Abstract
Oxidative stress from generation of increased reactive oxygen species or free radicals of oxygen has been reported to play an important role in the aging. To investigate the relationship between the oxidative stress and memory decline during aging, we have determined the level of lipid peroxidation, activities of antioxidant enzymes, and activity of acetylcholine esterase (AChE) in brain and plasma as well as biogenic amine levels in brain from Albino–Wistar rats at age of 4 and 24 months. The results showed that the level of lipid peroxidation in the brain and plasma was significantly higher in older than that in the young rats. The activities of antioxidant enzymes displayed an age-dependent decline in both brain and plasma. Glutathione peroxidase and catalase activities were found to be significantly decreased in brain and plasma of aged rats. Superoxide dismutase (SOD) was also significantly decreased in plasma of aged rats; however, a decreased tendency (non-significant) of SOD in brain was also observed. AChE activity in brain and plasma was significantly decreased in aged rats. Learning and memory of rats in the present study was assessed by Morris Water Maze (MWM) and Elevated plus Maze (EPM) test. Short-term memory and long-term memory was impaired significantly in older rats, which was evident by a significant increase in the latency time in MWM and increase in transfer latency in EPM. Moreover, a marked decrease in biogenic amines (NA, DA, and 5-HT) was also found in the brain of aged rats. In conclusion, our data suggest that increased oxidative stress, decline of antioxidant enzyme activities, altered AChE activity, and decreased biogenic amines level in the brain of aged rats may potentially be involved in diminished memory function.
Keywords: Aging, Memory, AChE, Antioxidant enzymes, Biogenic amines
Introduction
Free radicals of oxygen or reactive oxygen species (ROS) have been shown to play an important role in the aging process (Tian et al. 1998). The oxidative stress hypothesis offers a possible mechanism for aging-related Alzheimer’s (Montine et al. 2002) and other neurodegenerative diseases (Hald and Lotharius 2005). The most commonly used biomarker to investigate the oxidative damage is the measurement of malondialdehyde (MDA), the major lipid peroxidation product, which react with the free amino group of proteins, phospholipids, and nucleic acids and cause their structural modification (Pandey and Rizvi 2010). Lipids are sensitive to oxidation by ROS, which undergo oxidative disintegration generating MDA as end product. A high level of lipid peroxidation products is usually detected upon cell degradation after cell injury or disease (Kingsley et al. 2009).
Superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase, and catalase (CAT) are the main endogenous enzymatic defense systems of all aerobic cells (Fisher-Wellman et al. 2009). These enzymes protect from oxidative damage by directly scavenging superoxide radicals and hydrogen peroxide (H2O2), converting them into less reactive species (Milic et al. 2009). SOD catalyzes the dismutation of superoxide radical (•O2) to H2O2 (Husain and Somani 1998). Although H2O2 is not a radical, it is rapidly converted by Fenton reaction into •OH radical which is very reactive (Pandey and Rizvi 2010). CAT is one of the most active catalysts that rapidly degrade H2O2. It has been proposed that CAT plays an important role in systems which have evolved to allow organisms to live in aerobic environments (Scandalios 2005). Any factors that reduce the activities of antioxidant enzymes may lead to accumulation of ROS and subsequently oxidative damage to biological macromolecules (Cabalas-Picot et al 1992).
Age-related memory impairment is correlated with a decrease in brain and plasma antioxidants. Several authors suggested the possibility that the increase in oxidative stress may result in a relative decrease in antioxidant enzyme activities (Sigueira et al. 2005). An age-related decline in cholinergic function is thought to be partially responsible for short-term (Jha and Rizvi 2009) and long-term (Papandreou et al. 2011) memory disorders during senescence. The age-associated memory impairments in rats have also been directly correlated with decreased levels of brain biogenic amines in senescent rats (Lee et al. 2010). It is evident that dopamine (DA) (Peters 2006) and 5-hydroxytryptamine (5-HT) have a role in memory and learning (Meneses 1999). The age-associated memory impairment is also thought to be related to an increased oxidative stress in aging brain (Dias et al. 2007). Catecholamine oxidation, increased activity of monoamine oxidase B, and decreased activity of antioxidant enzymes (SOD and CAT) are the possible contributing factors that lead to increased oxidative stress during senescence (Alper et al. 1999).
The present study was conducted to investigate status of oxidative stress, levels of biogenic amines, and memory impairment in aged rats. We have determined the level of lipid peroxidation, activities of antioxidant enzymes (SOD, GPx, and CAT), activity of AChE, and biogenic amine levels in brain and plasma of both aged and young rats. Some of the hallmarks of aging are cognitive decline and impaired memory function, so the present study was particularly aimed to address the role of various factors involved in age-related decline in memory function.
Materials and methods
Reagents and chemicals
H2O2 stock (35 %) solution, thiobarbituric acid (TBA), nitro blue tetrazolium (NBT), and dithiobisnitrobenzoic acid (DTNB) were purchased from British Drug House (BDH, Dorset, UK). Hydroxylamine hydrochloride (H3NO · HCl), acetylthiocholine, and all other analytical grade reagents were purchased from Sigma Chemical Co. (St. Louis, USA).
Animals
Twelve locally bred Albino-Wistar rats of two different age groups, purchased from Dow University of Health Sciences, OJHA campus, Karachi, Pakistan, were used in the study. Animals were caged individually (to avoid effect of social interaction) with ad libitum access to cubes of standard rodent diet [A control diet (4.47 kcal/g) containing 25 % fat, 50 % carbohydrate, and 25 % protein] (Bocarsly et al. 2012) and tap water under a 12:12 h light/dark cycle (lights on at 7:00 am) at controlled room temperature (22 ± 2 °C). Prior to experiments, animals were subjected to 1 week of acclimation period and to various handling procedures in order to nullify the psychological affliction of environment for reducing the novelty and handling stress. All animal experiments were approved by the institutional ethics and animal care committee and performed in strict accordance with National Institute of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised1985). All treatment and behavioral monitoring were done in a balanced design to avoid order and time effect.
