Abstract
Clinically, heart failure is an age-dependent pathological phenomenon and displays sex-specific characteristics. The renin-angiotensin system mediates cardiac pathology in heart failure. This study investigated the sexually dimorphic functional effects of ageing combined with angiotensin II (AngII) on cardiac muscle cell function, twitch and Ca2+-handling characteristics of isolated cardiomyocytes from young (~13 weeks) and aged (~87 weeks) adult wild type (WT) and AngII-transgenic (TG) mice. We hypothesised that AngII-induced contractile impairment would be exacerbated in aged female cardiomyocytes and linked to Ca2+-handling disturbances. AngII-induced cardiomyocyte hypertrophy was evident in young adult mice of both sexes and accentuated by age (aged adult ~21–23 % increases in cell length relative to WT). In female AngII-TG mice, ageing was associated with suppressed cardiomyocyte contractility (% shortening, maximum rate of shortening, maximum rate of relaxation). This was associated with delayed cytosolic Ca2+ removal during twitch relaxation (Tau ~20 % increase relative to young adult female WT), and myofilament responsiveness to Ca2+ was maintained. In contrast, aged AngII-TG male cardiomyocytes exhibited peak shortening equivalent to young TG; yet, myofilament Ca2+ responsiveness was profoundly reduced with ageing. Increased pro-arrhythmogenic spontaneous activity was evident with age and cardiac AngII overexpression in male mice (42–55 % of myocytes) but relatively suppressed in female aged transgenic mice. Female myocytes with elevated AngII appear more susceptible to an age-related contractile deficit, whereas male AngII-TG myocytes preserve contractile function with age but exhibit desensitisation of myofilaments to Ca2+ and a heightened vulnerability to arrhythmic activity. These findings support the contention that sex-specific therapies are required for the treatment of age-progressive heart failure.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9630-7) contains supplementary material, which is available to authorized users.
Keywords: Angiotensin overexpression, Excitation-contraction coupling, Hypertrophy, Calcium, Ageing, Arrhythmogenesis, Failure
Introduction
Heart failure is an age-dependent pathological phenomenon, most prevalent in patients >65 years old (de Giuli et al. 2005). Age is the primary risk factor for cardiovascular disease with each 5-year increment over 65 years associated with 22 % increase in risk (Larson 1995). Despite this pivotal link between ageing and cardiovascular risk, studies investigating heart failure in aged animal models are lacking. Advances in treatment strategies over the past two decades have led to an improvement in heart failure survival. This improvement has been less marked in relation to outcomes for women (Roger et al. 2004) and attests to a sex disparity in the effectiveness of heart failure treatment. The epidemiology and clinical characteristics of heart failure are identified to be sex dependent (Barsheshet et al. 2012; Regitz-Zagrosek and Lehmkuhl 2005; Seeland and Regitz-Zagrosek 2012), and evidence from meta-analysis of clinical trials suggests that current heart failure therapies are more effective in men than in women (Barsheshet et al. 2012; Rabi et al. 2008; Shekelle et al. 2003). These data indicate important underlying sex differences in the cardiac pathology of ageing-associated failure. Experimentally, an age-dependent reduction in cardiomyocyte Ca2+ current influx with age has been identified in female but not in male rodents (Howlett 2010). Sex differences in cardiomyocyte dysfunction associated with the progression of heart failure have not yet been investigated.
It is well established that the renin-angiotensin system (RAS) is upregulated in heart failure (Unger and Li 2004) and usual treatment regimes include angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) (Ahmed 2003; Durand 2002). Experimental studies have identified that cardiac RAS upregulation appears to play an important role in ageing hearts. In addition to exerting systemic effects, local cardiac RAS actions have been well documented, and all components of the RAS are expressed in cardiac tissue (Lindpaintner and Ganten 1991). Left ventricular expression of angiotensinogen and ACE is selectively elevated in the hearts of aged (non-failing) male rodents despite an age-induced reduction in systemic RAS activity (Heymes et al. 1994). Myocardial expression of angiotensin II (AngII) receptor subtypes AT1R and AT2R is also upregulated in aged males, potentially mediating age-related cardiac pathology (Heymes et al. 1998). Experimentally, sex-specific vascular and renal effects of both systemic RAS stimulation and inhibition have been identified (Brown et al. 2012; Maric 2005; Sullivan 2008), but whether similar sex differences exist in the myocardial effects of RAS activation is unknown. Elevation of cardiac RAS activity in the aged female hearts would be expected, although this has not yet been demonstrated. An estrogen response element has been localised to the angiotensinogen promotor, and in relatively estrogen-suppressed aged females (where gonadal estrogen production is low), this may be instrumental in regulating local tissue AngII production (Feldmer et al. 1991).
A role for locally produced AngII in cardiac functional and structural remodelling has been demonstrated (Dostal and Baker 1993). We have previously reported that cardiac-specific AngII upregulation induces ventricular hypertrophy and modulates cardiomyocyte electromechanical coupling in male adult mice (Domenighetti et al. 2007; Domenighetti et al. 2005; Gusev et al.2009). In this transgenic mouse model, intracardiac AngII levels are elevated (Mazzolai et al. 2000), and progression to failure in males involves a transition from compensated, hypertrophic cardiomyopathy to a decompensated, dilated phenotype with altered intracellular Ca2+-handling dynamics (Domenighetti et al. 2005). No study to date has investigated these effects in females.
Given that (i) conventional heart failure treatments appear less effective in women than those in men, (ii) female (but not male) cardiomyocytes exhibit suppressed Ca2+ current with ageing and (iii) estrogen regulation of RAS components is evident, we hypothesised that AngII-induced contractile impairment would be exacerbated in aged female cardiomyocytes linked with Ca2+-handling disturbances. To elucidate the sex-specific effects of ageing and cardiac AngII elevation on myocardial function, isolated cardiomyocytes from young adult and aged adult male and female mice with genetically manipulated angiotensinogen overexpression (AngII-transgenic (TG)) were evaluated for contractile and Ca2+ disturbances. We demonstrate that ageing in AngII-TG females is associated with selective reduction in cardiomyocyte contractility. In contrast, cardiomyocytes of aged male AngII-TG maintain contractility but exhibit a marked decrease in myofilament Ca2+ responsiveness and heightened vulnerability to spontaneous Ca2+ release. This study provides the first mechanistic demonstration that ageing induces functionally disparate and sex-specific difference in heart failure progression in a setting of elevated cardiac AngII. The biology of ageing impacts on the development of cardiopathology differentially in males and females.
Methods
Animals and dietary treatments
The transgenic mouse line with cardiac overexpression of the angiotensinogen gene (Tg1306/1R) was created by Pedrazzini and colleagues (Lausanne, Switzerland) by the insertion of 30 copies of the rat angiotensinogen transgene into the mouse genome (Mazzolai et al. 1998). The genetic background of these mice is estimated to be ~99 % C57Bl/6. All mice were housed in temperature-controlled conditions in a 12-hr light/dark cycle and were cared for in accordance with the ‘Principles of laboratory animal care’ (NIH publication no. 85–23, revised 1985; http://grants1.nih.gov/grants/olaw/references/phspol.htm) and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes , and procedures were approved by the Animal Ethics Committee of the University of Melbourne (Approval no. 0703180 and no. 1112184). Male and female wild type (WT) and transgenic mice were used at young adult (~13 weeks old) and aged adult (~87 weeks old) age ranges.
