Abstract
Dichloroacetic acid (DCA), a water disinfection by-product, has attained emphasis due to its prospect for clinical use against different diseases including cancer along with negative impact on organisms. However, these reports are based on the toxicological as well clinical data using comparatively higher concentrations of DCA without much of environmental relevance. Here, we evaluate cellular as well as organismal effects of DCA at environmentally and mild clinically relevant concentrations (0.02–20.0 μg/ml) using an established model organism, Drosophila melanogaster. Flies were fed on food mixed with test concentrations of DCA for 12–48 h to examine the induction of reactive oxygen species (ROS) generation, oxidative stress (OS), heat shock genes (hsps) and cell death along with organismal responses. We also examined locomotor performance, ROS generation, glutathione (GSH) depletion, expression of GSH-synthesizing genes (gclc and gclm), and hsps at different days (0, 10, 20, 30, 40, 50) of the age in flies after prolonged DCA exposure. We observed mild OS and induction of antioxidant defense system in 20.0 μg/ml DCA-exposed organism after 24 h. After prolonged exposure to DCA, exposed organism exhibited improved survival, elevated expression of hsp27, gclc, and gclm concomitant with lower ROS generation and GSH depletion and improved locomotor performance. Conversely, hsp27 knockdown flies exhibited reversal of the above end points. The study provides evidence for the attenuation of cellular and functional decline in aged Drosophila after prolonged DCA exposure and the effect of hsp27 modulation which further incites studies towards the therapeutic application of DCA.
Keywords: DCA, Drosophila, Healthspan, ROS, GSH, hsp27, Prolonged exposure
Introduction
Dichloroacetic acid (DCA), a water disinfection by-product, is prospected as a potential environmental hazard and an investigational drug (Stacpoole 2011). Environmental sources of DCA include chlorinated drinking water, industrial solvents, and pharmaceuticals (Stacpoole et al. 1998). Levels of DCA detected in drinking water ranged from 4.5–7.5 μg/l (Japan) to 200 μg/l (Australia), while its level in surface water downstream from a paper mill in Austria was detected between <3 and 522 μg/l (Geist et al. 1991). Human population has been exposed to drinking water containing up to 160 μg/l DCA for many generations (Stacpoole 2011).
Xenobiotic interaction and response related studies on DCA have been aggravated by both environmental and therapeutic concerns. DCA has been reported as a possible pharmacological cure for chronic diseases such as lactic acidosis and other mitochondria associated diseases (Michelakis et al. 2010; Miquel et al. 2012; Stacpoole et al. 2008). In a number of in vitro studies, DCA is reported to enhance reactive oxygen generation (ROS) generation and oxidative stress leading to cell death (Ayyanathan et al. 2012; Hassoun and Ray 2003; Wong et al. 2008). In exposed in vivo models, mainly rodents, DCA is reported to cause developmental- (Smith et al. 1992), spermato- (Linder et al. 1997), immuno-, and hepatotoxicity (Cai et al. 2007; Hassoun and Dey 2008). However, concern about DCA adversity underlies on the data generated in rodents after their exposure to this chemical at doses (up to ~4,000 mg/kg) thousands of times higher than those to which humans are usually exposed (Stacpoole et al. 1998). In the same context, a dose range of 10–100 mg/kg of DCA is suggested as therapeutics for human use via intravenous and oral routes (Stacpoole 2011). On the other, studies regarding long-term exposure to environmentally relevant concentrations (~160–200 μg/l, detected in the environment and to which organisms including human are likely to be exposed) of DCA are elusive.
