Abstract
Currently, the oxidative stress (or free radical) theory of aging is the most popular explanation of how aging occurs at the molecular level. Accordingly, a stress-induced senescence-like phenotype of human dermal fibroblasts can be induced in vitro by the exposure of human diploid fibroblasts to subcytotoxic concentrations of hydrogen peroxide. However, several biomarkers of replicative senescence e.g. cell cycle arrest and enlarged morphology are abrogated 14 days after treatment, indicating that reactive oxygen species (ROS) rather acts as a trigger for short-term senescence (1–3 days) than being responsible for the maintenance of the senescence-like phenotype. Further, DNA-damaging factors are discussed resulting in a permanent senescent cell type. To induce long-term premature senescence and to understand the molecular alterations occurring during the aging process, we analyzed mitomycin C (MMC) as an alkylating DNA-damaging agent and ROS producer. Human dermal fibroblasts (HDF), used as model for skin aging, were exposed to non-cytotoxic concentrations of MMC and analyzed for potential markers of cellular aging, for example enlarged morphology, activity of senescence-associated-ß-galactosidase, cell cycle arrest, increased ROS production and MMP1-activity, which are well-documented for HDF in replicative senescence. Our data show that mitomycin C treatment results in a drug-induced accelerated senescence (DIAS) with long-term expression of senescence markers, demonstrating that a combination of different susceptibility factors, here ROS and DNA alkylation, are necessary to induce a permanent senescent cell type.
Keywords: Aging, Mitomycin C, DNA damage, Reactive oxygen species, Replicative senescence, Drug-induced accelerated senescence
Introduction
The in vitro aging of human dermal fibroblasts (HDF), first described by Hayflick and Moorhead (Hayflick and Moorhead 1961), has become a classical experimental model for cellular aging and has been used to indentify aging-associated changes in human cells. Human cells derived from different tissues irreversibly stop dividing after a certain number of cumulative population doublings (CPDs), if they were cultured in vitro. This “limit in replicative capacity” occurs after a characteristic number of cell divisions and results in terminally arrested cells with altered physiology and is termed replicative senescence (RS) (Cristofalo and Pignolo 1993).
Replicative senescence is a stable, irreversible loss of proliferative capacity with several phenotypical and biochemical changes, which are used as markers to identify senescent cells. Even though, senescent HDF irreversibly lose their proliferative activity but remain metabolically active and differ from their mitotic counterparts in cellular, molecular, and biochemical parameters like morphology (Bayreuther et al. 1988), senescence-associated ß-galactosidase activity (Dimri et al. 1995) or gene expression (de Magalhães et al. 2004).
Sub-cultivations of nearly all human somatic cells lead to a proliferation stop, which is called replicative senescence (RS) (Hayflick and Moorhead 1961). As an important factor for the occurrence of replicative senescence, the shortening of telomeres to a critical length during DNA replication was discussed (Campisi and d’Adda di Fagagna 2007). Furthermore, it is described that not alone the telomere length, but rather a telomere dysfunction is responsible for replicative senescence (Gilley et al. 2005). Telomere dysfunction and other factors, e.g., oxidative stress (Reaper et al. 2004) activate signaling pathways resulting in premature senescence, which is called “stress-induced premature senescence” (SIPS) (Toussaint et al. 1992; Campisi 2005). Pro-oxidants like H2O2 (Toussaint et al. 2000) and DNA-damaging substances like busulfan (Probin et al. 2007), a cytostatic, alkylating agent can induce transient premature senescence without the shortening of telomeres. A transient, temporary cell cycle arrest is described, after 3 days of H2O2 treatment (Frippiat et al. 2001) or 11 days after busulfan treatment (Probin et al. 2007). However, a permanent senescence resembling the in vivo situation is not described.
A very popular theory to explain the occurrence of RS is the oxidative stress theory, which was proposed at the end of the 1950s. This theory was first described by Harman as the “free radical theory of aging” and suggested that oxygen free radicals (OH·, HO2·) formed endogenously as byproducts from metabolic processes play an essential role in aging via oxidative damage and alteration of gene expression (Harman 1956). Over the years, that theory has been converted into the oxidative stress theory of aging, as non-radicals such as H2O2 or other peroxides play a role in oxidative damage as well. Oxidative damage accumulates in a variety of macromolecules like lipids, DNA, or proteins and results in a progressive decline in the function of cellular processes, finally resulting in the aging phenotype. Accordingly, a stress-induced premature senescence (SIPS)-like phenotype of dermal fibroblasts can be induced in vitro by exposure of the cells to subcytotoxic concentrations of H2O2 or other stressors such as doxorubicin (Robles et al. 1999), UV-A (Wlaschek et al. 2000), and UV-B irradiation (Brenneisen et al. 2002) or cis-platinum (Wang et al. 1998). SIPS possesses the standard features of replicative senescence: similar morphology, cell cycle arrest, SA-ß-galactosidase activity, among others (Toussaint et al. 2000). Even though the SIPS model of cellular aging resembles replicative senescence, SIPS is a transient phenomenon in contrast to RS. ROS alone do not result in a permanent senescent phenotype.
In that context, we herein report that the drug mitomycin C (MMC) is a valuable tool in the accelerated formation of a permanent senescent phenotype. MMC is an antibiotic isolated from Streptomyces caespitosus and other Streptomyces bacterial species. Bioreduced mitomycin C generates oxygen radicals, alkylates DNA, and produces interstrand as well as intrastrand DNA cross-links, thereby inhibiting DNA synthesis. Preferentially toxic to hypoxic cells, mitomycin C also inhibits RNA and protein synthesis at high concentrations. As a bifunctional alkylating agent, it is predominantly used in tumor therapy as a monotherapy or combination therapy for the treatment of gastrointestinal adenocarcinomas and lung cancer (Paz et al. 1999). The concentration of MMC used as an anti-cancer drug is significantly higher than the concentration of MMC to induce accelerated permanent senescence. Low levels of mitomycin C induce irreversible cell senescence in human non-small cell lung carcinoma A549 cells and in BRCA1 defective cells (McKenna et al. 2012; Santarosa et al. 2009). Stopping proliferation and inducing senescence in cancer cells is interesting, but undergoes completely other mechanisms than in normal cells. However, we would like to mention that our study has a focus on inducing accelerated senescence in human dermal fibroblasts to build up an aging model which is prepared quickly and shows identical characteristics of replicative senescent cells so that the process of aging could be analyzed and understood in more detail. MMC is both a known producer of reactive oxygen species and has the ability to crosslink DNA. So it combines at least two initiating factors described to be involved in cellular aging, ROS (Ames et al. 1993) and alkylation (Robles et al. 1999). MMC reacts at the N2-position of guanine forming crosslinked DNA adducts (Tomasz et al. 1988). The main mechanism of MMC as redox cycler is the reduction of the chinon to a semichinon (Tomasz 1995). During this reduction, the semichinon is able to react with O2, and superoxide anion will be generated (Wang et al. 2010). An accelerated differentiation of fibroblasts after MMC treatment is also described (Bayreuther et al. 1988; Brenneisen et al. 1994). Even if aging has been suggested to be a multicausal process linked to a variety of molecular and cellular alterations, almost all popular theories of aging focus on a single physiological cause of aging. MMC combines several theories of aging, the DNA damage theory (Failla 1958) and the theory of free radicals (Bayreuther et al. 1988). Therefore, it is a valuable drug to induce permanent senescence and to analyze the molecular mechanisms underlying cellular aging.
The aim of this study was to induce long-term senescence with MMC to analyze cellular aging in time lapse comparable with cells in replicative senescence.