Cardiomyocyte isolation
The hearts were excised from heparinised (100 IU, intraperitoneal (i.p.)) and anaesthetised mice (sodium pentobarbitone, 140 mg/kg i.p.), and the aorta was cannulated. The heart was retrogradely perfused with Ca2+-free HEPES-buffered Krebs (in millimolar, 150 NaCl, 5 KCl, 0.33 NaH2PO4, 1 MgCl2, 25 HEPES, 20 d-glucose, 3 Na pyruvate, 1 Na lactate, pH 7.4) at 2 ml/min at 37 °C for 10 min. Addition of type II collagenase (0.66 mg/ml, 295 U/mg, Worthington Biochemical Corporation, NJ, USA), CaCl2 (50 μM) and trypsin (33 μg/ml; Sigma-Aldrich, MO, USA) to the perfusate enabled heart digestion. The heart was then removed from the cannula, the atria dissected away, and the ventricles gently teased apart. Cells were dispersed in a high-potassium HEPES-buffered Krebs solution (in millimolar, 30 KCl, 90 KOH, 30 KH2PO4, 3 MgSO4, 50 Glutamate, 20 taurine, 0.5 EGTA, 10 d-glucose, 10 HEPES, pH 7.4) and resuspended in Ca2+-free HEPES-buffered Krebs with trypsin inhibitor (25 μg/ml; Sigma-Aldrich, MO, USA).
Cardiomyocyte Ca2+-handling and twitch analysis
Cardiomyocytes were loaded with the Ca2+ fluorescent dye, Fura2-AM (1 μM, 20-min incubation at 25 °C; Invitrogen, CA, USA). The Fura2 loading conditions provided an optimal signal-to-noise ratio without compromising myocyte inotropic status and responsiveness. Myocytes were superfused with 2-mM Ca2+ HEPES buffer (in millimolar, 146.2 NaCl, 4.69 KCl, 11 d-glucose, 0.35 NaH2PO4H2O, 1.05 MgSO47H2O, 10 HEPES) at 2.6 ml/min and field-stimulated at 4 Hz to establish steady state contractile performance (>5 min, 37 °C) followed by a low-frequency challenge (0.5 Hz) for assessment of spontaneous activity. Myocyte Ca2+ signals were measured by microfluorimetry (360:380 nm fluorescence ratio, 1,000-Hz sampling; IonOptix, MA, USA) as previously described (Mellor et al. 2012). Ca2+ transient time course was assessed by measurement of the time constant of decay (Tau; exponential curve set from 50 % decay). All fluorescent signals were corrected for background. Cardiomyocyte twitch properties were assessed by video-based edge detection (IonOptix, MA, USA) and analysed for peak shortening normalised to diastolic cell length (%S), maximum rate of shortening normalised to diastolic cell length (%MRS) and maximum rate of lengthening normalised to diastolic cell length (%MRL). All indices were analysed offline using IonWizard (IonOptix, MA, USA) and were determined after averaging ten steady state transients for each myocyte. Representative Ca2+-shortening phase loop plots were constructed as described (Bers 2001; Spurgeon et al. 1992). The Fura2 signal (F380:360) at 50 % myocyte relaxation (expressed as % change from diastolic Ca2+) was evaluated as an indicator of myofilament Ca2+ responsiveness.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from AngII-TG and WT male and female mouse cardiac ventricular tissues using the TRIzol® reagent in conjunction with the PureLinkTM Micro-to-Midi Total RNA Purification kit (Invitrogen, CA, USA) as per the manufacturer’s instructions and was reverse transcribed using SuperScript™ III First-Strand Synthesis System (Invitrogen, CA, USA). Real-time RT-PCR was used to determine the relative gene expression levels of AngII receptor type 1a (AT1aR). Real-time RT-PCR primer sequences for AT1aR are the following: Fwd 5′-TGCCATGCCCATAACCATCTG-3′ and Rev 5′-CGTGCTCATTTTCGTAGACAGG-3′. The comparative ΔΔCt method was utilised to analyse the genes of interest as described (26, 31).
Statistical analyses
Figures 1, 2 and 6 present raw data from all groups of mice. Figures 3–5 present data as a percentage of the young WT group for each sex. Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using SPSS V20 (SPSS Inc., IL, USA). Data were analysed by multi-way ANOVA with post hoc Fisher’s LSD tests where appropriate. Data were log transformed where appropriate to satisfy the assumption of equal variances. A p value < 0.05 was considered statistically significant.
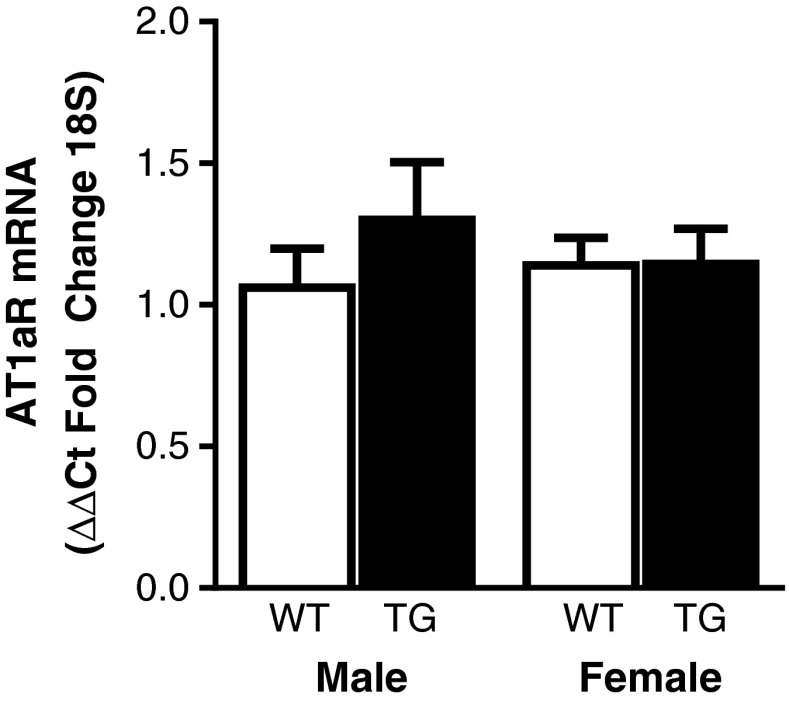
Fig. 7.
AngII type 1a receptor mRNA expression in cardiac ventricular tissue. AT1aR mRNA expression in AngII-TG and WT young adult male and female mouse cardiac ventricular tissue, n = 6–8. Data are presented as mean ± SEM
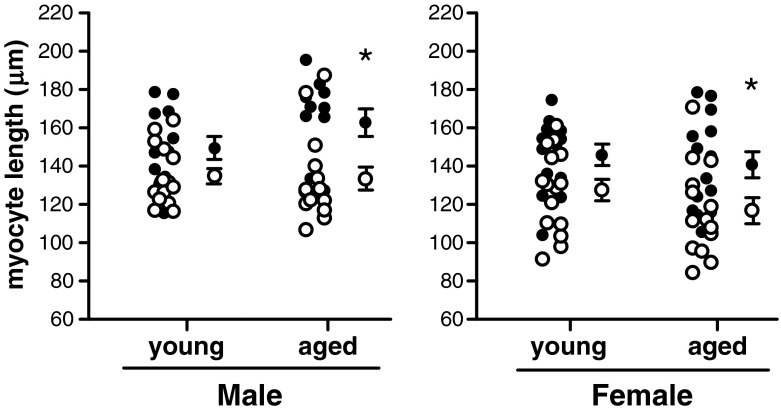
Fig. 1.
