significance was calculated using GraphPad Prism 4 (San Diego, CA, USA). Student’s t test and Mann–Whitney U test were used to determine differences between two populations. P values of <0.05 were considered statistically significant.
Results
Balb/c elderly-derived macrophages are similar to C57BL/6J elderly-derived macrophages
Our previous studies showed that IL-10-secreting M2 macrophages and MDSCs dominated lymphoid organs in elderly but not young adult C57BL/6J mice. Nonetheless, elderly-derived macrophages maintained their ability to respond to stimuli but lost their ability to induce T cells to secrete IFN-γ; a function that could be restored by activating macrophages using a combination of IL-2 with agonist anti-CD40 antibody (IL-2/CD40; Jackaman et al. 2013). However, we did not examine genetic differences between strains in that study. We now have data showing that, similar to C57BL/6J mice, healthy elderly Balb/c mice contain significantly more splenic IL-10-secreting M2-macrophages and MDSCs than young mice; these macrophages also responded to M1 and M2 stimuli. Importantly, exposure to conditioned media from mesothelioma tumor cells induced significantly greater IL-4 secretion relative to young-derived macrophages (data not shown) implying polarization into more potent suppressive M2 macrophages in the elderly when faced with a progressing tumor.
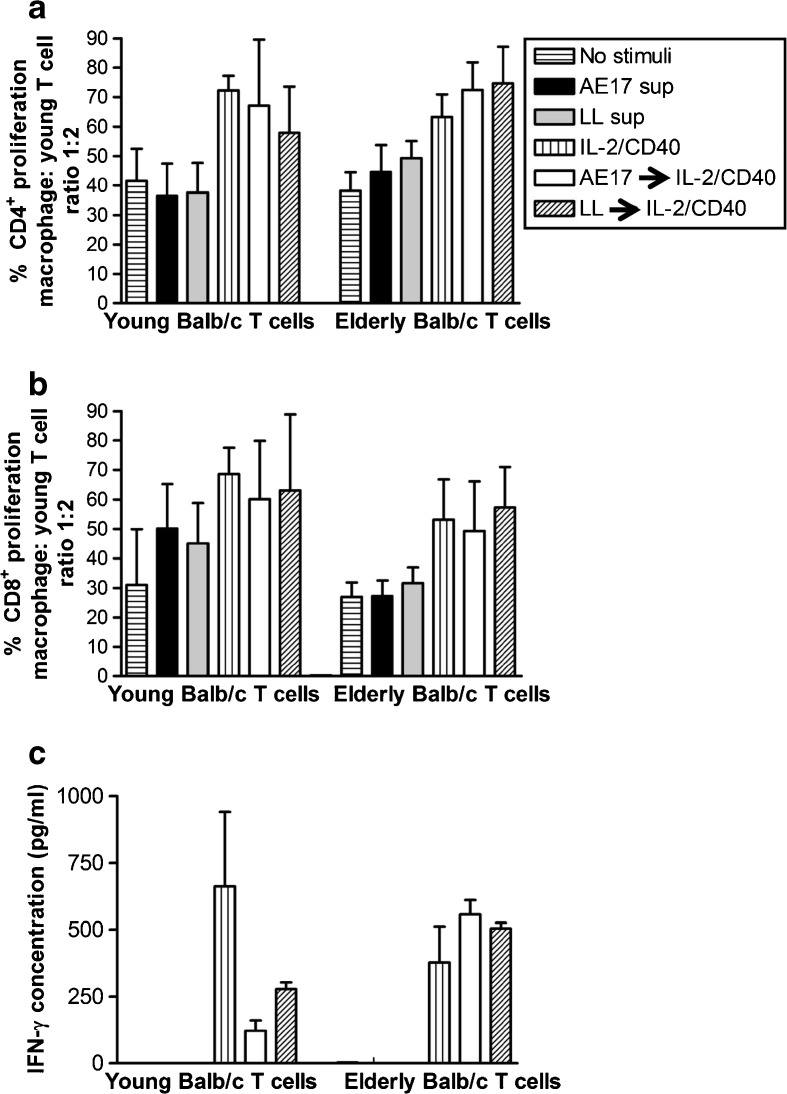
Similar to C57BL/6J mice (Jackaman et al., 2013), young and elderly-derived Balb/c M1 macrophages induced T cell proliferation, and again, only young-derived M1 macrophages could induce T cells to produce IFN-γ (data not shown); these data confirm the identification of an age-related defect at the macrophage/T cell interface in elderly mice. Importantly, we also confirmed that IL-2/CD40 activation restored the function of elderly-derived Balb/c macrophages with both age groups inducing increased CD4+ and CD8+ T cell proliferation resulting in more divisions than the M1 and M2 stimuli (data not shown). Finally, unlike M1-stimulation, IL-2/CD40-activated elderly-derived macrophages could induce T cells to secrete IFN-γ and upregulate the lymphocyte activation marker, CD44 (data not shown). These data imply that regardless of genetic strain, macrophages from healthy elderly mice are more likely to be immunosuppressive and that IL-2/CD40 activation overcomes age-related immunosuppression.