Experimental protocol
The animals (n = 12) were divided into two groups (n = 6): young (4–5 months) and old (22–24 months). After 1 week of acclimation, the behavioral tests for the assessment of memory were performed using Morris Water Maze Test (MWM) and Elevated Plus maze Test (EPM). Rats were then decapitated, and their brains and blood were collected. Brains were removed from the skull within 30 s after decapitation. Blood samples were taken in heparinized tubes and kept at the room temperature for 30 min and then centrifuged at 1,500 × g for 10 min. All samples were stored at −70 °C until analyzed for biochemical and neurochemical assays.
Determination of MDA content
Estimation of lipid peroxidation was performed as described by Chow and Tappel (1972) with slight modifications. Brain homogenate or plasma (100–500 μl) was mixed with 2 ml of TCA (15 %)–TBA (0.375 %) mixture. The mixture was boiled for 20 min in water bath, cooled with ice cold water at 4 °C, and then centrifuged at 2,000 × g for 10 min. Supernatant of light pink color was then collected, and the absorbance was recorded at 532 nm. Lipid peroxidation was quantified using molar extinction coefficient (1.56 × 105), and data are expressed as micromoles of MDA per gram of brain or micromoles of MDA per milliliter of plasma.
Determination of AChE activity
Activity of acetyl cholinesterase (AChE) in brain and plasma was determined according to the method of Ellman et al. (1961) using acetylthiocholine (ATC) as substrate. The reaction mixture contained 0.4 ml of brain homogenate (20 %) or 0.4 ml plasma, 2.6 ml phosphate buffer (0.1 M, pH 8.0), and 100 μl DTNB. The reaction mixture was mixed by bubbling air and placing in the spectrophotometer. Once the reaction is stable, the absorbance was recorded at 412 nm for the basal reading. The reaction was started by adding 5.2 μl of ATC to this cuvette, and change in absorbance was recorded at time zero and after 10 minutes at 25 °C. The activity of AChE was expressed for as micromoles per minute per gram of brain or micromoles per minute per milliliter of plasma.
Determination of superoxide dismutase (SOD) activity
Brain and plasma SOD activity was estimated by the method (Beauchamp and Fridovich 1971; Chidambara Murthy et al. 2002) based on the reduction of NBT to water-insoluble blue formazan. Brain homogenate (10 %, 0.5 ml) or plasma (0.5 ml) was mixed with 1 ml of 50 mM sodium carbonate, 0.4 ml of 24 μM NBT, and 0.2 ml of 0.1 mM EDTA. The reaction was initiated by adding 0.4 ml of 1 mM hydroxylamine hydrochloride. Change in absorbance was recorded at time zero and after 5 min at 560 nm at 25 °C. An appropriate control without brain homogenate or plasma was run along each batch of samples. Units of SOD activity were expressed as the amount of enzyme required to inhibit the reduction of NBT by 50 %. The specific activity was expressed as units per gram of brain or units per milliliter of plasma.
Determination of catalase (CAT) activity
Catalase was estimated as described previously (Sinha 1972). The reaction mixture contained 1.0 ml of 0.01 M Phosphate buffer (pH 7.4), 0.1 ml of brain homogenate (10 %) or plasma, and 0.4 ml of 0.2 M H2O2. The tubes were incubated at 37 °C for 90 s. The reaction was stopped by adding 2.0 ml of dichromatic–acetic acid reagent (5 %). Samples were further incubated at 100 °C for 15 min in a boiling water bath. An appropriate control was carried out without addition of H2O2, and the amount of H2O2 consumed was determined by recording absorbance at 570 nm. CAT activity was expressed as micromoles of H2O2 consumed per minute per gram of brain or micromoles per minute per milliliter of plasma.
Determination of glutathione peroxidase (GPx) activity
Glutathione peroxidase activity was measured by the procedure of Flohe and Gunzler (1984). One milliliter of reaction mixture was prepared which contained 0.3 ml of phosphate buffer (0.1 M, pH 7.4), 0.2 ml of GSH (2 mM), 0.1 ml of sodium azide (10 mM), 0.1 ml of H2O2 (1 mM), and 0.3 ml of brain supernatant or plasma. After incubation at 37 °C for 15 min, reaction was terminated by addition of 0.5 ml 5 % TCA. Tubes were centrifuged at 1,500 × g for 5 min, and supernatant was collected. Phosphate buffer 0.2 ml (0.1 M, pH 7.4) and DTNB 0.7 ml (0.4 mg/ml) were added to 0.1 ml of reaction supernatant. After mixing, absorbance was recorded at 420 nm. Activity of GPx was expressed as micromoles per minute per gram of brain or micromoles per minute per milliliter of plasma.
Behavioral analysis
Memory function tested by Morris Water Maze Test (MWM)
Morris Water Maze (MWM) test was performed to examine the effects on spatial memory (Morris 1981). It is a circular pool of water with a diameter of 45 cm, height of 37 cm, and depth of 12 cm. The pool is a metal cylinder painted white on the inner surface. The escape platform is also made of metal cylinder with flat metallic top having a surface diameter of 8 cm, and it is placed 2 cm below the surface of water during water maze training. The pool is filled with water (23 ± 2 °C) which was made opaque with milk in order to obscure the platform to allow proficient tracking of the swim paths of the rats (Haider et al. 2011). In our experiment, we have assessed the reference (long-term) memory and working (short-term) memory in terms of latency to locate the escape platform. The test is based on two phases: the training phase and the test phase. Memory functions of rats were tested by noting down the retention latency. The cut off time was 2 min for each session. Initially, the training session was performed during which each rat was placed into the water in such a way that their face was towards the wall of the tank. Each animal was given 120 seconds to find and mount onto the hidden platform. If the rat located the platform it was allowed to stay on it for 10 s. If it failed to locate the platform during the allocated time, then it was guided gently onto the platform (Haider et al. 2007). The test consisted of two trials: STM (short-term memory) and LTM (long-term memory). STM was assessed 60 min after training session, and LTM was measured after 24 h of training (Haider et al. 2012a).