Diastolic cardiomyocyte length. Young adult and aged adult male and female cardiomyocytes from wild type (open circles) and AngII-TG (closed circles) mice. Individual data points (left) and mean ± SEM (right) for each group. n = 11–15 cells/group. *p(genotype) < 0.05, two-way ANOVA
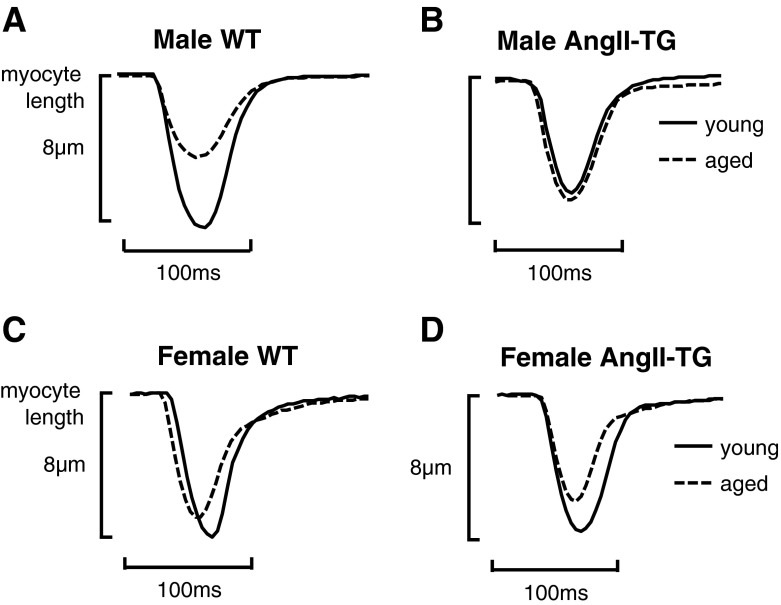
Fig. 2.
Cardiomyocyte twitch shortening profiles are modulated by age in AngII-TG male and female mice. Representative twitch profiles of young and aged adult male WT myocytes (a), young and aged adult male AngII-TG myocytes (b), young and aged adult female WT (c), and young and aged adult female AngII-TG myocytes (d)
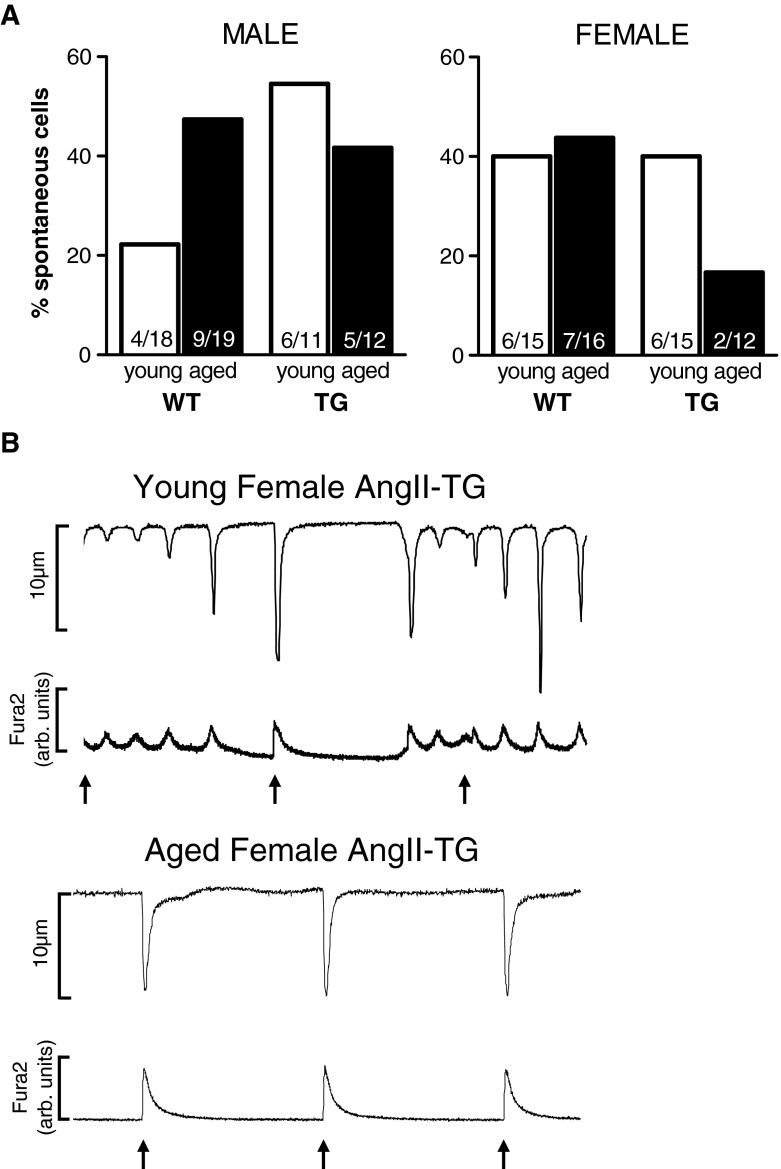
Fig. 6.
Incidence of spontaneous activity emergent at 0.5-Hz stimulation. a The percentage of cells that exhibited spontaneous activity (i.e. non-stimulated) when subjected to a low-frequency stimulation protocol (0.5-Hz stimulation). b Representative traces for young adult female AngII-TG and aged adult female AngII-TG cardiomyocytes stimulated at 0.5 Hz. Arrows depict stimulus
Fig. 3.
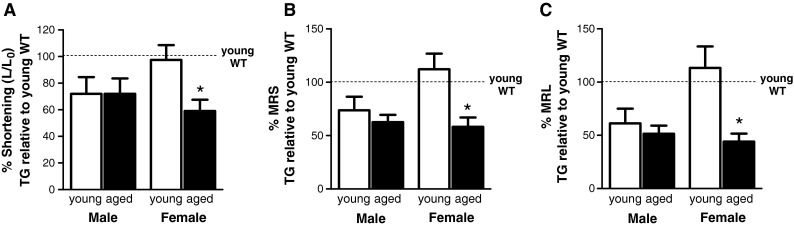
AngII-TG contractile performance—age- and sex-specific shifts compared with young adult WT. a Extent of twitch shortening normalised to diastolic length expressed as a percentage of young adult WT for each sex. b Maximum rate of shortening (MRS) normalised to cell length (L 0), expressed as a percentage of young adult WT for each sex. c Maximum rate of lengthening (MRL) normalised to cell length (L 0), expressed as a percentage of young adult WT for each sex. n = 11–14 cells/group. Data are presented as mean ± SEM. *p(age) < 0.05, p(interaction) < 0.05, two-way ANOVA
Fig. 5.