Material and methods
Cell culture media (Dulbecco’s Modified Eagle’s Medium (DMEM)) was purchased from Invitrogen (Karlsruhe, Germany), and the defined fetal calf serum (FCS gold) was from PAA Laboratories (Linz, Austria). All chemicals including protease as well as phosphatase inhibitor cocktail 1 and 2 were obtained from Sigma (Taufkirchen, Germany) or Merck Biosciences (Bad Soden, Germany) unless otherwise stated. The protein assay kit (Bio-Rad DC, detergent-compatible) was from Bio-Rad Laboratories (München, Germany). The enhanced chemiluminescence system (SuperSignal West Pico/Femto Maximum Sensitivity Substrate) was supplied by Pierce (Bonn, Germany).
Polyclonal rabbit antibody phospho-p53 (Ser15) was supplied by Cell Signaling (Frankfurt a. M., Germany). Monoclonal mouse anti-human Ki67 was supplied by Dako (Denmark). Monoclonal anti-GAPDH was produced in mouse (Sigma, Taufkirchen, Germany). The following secondary antibodies were used: goat anti-rabbit horseradish peroxidase (HRP) antibody from Dianova (Hamburg, Germany) and FITC rabbit anti-mouse from Dako (Denmark).
Cell culture
Human dermal fibroblasts were isolated from foreskin tissues from a 3-year-old boy. First, dermis and epidermis were separated by thermolysin in 10-mM Hepes buffer (2.5-mg/ml KCl, 2.9-mg/ml NaCl, 0.7-mg/ml CaCl2) treatment overnight at 4 °C (Sigma, Taufkirchen, Germany).
The connective tissue was dissociated by incubation in a 10,000-U/ml collagenase solution for 3 h at 37 °C (Sigma, Taufkirchen, Germany). The isolated fibroblasts were harvested by centrifugation (5 min, 1,000 rpm) and seeded with a density of 6,000 cells/cm2 in cell culture flasks containing a fibroblast growth medium. Fibroblasts were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with Glutamax supplemented with 10 % fetal calf serum, 100 IU/ml of penicillin, and 100 μg/ml of streptomycin in humidified 95 % air, 5 % CO2, and 37 °C.
Exponentially growing cells (mitotic cells) at passage 2–15, corresponding to cumulative population doubling levels (CPD) of 3–40, were used for all experiments. Replicative senescent HDF were prepared by serial subcultivation until complete exhaustion of the proliferative capacity at a CPD between 55 and 60. These fibroblasts had a growth rate less than 0.05 population doublings (PD) for three consecutive weeks. For the induction of an accelerated senescence, cells at about 70 % confluence were exposed twice to mitomycin C (200 nM) for 24 h. That means that the fibroblasts were exposed to MMC two times for 24 h. MMC-containing medium was added to subconfluent fibroblasts. After 24 h, the medium was replaced by a freshly prepared MMC-containing medium for additional 24 h. Afterwards, the cells were washed and cultured in fresh medium for 5 days, if not otherwise mentioned. The others stressors were incubated for the indicated time (see Table 1) and thereafter incubated as it was done for the MMC treatment, meaning that the cells were cultured in fresh medium for 5 days, if not otherwise mentioned.
Table 1.
Prospective substances for induction of senescence
Cell viability
The effect of mitomycin C (MMC; Sigma, Taufkirchen, Germany), busulfan (BU; Sigma, Taufkirchen, Germany), and H2O2 (Riedel de Häen, Seelze, Germany) on cell viability was measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) assay (Mosmann 1983). The activity of mitochondrial dehydrogenases, as indicator of cellular viability, results in the formation of a purple formazan dye. Briefly, MTT solution (0.5 mg/ml) was added to the fibroblasts for 3 h. The medium was removed, and the cells were lysed in dimethyl sulfoxide (Roth, Karlsruhe, Germany). The intensity of the formazan dye was measured at 590 nm. The results were presented as percentage of mock-treated control which was set at 100 %.
BrdU incorporation assay
DNA synthesis in untreated, SIPS, and RS cells was determined by measuring BrdU incorporation into DNA with the BrdU proliferation assay kit (Merck, Darmstadt, Germany). Shortly, the cells were incubated with BrdU-label for 12 h. After fixation and denaturation for 30 min, the fibroblasts were incubated with primary antibody for 4 h at RT. After washing three times with PBS, they were incubated with secondary antibody for 1 h at RT, and after another washing step, a substrate solution was added for 30 min at RT. After adding the stop solution, the extinction was immediately measured at 450 nm.
Immunocytochemistry
HDF monolayer cultures were grown in DMEM plus 10 % (v/v) FCS on coverslips in a 3.5-cm diameter tissue culture dishes before use. Cells were washed with PBS and fixed with methanol for 10 min at 4 °C. After washing with PBS, non-specific binding of antibodies was blocked with 3 % (v/v) NGS (normal goat serum) in Tris-buffered saline with Tween 20 (TBST) containing 0.3 % (v/v) Triton X-100 at room temperature (20 °C). Cells were incubated with polyclonal 53BP1 antibody (Cell Signaling, Frankfurt) and diluted with 1:1,000 in 1 % (v/v) NGS/TBST overnight at 4 °C. After washing, the cells were incubated with an Alexa 546-coupled goat anti-mouse IgG (1/1,000 diluted in TBST) for 1 h at 37 °C. For DAPI staining, cells were incubated for 10 min at room temperature with 1:500 diluted DAPI solution (Sigma, stock solution 0.5-mg/10 ml H2O) in McIlvaine’s buffer (100-mM citric acid, 200-mM Na2HPO4; pH 7.2). After washing and embedding, images were taken with a Zeiss Axiovert fluorescence microscope with a CCD camera.
Ki67 staining
HDFs were grown in a fibroblast growth medium at 37 °C in a 5 % CO2 atmosphere on chamber slides. For indirect immunofluorescent staining, subconfluent cells (∼70 %) were washed three times with PBS and then fixed with ice-cold methanol for 5 min. Cells were incubated with primary antibody (1:20 dilution in 10 % normal goat serum; Sigma, Taufkirchen, Germany) overnight at 4 °C in a humidified chamber. After washing three times in PBS for 5 min at RT, cells were incubated with secondary antibodies (1:20 dilution in 10 % normal goat serum; Sigma, Taufkirchen, Germany) and DAPI (1:1,000; Sigma, Taufkirchen, Germany) for 1 h at RT. Cells were then washed three times with PBS and mounted with Cytoseal (Thermo Scientific, Waltham, MA, USA). Slides were viewed using a fluorescence microscope (BX51, Olympus, Hamburg, Germany), and images were taken with the imaging software cellF (Olympus, Hamburg, Germany). To calculate the percentage of Ki67-positive cells, the number of positive cells was divided by the total number of cells counted indicated by nuclear staining with DAPI.
Senescent-associated ß-galactosidase activity
The proportion of HDFs positive for SA-ß-gal activity was determined as described by Dimri et al. (4). The cells were plated at a low density of 500/cm2 and fixed after 21 days with 4 % formaldehyde (Roth, Karlsruhe, Germany). The presence of SA-ß-gal activity was determined by incubation with 1-mg/ml solution of 5-bromo-4-chloro-3-indolyl ß-d-galactopyranoside in 0.04-M citric acid/sodium, 5-mM K3FeCN6 (MP Bio), 5-mM K4FeCN6 (MP Bio), 150-mM NaCl (Invitrogen), 2-mM MgCl2 (Invitrogen) diluted in phosphate-buffered saline (pH6). Blue color stained cells were identified as SA-ß-gal-positive cells by means of a standard light microscope.