Memory function tested by Elevated plus Maze Test
Elevated plus Maze Test (EPM) was used as behavioral model to evaluate memory function in rats (Haider et al. 2011). The apparatus used in the present study consisted of two open arms (50 × 10 cm) crossed with two closed arms of the same dimensions with walls of 40 cm high. The arms were connected with a central square (5 × 5 cm) to give the apparatus a plus sign appearance. The maze was elevated 60 cm high above the floor. The procedure and techniques were essentially the same as reported previously (Haider et al. 2012b). The memory test was assessed during elevated plus maze experimental session in two trials (first day: learning and second day: retention of memory). In the training session, on day 1, the rats were individually placed at one end of the open arm facing away from the central platform and the transfer latency (time taken in seconds by the rats to move into one of the closed arm with all its four paws) was recorded. The cut off time during the training session was 2 min for the rats to explore the maze. Test session to evaluate the retention of memory was performed after 60 min for STM and 24 h for LTM and was repeated two times. Significant increase in the transfer latency was taken as an index of impairment in memory.
Determination of biogenic amines
Frozen brains were homogenized in extraction medium using an electrical homogenizer (Polytron; Kinematica). The neurochemical analysis was performed to estimate NA, DA, and 5-HT concentrations in brain as reported by Haider et al. (2011). The biogenic amines were detected in a single sample by reversed-phase HPLC with electrochemical detector (Schimadzu LEC 6A detector) at an operating potential of +0.8. A 5-μ Shim-pack ODS separation column of 4.0 mm internal diameter and 150 mm length was used as the stationary phase. The mobile phase consist of 0.023 % octyl sodium sulfate in 0.1 M phosphate buffer at pH 2.9, which was passed through the column under a pressure of 2,000–3,000 psi.
Statistical analysis
The results are presented as mean ± SD for n = 6 animals in each group. The statistical significant differences were evaluated by Student’s t test, and values of p < 0.05 were taken as significant differences.
Result
The present study investigates the effect of aging on lipid peroxidation and activities of anti-oxidation enzymes (CAT, SOD, and GPx) along with AChE activity and levels of biogenic amines. In addition, the behavioral deficits, including memory impairment and related neurochemical alterations, were also analyzed. Data presented in Fig. 1a, b shows that lipid peroxidation (LPO) was significantly increased (p < 0.01) by 280 % and 45 % in the brain and plasma, respectively, in aged rats (189.133 ± 11.066 μmole/gm of brain, 12.23 ± 2.4 μmole/ml of plasma) when compared with that of the young rats (67.37 ± 1.053 μmole/gm of brain, 8.441 ± 1.6 μmole/ml of plasma).
Fig. 1.
Effect of age on brain and plasma lipid peroxidation in young and aged rats. Data are means ± SD (n = 6) and expressed as micromoles of MDA per gram of brain (a) or MDA per milliliter of plasma (b). Data are analyzed for significant difference by Student’s t test; *p < 0.01 compared with young rats
The AChE activity in brain of aged and young rats was found to be 4,364.4 ± 189.9 and 5,042.3 ± 280.2 μmole/min/gm, and in plasma, it was 0.695 ± 0.05 and 0.81 ± 0.05 μmole/min/ml, respectively. Figure 2a, b shows that aged rats exhibited a significant 13 % and 14 % decline (p < 0.01) in AChE activity in brain and plasma, respectively, compared with that of young rats.