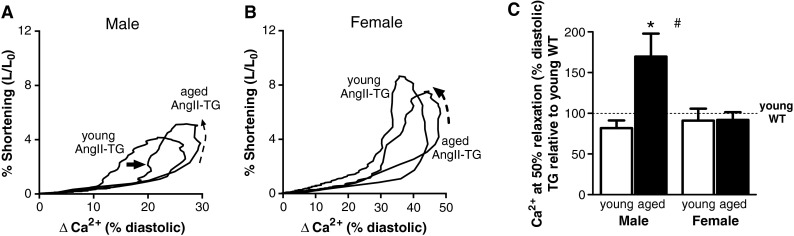
Myofilament Ca2+ responsiveness decreases with ageing in AngII-TG male mice only. a Representative Ca2+-shortening phase loops from young and aged male AngII-TG mouse cardiomyocytes. Dashed arrow shows the direction of twitch cycle; solid arrow highlights the right shift of the phase loop. b Ca2+-shortening phase loops from young and aged female AngII-TG mouse cardiomyocytes. Dashed arrow shows the direction of twitch cycle. c Intracellular Ca2+ change from diastolic Ca2+ at 50 % myocyte relaxation, expressed as percentage change from young adult wild type for each sex. n = 7–11 cells/group. Data are presented as mean ± SEM. *p(age) < 0.05, # p(sex) < 0.05, two-way ANOVA
Results
Animal and cardiomyocyte characteristics
WT and AngII-TG male and female mice were age matched for ‘young adult’ (13 ± 0.1 weeks) and ‘aged’ adult (87 ± 0.6 weeks) groups. The young adult age was chosen on the basis of previously established AngII-induced cardiac hypertrophy at this age (Mazzolai et al. 2000). The aged cohort was chosen to be within the last quartile of C57Bl/6 life span for females (average life span for females 113 weeks and males 125 weeks (Kunstyr and Leuenberger 1975)) and based on our previous report that significant AngII-induced mortality is evident at this age (Domenighetti et al. 2005).
In male WT, there was no ageing-associated increase in cardiomyocyte length dimension. In young males, cardiomyocytes of AngII-TG mice were elongated ~11 % relative to WT mice, and cellular hypertrophy was accentuated with age (aged male AngII-TG myocytes were approximately 22 % longer than aged male WT). In young female AngII-TG mice, cardiomyocytes were ~14 % longer relative to young WT. Interestingly, with ageing in females, neither WT nor AngII-TG myocytes elongated—indeed, myocytes of aged females were marginally (not significantly) shorter than myocytes from young adults (~8 % in WT and ~3 % in AngII-TG). Thus, cardiomyocytes of aged AngII-TG females were observed to be ~20 % longer than those of aged female WT mice (Fig. 1; diastolic cell length, two-way ANOVA p < 0.05). Cardiomyocyte hypertrophy was therefore confirmed for both male and female AngII-TG mice. The degree of cardiomyocyte hypertrophy induced by AngII overexpression increased with age in males due to AngII-TG myocyte enlargement whereas the apparent increase in degree of hypertrophy observed with age in females (20 % vs 14 % increase, aged vs young respectively) partially reflected by an age-associated decrement in myocyte length in WT mice.
Sex-specific effects of ageing and cardiac AngII on cardiomyocyte contractility
Representative cardiomyocyte twitch profiles from each group are depicted in Fig. 2. Male WT myocytes exhibited a diminished twitch contraction with age (Fig. 2a). In the males, no difference was observed in twitch contraction profiles between young and aged AngII-TG cardiomyocytes (Fig. 2b), primarily reflecting a reduced contractile state in the young AngII-TG compared with the young WT. Female WT mice exhibited a relatively modest reduction in twitch contraction amplitude with age (Fig. 2c). The twitch profile in young adult female myocytes was not modified by AngII overexpression, but in contrast to males, myocytes of aged AngII-TG females exhibited a marked reduction in the size of the twitch contraction (Fig. 2d).
A detailed analysis of ageing-related shifts in cardiomyocyte contractile parameters was undertaken to quantify mean group differences in twitch characteristics. The parameters employed to describe the twitch contraction are depicted in Supplementary Fig. S1a. The contractile parameters for AngII-TG mouse cardiomyocytes are presented normalised to young adult WT values for each sex (Fig. 3). Mean cardiomyocyte percent shortening (maximal change in length normalised to resting cell length) was significantly diminished by transgenic cardiac elevation of AngII in young male mice (~72 % of young WT levels, p < 0.05). A similar level of deficit was observed in aged male AngII-TG mice (p = 0.054 vs young WT). In myocytes of young females, percent shortening was not affected by cardiac AngII elevation, but aged female AngII-TG mice exhibited a significant and marked reduction in extent of myocyte shortening (Fig. 3a).
Similar patterns emerged for cardiomyocyte twitch kinetics. The maximum rate of myocyte shortening (expressed normalised to resting cell length, %MRS) was reduced in young male AngII-TG myocytes, ~74 % of WT values, but this effect did not reach statistical significance (p = 0.086). Similarly, a trend for reduced %MRS in aged male AngII-TG mice was observed, ~63 % of WT values (p = 0.054). Thus, %MRS in aged male AngII-TG myocytes was not different to young male AngII-TG. In AngII-TG female myocytes, %MRS was slightly faster than WT myocytes in the younger mice (difference not significant). In contrast, the aged AngII-TG female myocytes exhibited markedly slower %MRS (~58 % of young female WT values, p < 0.05; Fig. 3b). Cardiomyocyte maximum rates of lengthening (expressed normalised to resting cell length, %MRL) were significantly slower in young and aged male AngII-TG mice (~61 and ~51 % of young WT values, respectively, p < 0.05; Fig. 3c). Young female AngII-TG myocytes, however, exhibited preserved relaxation kinetics, but an age-related deficit in the rate of lengthening was observed (~44 % of WT values, p < 0.05). These data indicate that the age-dependent contractile response of females to AngII overexpression is distinctly different to males. No early deficit is observed, and a later substantial functional detriment is apparent. In contrast, male myocytes at both ages show an intermediate contractile dysfunction.
Refer to Supplementary material for full details of contractile parameter data from all groups of mice (Table S1).
Ageing selectively delays cytosolic Ca2+ removal in female myocytes of high-AngII mice
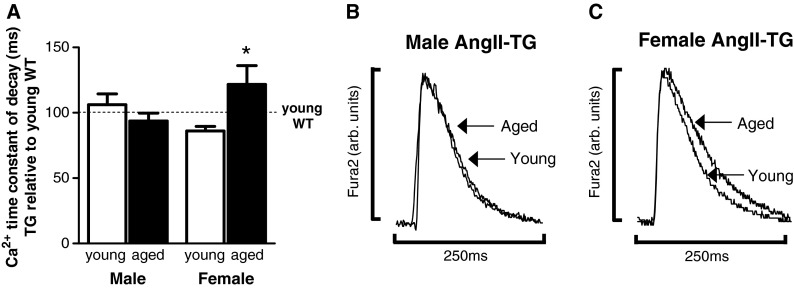
To determine whether the disparate sex effects of ageing on cardiomyocyte contractility were associated with underlying Ca2+-handling disturbances, Ca2+ transients were recorded from myocytes loaded with the intracellular Ca2+ fluorophore, Fura2. Diastolic Ca2+ levels were lower in male and female AngII-TG myocytes relative to WT in both age groups; no sex differences were apparent (Supplementary Fig. S2). The time constant of Ca2+ transient decay was similar in male WT and AngII-TG myocytes and was not different with age. In contrast, cardiac AngII overexpression slightly reduced the time constant of Ca2+ decay in young female mice (~86 % of young WT values), and this parameter was markedly increased in aged female AngII-TG mice (~122 % of young WT values, p < 0.05; Fig. 4a). Representative cardiomyocyte Ca2+ transients for male AngII-TG young and aged mice are shown in Fig. 4b and for female AngII-TG young and aged mice in Fig. 4c. The combined effects of ageing and elevated cardiac AngII appear to delay cytosolic Ca2+ removal selectively in females.
Fig. 4.