M1-stimulated macrophages cannot reverse tumor-induced and age-related suppression
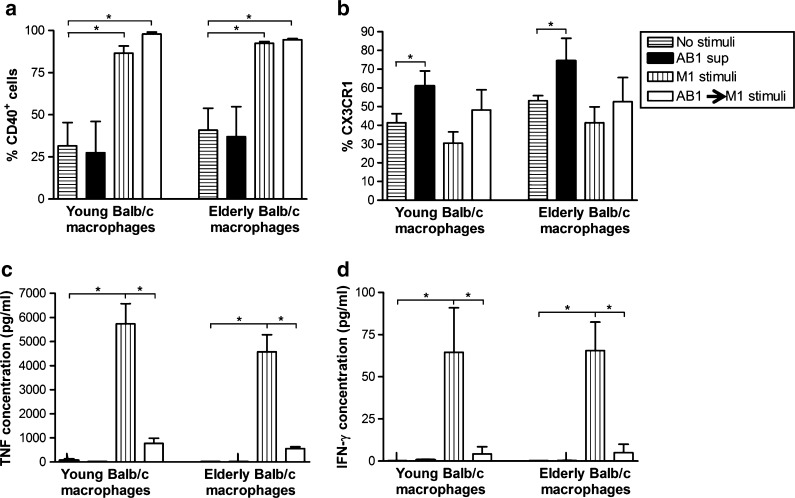
We did not attempt to rescue tumor-exposed macrophages in our previous study. Therefore, here we assessed whether the suppressive IL-4-secreting M2 phenotype induced by tumor-conditioned media could be reversed with M1 (LPS/IFNγ) or IL-2/CD40 stimulation. Peritoneal macrophages from young or elderly Balb/c mice were first exposed to AB1 mesothelioma-conditioned media overnight then cultured for further 24 h with either the M1 stimuli or IL-2/anti-CD40 Ab. Regardless of age, tumor-exposed, M1-stimulated macrophages upregulated CD40 (Fig. 1a) and appeared to downregulate CX3CR1 expression (Fig. 1b) implying polarization into M1 cells. However, supernatants collected for CBA analysis showed that the M1-associated cytokines TNF-α (Fig. 1c) and IFN-γ (Fig. 1d) were significantly decreased compared to M1 stimuli alone (i.e., not tumor-exposed). These data imply that prior exposure to tumor-derived factors diminishes the ability of macrophages to respond to LPS/IFNγ resulting in incomplete polarization into M1 cells.
Fig. 1.
Classical M1 stimulation does not override age-related and tumor-induced M2-like macrophage dysfunction. Peritoneal macrophages from young or elderly Balb/c mice were cultured overnight with AB1 tumor cell-conditioned media (AB1 sup) then activated with M1 stimuli (LPS/IFN-γ) for another 24 h (AB1 sup → M1 stimulus). Controls included no stimuli, AB1 sup only, and M1 stimuli only. CD11b+F4/80+ macrophages were analyzed by flow cytometry for surface expression of CD40 (a) and CX3CR1 (b). TNF-α (c) and IFN-γ (d) were measured in the supernatant by CBA. Data from two to four experiments is shown as mean ± SEM. *p < 0.05
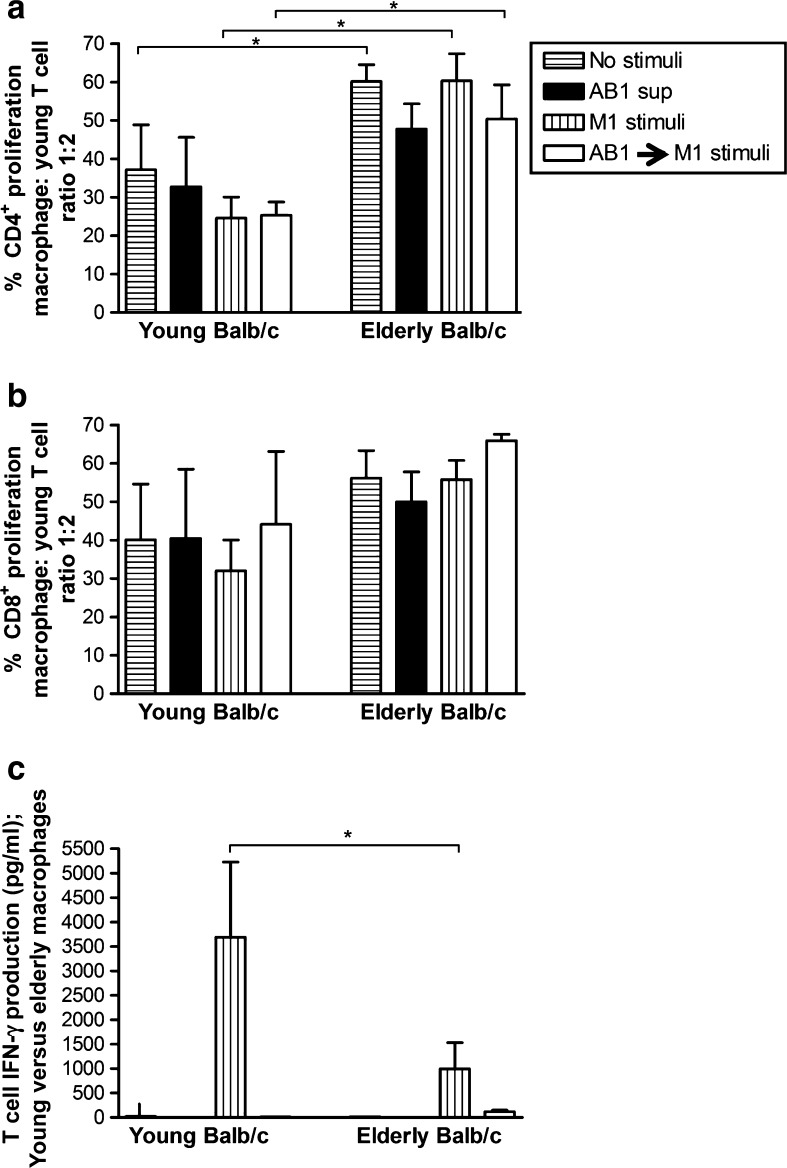
The macrophages were then co-cultured with varying numbers of CFSE-labelled allogeneic, non-adherent splenic lymphocytes from young C57BL/6J mice for 5 days and CD4+ and CD8+ T cell proliferation measured, as described above. Regardless of stimuli, macrophages from elderly mice induced greater proliferation of young CD4+ T cells than young-derived macrophages at all macrophage: T cell ratios (Fig. 2a and data not shown). Macrophages from young and elderly Balb/c mice first exposed to mesothelioma-conditioned media then activated with M1 stimuli did not lose their ability to induce young-derived CD4+ (Fig. 2a) and CD8+ T cells (Fig. 2b) to proliferate. However, in the presence of tumor-derived factors, macrophages from both age groups could not induce splenic T cells to secrete IFN-γ (Fig. 2c). In contrast, in the absence of tumor-derived factors, young-derived M1 macrophages induced significantly greater levels of IFN-γ secretion by young T cells than elderly-derived M1 macrophages (Fig. 2c). These data suggest that M1-stimulated macrophages from elderly mice lose their ability to induce functional IFN-γ secreting T cells and that exposure to tumor-derived factors disables young-derived macrophages such that they functionally resemble elderly-derived macrophages.