Fig. 2.
Effect of age on brain (a) and plasma (b) AChE activity in rats. Data are means ± SD (n = 6), and activity is expressed as micromoles per minute per gram in brain or micromoles per minute per milliliter in plasma. Data are analyzed for significant difference by Student’s t test; *p < 0.01 compared with young rats
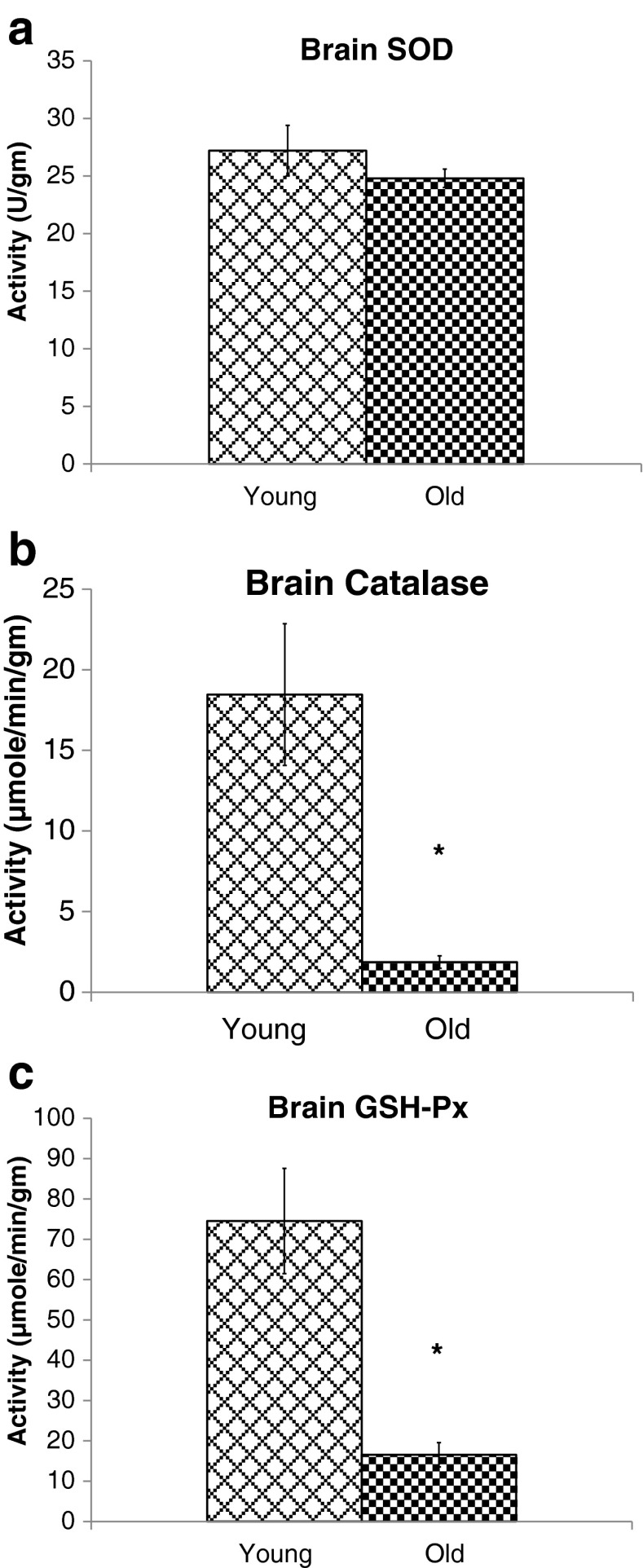
To examine the impact of aging on antioxidant defense system, we measured SOD, CAT, and GPx activities in the brain and plasma. Figure 3a shows that SOD activity in brain was decreased by 9 % in aged rats compared with that in young rats. However, this decrease was statistically not significant. Furthermore, as shown in Fig. 3b, an age-dependent significant decline (p < 0.01) in CAT activity by 90 % (1.867 ± 0.386 μmole/min/gm) was observed compared with that of young rats (18.46.2 ± 4.39 μmole/min/gm). Data also exhibited a significant decline (p < 0.01) in GPx activity in brain of aged rats. A 78 % decrease (16.553 ± 3.0 μmole/min/gm) in GPx activity was observed in aged rats compared with young animals (74.545 ± 13.08 μmole/min/gm) as shown in Fig. 3c.
Fig. 3.
Effect of age on activities of brain antioxidant enzymes SOD (a), CAT (b), and GPx (c) in rats. Data are means ± SD (n = 6). SOD activity is expressed as units per gram of brain whereas CAT and GPx activities are expressed as micromoles per minute per gram. Data are analyzed for significant difference by Student’s t test; *p < 0.01 compared with young rats
Figure 4a shows that SOD activity in the plasma was significantly decreased, by 22 % (p < 0.01) in aged rats (1.711 ± 0.22 U/ml), compared with that of young rats (2.264 ± 0.35 U/ml). Furthermore, as shown in Fig. 4b, an age-dependent decline in CAT activity, by 23 % (58.5 ± 6.9 μmole/min/ml) was also observed compared with that of young rats (75.2 ± 3.6 μmole/min/ml). Data also revealed that the GPx activity is significantly decreased (p < 0.01) in plasma of aged rats. A 25 % decline (0.285 ± 0.02 μmole/min/ml) in GPx activity was observed in aged rats compared with young animals (0.385 ± 0.039 μmole/min/ml) as shown in Fig. 4c.
Fig. 4.

Effect of age on activities of plasma antioxidant enzymes SOD (a), CAT (b), and GPx (c) in rats. Data are means ± SD (n = 6). SOD activity is expressed as units per milliliter of plasma whereas CAT and GPx activities are expressed as micromoles per minute per milliliter. Data are analyzed for significant difference by Student’s t test; *p < 0.01 compared with young rats
The effect of aging on memory function was analyzed by using two behavioral methods, MWM and EPM. The effect of senescence on STM and LTM in each behavioral test, as assessed by MWM and EPM, is presented in Figs. 5a, b and 6a, b, respectively. The results of MWM showed a significant impairment of STM (73.8 ± 16.9 s) and LTM (28.7 ± 3.8 s) in aged rats, which was evident by a significant (p < 0.01) increase in latency time compared with young rats [STM (17.83 ± 3.7 s) and LTM (8 ± 1.4 s)]. A three- and twofold increase in latency time in STM and LTM was exhibited by aged rats, respectively. Aged rats also exhibited a 56 % increase (p < 0.01) in transfer latency for STM (76 ± 23.23 s) and 65 % increase (p < 0.01) in transfer latency for LTM (27.33 ± 6.3 s) compared with that of STM (48.75 ± 16.2 s) and LTM (16.6 ± 2.63 s) of the young rats as assessed by EPM.
Fig. 5.
Effect of age on the memory of rats tested in MWM as STM (a) and LTM (b). Values are means ± SD (n = 6). Data are analyzed for significant difference by Student’s t test; *p < 0.01 compared with young rats
Fig. 6.