Age prolongs cardiomyocyte Ca2+ transient decay in AngII-TG female mice. Cardiomyocytes were loaded with 1-μM Fura2 Ca2+ dye to evaluate Ca2+ cycling. a Ca2+ transient time constant of decay (ms), expressed as a percentage of young adult WT for each sex. b Representative cardiomyocyte Ca2+ transient profiles for young and aged adult male AngII-TG mice. c Representative cardiomyocyte Ca2+ transient profiles for young and aged adult female AngII-TG mice. Traces have been normalised to match the amplitude to enable direct time course comparisons n = 9–11 cells/group. Data are presented as mean ± SEM. *p(genotype) < 0.05, p(interaction) < 0.05, two-way ANOVA
Refer to Supplementary material for full details of Ca2+ transient characteristics from all groups of mice (Table S1) and a schematic depicting the Ca2+ transient parameters (Fig. S1b).
Suppressed myofilament responsiveness to Ca2+ in aged AngII-TG male cardiomyocytes
Evidence of AngII-induced altered myofilament responsiveness to Ca2+ in young adult and aged adult male and female cardiomyocytes was sought. Examination of individual myocyte Ca2+-shortening relationships was undertaken using ‘phase loop’ analysis (Mellor et al. 2012; Spurgeon et al. 1992). These analyses map myocyte Ca2+ levels against cell length (expressed as % change from diastolic Ca2+ and cell length respectively) throughout the contraction and relaxation phases of the activation cycle. During the relaxation phase, the descending portion of the ‘loop’ provides a dynamic index of myofilament Ca2+ sensitivity (Bers 2001; Spurgeon et al. 1992). Exemplar myocyte Ca2+-shortening phase loop plots show a right shift in the relaxation phase in the aged male AngII-TG compared with young adult male AngII-TG (Fig. 5a), indicative of decreased myofilament responsiveness to Ca2+ (Spurgeon et al. 1992). Young and aged female AngII-TG myocytes exhibit similar Ca2+-shortening phase loop plots (Fig. 5b). The phase loop shift was quantified by assessment of intracellular Ca2+ at 50 % myocyte relaxation (expressed relative to the diastolic Ca2+ level). Aged male AngII-TG mouse cardiomyocytes exhibited an ~70 % increase in Ca2+ levels at 50 % myocyte relaxation relative to young male AngII-TG (p < 0.05; Fig. 5c). Female cardiomyocytes did not exhibit significant changes in Ca2+ at 50 % myocyte relaxation with age or AngII overexpression. These data are consistent with an age-related decrease in myofilament Ca2+ responsiveness in male cardiomyocytes but not in female cardiomyocytes, in a setting of elevated cardiac AngII.
Aged female cardiomyocytes with elevated cardiac AngII exhibit lower spontaneous activity
Progression to failure and arrhythmogenic predisposition is frequently linked with disturbed cardiomyocyte diastolic Ca2+ handling. Thus, evidence of an ageing-related, sex-specific difference in cardiomyocyte diastolic Ca2+ management was sought. Manipulation of diastolic interval was used to potentiate intracellular sarcoplasmic reticulum store Ca2+ uptake and evaluate Ca2+ overload predisposition. Myocyte pacing at a subphysiological frequency (0.5 Hz) and at physiological temperature (37 °C) was employed as a low-frequency challenge to evaluate the emergence of spontaneous (i.e. potentially arrhythmogenic) myocyte activity. The proportion of male WT cardiomyocytes that exhibited spontaneous Ca2+ release at 0.5-Hz stimulation increased with age (Fig. 6a). Forty-seven percent of young male myocytes with cardiac AngII overexpression were spontaneous at low frequency compared with 22 % of young male WT myocytes. Young and aged AngII-TG male myocytes exhibited similar levels of spontaneous activity. More young female WT myocytes (40 %) exhibited spontaneous activity than young male WT myocytes, but surprisingly, 90-week-old female myocytes with elevated cardiac AngII had the lowest proportion of cells exhibiting spontaneous activity (17 %) of all groups (Fig. 6a). Representative twitch and Ca2+ traces depicting the relative levels of spontaneous activity in young adult and aged adult female AngII-TG myocytes are presented in Fig. 6b. The time to first spontaneous Ca2+ release was not significantly different between groups (Table S2). These data suggest that the female cardiomyocyte adaptations to age and AngII may act to stabilise the sarcoplasmic reticulum Ca2+ store during a low-frequency challenge.
Sex-specific AngII-induced cardiomyocyte dysfunction is not linked to altered AT1aR expression
To determine whether sex differences in cardiomyocyte function observed with high AngII at the young adult stage were due to differences in the receptor for AngII, messenger RNA (mRNA) expression of AT1aR was measured in heart tissue of AngII-TG and WT male and female young adult mice using real-time RT-PCR. Assessment of mRNA expression (vs protein expression) is the preferred option for AngII receptors due to limitations relating to antibody specificity. AT1aR mRNA expression was similar in all groups suggesting that the sex-specific effects of elevated cardiac AngII on cardiomyocyte function were not linked to altered expression of the AT1aR.
Discussion
This is the first investigation to demonstrate distinctly different patterns of ‘functional ageing’ in cardiomyocytes in the presence and absence of genetic influence of heightened cardiac RAS activity. Comparing adult cellular functional phenotype in cardiomyocytes of rodents in the first and last life span quartiles, these data provide novel evidence that ageing is selectively associated with reduced contractility of female (but not male) cardiomyocytes when cardiac AngII is chronically elevated. Specifically, in mice genetically manipulated to overexpress angiotensinogen in the heart, ageing was associated with decreased extent of myocyte shortening and slower twitch contraction and relaxation rates in females. These kinetic shifts in myocytes of aged females were observed concomitant with delayed cytosolic Ca2+ removal during the relaxation phase of the twitch cycle and preserved myofilament responsiveness to Ca2+. In contrast, in male cardiomyocytes, AngII overexpression caused a similar extent of contractile suppression in young and aged myocytes. The observation that the contractile deficit in male myocytes was not accentuated with ageing suggests that ageing and AngII act by a convergent mechanism in male but not in female myocytes. Myofilament Ca2+ responsiveness was reduced with age in male AngII-TG myocytes only; yet, contractility was preserved. In males, an expected increase in spontaneous activity was observed with age and cardiac AngII overexpression, but surprisingly, aged female AngII-TG myocytes display a remarkable stability of SR Ca2+ stores during a low-frequency challenge. Female myocytes with elevated AngII appear more susceptible than male myocytes to an age-related contractile deficit whereas male AngII-TG myocytes preserve contractile function with age but exhibit desensitisation of myofilaments to Ca2+ and a heightened vulnerability to spontaneous activity. These findings validate that the hypothesis advanced.
Sex-specific progression of AngII-induced cellular hypertrophy with ageing
Cardiac RAS upregulation has been identified clinically and experimentally in settings of ageing-associated cardiopathology. Cardiac hypertrophy is a well-established structural phenotype linked with AngII elevation. The cardiac-specific angiotensinogen overexpressing transgenic mouse is a normotensive model of cardiac hypertrophy previously characterised to exhibit in vivo cardiac dysfunction and cardiomyocyte electromechanical coupling abnormalities evident by 15–20 weeks of age and transition to dilated failure with ~45 % reduced mortality at 94 weeks (Domenighetti et al. 2005; Gusev et al. 2009). Using this model, it is possible to investigate the direct hypertrophic and functional impacts of AngII excess. Cardiomyocyte hypertrophy was established in myocytes from aged AngII-TG animals, with the differential in myocyte size relatively similar in male and female cell populations (21–23 % myocyte elongation, Fig. 1). This is consistent with our previous finding that the extent of cardiac hypertrophy is similar in male and female AngII-TG mice (Domenighetti et al. 2005; Huggins et al. 2009). There is indication (non-significant finding) that in males, the pro-growth AngII influence is more marked with ageing, whereas for female myocytes, the effect of local AngII offsets a reduction in myocyte size that occurs with ageing in WT. This may suggest that there are underlying sex differences in ageing trophic responses in WT mice and this requires further investigation mechanistically. It has been previously reported that myocyte hypertrophy in this transgenic model (male mice at 8 and 20 weeks of age) is driven by an AngII-induced intrinsic mitogen-activated protein kinase (MAPK) signalling activation, rather than systemic influence (Pellieux et al. 2000). Whether this effect is mediated by autocrine/paracrine actions of AngII at sarcolemmal receptors (subtypes AT1R, AT2R) has not been determined. Baker et al. demonstrated that AngII has intracrine effects in neonatal cardiomyocytes and in vivo mouse hearts (Baker et al. 2004) and receptor-independent intracellular AngII actions on myocyte functional remodelling may play a role in the present study.