Enzyme-linked immunosorbent assay for MMP1
The presence of matrix metalloproteinase-1 (MMP1) in culture media was quantified using the Quantikine ELISA Human Pro-MMP1 (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Shortly, the supernatants of mitotic and senescent fibroblasts were collected after 48 h in a serum-free fibroblast medium and incubated for 24 h in a microplate at 37 °C and 5 % CO2, which was precoated with MMP-1 antibody. After a washing step, the secondary antibody was added and incubated for 2 h at RT. After washing away the secondary antibody, the substrate solution was added for 20 min, and the absorption was measured at 450 nm.
Determination of cell size
HDFs were seeded at a low density of 500/cm2 and cultured until they express their cell type-specific morphology (3). Thereafter, they were washed with phosphate-buffered saline (PBS), fixed with 4 % formaldehyde (Roth) for 5 min at RT, and stained for 1 min with 0.1 % Coomassie Brilliant Blue (Merck). Cells were analyzed by standard light microscopy and photographed to document morphological characteristics. Cell size was quantified by encircling a minimum of 10 single cells of mitotic or senescent fibroblasts and measuring the area with ImageJ (NIH).
Measurement of cell size
The fibroblasts were seeded with a cell density of 1 × 104 cells per chamber of a 4-well chamber slide (Nunc, Wiesbaden, Germany). After 3 days, the medium was replaced by a fresh culture medium, and 7 days after seeding the cells, a morphology staining was performed. Therefore, the cells were fixed for 5 min in 4 % formaldehyde (Roth) and afterwards stained with Coomassie Brilliant Blue (Merck, Darmstadt, Germany). After washing with PBS, the slides were embedded with Cytoseal (Thermo Scientific, Waltham, MA, USA) and microscopically examined. For the analysis of the cell size, three image sections per cell type of three independent experiments were analyzed. The size of five cells per image was measured by encircling with the ImageJ software.
Cell cycle analysis
The analysis of the cell cycle of fibroblasts was performed by flow cytometry after propidium iodine staining. Propidium iodide is a DNA dye that intercalates into the DNA and thus shifts its emission maximum of 519 nm to 617 nm. The stained cells are individually drawn through a capillary tube and passes through a laser beam whose light is differently scattered depending on the cell characteristics. The emission of the fluorescent light is detected, and the intensity gives information on the DNA content of the cell and thus over the cell cycle.
For analysis, the cells were cultured in 175-cm2 tissue culture flasks and trypsinized with a trypsin-EDTA solution. The cell suspension was mixed 1:1 with FB medium to stop the reaction and centrifuged (1,000 rpm, 5 min). After centrifugation, the cell pellet was first washed twice with PBS (2 ml), and then ice-cold methanol (1 ml) was added dropwise. The fixed cells could now be stored for several weeks in the refrigerator. After fixation, the cells were centrifuged (1,000 rpm, 5 min), the supernatant was discarded, washed with 2 ml of PBS, and then incubated with 350 ul of RNase (50 μg/ml) for 30 min at 37 ° C. After RNase digestion, 20 μl of propidium iodide was added and measured after 5 min by flow cytometry. In a flow cytometer (Epics XL-MCL), the intensity of the fluorescent light emitted from each cell is measured and the DNA content per cell determined. Ten thousand cells were measured per batch, and then the measured values using the software Expo32 MultiComp were evaluated.
Measurement of intracellular ROS
Generation of ROS was determined using 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA; Sigma), a dye that diffuses across the lipid membranes into cells and is subsequently oxidized by intracellular ROS forming the highly fluorescent dichlorodihydrofluorescein (DCF).
Subconfluent HDF were exposed to H2O2 (150 μM; 2 h), busulfan (BU; 120 μM; 24 h), and mitomycin C (MMC; 200 nM; 2 × 24 h) and cultured for different time points in serum-free DMEM at 37 °C and 5 % CO2. Untreated subconfluent mitotic HDF were used as negative controls. Afterwards, a medium has been exchanged by 100-μm H2DCF-DA containing phosphate-buffered saline (PBS), and cells were incubated for 30 min at 37 °C before the solution was replaced by PBS. DCF fluorescence was detected at an excitation wavelength of 485 nm and emission wavelength of 520 nm after 90 min in a fluorescence plate reader (Tecan, Männedorf, Schweiz, Germany). Mean fluorescence intensities and standard error of mean (s.e.m.) were determined.
SDS-PAGE and Western blotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the standard protocols published elsewhere (Laemmli 1979) with minor modifications. Briefly, cells were lysed 5 days after incubation with MMC in 1 % SDS with 1:1,000 protease inhibitor cocktail (Sigma; Taufkirchen, Germany). After sonication, the protein concentration was determined by using a modified Lowry method (Bio-Rad DC). 4x SDS-PAGE sample buffer (1.5 M Tris-HCl pH 6.8, 6 ml 20 % SDS, 30 ml glycerol, 15 ml ß-mercaptoethanol and 1.8 mg bromophenol blue) was added, and after heating, the samples (3 5 μg total protein/lane) were applied to 10 % (w/v) SDS-polyacrylamide gels. After electroblotting, immunodetection was carried out (1:1,000 dilution of primary antibodies (polyclonal rabbit anti-phospho-p53Ser15 (Cell Signaling), 1:10,000 dilution of goat anti-rabbit HRP antibody from Dianova (Hamburg, Germany). Antigen-antibody complexes were visualized by an enhanced chemiluminescence system. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control for equal loading.
Statistical analysis
Means were calculated from at least three independent experiments, and error bars represent standard error of the mean (s.e.m.). Analysis of statistical significance was done by Student’s t test or ANOVA with *p < 0.05, **p < 0.01, and ***p < 0.001 as levels of significance.
Results
In this study, the question was addressed of whether mitomycin C is able to induce a premature and permanent senescence in human dermal fibroblasts.
To induce telomere-independent, accelerated, and permanent senescence, the following substances were tested (s. Table 1): hydrogen peroxide (H2O2), mitomycin C (MMC), and busulfan (BU). The concentration as well as the incubation time was adjusted from literature. As a control (ct) for mitotic cells, subconfluent fibroblasts up to passage 15 were used, reflecting a cumulative population doubling of a maximum of 40.
Cytotoxicity of senescence-inducing substances in fibroblasts
It is described that subtoxic concentrations of H2O2 or busulfan induce premature cellular aging (Probin et al. 2007; Frippiat et al. 2001). The impact on cell viability of the substances used herein to induce senescence was analyzed with a viability assay (MTT assay).
The cell viability H2O2, busulfan (BU), and mitomycin C (MMC) was tested after 5 days of incubation, which showed an increase of 145 ± 20 % (H2O2), 160 ± 30 %, or 180 ± 35 % (MMC) (Fig. 1a). The viability was analyzed immediately after treatment as well which resulted in no toxic effect (data not shown).