Effect of age on the memory of rats tested in EPM as STM (a) and LTM (b). Values are means ± SD (n = 6). Data are analyzed for significant difference by Student’s t test *p < 0.01 versus young rats
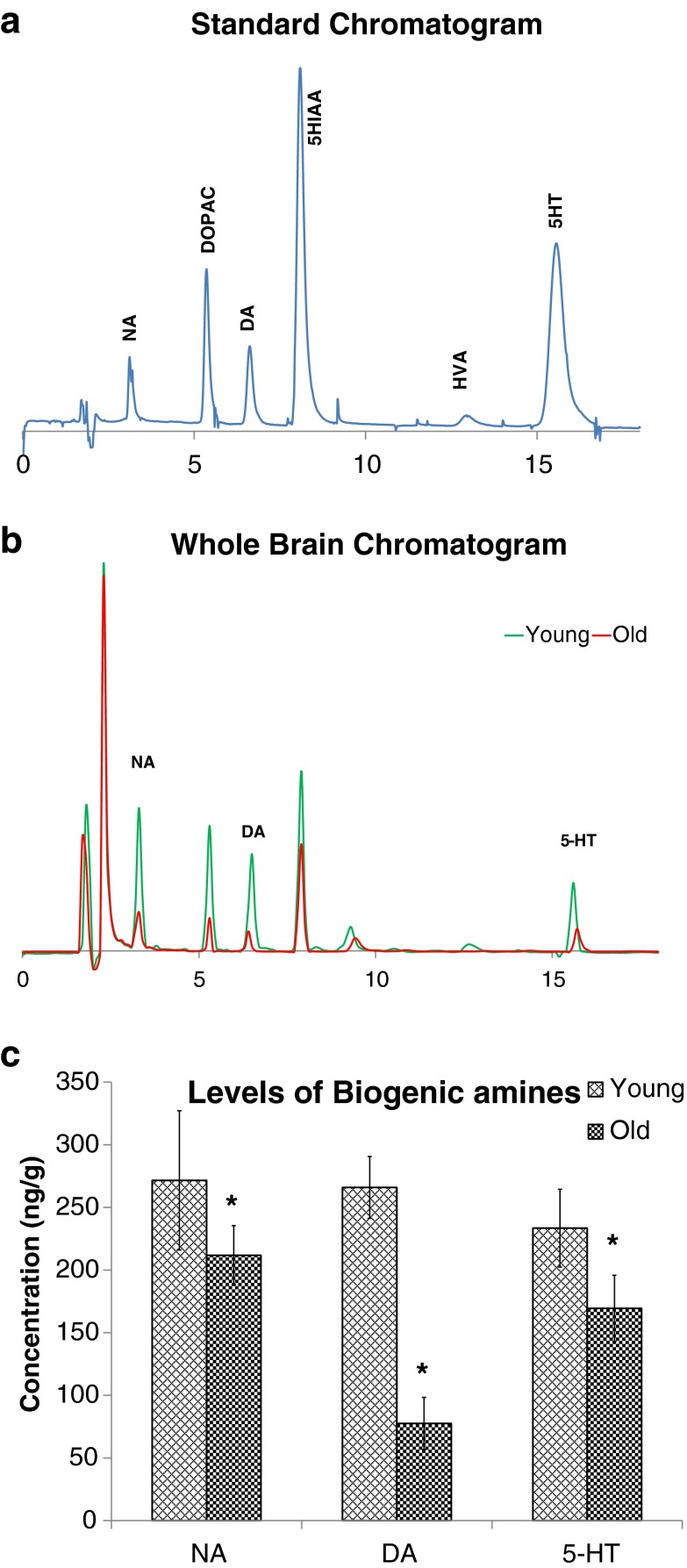
The HPLC separation profile of standard solution and brain samples are shown in Fig. 7. Data in Fig. 7a show the chromatogram of standard solution containing NA, DA, and 5-HT, whereas Fig. 7b shows the chromatogram of biogenic amines in the whole brain of young and aged rats. The results of neurochemical estimation in Fig. 7c show the changes in brain biogenic amine levels. The 5-HT levels in aged rats (169.4 ± 26.5 ng/g) showed a significant 27 % decline (p < 0.01) as compared with that of young rats (233.47 ± 31.04 ng/g). Aged rats also exhibited significant 22 % and 77 % decrease (p < 0.01) in NA (211.8 ± 23.6) and DA (77.513 ± 20.86 ng/g) levels, respectively, compared with the levels of NA (271.6 ± 55.6 ng/g) and DA (265.96 ± 24.7 ng/g) of young rats.
Fig. 7.
HPLC chromatogram of standard solution (a), biogenic amines in the whole brain of young and aged rats (b) and effect of age on concentration of brain monoamine levels in rats (c). Values are means ± SD (n = 6). Data are analyzed for significant difference by Student’s t test *p < 0.01 versus young rats
Discussion
The present study was conducted to investigate the status of free radical generation, activities of anti-oxidation and neurotransmission systems, biogenic amine concentrations, and short- and long-term memories in aged and young animals. Our results showed increased MDA levels in brain and plasma of aged rats compared with that of young rats, indicating increased lipid peroxidation during aging. It should be noted that the amount of MDA in the brain was markedly increased compared with that of plasma. The brain is an aerobic organ that has one of the highest oxygen consumption rates on the basis of the weight. Thus, the brain may be considered as the tissue more susceptible to oxidative damage by free radicals. Our data are also consistent with other studies showing an increased lipid peroxidation in aged animals (Rizvi and Maurya 2007). The neuronal and plasma membrane is a direct target of lipid peroxidation under oxidative stress. The polyunsaturated fatty acids (PUFAs) are very sensitive to oxidative cleavage at their double bonds and generate MDA as an end product (Jha and Rizvi 2009). PUFAs are abundant in the brain and are highly susceptible to free radical attack. ROS are usually derived from abnormally interrupted metabolism of oxygen and is thought to play an important role in oxidative damage to biological macromolecules (Tian et al. 1998). Oxygen radical production in the cell increases with age in mammals and insects (Smith et al. 1991) as well as in humans (Singh et al. 2009). The age-related increase in production of prooxidants is derived from the membrane damage by O2˙ˉ and H2O2 (Starke-Reed and Oliver 1989). This leads to an accumulation of oxidatively damaged macromolecules, including DNA, RNA, lipids, and crucial enzyme proteins in senescent cells (Tian et al. 1998).
It has been documented that the process of aging is associated with memory impairment. The age-associated memory impairment is thought to be due to an increased oxidative stress in aging brain (Bagheri et al. 2011), which plays an important role in the development and progression of Alzheimer’s disease (AD) (Reddy 2006). The present results showed significant impaired effect of senescence on STM and LTM in aged rats. Aged rats displayed spatial memory impairment, which was evident by increase in latency time to locate the submerged platform. The cognitive impairment in rodents is a consequence of advancing chronological age (Haider et al. 2011). Various reports have shown that, compared with young rats, aged rats perform worse on a broad range of learning and memory tasks (Okuda et al. 2004). Normal aging is associated with a slow decline in brain functions such as sensory and motor performance, and at times, this decline is accompanied by progressive memory loss, dementia, and cognitive dysfunctions, ultimately resulting in limited functionality (Papandreou et al. 2011). In both aged humans and rodents, cognitive impairment has been correlated to the accumulation of oxidative damage to lipids, proteins, and nucleic acids (Butterfield et al. 2006). Possible cause of age-related brain dysfunction is oxidative damage as it is principally susceptible to oxidative stress by free radical generation (Albert 1997). Therefore, the impaired memory function exhibited by the aged rats in the current study might be due to increased oxidative damage and LPO. Furthermore, increased ROS generation in aging has been shown to be associated with increased blood–brain barrier (BBB) permeability (Enciu et al. 2013). The BBB changes increase the chances of neurodegenerative diseases in old age.