Organismal susceptibility to chronic and degenerative diseases is casually linked to aging. Age-related behavioral insufficiencies are one of the main functional changes that occur in aged individuals. In human, progressive decline in locomotor ability, memory function, and olfactory sensitivity is imperative age-related changes (Grotewiel et al. 2005). Thus, life expectancy may be improved by the amelioration of age-related behavioral declines (Jones and Grotewiel 2011). Strategies for promoting heathspan via reduced ROS generation and increased antioxidants level have gained much interest (Gruber et al. 2008, 2009). Among the antioxidants, glutathione (GSH) is the most abundant nonprotein thiol that protects the organism against oxidative stress and participates in the detoxification of xenobiotics and/or their metabolites, antioxidant defense, maintenance of redox potential, regulation of cell cycle progression, and apoptosis (Lu 2013). Reduced GSH levels are reported in aged organisms (Jiang et al. 2013; Suh et al. 2004). The rate-limiting enzyme, glutamate cysteine ligase (GCL), is one of the key determinants of GSH biosynthesis and is composed of catalytic (GCLc) and modifier (GCLm) subunits. In in vitro studies, altered expression of GCL against xenobiotic exposure was reported (Ha et al. 2006; Thompson et al. 2009; Zheng et al. 2007). Although DCA has been reported to upregulate GSH biosynthesis via the induction of GCL in mice (Theodoratos et al. 2012), no information is available on the modulation of GSH level and the status of GCL expression (GCLc and GCLm) in DCA-exposed organism in the context of aging.
Premature aging of an organism has been implicated by the alteration in the expression and regulation of a number of genes concurrent with factors like xenobiotics exposure, dietary habits, etc. Heat shock proteins (HSPs) are reported to function as chaperones by orchestrating correct folding and unfolding of proteins. Levels of HSPs increase in response to different types of stresses that also include xenobiotic exposure. HSPs can counteract proteo-toxicity and favor stress resistance to the organism which may be causally linked to an increase in life span vis-à-vis positive impact on the aging-related functional declines (Tower 2009, 2011). Genome-wide studies on age-associated gene expression changes in flies have shown the upregulation of heat shock genes (hsps) (Curtis et al. 2007; Landis et al. 2012). In addition, modulated expression of hsps (hsp70, hsp27, and hsp22) has been reported to alter life span in flies (Kim et al. 2010; Liao et al. 2008; Tatar et al. 1997) and higher level of HSPs is reported in longer-lived mammals and birds (Salway et al. 2011). In the same context, improved health- and life span were observed in DCA-exposed Caenorhabditis elegans (Schaffer et al. 2011). However, studies regarding expression of hsps in DCA-exposed organisms are inadequate.
Model organisms provide ample opportunities to examine the underlying mechanism of xenobiotic-induced effects on exposed organism. Drosophila, an insect model, with well-documented genetics and developmental biology and high degree of homology of its genes with that of higher mammals, is the closest invertebrate to the humans and has been used for toxicological studies and for studying human diseases (Jeibmann and Paulus 2009). Relevant to the higher mammals, Drosophila exhibits an age-related decline in several behaviors such as senescence of motor activity, olfaction, olfactory memory, and noncircadian rest (Grotewiel et al. 2005). Assay for locomotor performance is one of the reliable assays to examine the senescence of motor activity in flies (Lliadi et al. 2012). Further, this model has been a key to comprehend the association between hsps and aging process since the discovery of heat shock response and hsps (Tower 2011). It raises fewer ethical concerns and falls within the recommendations of the European Centre for the Validation of Alternative Methods (ECVAM) and aims to prop up the scientific and regulatory acceptance of alternative methods that are important in the field of biological science and towards reducing, refining, and replacing the use of laboratory animals (Benford et al. 2000).
The present study, therefore, aims to examine the cellular stress inducing potential of DCA in exposed nontarget organism, Drosophila melanogaster, and its effect on life span and age-dependent impairments. Further, the study is intended to provide evidence on the modulatory effect of small hsp, viz., hsp27, on DCA-exposed organism.
Materials and methods
Fly strain and culture
Wild-type D. melanogaster (Oregon R+), w1118, Gal4-UAS transgenic lines, namely, Act-Gal4/Cyo, UAS-hsp27, UAS-hsp27RNAi, and UAS-hsp22RNAi, were used in this study. The fly strains were reared on a standard Drosophila food medium (consisting of agar-agar, maize powder, sugar, yeast, nepagin, and propionic acid) at 24 ± 1 °C. Additional yeast supplement was provided for healthy growth of the organisms.