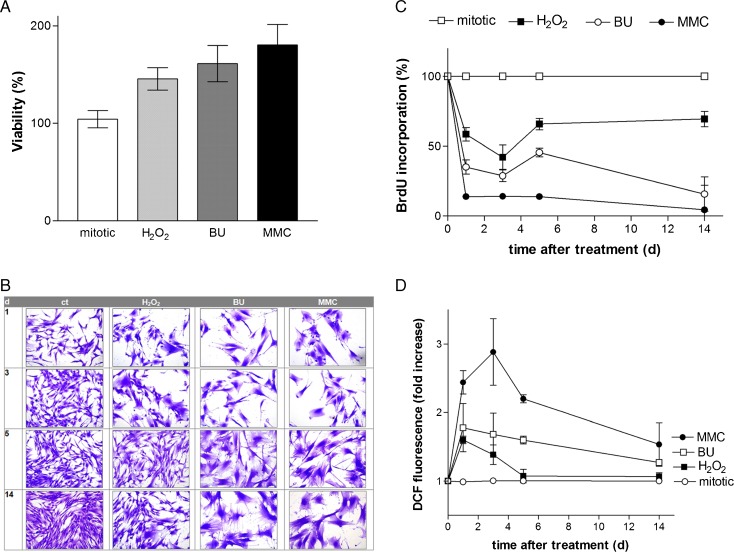
Fig. 1.
a Cytotoxicity of senescence-inducing substances. Subconfluent human dermal fibroblasts (passages 2–15) were incubated with H2O2 (150 μM; 2 h), busulfan (BU; 120 μM; 24 h), and mitomycin C (MMC; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium (DMEM, 10 % FCS, 100-IU/ml penicillin, 100-μg/ml streptomycin). The percentage of living cells was measured 5 days after treatment using MTT assay. The results were presented as a percentage of mock-treated control which was set at 100 %. The experiments were performed in three independent experiments. The data represent the mean ± s.e.m. of three independent experiments. b Morphological changes of cell structure. Subconfluent fibroblasts (passages 2–15) were incubated with H2O2 (150 μM; 2 h), busulfan (BU; 120 μM; 24 h), and mitomycin C (MMC; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2. After 1, 3, 5, and 14 days, the cells were fixed with 4 % formaldehyde (Roth) and stained with Coomassie Brilliant Blue (Merck). The experiments were performed in three independent experiments, and representative images were shown. Magnification is 40-fold. c Proliferation of mitotic and senescent fibroblasts. Subconfluent fibroblasts (passages 2–15) were incubated with H2O2 (150 μM; 2 h), busulfan (BU; 120 μM; 24 h), and mitomycin C (MMC; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium. The proliferative capacity was measured at days 1, 3, 5, and 14 after treatment with the BrdU assay kit (Merck) in comparison to mitotic fibroblasts which were used as control (ct) and set to 100 %. The data represent the mean ± s.e.m. of three independent experiments. d Time course analysis of the ROS production. Subconfluent fibroblasts (passages 2–15) were treated with H2O2 (150 μM; 2 h), busulfan (BU; 120 μM; 24 h), or mitomycin C (MMC; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium. Subsequently, the intracellular ROS level was determined 1, 3, 5, and 14 days after treatment using the DCF measurement. The data represent the mean ± s.e.m. of three independent experiments. The ROS level represents the fold increase over control, which was set at 1
Effect of senescence-inducing agents on cell morphology
Replicative senescence in fibroblasts results in an enlarged cell size (Mitsui and Schneider 1976). To check whether the treatment with H2O2, busulfan, or MMC leads to a senescent cell morphology, fibroblasts were stained with Coomassie Brilliant Blue. In Fig. 1b, representative images are shown. An increase in cell size was observed until 3 days after treatment with H2O2 (Fig. 1b, H2O2, day 1 + 3). Five and 14 days after treatment, only a few fibroblasts showed an enlarged size (Fig. 1b, H2O2, day 5 + 14). At these time points, most fibroblasts showed a spindle-shaped cell form, a characteristic of mitotic (proliferating) fibroblasts. By contrast, treatment with busulfan or MMC resulted in an enlarged cell type even after 14 days of treatment, which is in line with the described morphological criteria of “differentiated” (aged) fibroblasts (Tomasz 1995). Mock-treated (mitotic) fibroblasts (ct) had a spindle-shaped cell form with bulges around the nucleus (Fig. 1b, ct, day 1–14). A typical indicator of senescent fibroblasts is a 10–20-fold increase of the cell surface with a big, flat, and oval nucleus. Cells treated with busulfan (Fig. 1b, busulfan, day 5 + 14) or mitomycin C (Fig. 1b, MMC, day 5 + 14) showed this morphology after 5 or 14 days, whereas with H2O2 alone had no effect (Fig. 1b, H2O2, day 5 + 14), indicating that H2O2 does not result in a permanent senescent cell type. Even after 6 months, MMC-treated fibroblasts did not start to proliferate, and they maintained their senescent morphology (data not shown).
Impact of senescence-inducing substances on cell proliferation
An important marker of replicative senescent fibroblasts is the permanent cell cycle stop. How H2O2, busulfan, and mitomycin C can induce the inhibition of proliferation, and thereby inducing the cell cycle arrest, was measured by means of the bromodeoxyuridine-5-bromo-2′-deoxyuridine (BrdU) assay. The incorporation of the thymidine analog into newly synthesized DNA can be used as a marker for the proliferative capacity of cells. Figure 1c shows the proliferation over time after treatment. H2O2 induced a temporary inhibition of proliferation. Immediately after treatment (H2O2, 1d), a decrease to 58 % compared to mock-treated control cells was observed (set to 100 %). After 3 days of treatment, the proliferation decreased to 42 %, but increased already after 5 days to 66 %.
After 14 days, the growth arrest was abrogated, and the proliferation rate reached 70 % again. The treatment with busulfan resulted in a lowered proliferation rate of 35 % at day 3 and a minimum after 14 days to a level of 15 %. However, mitomycin C induced a permanent proliferation stop during cultivation of 14 days. MMC-treated fibroblasts showed no proliferative activity over the studied time period (Fig. 1c).
Reactive oxygen species (ROS) play a central role in the aging process of fibroblasts (Robles et al. 1999). To investigate the influence of H2O2, busulfan, and mitomycin C on intracellular ROS level of fibroblasts, fibroblasts were stained with 2,7 dichlorodihydrofluorescein diacetate (H2DCF-DA), which is oxidized intracellularly by ROS to the fluorescent dichlorofluorescein (DCF). The fluorescence intensity of the dye is proportional to the amount of ROS.
After treatment with H2O2, the reactive oxygen species level increased already after 1 day with a maximum of 1.5-fold increase compared with the mock-treated control (Fig. 1d). After 14 days, there was no significant increase detectable. The ROS level increased immediately after treatment with busulfan (BU), and after 3 and 5 days, a 1.7-fold increase was measured compared to untreated control. After 14 days, a not significant 1.3-fold increase was measured. Treatment with mitomycin C (MMC) showed a 3-fold increase of ROS level after 3 days compared to mock-treated control. After 14 days, the ROS level was 1.5-fold higher compared to untreated control cells.
In summary, treatment with H2O2 induced a transient senescence. This senescence was maintained over 3 days posttreatment accompanied by an increased level of intracellular ROS as well as an enlargement of the cell morphology, which was abolished 14 days after H2O2 treatment. Thus, ROS alone appears to be insufficient to sustain a permanent senescent cell type. Both treatment with busulfan and mitomycin C resulted in an increase in cell size over the studied time period. No increased level of reactive oxygen species was measured 14 days after treatment with busulfan and MMC.
However, treatment with mitomycin C resulted in a permanent exhaustion of the proliferative capacity in contrast to busulfan-treated cells, which is a characteristic of premature senescent and replicative senescent cells.
The data prompt at least different senescence-initiating factors to induce accelerated permanent cellular senescence. The redox cycler and alkylating drug mitomycin C fulfills that criteria and therefore was used for further experiments to study the mechanisms of cellular aging (DIAS = drug-induced accelerated senescence). By the use of MMC, at least two aging theories are combined, the free radical theory of aging and DNA damage theory of aging.