Fig. 2.
Tumor-exposed macrophages from elderly mice show impaired ability to stimulate T cell production of IFN-γ. Peritoneal macrophages from young or elderly Balb/c mice were cultured overnight with AB1 tumor cell-conditioned media (AB1 sup) then activated with M1 stimuli (LPS/IFN-γ) for another 24 h (AB1 sup → M1 stimulus). Controls included no stimuli, AB1 sup only, and M1 stimuli only. CFSE-labelled young C57BL/6J-derived splenic lymphocytes were added to macrophages at varying ratios and 5 days later cells stained for CD4 and CD8, and the percentage of proliferating CFSE+CD4+ T cells (a) and CFSE+CD8+ T cells calculated (b). MLR supernatants were assayed for T cell-derived IFN-γ by CBA; calculated by subtracting IFN-γ secreted by macrophages (c). Data from two to four experiments is shown at the macrophage: T cell ratio of 1:2 as mean ± SEM. *p < 0.05
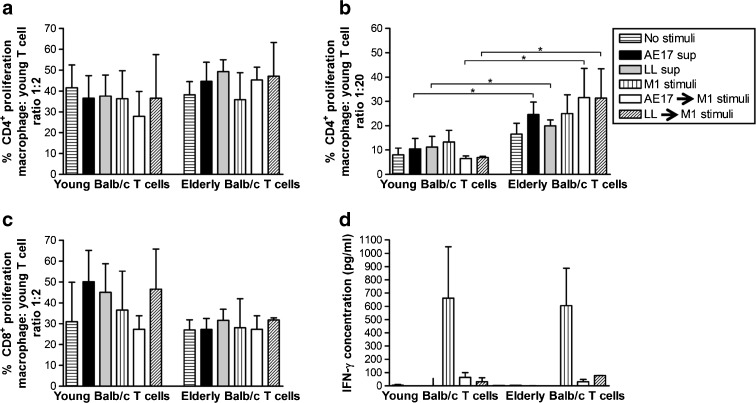
In a further experiment analyzing the T cell compartment, macrophages from young C57BL/6J mice were first exposed to conditioned media from syngeneic AE17 mesothelioma or LL tumor cell lines then activated with M1 stimuli. These tumor-exposed M1 macrophages induced young and elderly-derived Balb/c CD4+ (Fig. 3a, b) and CD8+ T cells (Fig. 3c) to proliferate. Interestingly, elderly-derived CD4+ T cells proliferated more readily than young-derived CD4+ T cells at lower macrophage: T cell ratios (Fig. 3b and data not shown). In contrast, young-derived CD8+ T cells appeared to proliferate more readily at the higher macrophage: T cell ratios (Fig. 3c); however, the differences are not statistically significant. There was no difference in CD8+ T cell proliferation between young and elderly groups at lower macrophage: T cell ratios (data not shown). Again, this did not lead to restoration of young or elderly splenocyte-derived IFN-γ production (Fig. 3d). The data for Fig. 3d was calculated by subtracting IFN-γ secreted by macrophages to exclude macrophage-derived cytokines (i.e., young M1-stimulated macrophages; 193 ± 142 pg/ml: elderly M1-stimulated macrophages; 152 ± 88 pg/ml: young AB1 sup → M1 stimulated macrophages; 4 ± 4 pg/ml: elderly AB1 sup → M1 stimulated macrophages: 5 ± 5 pg/ml).
Fig. 3.