Cardiac AngII-induced contractile dysfunction is age-dependent in female myocytes
In the setting of elevated cardiac AngII, we determined that myocyte contractility in female mice was markedly reduced with age. AngII elevation was associated with functional deficit in young and aged male myocytes, but age per se did not confer additional detriment (Fig. 2). These findings demonstrate that there are fundamental differences in the mechanistic progression of cardiac ageing and functional deterioration in males and females. A significant reduction in cardiomyocyte extent of twitch shortening and rate of relaxation by cardiac AngII elevation was observed in male mice, but young and aged AngII-TG myocytes exhibited similar contractile deficit indicating that ageing and AngII may act via a convergent mechanism in male myocytes. These data are consistent with our previous study which demonstrated that significant detriment in cardiomyocyte contractile kinetics is observed in male AngII-TG, evident at the cellular and ex vivo heart level in the absence of fibrosis (Domenighetti et al.2005; Huggins et al. 2003). In the present study, a trend for lower extent of shortening and slower shortening kinetics was observed in young female versus male WT cardiomyocytes (Supplementary Table S1). These data are consistent with the findings reported in the literature (Ceylan-Isik et al. 2011; Howlett 2010). In contrast to Ceylan-Isik et al. (but consistent with Howlett et al.), we did not observe a difference in the time to peak myocyte shortening in young female and male myocytes. In WT mice, Howlett et al. reported that age-related cardiomyocyte functional impairment was less marked in female mice than that in male mice (Howlett 2010). Our findings extend this work by demonstrating that age-related female myocyte functional detriment emerges in a setting of high cardiac AngII, to a level which is comparable with that of males. At the young adult stage, when sex differences in AngII-TG cardiomyocyte function are most marked, AT1aR expression is not different in male or female AngII-TG versus WT hearts suggesting that the sex-specific effects of AngII are not associated with changes in receptor density.
The cardiac RAS is known to mediate many of the remodelling characteristics of heart failure and has also been shown to progressively increase in activity with age (Heymes et al. 1998; Heymes et al. 1994; Unger and Li 2004). Elevated cardiac AngII suppresses the fatty acid oxidation pathway and reduces insulin-dependent glucose transporter 4 (GLUT4) expression, albeit with a concomitant increase in GLUT1 (Pellieux et al. 2006). Thus, the provision and metabolism of both glycolytic and non-glycolytic substrates for ATP supply may be limiting in relation to maintenance of contractile function when cardiac AngII is chronically elevated. We have previously reported that AngII-TG male mice exhibit altered cardiomyocyte Ca2+ handling at 15–20 weeks of age. Reduced expression of SERCA2a, the ATP-dependent transporter responsible for loading/reloading activator Ca2+ from the cytosol into the sarcoplasmic reticulum stores for release during each twitch cycle is observed, associated with lower sarcoplasmic reticulum Ca2+ load (Domenighetti et al. 2005; Gusev et al. 2009). Similarly, AT1R overexpression is linked with a reduction in cardiac SERCA2 expression (Rivard et al. 2011). There is evidence that SERCA2 function may be specifically modulated by glycolytically derived ATP (Kockskamper et al.2005). In the present study, we observed delayed Ca2+ removal from the cytosol during twitch relaxation in aged AngII-TG female myocytes. This finding is consistent with AngII-induced SERCA2 downregulation and may explain the slower rate of twitch relaxation observed in this group (Fig. 2c). Recently, sex differences in cardiomyocyte Ca2+ handling have been reviewed in detail (Bell et al. 2013; Parks and Howlett 2013). In WT mice, SERCA2 expression has been reported to be not different or lower in female hearts relative to male (Ceylan-Isik et al. 2011; Parks and Howlett 2013). The effect of ovariectomy on SERCA2 expression decreases SERCA2 expression only with long-term hormone withdrawal (10-week duration (Bupha-Intr et al. 2009; Bupha-Intr and Wattanapermpool 2006)). Treatment of H9c2 cells with 17β-estradiol increases SERCA2 expression (Liu et al. 2007). Findings from the present study indicate that with ageing, female cardiomyocytes become more vulnerable to sarcoplasmic reticulum Ca2+ uptake impairment, a condition which has been generally linked to diastolic dysfunction (Asp et al. 2012). Age-related estrogen decline in females may underlie a sex-specific susceptibility to Ca2+ mishandling with high AngII and warrants further investigation.
Cardiac AngII elevation selectively decreases myofilament Ca2+ responsiveness in aged males
Aged male cardiomyocytes with elevated cardiac AngII exhibited a marked decrease in cardiomyocyte myofilament responsiveness to Ca2+, demonstrated by a right shift in the Ca2+-shortening phase loop plot (Fig. 5a). This effect was not observed in females (Fig. 5b). No previous study has investigated the effect of age and chronic cardiac AngII elevation on cardiomyocyte Ca2+ sensitivity. Interestingly, these findings contrast with the known myofilament ‘sensitisation’ to Ca2+ that occurs with acute AngII exposure by ex vivo perfusion (Ikenouchi et al. 1994; Mattiazzi 1997). An early study using skinned cardiomyocyte preparations investigated myofilament Ca2+ sensitivity in aged male rats and demonstrated that, although myofibrillar ATPase activity was reduced relative to young adult rats, no change in Ca2+ sensitivity was observed (Bhatnagar et al.1984). The findings from the present study suggest that elevated cardiac AngII reduces cardiomyocyte Ca2+ sensitivity with age in male mice only. The sex specificity of this action is very marked. While myocytes of both young and aged females preserved a Ca2+ responsiveness equivalent to young WT, in the male aged AngII-TG, there was a 70 % upward shift in the Ca-shortening relationship (Fig. 5c). These findings suggest that ageing and cardiac AngII act synergistically to suppress Ca2+ sensitivity in male cardiomyocytes only. The mechanism by which ageing and chronic AngII elevation modulate myofilament Ca2+ responsiveness is not clear but may involve a reduction in pHi, shift in the α:β-myosin heavy chain ratio or an increase in troponin-I phosphorylation. We have previously reported that an age-dependent reduction in Na+–H+ exchanger expression is evident in AngII-TG male mouse hearts (Domenighetti et al. 2001) thus impaired extrusion of intracellular H+ ions may contribute to a decrease in pHi and consequent decrease in myofilament Ca2+ responsiveness. Other investigators have identified a role for AngII-induced phosphorylation of troponin-I in reducing Ca2+ sensitivity in hyperglycaemic cardiomyocytes in vitro (Malhotra et al.2001). Whether this AngII action can be extrapolated to other disease settings (i.e. ageing-associated chronic AngII elevation) has not yet been established.