The age of a cell culture can be expressed by the cumulative population doubling (CPD) as the sum of the traversed total population doublings. Figure 2a shows the evolution of population doublings of untreated cells up to the status of replicative senescence (RS) compared to the MMC-induced senescent cells (DIAS).
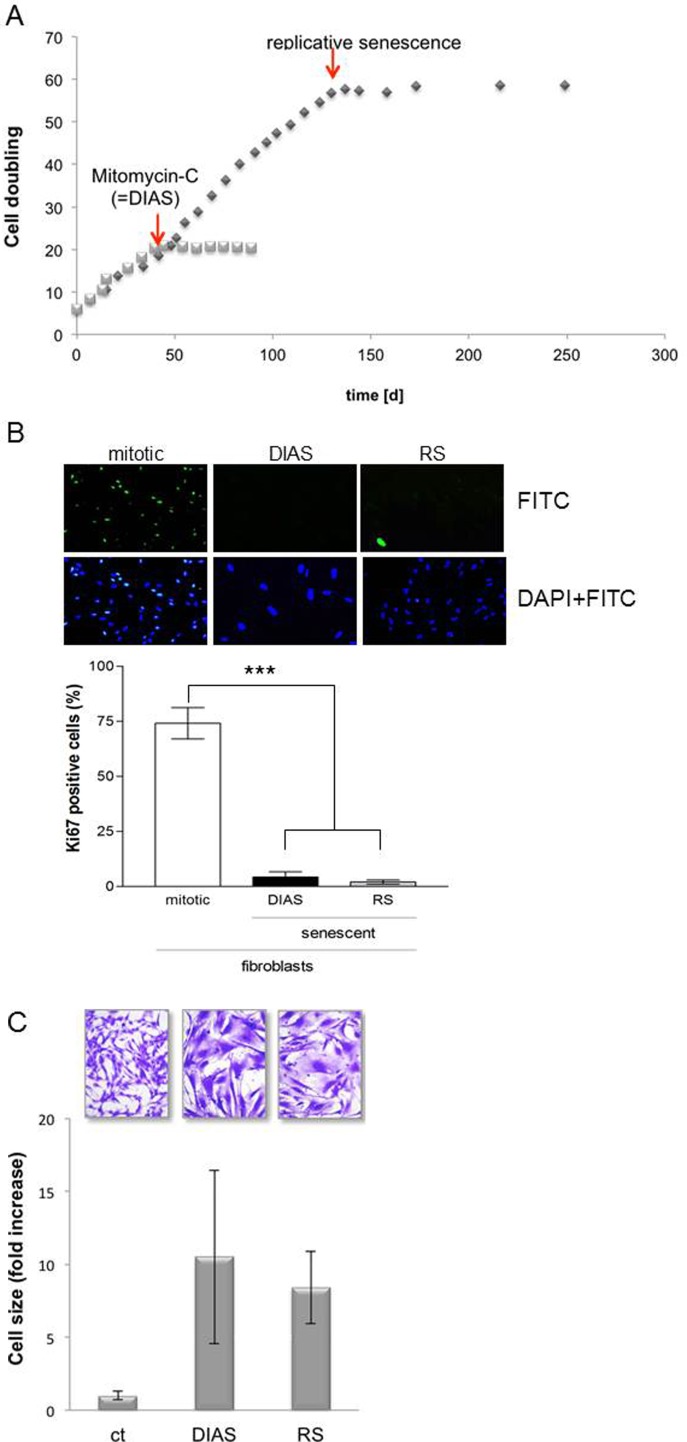
Fig. 2.
a Time course analysis of population doublings. To receive replicative senescent cells (passages 55–60), dermal fibroblasts (passages 2–15) were serially subcultured over a period of 250 days at 37 °C and 5 % CO2 in a fibroblast growth medium. For the induction of accelerated senescence, mitotic fibroblasts in the exponential phase were treated with mitomycin C (DIAS; 200 nM, 2 × 24 h). By calculating the cell population doublings (CPD), the growth behavior has been documented. b Ki67 staining of fibroblasts. Subconfluent fibroblasts (passages 2–15) were incubated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium, and proliferative capacity was analyzed in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts (passages 2–15) were used as control (ct). Five days after treatment, the percentage of Ki67-positive cells related to total number of cells was determined. The data represent means ± s.e.m. of three independent experiments with a minimum of three images of ct, DIAS, and RS cells. Representative images are shown (***p < 0.001; one-way ANOVA). Magnification is 20-fold. c Morphology and cell size of mitotic and senescent fibroblasts. Subconfluent fibroblasts (passages 2–15) were incubated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium, and morphology was analyzed in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts (passages 2–15) were used as control (ct). A minimum of five cells per image were analyzed by encircling single cells and measuring the area with ImageJ. The cell size of the mitotic fibroblasts was normalized to 1. Shown are representative images (×20 magnification) and the mean values ± standard deviation of three independent experiments (n = 3)
At the beginning of the growth curve, the fibroblasts proliferated exponentially and had a PD of average 3/week. After approximately 170 days and a total of 57 population doublings in the exponential phase, the stationary phase was reached with a PD at 0.02/week. This stagnancy of proliferation after approximately 170 days designated the beginning of replicative senescence. Treatment with mitomycin C induced a premature permanent growth arrest with a PD 0.08/week. The MMC treatment of mitotic fibroblasts resulted immediately in a growth arrest, which was observed over a period of 2 months and thus could be permanently maintained.
Effect of senescence on proliferation
The proliferation of replicative senescent fibroblasts (RS) is similar to MMC-induced senescent (DIAS) fibroblasts (data not shown). Both cell types showed an inhibitory effect regarding BrdU incorporation with less than 50 % proliferative capacity and thus a significant decrease compared to mock-treated, mitotic fibroblasts (ct). To confirm the results of the BrdU assay, the expression of the protein Ki67 was evaluated using immunofluorescent technique. Ki67 is expressed in the nucleus of mitotic fibroblasts, but not in senescent cells. Thus, Ki67 is a good marker to check the proliferative capacity of cells. The Ki67 staining showed a significantly decreased Ki67 expression in replicative senescent (RS) as well as in MMC-induced senescent (DIAS) cells compared to mitotic fibroblasts (ct) (Fig. 2b).
With the increasing replicative senescence of fibroblasts, an increase in cell size was described (Mitsui and Schneider 1976). To determine whether the MMC-induced senescent (DIAS) as well as the replicative senescent (RS) fibroblasts shows an increase in cell size, the average cell size of the total population was determined. Mitotic control cells and senescent cells (DIAS and RS) were stained with Coomassie Brilliant Blue (Merck, Darmstadt). For microscopic evaluation, for each independent experiment, three image sections per cell type were analyzed, and the size of five cells was exemplarily measured using the software ImageJ (Fig. 2c). The cell size of replicative senescent fibroblasts as well as the MMC-induced senescent fibroblasts is increased compared to the mitotic fibroblasts. The area is significantly increased by a factor of 8 ± 2 in RS fibroblasts and 10 ± 5 in DIAS fibroblasts compared to the mitotic fibroblasts. The mitotic fibroblasts are characterized by a spindle-like cell shape, mostly with bulges around the nucleus. A typical characteristic of senescent fibroblasts is a 10 to 20-fold increase in the cell surface with a large, flat, and oval nucleus. Both, the RS and the DIAS fibroblasts showed the morphological characteristics of senescent fibroblasts, which were accompanied by a pleomorphic cell shape (Fig. 2c). In summary, a replicative senescence-resembling phenotype could be induced by treatment with mitomycin C, characterized by the high increase in cell size and a change in cell morphology in addition to growth arrest.