Classical M1 stimulation does not restore tumor-induced macrophage dysfunction or rescue elderly T cell production of IFN-γ. Macrophages from young C57BL/6J mice were cultured overnight with AE17 tumor cell-conditioned media (AE17 sup) or LL tumor cell-conditioned media (LL sup), then stimulated overnight with M1 stimuli before co-culture with CFSE-labelled young or elderly Balb/c splenic lymphocytes and analyzed for proliferating CFSE+CD4+ T cells (a and b) and CFSE+CD8+ T cells (c). Controls included no stimuli, AE17 sup only, LL sup only, and M1 stimuli only. MLR supernatants were assayed for T cell-derived IFN-γ by CBA, calculated by subtracting IFN-γ secreted by macrophages (d). Data from two to four experiments is shown at the macrophage: T cell ratio of 1:2 (a, c, and d) and 1:20 (b) as mean ± SEM. *p < 0.05
IL-2/CD40 activated macrophages reverse tumor-induced and age-related T cell dysfunction
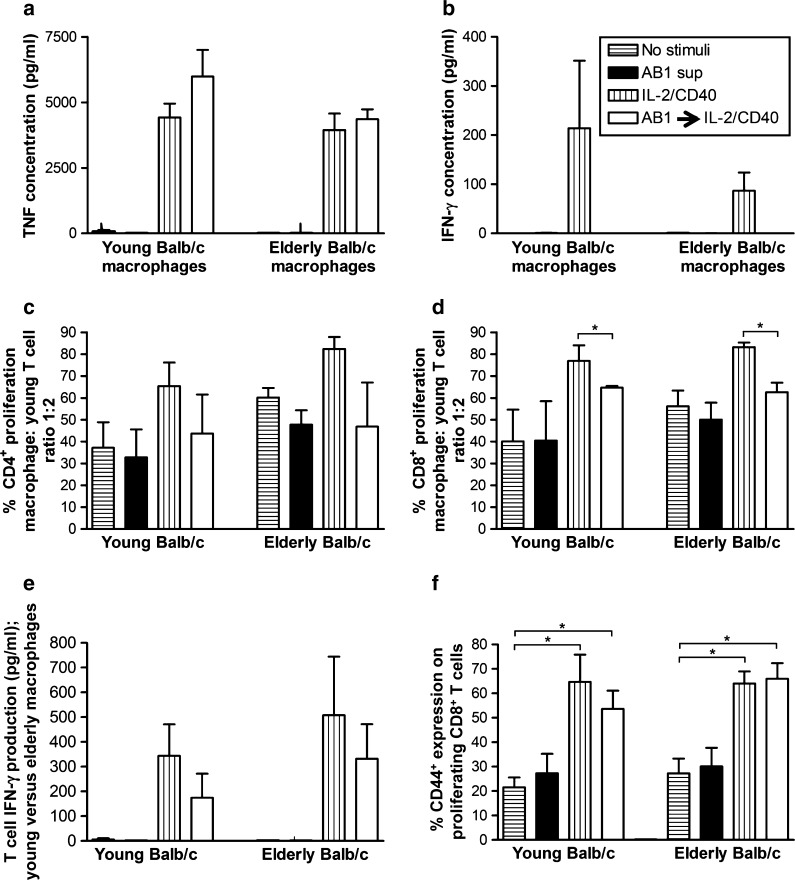
Given M1 stimuli could not completely rescue the suppressive M2 phenotype induced by tumor-conditioned media, we next assessed the effect of IL-2/CD40 activation in similar experiments. Regardless of age, tumor-exposed, IL-2/CD40-stimulated macrophages demonstrated partial polarization into M1 cells by secretion of the M1 cytokine, TNF-α (Fig. 4a), but not IFN-γ (Fig. 4b) or IL-4 (an M2 cytokine; data not shown). Macrophages were then co-cultured with varying numbers of CFSE-labelled allogeneic splenic lymphocytes from young C57BL/6J mice for 5 days and CD4+ and CD8+ T cell proliferation measured. Macrophages from young and elderly mice first exposed to mesothelioma-conditioned media then activated with IL-2/anti-CD40 Ab-induced young CD4+ (Fig. 4c) and CD8+ T cells (Fig. 4d) to proliferate. However, CD8+ T cell proliferation was decreased for both young and elderly-derived mesothelioma-exposed, IL-2/CD40 activated macrophages compared to IL-2/CD40 stimuli alone (Fig. 4d). Importantly, mesothelioma-exposed, IL-2/anti-CD40-stimulated macrophages from young and elderly mice induced young-derived splenic lymphocytes to secrete IFN-γ (Fig. 4e) and upregulate CD44 similar to the IL-2/CD40 alone controls (Fig. 4f). These data imply that IL-2/CD40 activation overrides tumor-induced suppressive effects on macrophages regardless of age; thus, age-related, tumor-induced macrophage defects can be rescued.
Fig. 4.
IL-2/anti-CD40 Ab overrides age-related and tumor-induced macrophage dysfunction leading to T cell proliferation and IFN-γ production. Peritoneal macrophages from young or elderly Balb/c mice were cultured overnight with AB1 tumor cell-conditioned media (AB1 sup) then activated with IL-2/anti-CD40 Ab for another 24 h (AB1 → IL-2/CD40). Controls included no stimuli, AB1 sup only, and M1 stimuli only. Supernatants were analyzed for TNF-α (a) and IFN-γ (b) by CBA. CFSE-labelled young C57BL/6J splenocytes were added to macrophages, 5 days later cells stained with CD4, CD8, and CD44 and the percentage of proliferating CFSE+CD4+ T cells (c), CFSE+CD8+ T cells (d), and CD44 expression on proliferating CFSE+CD8+ cells (f) calculated using flow cytometry. MLR supernatants were assayed for T cell-derived IFN-γ by CBA; calculated by subtracting IFN-γ secreted by macrophages (e). Data from two to four experiments is shown at the macrophage: T cell ratio of 1:2 as mean ± SEM. *p < 0.05
To better understand the T cell compartment in elderly mice, peritoneal macrophages from young C57BL/6J mice were first exposed to AE17 mesothelioma-conditioned media or LL tumor-conditioned media overnight, then cultured with IL-2/anti-CD40 Ab for further 24 h before co-culture with CFSE-labelled allogeneic splenic lymphocytes from young or elderly Balb/c mice. Equivalent levels of proliferation in CD4+ and CD8+ T cells (Fig. 5a, b) from young and elderly mice were observed. Macrophages from young and elderly mice first exposed to mesothelioma-conditioned media then activated with IL-2/anti-CD40 Ab restored T cell proliferation to similar levels as IL-2/CD40 alone controls. Furthermore, the data show that IL-2/CD40-activated macrophages from young mice induced young-derived splenocytes to secrete significant levels IFN-γ (Fig. 5c). However, prior exposure of these young-derived macrophages to AE17 or LL tumor cell-conditioned media, followed by IL-2/anti-CD40 Ab only partially rescued IFN-γ production by splenocytes from young mice (Fig. 5c). In contrast and importantly, IL-2/CD40-driven rescue of IFN-γ secretion by splenocytes was more effective in elderly derived T cells (Fig. 5c). CD44 expression appeared to be upregulated to a greater extent by elderly-derived CD4+ T cells, but not C84+ T cells (data not shown); however, the age-related differences were not statistically significant. Overall, these data suggest that IL-2/anti-CD40 Ab may restore anti-tumor T cell immunity in elderly mice via activated macrophages.