Chemical and treatment schedule
DCA (PESTANAL® analytical standard, 99.3 %) obtained from Sigma Chemicals, St. Louis, MO, USA, was used in the study. Of the four different concentrations of DCA used (0.02, 0.2, 2.0, and 20.0 μg/ml), lower concentrations (0.02 and 0.2 μg/ml) are environmentally relevant (IARC 2004) while the other two higher concentrations (2.0 and 20.0 μg/ml) are less than clinically relevant concentrations (up to ~100 mg/kg). Flies were allowed to feed on food contaminated with different concentrations of DCA. Control group received standard Drosophila food.
Chemical estimation
Quantification of DCA in exposed organism was carried out by gas chromatography (GC) with an electron capture detector (ECD). In brief, control and exposed flies were homogenized in Milli-Q water and then treated with pyridine and methyl chloroformate to get volatile and nonpolar methyl ester of DCA (Mudiam et al. 2013). The ester derivative after its extraction in hexane was applied on an Agilent GLC7890A GC (Foster City, CA, USA) equipped with an ECD.
Emergence pattern of flies
First instar larvae were transferred to normal food medium (control) and to food containing different concentrations of the DCA (50 larvae/vial, 10 vials/group). The number of flies emerging from different groups was counted until all the flies emerged (Gayathri and Krishnamurthy 1981).
Survivorship assay
To examine the effect of DCA on the life span, male flies were fed on the food mixed with different concentrations of DCA from day 1 of their emergence. For each group, 250 flies (maximum 25 flies were maintained per vial) were scored. Every alternate day, flies were transferred to fresh vials and the number of dead flies was scored till the death of the last fly (Nazir et al. 2001).
Reproductive assay
Reproductive assay was performed using a previously published method (Gayathri and Krishnamurthy 1981). Briefly, freshly eclosed first instar larvae were transferred to control and chemical-contaminated food and they were allowed to grow throughout their development. Virgin male and female flies emerging from control and treated food were separated and mated in vials containing normal food. For each group, 10 pair of flies in 10 individual vials were taken and transferred to fresh vials everyday for the next 10 days and the number of eggs laid during this period was scored. The total number of flies emerging from the eggs laid during these 10 days was counted; the mean number of flies emerged per pair for 10 days gave a measure of reproductive performance.
RNA isolation and quantitative real-time polymerase chain reaction (qPCR)
Total RNA from control and treated flies was extracted using TRI reagent (Ambion, Austin, TX, USA). Purity and concentration of the isolated RNA were determined by measuring the absorbance ratio at 260/280 and 230/260 nm on a NanoDrop spectrophotometer (Wilmington, DE, USA). RNA was reverse-transcribed into cDNA using a cDNA synthesis kit (Fermentas, MD, USA) essentially following the manufacturer’s instructions. qPCR was performed in 96-well PCR plates on a 7900 HT Fast Real-Time PCR (Applied Biosystems, CA, USA) using Power SYBR Green Master Mix (Applied Biosystems CA, USA) with gene-specific primers for the target genes (hsp22, hsp23, hsp26, hsp27, hsp60, hsp70, hsp83, gclc, and gclm) (primer details in Table 1). Relative quantification of gene expression was carried out by concurrent amplification of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an endogenous control.
Table 1.