Detection of reactive oxygen species in senescent fibroblasts
Reactive oxygen species are an important marker of replicative senescent cells (Kregel and Zhang 2007). Possible causes for growth arrest are reactive oxygen species (ROS) (Frippiat et al. 2001). To check if drug-induced and accelerated senescent (DIAS) fibroblasts resemble replicative senescent cells in the level of ROS, ROS were measured with a fluorescent dye 5 days after MMC treatment (Fig. 3a).
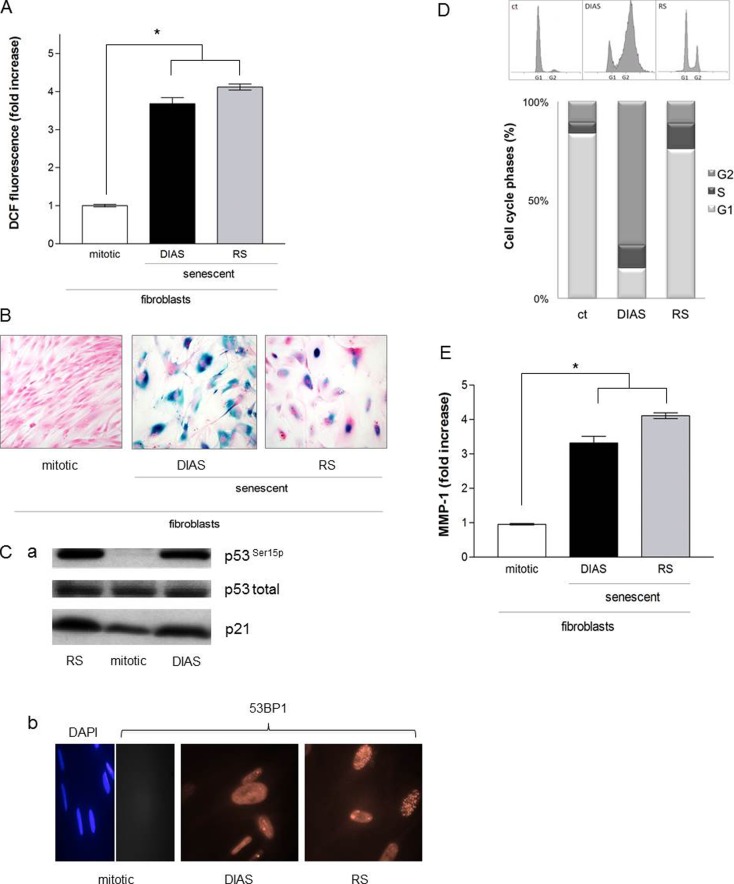
Fig. 3.
a Reactive oxygen species in senescent cells. Subconfluent fibroblasts (passages 2–15) were treated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium. The intracellular ROS level was measured 5 days after treatment in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts were used as control (ct). The ROS level represents the fold increase over control, which was set to 1. The data represent the mean ± s.e.m. of three independent experiments (*p < 0.05; one-way ANOVA). b SA-ß-galactosidase activity in senescent fibroblasts. Subconfluent fibroblasts (passages 2–15) were incubated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium, and the SA-ß-galactosidase activity was measured 21 days after treatment in comparison to replicative senescent cells (RS). Mock-treated, mitotic fibroblasts were used as control (ct). Representative images of three independent experiments are shown. Magnification is 40-fold. c (a) p53 in senescent fibroblasts. Subconfluent fibroblasts were incubated with MMC (200 nM, 2 × 24 h, DIAS) at 37 °C and 5 % CO2 in a fibroblast growth medium, and phosphorylation of p53 was examined in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts were used as control (ct). The level of phosphorylated and total p53 and p21 was determined by Western blot, and representative blots are shown. (b) Formation of 53BP1 foci in senescent fibroblasts. Subconfluent fibroblasts (passages 2–15) were treated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium. The 53BP1 staining was performed 5 days after treatment in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts were used as control (ct). Cells were stained for 53BP1 expression with a polyclonal antibody followed by incubation with an Alexa 546-coupled secondary antibody (red). Nuclei of control cells were visualized with 4′,6′-diamidin-2-phenylindol (DAPI; blue). Images were acquired by fluorescence microscopy. Representative images of two independent experiments are shown. Magnification is 40-fold. d Cell cycle regulation in senescent fibroblasts. Subconfluent fibroblasts (passages 2–15) were treated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium. The cell cycle phases were analyzed 5 days after treatment in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts were used as control (ct). Cells were fixed with ice-cold methanol, and cell cycle phases were analyzed with flow cytometry by staining with propidium iodide. Untreated mitotic fibroblasts (ct) served as control. Shown are representative histograms of three independent experiments (n = 3) and the mean values of the percentage distribution of the cell cycle phases (*p < 0.05, one-way ANOVA). e Release of pro-MMP-1 of fibroblasts. Subconfluent fibroblasts (passages 2–15) were incubated with MMC (DIAS; 200 nM; 2 × 24 h) at 37 °C and 5 % CO2 in a fibroblast growth medium, and 5 days after treatment, the concentration of extracellular MMP-1 was measured by ELISA in comparison to replicative senescence cells (RS; passages 55–60). Mock-treated, mitotic fibroblasts were used as control (ct). Concentration of Pro-MMP-1/cell presents the fold increase over control, which was set to 1. The data represent the mean ± s.e.m. of three independent experiments (*p < 0.05; one-way ANOVA)
The ROS level increased significantly in RS as well as in DIAS fibroblasts compared with mitotic fibroblasts. Figure 3a shows in replicative senescence (RS) a 4.1 ± 1.1-fold and in MMC-induced senescent (DIAS) fibroblasts a 3.5 ± 0.7-fold increase of the ROS level. The analysis of intracellular ROS content 5 days after treatment showed a further common feature between RS and DIAS.
ß-Galactosidase-activity in senescent fibroblasts
Replicative senescent fibroblasts show the activity of the lysosomal senescence-associated enzyme ß-galactosidase (SA-ß-gal) as a biomarker (Dimri et al. 1995). RS and DIAS fibroblasts were compared in context of ß-galactosidase activity. After MMC treatment, DIAS cells were cultivated for additional 21 days to allow the accumulation of the enzyme. Enzyme activity was measured by means of a colorimetric method indicating blue-colored cells with enhanced enzyme activity. Figure 3b shows that both RS and DIAS cells revealed a blue staining at pH 6.0 indicating SA-ß-gal-activity. The number of cells with an elevated enzyme activity was similar in both senescent cell types. By contrast, the mitotic fibroblasts showed no blue staining indicating no ß-galactosidase activity at pH 6.0.
Activation of p53 and cell cycle phases
Replicative senescent fibroblasts show a cell cycle arrest in the G1 phase (Stein and Dulic 1995). The transition from the G1 to the S phase of the cell cycle is regulated by the G1 checkpoint. The tumor suppressor protein p53 plays an inhibitory role at this checkpoint (Cox and Lane 1995).