In addition, an age-related decline in cholinergic function is thought to be partially responsible for the memory disorders occurring during senescence. One of the major markers of cholinergic function is the activity of the enzyme AChE, which is decreased with aging in brain (Papandreou et al. 2009; Gorini et al. 1996) and plasma (Cerejeira et al. 2011). Our results showed a significant decline in brain and plasma AChE activity in aged rats compared with that of young rats. The relationship between aging and AChE activity in plasma and different brain regions has been reported earlier (Cerejeira et al. 2011; Das et al. 2001; Papandreou et al. 2009). Decline in cholinergic indices such as choline acetyltransferase, AChE, muscarinic, and nicotinic acetylcholine receptors has been reported during normal aging process (Zhang 2004; Barnes et al. 2000). The cholinergic hypothesis of geriatric memory dysfunction suggests that the reduction of cholinergic markers is critical components for the age and dementia-associated memory deficits (Terry and Buccafusco 2003). Aging is the most important risk factor for AD, and many studies have assessed plasma AChE as a marker for AD (Garcıa-Ayllon et al 2010). Changes in cholinergic function have been characterized, and a strong correlation has been observed with cognitive decline associated with aging. A low activity of AChE or cholinesterase present in cerebrospinal fluid of a non-demented individual may indicate a brain at risk or in the preclinical stage of dementia (Shen 1996). AChE activity is decreased by free radicals and increased oxidative stress (Molochkina et al. 2005). The decrease in AChE has also been correlated with increase in lipid peroxidation during human aging (Jha and Rizvi 2009). It is therefore possible that the age-related decrease in brain and plasma AChE activity in the present study may be due to increased lipid peroxidation in aged rats. Previous studies in both rodents and humans have shown that there is a close correlation between age-related alterations in AChE and increased oxidative stress (Molochkina et al. 2005), as well as between age-related losses of cognitive function with oxidative protein damage in the brain (Nicolle et al. 2001). It has been suggested earlier that the uptake of circulatory choline decreases with age (Cohen et al. 1995). Therefore, in the present study, the impaired memory exhibited by the aged rats may be attributed to the observed decreases in AChE activity in plasma and brain. This decrease in AChE activity both in brain and plasma could be taken as an important biomarker for age-related decrements in learning and memory which may lead to neurodegenerative disorders such as AD and dementia in senescent subjects.
Antioxidant defense system of plasma is also an important marker to evaluate the state and potential of oxidative stress in aging and other aging-induced pathological events such as diabetes, cardiovascular diseases, and cancer. Since imbalance between antioxidant and oxidant generates oxidative stress, estimation of the reducing power/antioxidant capacity in plasma is the first step in the prediction of oxidative stress in aging process (Pandey and Rizvi 2010). Catecholamine oxidation, increased activity of monoamine oxidase B, and decreased activity of antioxidant enzymes (SOD and CAT) are few of the causative factors of increased oxidant stress during aging (Alper et al. 1999). In support of this observation, antioxidant supplementation prevented the decline in cognitive performance that accompanied normal aging in mice (Tchantchou et al. 2005). The present findings also demonstrate that the antioxidant defense systems are altered in senescence as the levels of antioxidant enzymes CAT, SOD, and GPx are decreased in brain and plasma of aged rats compared with those of young controls. Our results are in agreement with previous studies showing decreased levels of SOD, CAT, and GPx in different tissue of senescent animals (Navarro et al. 2004). Decreased CAT activity may compromise the overall antioxidant enzyme defense system (Tian et al. 1998). Therefore, the decrease activities of antioxidant enzymes in our study and an increase level of lipid peroxidation suggest a role of ROS in the pathogenesis of aging. Leutner et al. (2001) and Smith et al. (1991) observed the reaction products of active oxygen such as lipofuscin and lipid peroxide increases with aging. These effects are due to continuous presence of small concentration of oxygen free radicals in the tissues during aging (Leutner et al. 2001), which eventually leads to the imbalance in cellular redox status. In the present study, these changes were further reflected in the activities of antioxidant enzymes which decreased during aging, and more importantly, the decrease in antioxidant enzymes in brain is due to a relatively deficient antioxidative enzyme’s defense against ROS exist (Tian et al. 1998). Alterations in the normal antioxidant status influence the normal redox status of the cell and hence may lead to lipid peroxidation (Dringen 2000).