Spontaneous activity is attenuated with age in female AngII-TG myocytes
In this study, it is demonstrated that with ageing, male (but not female) WT cardiomyocytes exhibit increased spontaneous Ca2+ release. Young male AngII-TG myocytes also exhibit a similar increase in spontaneity relative to WT— but in the aged AngII-TG, there is no compounding of the effects of AngII and age to exacerbate the level of spontaneous activity (Fig. 6). It has been previously shown that cardiac angiotensinogen overexpression induces a prolonged QT interval coincident with reduced expression of IK1-related KCNJ2 and KCNJ12 potassium channels in male mice at 50–60 weeks of age. Isolated cardiomyocytes from these male AngII-TG mice also exhibited prolonged repolarisation phase of the action potential. This earlier study reported that male transgenic mice are more susceptible to arrhythmias, as measured by in vivo ECG analysis (Domenighetti et al.2007). Electrical disturbances may partially underlie our finding of heightened spontaneous activity in isolated male AngII-TG cardiomyocytes. Additionally, the observed increased time to peak Ca2+ transient in male AngII-TG myocytes may reflect prolonged Ca2+ release from the SR by ryanodine receptors, previously shown to contribute to arrhythmogenesis (Gomez and Richard 2004). Diastolic Ca2+ levels were lower in AngII-TG mice relative to WT in both age groups; thus, spontaneous activity is not related to elevated cytosolic Ca2+ during diastole.
Interestingly, female myocytes exhibit a markedly different response. Aged female myocytes with elevated cardiac AngII exhibit lower spontaneous activity in association with a marked contractile deficit. In females, irregular Ca2+ release from the SR may be prevented by the observed AngII-induced electromechanical coupling alterations (in particular, the prolonged Ca2+ transient observed in aged female AngII-TG mice), likely indicative of suppressed sarcoplasmic reticulum Ca2+ loading. It is notable that the marked reduction in spontaneous activity is observed in the presence of prolonged Ca2+ release (increased time to peak Ca2+ transient) suggesting that female aged AngII myocytes may exhibit compensatory mechanisms to prevent spontaneity in this pro-arrhythmogenic setting. Our previous study reported that female AngII-TG mice (34 weeks) exhibit significantly less percent ectopic beats relative to female WT mice during the first 10-min reperfusion post-ischaemia. Detailed analysis of arrhythmic events revealed that female AngII-TG mice exhibited lower incidence of ventricular premature beats and ventricular tachycardia and shorter duration of bigeminy relative to female WT mice. Ventricular fibrillation was detected in the female WT hearts but not in AngII-TG hearts (Huggins et al.2009). The lower level of spontaneous activity observed in aged female AngII-TG myocytes in the present study is consistent with the lower incidence of arrhythmias identified in vivo in Huggins et al. (2009). Interestingly, these findings are consistent with clinical observations of arrhythmia-related mortality. The Framingham Heart Study reported that women with heart failure have lower incidence (approximately one quarter) of sudden cardiac death than men (Kannel et al.1998). Thus, female-specific cardiac functional adaptations in aged failing hearts may limit occurrence of arrhythmogenic events—yet confer failure liability.
In conclusion, this is the first study to demonstrate that cardiomyocyte functional state deteriorates with age in a sex- and AngII-dependent manner. Female myocytes appear more susceptible to an age-related contractile deficit in a setting of high cardiac AngII but exhibit a low level of spontaneous activity. In contrast, aged male myocytes exposed to elevated cardiac AngII maintain contractile function relative to young adult myocytes, but a profound desensitisation of myofilaments to Ca2+ is apparent, associated with a heightened vulnerability to arrhythmic activity. The findings from this study provide important mechanistic insight into the progression of ageing- and AngII-related cardiac pathology in males and females and support the contention that sex-specific therapies are required for the treatment of age-progressive heart failure. This may be particularly relevant to the clinical targeted use of AngII-directed pharmacological intervention.
Electronic supplementary material
(DOC 71 kb)
Contributor Information
Kimberley M. Mellor, Email: k.mellor@auckland.ac.nz
Claire L. Curl, Email: ccurl@unimelb.edu.au
Chanchal Chandramouli, Email: c.chandramouli@student.unimelb.edu.au.
Thierry Pedrazzini, Email: thierry.pedrazzini@chuv.ch.
Igor R. Wendt, Email: igor.wendt@monash.edu.au
Lea M. D. Delbridge, Phone: +61-3-83445853, FAX: +61-3-83445818, Email: lmd@unimelb.edu.au
References
- Ahmed A. American College of Cardiology/American Heart Association Chronic Heart Failure Evaluation and Management guidelines: relevance to the geriatric practice. J Am Geriatr Soc. 2003;51:123–126. doi: 10.1034/j.1601-5215.2002.51020.x. [DOI] [PubMed] [Google Scholar]
- Asp ML, Martindale JJ, Heinis FI, Wang W, Metzger JM (2012) Calcium mishandling in diastolic dysfunction: mechanisms and potential therapies. Biochim Biophys Acta [DOI] [PMC free article] [PubMed]
- Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Barsheshet A, Brenyo A, Goldenberg I, Moss AJ. Sex-related differences in patients' responses to heart failure therapy. Nat Rev Cardiol. 2012;9:234–242. doi: 10.1038/nrcardio.2012.10. [DOI] [PubMed] [Google Scholar]
- Bell JR, Bernasochi GB, Varma U, Raaijmakers AJ, Delbridge LM (2013) Sex and sex hormones in cardiac stress-mechanistic insights. J Steroid Biochem Mol Biol [DOI] [PubMed]
- Bers DM (2001) Excitation-contraction coupling and cardiac contractile force, 2nd edn. Kluwer Academic, Dordrecht
- Bhatnagar GM, Walford GD, Beard ES, Humphreys S, Lakatta EG. ATPase activity and force production in myofibrils and twitch characteristics in intact muscle from neonatal, adult, and senescent rat myocardium. J Mol Cell Cardiol. 1984;16:203–218. doi: 10.1016/S0022-2828(84)80587-8. [DOI] [PubMed] [Google Scholar]
- Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension. 2012;59:129–135. doi: 10.1161/HYPERTENSIONAHA.111.178715. [DOI] [PubMed] [Google Scholar]
- Bupha-Intr T, Laosiripisan J, Wattanapermpool J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol. 2009;107:1105–1112. doi: 10.1152/japplphysiol.00407.2009. [DOI] [PubMed] [Google Scholar]
- Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2006;291:H1101–H1108. doi: 10.1152/ajpheart.00660.2005. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Li Q, Ren J. Insulin-like growth factor I (IGF-1) deficiency ameliorates sex difference in cardiac contractile function and intracellular Ca(2+) homeostasis. Toxicol Lett. 2011;206:130–138. doi: 10.1016/j.toxlet.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Giuli F, Khaw KT, Cowie MR, Sutton GC, Ferrari R, Poole-Wilson PA. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur J Heart Fail. 2005;7:295–302. doi: 10.1016/j.ejheart.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Boixel C, Cefai D, Abriel H, Pedrazzini T. Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation. J Mol Cell Cardiol. 2007;42:63–70. doi: 10.1016/j.yjmcc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Pedrazzini T, Delbridge LMD. Abnormal contractile function in cardiomyocytes from transgenic mice overexpressing AngII in the heart. Proc. Renin Angiotensin-Aldosterone System Satellite of the XXXIV. Melbourne: Congress of the International Union of Physiological Science; 2001. [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. Evidence for a role of an intracardiac renin-angiotensin system in normal and failing hearts. Trends Cardiovasc Med. 1993;3:67–74. doi: 10.1016/1050-1738(93)90039-9. [DOI] [PubMed] [Google Scholar]