Therefore, it has been analyzed whether the detected inhibition of cell proliferation was attended by a change in activity of the p53 and p21 proteins by Western blot analysis (Fig. 3c(a)). The phosphorylation of p53 is a marker for the activity of the protein and can trigger a cell cycle shift. The activity of p53 was significantly increased in both the replicative senescence (RS) as well as in MMC-induced senescent (DIAS) fibroblasts. The phosphorylated p53 at serine-15 was increased 9.8 ± 2.3-fold in the DIAS fibroblasts and 7.9 ± 0.32-fold in the replicative senescent cells. p53 is activated by phosphorylation and consequently initiates different signaling pathways, which can lead to the detected growth arrest. This suggests that the growth arrest in replicative senescence as well as in MMC-induced senescent fibroblasts is due to the activation of p53. Total p53 was in all samples constitutive active. For the control of the cell cycle is also p21 responsible (Herbig et al. 2004). The p21 expression increased in RS and DIAS fibroblasts (Fig. 3c(a)). We also analyzed the formation of p53-binding protein 1 (53BP1) in senescent fibroblasts by fluorescence microscopy. 53BP1 is known to accumulate at DSBs and is critically involved in DSB repair (Schultz et al. 2000; Noon and Goodarzi 2011). 53BP1 is induced in DIAS and RS fibroblasts. The analysis of 53BP1 in mitotic fibroblasts showed not 53BP1-positive staining. The DAPI staining was used as control to show the mitotic cells.
The cell cycle was examined in more detail with the help of flow cytometry. Mitotic (ct), replicative senescent (RS) and MMC-induced senescent (DIAS) fibroblasts were stained with the DNA dye propidium iodide (PI). The dye is intercalated into double-stranded nucleic acids, thereby enabling a quantitative determination of nucleic acids which is characteristic for the individual phases of the cycle. The analysis of the cell cycle of mitotic fibroblasts revealed a typical distribution of the cell cycle phases (Fig. 3d). The G1 phase contained 83 %, the S phase 6 %, and the G2 phase 11 %. By treatment of the mitotic fibroblasts with mitomycin-C (DIAS), a redistribution of the cell cycle phases was analyzed 5 days after the treatment, so that 14 % were in the G1 phase, 11 % in S phase, and 68 % in G2 phase. Thus, G2 arrest induced by MMC treatment could not be detected in replicative senescent fibroblasts (RS). The RS gives the following picture: 72 % of the cells remained in the G1 phase, 13 % in S phase, and 11 % in the G2 phase. A difference in the cell cycle arrest-phase could be detected in both senescent cell types (RS and DIAS) (Fig. 3d). However, both senescent cell types arrest.
Effect of mitomycin C on the expression of matrix metalloproteinase 1
Replicative senescent fibroblasts are characterized by an increased expression of matrix-metalloproteinases (MMP) (Coppe et al. 2010). That MMP expression is described to be one of the essential triggers for wrinkle formation in aged skin and is therefore a target for potential anti-aging strategies (Griffiths 2001). To address the question of whether the expression of matrix-metalloproteinases after treatment with MMC is comparable with the expression in replicative senescent cells, the supernatant of these fibroblasts were analyzed with a commercially available ELISA assay and compared with mitotic cells. The MMP-1 protein level was significantly increased in the supernatant of both replicative (RS) and drug-induced senescent (DIAS) fibroblasts. A 3.6 ± 1.2-fold increase in the MMP-1 steady-state protein level of MMC-induced (DIAS) and a 4 ± 0.2-fold increase in the MMP-1 protein amount of replicative senescent (RS) fibroblasts were detected compared with the MMP-1 level of mitotic fibroblasts (Fig. 3e).
In conclusion, the data herein confirm that mitomycin C induces a premature, accelerated, and permanent senescent cell type, which mimics replicative senescence. Therefore, MMC appears to be an appropriate tool to study cellular aging in time lapse which is comparable with replicative senescence.
Discussion
Aging is the result of several mechanisms, which operate simultaneously. Among them, it is postulated that reactive oxygen species are mainly involved (Robles et al. 1999). Recently, the free radical theory of aging comes under criticism. Replicative senescent fibroblasts (Hutter et al. 2002), senescent keratinocytes (Bernard et al. 2004), or senescent endothelial cells (Haendeler et al. 2004) show increased level of reactive oxygen species as well. Thus, senescence could be counteracted in vitro with antioxidants (Atamna et al. 2000). Many publications show a premature senescence (SIPS) caused by oxidative stress, e.g., by H2O2 (Toussaint et al. 2000; Frippiat et al. 2001). Subtoxic concentrations of H2O2 induce premature senescence in fibroblasts. However, this senescence is only described for 3 days after treatment with a characteristic marker like proliferation stop, increased level of ROS, and altered cell morphology. Fourteen days after treatment, the senescent status of the cells was abrogated, the ROS level decreased, and the cells start to proliferate. Thus, it becomes clear that the H2O2-induced senescence is only a transient phenomenon. Duan and co-workers also induced a premature senescence with H2O2. A H2O2-initiated senescence was maintained over 14 days, but only if the fibroblasts were treated with H2O2 over the entire time (Duan et al. 2005).
Furthermore, even if the manganese superoxide dismutase (SOD) in C. elegans, an important anti-oxidative enzyme in the mitochondria, was inactivated, no effect on life-time was detectable (Gems and Doonan 2009). Also, many studies with antioxidants had no striking success regarding the life-time of mammalian, e.g., mice (Huang et al. 2000). Among others, Perez et al. showed that even though the ROS level was lowered, there was no effect on the life-time of mice (Perez et al. 2009).
It is doubtless that oxidative stress plays an important role during aging; but until now, it remains obscure whether elevated ROS level triggers the aging process or ROS are a consequence of other aging-related factors described in the different aging theories. However, our data show that ROS alone are not enough to initiate a permanent senescent cell type. In fact, a combination of different theories of aging and, therefore, different exogenous and endogenous factors should be considered to clarify the mechanisms underlying the aging process. In addition to oxidative stress, alkylation of DNA could be a source for an on-going aging process. Alkylating substances are among the most common environmental toxins, and the organism is only slightly protected (Tubbs et al. 2009). The alkylation of the DNA leads, most frequently, to the addition of a methyl group at the seventh position of guanine (N7-meGua) or to the phosphate backbone of the DNA. More toxic is the incorporation of a methyl group at the sixth position of guanine (O6-meGua). This form is often accompanied by secondary reactions of DNA with metabolites or other toxins, e.g., cigarette smoke. In cell culture experiments, a senescent cell type was induced within lung fibroblasts by incubation with cigarette smoke (Nyunoya et al. 2006). In addition, in vitro studies showed that lung fibroblasts of emphysema patients compared with healthy smokers were stained positive on SA-ß-galactosidase (Muller et al. 2006). Furthermore, there are a number of publications showing that treatment with alkylating agents might induce premature senescence (Robles et al. 1999; Meng et al. 2003; Jaiswal et al. 2004). Colon cancer cells were transformed by treatment with Methylnitronitrosoguanine, which incorporates methyl groups on the sixth position of guanine to get premature senescent colon cells (Jaiswal et al. 2004). Human fibroblasts showed numerous senescence markers after treatment with busulfan (Probin et al. 2007; Meng et al. 2003). These results clearly show that not only oxidative stress is capable to induce (transient) premature senescence but also that the alkylation of the DNA seems to be a key mechanism on the way to a senescent cell. Mitomycin C was chosen as an appropriate substance to generate permanent senescence because it combines both the generation of ROS and the direct reaction with DNA as an alkylating agent (Tomasz and Palom 1997).
In our study mitomycin C treated cells show characteristics of senescent cells, such as abrogated proliferation, a stable change in cell morphology, intensified SA-ß-galactosidase activity, and a transient increase in the ROS level. The results of this work show for the first time that ROS alone is not sufficient to induce a permanent senescence. Rather, a second stimulus such as DNA damage by alkylating agents is needed to induce the permanent senescent cell type. This drug-induced accelerated senescence (DIAS) is comparable with replicative senescence (RS).