Present study also revealed age related changes in brain tissue level of biogenic amines, i.e., NA, DA, and 5-HT. These neurotransmitters have been linked to cognitive processes such as attention and learning (Tang et al. 1999). Several studies have reported that brain neurotransmitter levels reduced in association with aging (Morgan and May 1990). Long-term potentiation can be affected by changes in ACh, DA, NA, and 5-HT systems (Ohashi et al. 2002). 5-HT and DA transmission declined during aging because of decreased 5-HT and DA turnover in hippocampus (Míguez et al. 1999), striatum, and other limbic regions (Venero et al. 1991). Research indicates a combined effect of inefficient phosphorylation and oxidative damage of tryptophan hydroxylase (TrpH) enzyme which may be responsible for lower TrpH activity in aging brain. Such alterations in TrpH activity may reduce the level of 5-HT in brain and may be linked to late-life depression and other brain disorders, such as AD and Parkinson diseases (Hussain and Mitra 2000). Decreases in 5-HT levels in our study could be due to decreased TrpH activity in aged rats as reported earlier. Our data are also consistent with other studies reporting reduced level of 5-HT and NA and their metabolites in cortex, hippocampus, and hypothalamus of aged rats (Tsunemi et al. 2005; Hegazy and Ali 2011). Studies have found that, with the increasing age, dopamine level and dopaminergic pathways decline because number of dopamine synapses/receptors or its binding affinity to receptors is decreased (Peters 2006). Moreover, the level of 5HT is also reduced with aging (Koprowska et al. 2004), which may be involved in the regulation of synaptic plasticity and neurogenesis (Mattson et al. 2004). The decreased level of monoamines in whole brain tissue of senescent rats can be directly correlated to the age associated memory impairment in rats (Luine et al. 1990). Serotonergic system has an important role in memory function (Schmitt et al. 2006). We have previously shown that increase brain 5-HT improves cognitive performance (Haider et al. 2006). The age-related decline in brain DA activity is also associated with reduction in cognitive and motor performance (Volkow et al. 1998). A 10 % decline in DA level at every decade from early adulthood is associated with decreased cognitive and motor ability (Peters 2006). In addition, NA is critical for memory consolidation, and a disturbance of its concentration in brain during development could compromise cognitive ability (Tsunemi et al. 2005).
Conclusions
In conclusion, we report that aged rats exhibit an increased oxidative stress, decline in anti-oxidation system, decreased AChE activity, reduced biogenic amine levels, and both short- and long-term memory impairment. During present investigation, we have not examined a direct association of oxidative stress to memory impairment; however, our data suggest that age-related impaired memory functions may possibly be attributed to increased oxidative stress, which may lead to neurodegenerative disorders such as AD and dementia.
Acknowledgments
Authors are grateful to Dr. Rafat Siddiqui for help in writing the manuscript. The study was financially supported from a grant from the University of Karachi, Karachi, Pakistan.
Conflict of interest
The authors declare no conflict of interest.
References
- Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper G, Girgin FK, Ozgonul M, Mentes G, Ersoz B. MAO inhibitors and oxidant stress in aging brain tissue. Eur Neuropsychopharmacol. 1999;9:247–252. doi: 10.1016/S0924-977X(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid beta((1-40)) rat model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;3:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Meltzer J, Houston F, Orr G, Mcgann K, Wenk GL. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/S0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav. 2012;107:568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Abdul HM, Newman S, Reed T. Redox proteomics in some age-related neurodegenerative disorders or models thereof. Neuro Rx. 2006;3:344–357. doi: 10.1016/j.nurx.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabalas-Picot I, Nicole A, Clement M, Bourke J, Signet P. Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice overexpressing copper–zinc superoxide dismutase. Mutat Res. 1992;275:281–293. doi: 10.1016/0921-8734(92)90032-K. [DOI] [PubMed] [Google Scholar]
- Cerejeira J, Batista P, Nogueira V, Firmino H, vaz-Serra A, Mukaetova-ladinska EB. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing. 2011;40:621–626. doi: 10.1093/ageing/afr053. [DOI] [PubMed] [Google Scholar]
- Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punicagranatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Chow CK, Tappel AL. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972;7:518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Renshaw PF, Stoll AL, Wurtman RJ, Yurgelun-Todd D, Babb SM. Decreased choline uptake in older adults: and in vivo proton magnetic resonance spectroscopy study. JAMA. 1995;274:902–907. doi: 10.1001/jama.1995.03530110064037. [DOI] [PubMed] [Google Scholar]
- Das A, Dikshit M, Nath C. Profile of acetylcholine esterase in brain areas of male and female rats of adult and old age. Life Sci. 2001;68:1545–1555. doi: 10.1016/S0024-3205(01)00950-X. [DOI] [PubMed] [Google Scholar]
- Dias CP, De Lima MN, Torres JP, Dormelles A, Garcia VA, Scalco F, Guimarães M, Constantino L, Budni P, Dal-Pizzol F, Schöder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholine esterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Enciu AM, Gherghiceanu M, Popescu BO (2013) Triggers and effectors of oxidative stress at blood–brain barrier level: relevance for brain ageing and neurodegeneration. Oxidative Med Cell Longev, 297512. doi:10.1155/2013/297512. [DOI] [PMC free article] [PubMed]
- Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxidative Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Garcıa-Ayllon M-S, Riba-Llena I, Serra-Basante C, Alom J, Boopathy R, Saez-Valero J. Altered levels of acetylcholinesterase in alzheimer plasma. PLoS ONE. 2010;14:e8701. doi: 10.1371/journal.pone.0008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Ghingini B, Villa RF. Acetylcholinesterase activity of synaptic plasma membranes during aging: effect of l-acetylcarnitine. Dementia. 1996;7:147–154. doi: 10.1159/000106870. [DOI] [PubMed] [Google Scholar]
- Haider S, Khaliq S, Ahmed SP, Haleem DJ. Long-term tryptophan administration enhances cognitive performance and increases 5HT metabolism in the hippocampus of female rats. Amino Acids. 2006;31:421–425. doi: 10.1007/s00726-005-0310-x. [DOI] [PubMed] [Google Scholar]
- Haider S, Khaliq S, Haleem DJ. Enhanced serotonergic neurotransmission in the hippocampus following tryptophan administration improves learning acquisition and memory consolidation in rats. Pharmacol Rep. 2007;59:53–57. [PubMed] [Google Scholar]