Genes and their primer sequences used in RT-PCR amplification
| hsp22 | Forward (F) 5′ GGATGAACTGGACAAGGCTCTAAA 3′ |
| Reverse (R) 5′ ATATGATTGGCGACTGCTTCTCC 3′ | |
| hsp23 | Forward (F) 5′ GAGCCTTGCCGACGATTTG 3′ |
| Reverse (R) 5′ GGCGCCCACCTGTTTCTC 3′ | |
| hsp26 | Forward (F) 5′ GAGCCCCGCAGCCCCATCTA 3′ |
| Reverse (R) 5′ GGAACGGCGCTGCTGAGTGC 3′ | |
| hsp27 | Forward (F) 5′ GCGTCGCCTGCTACTGCCCAACAC 3′ |
| Reverse (R) 5′ CTCCATTTTCTCGCCGCTGCCATTT 3′ | |
| hsp60 | Forward (F) 5′ ATGTCGCGCCCCGTTAGCAC 3′ |
| Reverse (R) 5′ GCCATCGCGTCCCACCTTCT 3′ | |
| hsp70 | Forward (F) 5′ ACGGGCCAAGCGCACACTCTC 3′ |
| Reverse (R) 5′ CCGCGCACAGTCCCTCAAACC 3′ | |
| hsp83 | Forward (F) 5′ TGCGCACTTTTCGACCGTATCA 3′ |
| Reverse (R) 5′ GAAAAACCCGACCCGAACTGGA 3′ | |
| gclc | Forward (F) 5′ CGCCGGCGAGCTAATCACC 3′ |
| Reverse (R) 5′ CTCCACCTGCTTGCCCTCCTG 3′ | |
| gclm | Forward (F) 5′ GGAGCGCGTGGTGGTTGA 3′ |
| Reverse (R) 5′ TGTGGAGTGGCGATTGAGGAA 3′ | |
| gapdh | Forward (F) 5′ CTGGGCTACACCGATGAGGAG 3′ |
| Reverse (R) 5′ CCACGAGATTAGCTTGACGAA 3′ |
Assay for oxidative stress markers
To examine the oxidative stress, ROS generation, superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST) activities, GSH content, protein carbonyl (PC) content, and lipid peroxidation (LPO) product were assayed in control and DCA-exposed flies. The above-mentioned assays were carried out in 10 % fly homogenate.
Preparation of fly homogenate
Flies from control and treated groups were homogenized in cold 0.1 M phosphate buffer (pH 7.4) containing 0.15 M KCl to obtain 10 % homogenate. The supernatant after centrifugation at 12,000 × g for 10 min was used for different assays and protein estimation.
Measurement of ROS generation
ROS generation was estimated by using the dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma, St. Louis, MO, USA). Assay was performed as described by Zhao et al. (2010). In brief, 100 μl of fly homogenate was incubated with 10 μM DCFH-DA (in dimethyl sulfoxide) and fluorescence was measured using a microplate reader at an excitation and emission wavelength of 485 and 535 nm, respectively. The fluorescence intensities were normalized to the protein concentration.
Superoxide dismutase (SOD) (superoxide: superoxide oxidoreductase EC 1.15.1.1)
The method for estimating SOD described previously by Nishikimi et al. (1972) was followed with minor modification (Gupta et al. 2005). The assay mixture consisted of 0.052 M sodium pyrophosphate buffer (pH 8.3), 186 μM phenazine methosulphate, 300 μM nitroblue tetrazolium, 780 μM reduced nicotinamide adenine dinucleotide, and the homogenate. One unit of enzyme activity is defined as the enzyme concentration required for inhibiting chromogen production (optical density 560 nm) by 50 % in 1 min under assay condition, and the results were expressed as specific activity in units per minute per milligram protein.
Catalase (CAT (H2O2: H2O2 oxidoreductase EC 1.11.1.6)
CAT activity in the control and treated flies was measured by following the ability of the enzyme to split H2O2 within 1 min of incubation time. After incubation, the reaction was stopped by adding dichromate/acetic acid reagent (5 % solution of K2Cr2O7/glacial acetic acid, 1:3 by volume) and the remaining H2O2 was determined by measuring chromic acetate at 570 nm as described previously by Sinha (1972). Enzyme activity was expressed as micromoles H2O2 decomposed per minute per milligram protein.