Fig. 5.
IL-2/anti-CD40 Ab restores tumor-induced macrophage dysfunction leading to elderly T cell production of IFN-γ. Peritoneal macrophages from young C57BL/6J mice were cultured overnight with AE17 tumor cell-conditioned media (AE17 sup) or LL tumor cell-conditioned media (LL sup), stimulated overnight with IL-2/anti-CD40 Ab then co-cultured with CFSE-labelled young or elderly Balb/c splenic lymphocytes and proliferating CFSE+CD4+ T cells (a) and CFSE+CD8+ T cells calculated (b); note only macrophage: T cell ratio of 1:2 shown. Controls included no stimuli, AE17 sup only, LL sup only and M1 stimuli only. MLR supernatants were assayed for T cell-derived IFN-γ by CBA, as described above (c). The data from two to four experiments is shown at the macrophage: T cell ratio of 1:2 as mean ± SEM. *p < 0.05
IL-2/CD40 activated elderly-derived macrophages reverse tumor-induced and age-related elderly T cell dysfunction
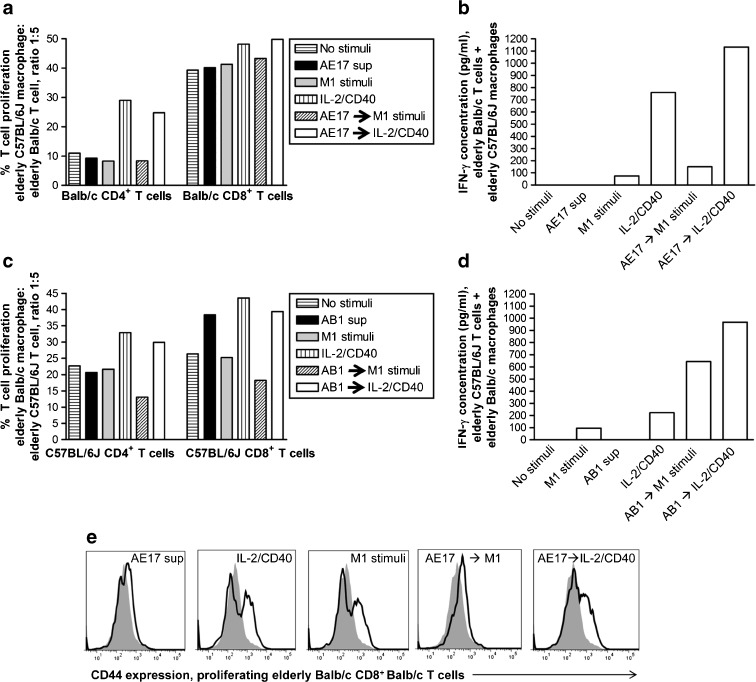
In our previous study, we did not attempt cross-over experiments with elderly-derived Balb/c macrophages to elderly-derived C57BL/6J mice and vice versa. Balb/c mice are more difficult to manage during aging than C57BL/6J mice (Yuan et al. 2009), which results in limited access to sufficient numbers of elderly C57BL/6J mice and elderly Balb/c mice that are the same age at the same time. Therefore, we have only been able to perform two experiments co-culturing elderly C57BL/6J-derived macrophages with elderly Balb/c-derived T cells and vice versa (Fig. 6a–e). Elderly-derived, mesothelioma-exposed, M1 stimulated macrophages did not increase elderly-derived CD4+ or CD8+ T cell proliferation (Fig. 6a, c) or lead to T cell upregulation of CD44 (Fig. 6e) compared to M1 stimuli only. However, M1 stimuli appeared to partially reverse tumor-induced suppression leading to increased levels of IFN-γ secretion by T cells from elderly mice (Fig. 6b, d) compared to M1 stimuli only; this was particularly clear when elderly-derived C57BL/6J T cells were cultured with elderly-derived, AB1-exposed, M1-stimulated Balb/c macrophages. Macrophages from elderly mice first exposed to mesothelioma-conditioned media then activated with IL-2/anti-CD40 Ab restored elderly-derived T cell proliferation to similar levels as IL-2/CD40 alone (Fig. 6a, c). Importantly, IL-2/CD40 but not M1-stimulation overcame tumor-induced and age-related macrophages suppression leading to the restoration of the ability of T cells from elderly mice to secrete IFN-γ (Fig. 6b, d) and upregulate CD44 expression; the latter seen in proliferating CD8+ T cells (Fig. 6e).