- Haider S, Tabassum S, Ali S, Saleem S, Khan AK, Haleem DJ. Age-related decreases in striatal DA produces cognitive deficits in male rats. J Pharmacol Nutri Sci. 2011;1:20–27. doi: 10.6000/1927-5951.2011.01.01.05. [DOI] [Google Scholar]
- Haider S, Khaliq S, Tabassum S, Haleem DJ. Role of Somatodendritic and postsynaptic 5-HT1A receptors on learning and memory functions in rats. Neurochem Res. 2012;37:2161–2166. doi: 10.1007/s11064-012-0839-5. [DOI] [PubMed] [Google Scholar]
- Haider S, Naqvi F, Batool Z, Tabassum S, Perveen T, Saleem S, Haleem DJ. Decreased hippocampal 5-HT and DA levels following sub-chronic exposure to noise stress: impairment in both spatial and recognition memory in male rats. Sci Pharm. 2012;80:1001–1011. doi: 10.3797/scipharm.1207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hegazy HG, Ali EHA. Modulation of monoamines and neurotransmitters in cerebral cortex and hippocampus of female senile rats by ginger and lipoic acid. Afr J Pharm Pharmacol. 2011;5:1080–1085. [Google Scholar]
- Husain K, Somani SM. Interaction of exercise training and chronic ethanol ingestion on testicular antioxidant system in rat. J Appl Toxicol. 1998;18:421–429. doi: 10.1002/(SICI)1099-1263(199811/12)18:6<421::AID-JAT532>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hussain AM, Mitra AK. Effect of aging on tryptophan hydroxylase in rat brain: implications on serotonin level. Drug Metab Dispos. 2000;28:1038–1042. [PubMed] [Google Scholar]
- Jha R, Rizvi SI. Age-dependent decline in erythrocyte acetylcholine esterase activity: correlation with oxidative stress. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:195–198. doi: 10.5507/bp.2009.032. [DOI] [PubMed] [Google Scholar]
- Kingsley M, Cunningham D, Mason L, Kilduff LP, McEneny J. Role of creatine supplementation on exercise-induced cardiovascular function and oxidative stress. Oxidative Med Cell Longev. 2009;2:247–254. doi: 10.4161/oxim.2.4.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowska M, Krotewicz M, Romaniuk A, Strzelczuk M. Age-related changes in fear behavior and regional brain monoamines distribution in rats. Acta Neurobiol Exp (Wars) 2004;64:131–142. doi: 10.55782/ane-2004-1499. [DOI] [PubMed] [Google Scholar]
- Lee CH, Hwang IK, Choi JH, Yoo K, Park OK, Huh S, Lee YL, Shin H, Won M. Age-dependent changes in calretinini and its protein level in the gerbil hippocampus. Neurochem Res. 2010;35:122–129. doi: 10.1007/s11064-009-0037-2. [DOI] [PubMed] [Google Scholar]
- Leutner S, Eckert A, Muller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm. 2001;108:955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: contributions of the cholinergic system assessed by ChAT. Brain Res. 1990;523:321–324. doi: 10.1016/0006-8993(90)91507-D. [DOI] [PubMed] [Google Scholar]
- Mattson M, Maudsleey S, Martin B. BDNF and 5-HT; a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–1125. doi: 10.1016/S0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Míguez JM, Aldegunde M, Paz-Valiñas L, Recio J, Sánchez-Barceló E (1999) Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Neural Transm 106:1089–1098 [DOI] [PubMed]
- Milic VD, Stankov K, Injac R, Djordjevic A, Srdjenovic B, Govedarica B. Activity of antioxidative enzymes in erythrocytes after a single dose administration of doxorubicin in rats pretreated with fullerenol C(60)(OH)(24) Toxicol Mech Methods. 2009;19:24–28. doi: 10.1080/01612840802203098. [DOI] [PubMed] [Google Scholar]
- Molochkina EM, Zorina OM, Fatkullina LD, Goloschapov AN, Burlakova EB. H2O2 modifies membrane structure and activity of acetylcholine esterase. Chem Biol Interact. 2005;157:401–404. doi: 10.1016/j.cbi.2005.10.075. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ. Lipid peroxidation in aging brain and alzheimer's disease. Free Radic Biochem Med. 2002;33:620–626. doi: 10.1016/S0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- Morgan DG, May PC. Age-related changes in synaptic neurochemistry. In: Schneider EL, Rowe JW, editors. Handbook of the biology of aging. New York: Academic Press; 1990. pp. 219–254. [Google Scholar]
- Morris RG. Spatial localization does not depend on the presence of local cues. Learn Motiv. 1981;12:239–260. doi: 10.1016/0023-9690(81)90020-5. [DOI] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol. 2004;286:505–511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/S0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno KI, Kaku A, Yoshiokaa M. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/S0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh LJ. Glucocorticoid effects on object recognition memory requires training-associated emotional arousal. PNAS. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxidative Med Cell Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholine esterase activity. Behav Brain Res. 2009;198:352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of alzheimer's disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Br J Med Biol Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Curr Pharm Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- Shen ZX. The significance of the activity of CSF cholinesterases in dementias. Med Hypotheses. 1996;47:363–376. doi: 10.1016/S0306-9877(96)90216-9. [DOI] [PubMed] [Google Scholar]
- Sigueira IR, Fochesatto C, Lucena da Silva Torres I, Dalmaz C, Netto CA. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Singh K, Kaur S, Kumari K, Singh G, Kaur A. Alterations in lipid peroxidation and certain antioxidant enzymes in different age groups under physiological conditions. J Hum Ecol. 2009;27(2):143–147. [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carnry JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke-Reed PE, Oliver CN. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ (1999) Genetic enhancement of learning and memory in mice. Nature 401:63–69 [DOI] [PubMed]
- Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis. 2005;8:283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med. 1998;24:1477–1484. doi: 10.1016/S0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Tsunemi A, Utsuyama M, Seidler BK, Kobayashi S, Hirokawa K. Age-related decline of brain monoamines in mice is reversed to young level by Japanese herbal medicine. Neurochem Res. 2005;30:75–81. doi: 10.1007/s11064-004-9688-1. [DOI] [PubMed] [Google Scholar]
- Venero JL, Machado A, Cano J. Turnover of dopamine and serotonin and their metabolites in the striatum of aged rats. J Neurochem. 1991;56:1940–1948. doi: 10.1111/j.1471-4159.1991.tb03451.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Zhang X. Cholinergic activity and amyloid precursor protein processing in aging and Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2004;3:137–152. doi: 10.2174/1568007043482499. [DOI] [PubMed] [Google Scholar]