- Durand JB (2002) Current guidelines in heart failure management. Ethn Dis 12:S1-3-11 [PubMed]
- Feldmer M, Kaling M, Takahashi S, Mullins JJ, Ganten D. Glucocorticoid- and estrogen-responsive elements in the 5'-flanking region of the rat angiotensinogen gene. J Hypertens. 1991;9:1005–1012. doi: 10.1097/00004872-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Richard S. Mutant cardiac ryanodine receptors and ventricular arrhythmias: is 'gain-of-function' obligatory? Cardiovasc Res. 2004;64:3–5. doi: 10.1016/j.cardiores.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Gusev K, Domenighetti AA, Delbridge LM, Pedrazzini T, Niggli E, Egger M. Angiotensin II-mediated adaptive and maladaptive remodeling of cardiomyocyte excitation-contraction coupling. Circ Res. 2009;105:42–50. doi: 10.1161/CIRCRESAHA.108.189779. [DOI] [PubMed] [Google Scholar]
- Heymes C, Silvestre JS, Llorens-Cortes C, Chevalier B, Marotte F, Levy BI, Swynghedauw B, Samuel JL. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139:2579–2587. doi: 10.1210/endo.139.5.6023. [DOI] [PubMed] [Google Scholar]
- Heymes C, Swynghedauw B, Chevalier B. Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation. 1994;90:1328–1333. doi: 10.1161/01.CIR.90.3.1328. [DOI] [PubMed] [Google Scholar]
- Howlett SE. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol Heart Circ Physiol. 2010;298:H659–H670. doi: 10.1152/ajpheart.00214.2009. [DOI] [PubMed] [Google Scholar]
- Huggins CE, Curl CL, Patel R, McLennan PL, Theiss ML, Pedrazzini T, Pepe S, Delbridge LM. Dietary fish oil is antihypertrophic but does not enhance postischemic myocardial function in female mice. Am J Physiol Heart Circ Physiol. 2009;296:H957–H966. doi: 10.1152/ajpheart.01151.2008. [DOI] [PubMed] [Google Scholar]
- Huggins CE, Domenighetti AA, Pedrazzini T, Pepe S, Delbridge LM. Elevated intracardiac angiotensin II leads to cardiac hypertrophy and mechanical dysfunction in normotensive mice. J Renin Angiotensin Aldosterone Syst. 2003;4:186–190. doi: 10.3317/jraas.2003.030. [DOI] [PubMed] [Google Scholar]
- Ikenouchi H, Barry WH, Bridge JH, Weinberg EO, Apstein CS, Lorell BH. Effects of angiotensin II on intracellular Ca2+ and pH in isolated beating rabbit hearts and myocytes loaded with the indicator indo-1. J Physiol. 1994;480(Pt 2):203–215. doi: 10.1113/jphysiol.1994.sp020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- Kockskamper J, Zima AV, Blatter LA. Modulation of sarcoplasmic reticulum Ca2+ release by glycolysis in cat atrial myocytes. J Physiol. 2005;564:697–714. doi: 10.1113/jphysiol.2004.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstyr I, Leuenberger HG. Gerontological data of C57BL/6 J mice. I. Sex differences in survival curves. J Gerontol. 1975;30:157–162. doi: 10.1093/geronj/30.2.157. [DOI] [PubMed] [Google Scholar]
- Larson MG. Assessment of cardiovascular risk factors in the elderly: the Framingham Heart Study. Stat Med. 1995;14:1745–1756. doi: 10.1002/sim.4780141604. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K, Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991;68:905–921. doi: 10.1161/01.RES.68.4.905. [DOI] [PubMed] [Google Scholar]
- Liu CG, Xu KQ, Xu X, Huang JJ, Xiao JC, Zhang JP, Song HP. 17Beta-oestradiol regulates the expression of Na+/K + -ATPase beta1-subunit, sarcoplasmic reticulum Ca2 + -ATPase and carbonic anhydrase iv in H9C2 cells. Clin Exp Pharmacol Physiol. 2007;34:998–1004. doi: 10.1111/j.1440-1681.2007.04675.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Kang BP, Cheung S, Opawumi D, Meggs LG. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes. 2001;50:1918–1926. doi: 10.2337/diabetes.50.8.1918. [DOI] [PubMed] [Google Scholar]
- Maric C. Sex differences in cardiovascular disease and hypertension: involvement of the renin-angiotensin system. Hypertension. 2005;46:475–476. doi: 10.1161/01.HYP.0000178600.88820.b2. [DOI] [PubMed] [Google Scholar]
- Mattiazzi A. Positive inotropic effect of angiotensin II. Increases in intracellular Ca2+ or changes in myofilament Ca2+ responsiveness? J Pharmacol Toxicol Methods. 1997;37:205–214. doi: 10.1016/S1056-8719(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.HYP.31.6.1324. [DOI] [PubMed] [Google Scholar]
- Mazzolai L, Pedrazzini T, Nicoud F, Gabbiani G, Brunner HR, Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000;35:985–991. doi: 10.1161/01.HYP.35.4.985. [DOI] [PubMed] [Google Scholar]
- Mellor KM, Wendt IR, Ritchie RH, Delbridge LM. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am J Physiol Heart Circ Physiol. 2012;302:H964–H972. doi: 10.1152/ajpheart.00797.2011. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch. 2013;465:747–763. doi: 10.1007/s00424-013-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellieux C, Aasum E, Larsen TS, Montessuit C, Papageorgiou I, Pedrazzini T, Lerch R. Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol. 2006;41:459–466. doi: 10.1016/j.yjmcc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Pellieux C, Sauthier T, Aubert JF, Brunner HR, Pedrazzini T. Angiotensin II-induced cardiac hypertrophy is associated with different mitogen-activated protein kinase activation in normotensive and hypertensive mice. J Hypertens. 2000;18:1307–1317. doi: 10.1097/00004872-200018090-00017. [DOI] [PubMed] [Google Scholar]
- Rabi DM, Khan N, Vallee M, Hladunewich MA, Tobe SW, Pilote L. Reporting on sex-based analysis in clinical trials of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker efficacy. Can J Cardiol. 2008;24:491–496. doi: 10.1016/S0828-282X(08)70624-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Lehmkuhl E. Heart failure and its treatment in women. Role of hypertension, diabetes, and estrogen. Herz. 2005;30:356–367. doi: 10.1007/s00059-005-2718-1. [DOI] [PubMed] [Google Scholar]
- Rivard K, Grandy SA, Douillette A, Paradis P, Nemer M, Allen BG, Fiset C. Overexpression of type 1 angiotensin II receptors impairs excitation-contraction coupling in the mouse heart. Am J Physiol Heart Circ Physiol. 2011;301:H2018–H2027. doi: 10.1152/ajpheart.01092.2010. [DOI] [PubMed] [Google Scholar]
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. Jama. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- Seeland U and Regitz-Zagrosek V (2012) Sex and gender differences in cardiovascular drug therapy. Handb Exp Pharmacol 211–236 [DOI] [PubMed]
- Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. doi: 10.1016/S0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- Spurgeon HA, duBell WH, Stern MD, Sollott SJ, Ziman BD, Silverman HS, Capogrossi MC, Talo A, Lakatta EG. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1220–R1226. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5(Suppl 1):S7–S10. doi: 10.3317/jraas.2004.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 71 kb)