Fig. 6.
IL-2/CD40 activated elderly-derived macrophages reverse tumor-induced and age-related elderly T cell dysfunction. Peritoneal macrophages from elderly C57BL/6J mice (a) or elderly Balb/c mice (c) were cultured overnight with AE17 (a) or AB1 (c) tumor cell-conditioned media (AE17 sup or AB1 sup), stimulated overnight with M1 (LPS/IFN-γ) or IL-2/anti-CD40 Ab then co-cultured with CFSE-labelled, elderly Balb/c (a) or C57BL/6J (c) splenic lymphocytes and proliferating CFSE+CD4+ T cells and CFSE+CD8+ T cells calculated; only macrophage: T cell ratio of 1:5 shown (a and c). Controls included no stimuli, AE17 sup only, M1 stimuli only, and IL-2/CD40 Ab only. MLR supernatants at the macrophage: T cell ratio of 1:2 were assayed for T cell-derived IFN-γ by CBA, as described above (b and d). Representative histograms for CD44 expression on proliferating Balb/c CD8+ T cells are shown in e; grey filled histogram indicates no stimuli, bolded line histogram indicates relevant stimuli
Discussion
Many cancers, including mesothelioma and lung carcinomas, emerge in aging populations. Yet little is known about the effect of aging on macrophage function in healthy hosts. Even less is known about their interactions with T cells and how this might impact on tumorigenesis and/or anti-cancer therapies in the elderly. This study furthers our previous work (Jackaman et al. 2013) and addresses lung carcinoma and mesothelioma-induced defects that are superimposed on age-related defects in macrophages and their interactions with T cells.
We report here that macrophage and MDSC proportions were significantly increased in spleens from elderly Balb/c relative to their younger counterparts and that most of these cells were immunosuppressive M2 cells. These data are in agreement with other studies (Dace and Apte 2008; Ly et al. 2010) including our recent study in C57BL/6J mice (Jackaman et al. 2013) and confirm that M2 and MDSC cells dominate key lymphoid organs in elderly mice in genetically different strains. Therefore, increasing immunosuppression during healthy aging could be a normal phenomenon in mice and, as shown by others, likely in humans as well (Gregg et al. 2005; Verschoor et al. 2013).
In agreement with our previous study in C57BL/6J mice, macrophages from young and elderly Balb/c mice responded similarly to M1 and M2 stimuli by upregulating surface CD40 or CX3CR1 molecules, respectively, and secreting the appropriate cytokines. These data are different to others who have reported decreased cytokine secretion from elderly macrophages (Corsini et al. 1999; Wallace et al. 1995). However, a recent conflicting study also showed that macrophages from aged mice produce higher cytokine levels (TNF and IL-6) following in vitro LPS stimulation (Bouchlaka et al. 2013). This discrepancy may reflect different technologies, different mouse strains or species, and/or macrophages from different tissue sites; the latter highlighted in a study showing that LPS-stimulated splenic macrophages, but not bone marrow-derived macrophages, from aging mice demonstrated an aberrant response (Mahbub et al. 2012). Importantly, our data from two different mouse strains suggest that despite a natural skewing towards M2 immunity, macrophages from aging hosts retain the capacity to respond to exogenous, and likely endogenous, signals. The latter was shown by studies examining rescue of geriatric muscle regeneration by the young host which is likely to be mediated by macrophages (Shavlakadze et al. 2010). Furthermore, a recent young-to-old parabiotic study (Ruckh et al. 2012) showed that macrophages from young mice reversed inefficient central nervous system remyelination in elderly mice. These studies suggest that intrinsic defects during aging can be reversed if given the correct stimuli. Thus, the data imply the potential of immune rescue by appropriate macrophage activation.
In our studies, examination of macrophage-T cell interactions showed that M1-stimulated macrophages from young and elderly mice induced CD8+ T cells from young and elderly mice to proliferate at similar levels. However, M1-stimulated macrophages from elderly animals could not induce young T cells to secrete the same levels of IFN-γ seen when using young-derived macrophages. These data identify an important age-related macrophage defect and imply that young but not elderly-derived macrophages present antigen in association with the correct co-stimulation required to induce functional T cells. These data are in agreement with others describing reduced antigen presentation capacity in macrophages from elderly hosts (Seth et al. 1990; Zissel et al. 1999).
We are particularly interested in the role of macrophages in solid tumors as, similar to normal healthy tissue, tumors including mesothelioma and lung carcinomas contain large numbers of macrophages (Burt et al. 2011; Jackaman et al. 2009). When we examined the potential role of macrophages in tumor development, we found that tumor-derived soluble factors polarized M2 macrophages, regardless of age. However, macrophages from elderly mice secreted higher levels of IL-4 in response to tumor-derived factors relative to their younger counterparts. These data imply that elderly-derived macrophages are particularly sensitive to signals that induce their development into highly immunosuppressive macrophages that could promote tumor development. Mesothelioma-exposed macrophages from young and elderly mice induced allogeneic CD8+ T cells to proliferate. However, these T cells, unlike those interacting with macrophages not exposed to mesothelioma factors, did not produce IFN-γ. One possible interpretation of these data is that CD8+ T cells activated by tumor-exposed macrophages, particularly macrophages in the tumor microenvironment, are unlikely to be functional and may not be able to lyse tumor cells. Taken together, these data suggest that, regardless of age, local macrophages will respond to tumor-derived signals by adopting an immunosuppressive phenotype that promotes tumor growth. However, macrophages from elderly hosts may provide an even more suppressed environment that readily promotes cancer development. Tumor-infiltrating CD8+ T cells could be compromised by these macrophages and lose their ability to kill target tumor cells.