Acknowledgments
This work is part of the PhD thesis of J.D. at the Heinrich-Heine-University of Düsseldorf. We thank C. Wyrich for the excellent technical assistance.
These authors contributed equally to this work
Lirija Alili and Johanna Diekmann.
Competing financial interests
The authors declare no competing financial interests.
The authors declare no conflict of interest.
References
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Paler-Martinez A, Ames BN. N-t-butyl hydroxylamine, a hydrolysis product of alpha-phenyl-N-t-butyl nitrone, is more potent in delaying senescence in human lung fibroblasts. J Biol Chem. 2000;275:6741–6748. doi: 10.1074/jbc.275.10.6741. [DOI] [PubMed] [Google Scholar]
- Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Cell Biol. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard D, Gosselin K, Monte D, Vercamer C, Bouali F, Pourtier A, Vandenbunder B, Abbadie C. Involvement of rel/nuclear factor-κB transcription factors in keratinocyte senescence. Cancer Res. 2004;64:472–481. doi: 10.1158/0008-5472.CAN-03-0005. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Gogol J, Bayreuther K. DNA synthesis and Fos and Jun protein expression in mitotic and postmitotic WI-38 fibroblasts in vitro. Exp Cell Res. 1994;211:219–230. doi: 10.1006/excr.1994.1081. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Lane DP. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995;17:501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993;73:617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Chainiaux F, de Longueville F, Mainfroid V, Migeot V, Marcq L, Remacle J, Salmon M, Toussaint O. Gene expression and regulation in H2O2-induced premature senescence of human foreskin fibroblasts expressing or not telomerase. Exp Gerontol. 2004;39:1379–1389. doi: 10.1016/j.exger.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dimri G, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Failla G. The aging process and cancerogenesis. Ann N Y Acad Sci. 1958;71:1124–1140. doi: 10.1111/j.1749-6632.1958.tb46828.x. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Gems D, Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- Gilley D, Tanaka H, Herbert BS. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol. 2005;37:1000–1013. doi: 10.1016/j.biocel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Griffiths CE. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613–618. doi: 10.1046/j.1365-2230.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J Gerontol A Biol Sci Med Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.B5. [DOI] [PubMed] [Google Scholar]
- Hutter E, Unterluggauer H, Uberall F, Schramek H, Jansen-Durr P. Replicative senescence of human fibroblasts: the role of Ras-dependent signaling and oxidative stress. Exp Gerontol. 2002;37:1165–1174. doi: 10.1016/S0531-5565(02)00136-5. [DOI] [PubMed] [Google Scholar]
- Jaiswal AS, Multani AS, Pathak S, Narayan S. N-methyl-N′-nitro-N-nitrosoguanidine-induced senescence-like growth arrest in colon cancer cells is associated with loss of adenomatous polyposis coli protein, microtubule organization, and telomeric DNA. Mol Cancer. 2004;3:3. doi: 10.1186/1476-4598-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1979;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKenna E, Traganos F, Zhao H, Darzynkiewicz Z. Persistent DNA damage caused by low levels of mitomycin C induces irreversible cell senescence. Cell Cycle. 2012;11:3132–3140. doi: 10.4161/cc.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- Mitsui Y, Schneider EL. Increased nuclear sizes in senescent human diploid fibroblast cultures. Exp Cell Res. 1976;100:147–152. doi: 10.1016/0014-4827(76)90336-0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck V, Hohlfeld J, Krug N, Nakashima M, Branscheid D, Magnussen H, Jorres R, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon AT, Goodarzi AA. 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair. 2011;10:1071–1076. doi: 10.1016/j.dnarep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35:681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz MM, Das TA, Tomasz M. Mitomycin C linked to DNA minor groove binding agents: synthesis, reductive activation, DNA binding and cross-linking properties and in vitro antitumor activity. Bioorg Med Chem. 1999;7:2713–2726. doi: 10.1016/S0968-0896(99)00223-0. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;10:11. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probin V, Wang Y, Zhou D. Busulfan-induced senescence is dependent on ROS production upstream of the MAPK pathway. Free Radic Biol Med. 2007;42:1858–1865. doi: 10.1016/j.freeradbiomed.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaper PM, di Fagagna F, Jackson SP. Activation of the DNA damage response by telomere attrition: a passage to cellular senescence. Cell Cycle. 2004;3:543–546. doi: 10.4161/cc.3.5.835. [DOI] [PubMed] [Google Scholar]
- Robles SJ, Buehler PW, Negrusz A, Adami GR. Permanent cell cycle arrest in asynchronously proliferating normal human fibroblasts treated with doxorubicin or etoposide but not camptothecin. Biochem Pharmacol. 1999;58:675–685. doi: 10.1016/S0006-2952(99)00127-6. [DOI] [PubMed] [Google Scholar]
- Rodemann HP. Differential degradation of intracellular proteins in human skin fibroblasts of mitotic and mitomycin-C (MMC)-induced postmitotic differentiation states in vitro. Differentiation. 1989;42:37–43. doi: 10.1111/j.1432-0436.1989.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Santarosa M, DelCol L, Tonin E, Caragnano A, Viel A, Maestro R. Premature senescence is a major response to DNA cross-linking agents in BRCA1-defective cells: implication for tailored treatments of BRCA1 mutation carriers. Mol Cancer Ther. 2009;8:844–854. doi: 10.1158/1535-7163.MCT-08-0951. [DOI] [PubMed] [Google Scholar]
- Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GH, Dulic V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Tomasz M, Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol Ther. 1997;76:73–87. doi: 10.1016/S0163-7258(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Tomasz M, Chawla AK, Lipman R. Mechanism of monofunctional and bifunctional alkylation of DNA by mitomycin C. Biochemistry. 1988;27:3182–3187. doi: 10.1021/bi00409a009. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Houbion A, Remacle J. Aging as a multi-step process characterized by a lowering of entropy production leading the cell to a sequence of defined stages. II. Testing some predictions on aging human fibroblasts in culture. Mech Ageing Dev. 1992;65:65–83. doi: 10.1016/0047-6374(92)90126-X. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Tubbs JL, Latypov V, Kanugula S, Butt A, Melikishvili M, Kraehenbuehl R, Fleck O, Marriott A, Watson AJ, Verbeek B, McGown G, Thorncroft M, Santibanez-Koref MF, Millington C, Arvai AS, Kroeger MD, Peterson LA, Williams DM, Fried MG, Margison GP, Pegg AE, Tainer JA. Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature. 2009;459:808–813. doi: 10.1038/nature08076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wong SC, Pan J, Tsao SW, Fung KH, Kwong DL, Sham JS, Nicholls JM. Evidence of cisplatin-induced senescent-like growth arrest in nasopharyngeal carcinoma cells. Cancer Res. 1998;58:5019–5022. [PubMed] [Google Scholar]
- Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Distinct roles of cytochrome P450 reductase in mitomycin C redox cycling and cytotoxicity. Mol Cancer Ther. 2010;9:1852–1863. doi: 10.1158/1535-7163.MCT-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlaschek M, Hommel C, Wenk J, Brenneisen P, Ma W, Herrmann G, Scharffetter-Kochanek K. Isolation and identification of psoralen plus ultraviolet A (PUVA)-induced genes in human dermal fibroblasts by polymerase chain reaction-based subtractive hybridization. J Investig Dermatol. 2000;115:909–913. doi: 10.1046/j.1523-1747.2000.00120.x. [DOI] [PubMed] [Google Scholar]