Assay for lipid peroxidation (LPO)
We followed the method of Ohkawa et al. (1979) for assaying malondialdehyde (MDA) as a measurement of LPO using tetraethoxypropane as an external standard. The assay mixture consisted of 10 % sodium dodecyl sulphate (SDS), 0.8 % thiobarbituric acid (TBA), and fly homogenate. Level of lipid peroxidation was expressed in terms of nanomoles MDA content per hour per milligram protein.
Glutathione S-transferase (GST, EC 2.5.1.18)
GST activity was determined by the method of Habig et al. (1974) with minor modifications. The reaction mixture consisted of 0.2 M sodium phosphate buffer, reduced glutathione (1.0 mM) and 1-chloro-2,4-dinitrobenzene (CDNB, 5.0 mM). An increase in absorbance (340 nm) was measured for 3 min at 30-s interval, and the enzyme activity was calculated as nanomoles CDNB reduced per minute per milligram protein using a molar extinction coefficient of 6.25 × 103 M−1 cm−1.
Glutathione (GSH) content
GSH content in the exposed flies was quantified using Ellman’s reagent (Ellman 1959). The assay mixture consisted of 0.2 M phosphate buffer (pH 8.0), 0.01 % 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), and the homogenate. The reaction was monitored at 412 nm, and the amount of GSH was expressed in terms of nanomoles per milligram protein.
Determination of protein carbonyl (PC) content
PC content was determined by following the method of Levine et al. (1990) with minor modifications. Two equal aliquots of supernatant fraction were taken, one treated with equal volume of 2,4-dinitrophenylhydrazine (10 mM dissolved in 2 M HCl) (test sample) and the other with 2 M HCl (blank). Each mixture was incubated for 1 h, followed by precipitation with 20 % TCA and subsequently extracted with ethanol/ethylacetate mixture (1:1). The pellets were then dissolved in 1.0 ml of 6 M guanidine hydrochloride. The spectrum of DNPH-treated sample versus the HCl blank was determined at 370 nm, and the results were expressed in terms of nanomoles DNPH incorporated per milligram protein based on a molar absorption coefficient of 22,000 M−1 cm−1.
Protein estimation
Protein concentration was determined by the method of Lowry et al. (1951) using a protein estimation kit (Bangalore Genei, Bangalore, India) essentially following the manufacturer’s protocol and bovine serum albumin (BSA) as the standard.
Cell death assay
To examine cell death in control and DCA-exposed flies, DEVDase (caspase-3 like) and IETDase (caspase-9 like) activities were examined. The assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) obtained after specific action of caspase-3 and caspase-9 on tetrapeptide substrates, DEVD-pNA and IETD-pNA, respectively. The assay mixture consisted of fly homogenate, chilled cell lysis buffer, 2× reaction buffer containing dithiothreitol, and 200 μM substrate (BioVision caspase assay kit, CA, USA). The reaction mixture was incubated at 37 °C for 1.5 h, and absorbance of the product was measured at 405 nm on a Cintra 20 ultraviolet spectrophotometer (GBC Scientific Equipment, Australia).
Locomotor assay
Locomotor assay was performed as described previously by Feany and Bender (2000). In brief, 20 flies in a vertical plastic tube (18 cm × 2 cm) were gently tapped to the bottom and kept for 1 min in order to get acclimatized with their environment. Flies that crossed the 15-cm line within 30 s from the time they were tapped to the bottom of vials were scored. One hundred male flies were used for locomotor assay per group (5 replicates, 20 flies in each). The locomotor performance was represented in terms of mean percentage of flies that crossed the 15-cm line among the total number of flies per experiment.
Measurement of body weight
Growth of flies was examined in terms of body weight. For each group, 100 male flies (4 replicates of 25 flies in each) were weighed. Body weights were expressed in milligrams.