Our previous studies showed that targeting large mesothelioma and lung carcinoma tumors with IL-2/anti-CD40 Ab-based immunotherapy induces a permanent cure (Jackaman et al. 2012a; Jackaman et al. 2008; Jackaman and Nelson 2012) mediated by T cells, neutrophils (Jackaman et al. 2008; Jackaman and Nelson 2012), NK cells (Jackaman et al. 2012a), and macrophages (manuscript submitted). However, those proof-of-principle studies were conducted in young adult mice, and both cancers generally emerge in elderly populations when T cell immunity is declining (Haynes and Maue 2009). Here, we showed that IL-2/anti-CD40 Ab-stimulated macrophages from young and elderly Balb/c mice secreted IFN-γ and TNF-α indicating polarization to an M1-like phenotype. Importantly, CD8+ T cells from elderly mice readily proliferated in response to interaction with IL-2/CD40-activated young-derived macrophages resulting in more cellular divisions than other stimuli including LPS/IFN-γ-activated M1 cells. Furthermore, the ability of elderly-derived CD8+ T cells to secrete IFN-γ was restored.
We extended these studies to examine the capacity of LPS/IFN-γ or IL-2/anti-CD40 Ab treatment to override age-induced and cancer (focussing on mesothelioma) driven macrophage dysfunction. To the best of our knowledge, no other similar study has been conducted. We showed that M1 stimuli only restored surface phenotype with no rescue of cytokine production. Furthermore, tumor-exposed, M1-stimulated macrophages could not induce T cells IFN-γ production. Interestingly, tumor-exposed, IL-2/CD40-activated macrophages demonstrated an incomplete skewing towards M1 cells as TNF-α, but not IFN-γ, was produced. This response was different to macrophages that had not been exposed to tumor-derived factors as their activation via IL-2/CD40 induced both TNF-α and IFN-γ production; IL-4 was not induced. Importantly, tumor-exposed, IL-2/CD40-activated macrophages from young and elderly mice induced CD4+ and CD8+ T cells to proliferate and secrete IFN-γ.
When we focussed on T cell dysfunction in elderly mice, we found that mesothelioma-exposed, IL-2/CD40-activated macrophages from young C57BL/6J mice induced equivalent levels of proliferation in CD4+ and CD8+ T cells from young and elderly mice. Interestingly, IL-2/CD40-driven macrophage rescue of IFN-γ secretion was more effective in elderly-derived than in young-derived T cells. This may be, at least in part, due to the increased CD4+ T cell proliferation seen in elderly-derived T cells relative to young-derived T cells. These data show that age- and tumor-induced macrophage defects can be partially but sufficiently, reversed to rescue T cell function measured by IFN-γ secretion. Further studies are required to address CD8+ cytolytic function.
These results are similar to others who showed that monocytes from aged hosts maintain their ability to differentiate into dendritic cells and activate effector function in senescent T cells (Lung et al. 2000). These data are also in agreement with studies suggesting that cytokines improve function of macrophages in the elderly (Hayakawa et al. 1995; Stout and Suttles 2005). Our data showed that use of an IL-2/anti-CD40 Ab-based immunotherapy could be effective in elderly cancer-bearing hosts. However, a recent study showed that systemic IL-2/anti-CD40 Ab induces rapid and lethal toxicity in aged mice, mediated by pro-inflammatory macrophages (Bouchlaka et al. 2013). Our previous studies observed high lethality in young mice treated systemically with IL-2/anti-CD40 Ab (Jackaman et al. 2008). This was overcome by targeting the treatment directly into the tumor (Jackaman et al. 2008). Therefore, future in vivo studies using intra-tumoral administration of IL-2/anti-CD40 Ab are required to delineate the direct and indirect (via CD8+ T cells and other effector cells) role of tumor-associated macrophages in elderly hosts.
Our data suggests that macrophages from elderly hosts are hypersensitive to environmental M1, M2, and tumor-derived stimuli. In particular, they are sensitive to immunosuppressive stimuli and therefore likely to provide a highly tumorigenic microenvironment. Nonetheless, macrophages from elderly mice maintain their capacity to transition into M1 or M2 cells. Activation via IL-2/CD40 not only restores the dual dilemma of tumor-induced and age-related macrophage dysfunction but also rescues age-related/tumor-induced T cell dysfunction. Therefore, targeting IL-2/CD40 activation overrides tumor-induced suppressive effects on macrophages regardless of age; thus, age-related macrophage defects can be rescued.
Acknowledgments
The authors acknowledge the provision of research facilities and the scientific and technical assistance of the staff of CHIRI Biosciences Research Precinct core facility, Curtin University. This work was carried out during the tenure of a grant from Cancer Council Western Australia.
References
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379–387. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DEC, Alderson KL, Hsiao H-H, Weiss JM, Monjazeb AM, Hesdorffer C, Ferruci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundell CS, Jackaman C, Suhrbier A, Robinson BW, Nelson DJ. Functional endogenous cytotoxic T lymphocytes are generated to multiple antigens co-expressed by progressing tumors; after intra-tumoral IL-2 therapy these effector cells eradicate established tumors. Cancer Immunol Immunother. 2006;55:933–947. doi: 10.1007/s00262-005-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117:5234–5244. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
- Chamoto K, Kosaka A, Tsuji T, Matsuzaki J, Sato T, Takeshima T, Iwakabe K, Togashi Y, Koda T, Nishimura T. Critical role of the Th1/Tc1 circuit for the generation of tumor-specific CTL during tumor eradication in vivo by Th1-cell therapy. Cancer Sci. 2003;94:924–928. doi: 10.1111/j.1349-7006.2003.tb01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappert P, Leboeuf M, Rameau P, Lalfer M, Desbois S, Liblau RS, Danos O, Davoust JM, Gross DA. Antigen-specific Treg impair CD8(+) T-cell priming by blocking early T-cell expansion. Eur J Immunol. 2010;40:339–350. doi: 10.1002/eji.200839107. [DOI] [PubMed] [Google Scholar]
- Corsini E, Battaini F, Lucchi L, Marinovich M, Racchi M, Govoni S, Galli CL. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-alpha production in rat macrophages. J Immunol. 1999;163:3468–3473. [PubMed] [Google Scholar]
- Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. Int J Cancer. 1992;52:881–886. doi: 10.1002/ijc.2910520609. [DOI] [PubMed] [Google Scholar]
- Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, Norwood C, Wang E, Marincola FM, Stroncek DF. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC, Singhal S, Snyder LA, Albelda SM. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol. 2011;44:230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H, Sato A, Yagi T, Uchiyama H, Ide K, Nakano M. Superoxide generation by alveolar macrophages from aged rats: improvement by in vitro treatment with IFN-gamma. Mech Ageing Dev. 1995;80:199–211. doi: 10.1016/0047-6374(95)01573-I. [DOI] [PubMed] [Google Scholar]
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, Nelson DJ. Cytokine-armed vaccinia virus infects the mesothelioma tumor microenvironment to overcome immune tolerance and mediate tumor resolution. Cancer Gene Ther. 2010;17:429–440. doi: 10.1038/cgt.2009.85. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Nelson DJ. Intratumoral interleukin-2/agonist CD40 antibody drives CD4(+)-independent resolution of treated-tumors and CD4 (+)-dependent systemic and memory responses. Cancer Immunol Immunother. 2012;61:549–60. doi: 10.1007/s00262-011-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20:1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Cornwall S, Lew AM, Zhan Y, Robinson BW, Nelson DJ. Local effector failure in mesothelioma is not mediated by CD4+ CD25+ T-regulator cells. Eur Respir J. 2009;34:162–175. doi: 10.1183/09031936.00101008. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Lansley S, Allan JE, Robinson BW, Nelson DJ. IL-2/CD40-driven NK cells install and maintain potency in the anti-mesothelioma effector/memory phase. Int Immunol. 2012;24:357–68. doi: 10.1093/intimm/dxs005. [DOI] [PubMed] [Google Scholar]
- Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012;61:2343–56. doi: 10.1007/s00262-012-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age-related immune deficiencies in C57BL/6 J geriatric mice. Aging Cell. 2013;12:345–357. doi: 10.1111/acel.12062. [DOI] [PubMed] [Google Scholar]
- Kapp JA, Honjo K, Kapp LM, Goldsmith K, Bucy RP. Antigen, in the presence of TGF-beta, induces up-regulation of FoxP3gfp + in CD4+ TCR transgenic T cells that mediate linked suppression of CD8+ T cell responses. J Immunol. 2007;179:2105–2114. doi: 10.4049/jimmunol.179.4.2105. [DOI] [PubMed] [Google Scholar]
- Leibovici J, Itzhaki O, Kaptzan T, Skutelsky E, Sinai J, Michowitz M, Asfur R, Siegal A, Huszar M, Schiby G. Designing ageing conditions in tumour microenvironment-a new possible modality for cancer treatment. Mech Ageing Dev. 2009;130:76–85. doi: 10.1016/j.mad.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lung TL, Saurwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/S0264-410X(99)00494-6. [DOI] [PubMed] [Google Scholar]
- Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van Rooijen N, van Hall T, van der Velden PA, Jager MJ. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol. 2010;185:3481–3488. doi: 10.4049/jimmunol.0903479. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther. 2000;7:624–632. doi: 10.1038/sj.gt.3301131. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Nagarkatti M, Nagarkatti PS, Subbarao B, Udhayakumar V. Macrophages but not B cells from aged mice are defective in stimulating autoreactive T cells in vitro. Mech Ageing Dev. 1990;52:107–124. doi: 10.1016/0047-6374(90)90118-Y. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 2010;11:363–376. doi: 10.1007/s10522-009-9260-0. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Smallwood HS, Lopez-Ferrer D, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50:9911–9922. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen I, Nelson DJ, Currie AJ, Jackaman C, Robinson BW. Intratumoral poly-N-acetyl glucosamine-based polymer matrix provokes a prolonged local inflammatory response that, when combined with IL-2, induces regression of malignant mesothelioma in a murine model. J Immunother. 2005;28:359–367. doi: 10.1097/01.cji.0000170361.37832.ec. [DOI] [PubMed] [Google Scholar]
- Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, Bowdish DM. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol. 2013;93:633–637. doi: 10.1189/jlb.0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace PK, Eisenstein TK, Meissler JJ, Jr, Morahan PS. Decreases in macrophage mediated antitumor activity with aging. Mech Ageing Dev. 1995;77:169–184. doi: 10.1016/0047-6374(94)01524-P. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissel G, Schlaak M, Muller-Quernheim J. Age-related decrease in accessory cell function of human alveolar macrophages. J Investig Med. 1999;47:51–56. [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]