Statistical analysis
Statistical significance of the mean values for different parameters was monitored in control and exposed flies using ANOVA followed by post hoc tests after ascertaining the homogeneity of variance and normality of data. For multiple comparisons, one-way and two-way ANOVAs were followed by Dunnett’s and Bonferroni’s test, respectively. Each end point was considered as a dependent variable while concentration and time of exposure as independent variables. Statistical significance level was ascribed as P < 0.05. Pearson’s correlations were calculated, and then, linear regression analysis was carried out. Prism computer program (GraphPad version 5.0, San Diego, CA, USA) was used for the statistical analysis. Kaplan-Meier analysis was used for survivorship analysis with stratified log rank tests using SPSS software version 13.0, (SPSS Inc., Chicago, IL, USA).
Results
Detection of DCA in exposed flies
DCA residues were detected in the exposed organism at its 2.0- and 20.0-μg/ml concentrations, while for the rest two lower concentrations, DCA residues were beyond the detection limit. We could recover 7.17 ± 0.04 and 4.51 ± 0.29 ng/g DCA from 2.0 μg/ml DCA-treated flies and 53.46 ± 0.51 and 31.62 ± 0.96 ng/g DCA from 20.0 μg/ml DCA-exposed flies after 24 and 48 h, respectively.
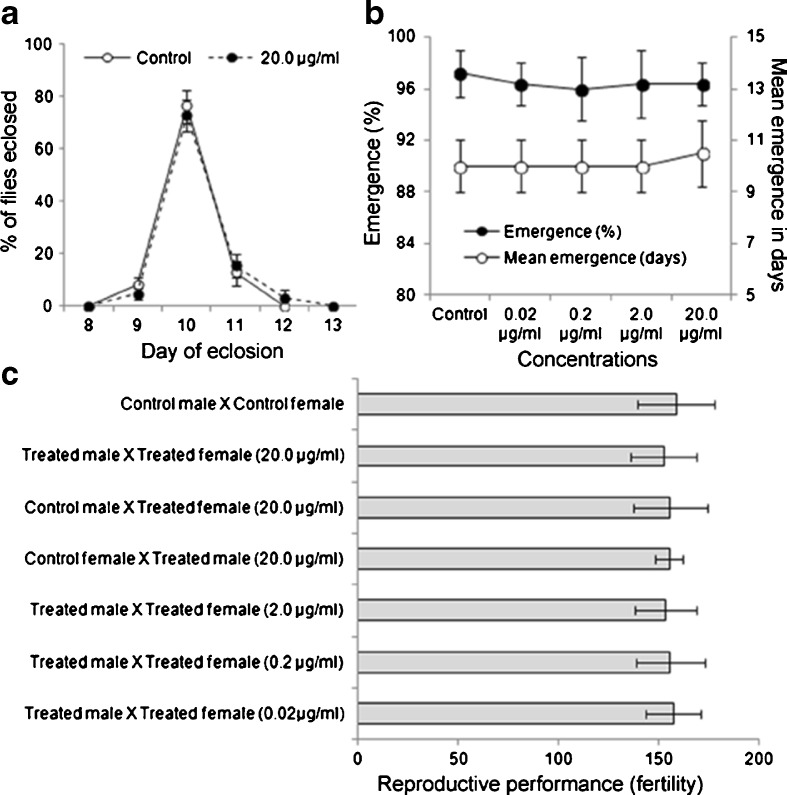
Effect of DCA on emergence pattern of flies (Oregon R+)
Figure 1a, b shows the emergence pattern and percent emergence in control and DCA-treated groups. We observed a nonsignificant (P < 0.05) change in the emergence and larval development of flies in DCA-exposed groups as compared to control.
Fig. 1.
Lack of any adverse effect on emergence, development, and reproductive performance of DCA exposed D. melanogaster (Oregon R +). Graphs showing emergence pattern (a), percent emergence and mean emergence in days (b), and reproductive performance in terms of fertility (total progeny per female in 10 days) (c) in control and DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 vs. control
Effect of DCA on reproductive performance
Figure 1c shows the reproductive performance in control and DCA-exposed groups. We observed a nonsignificant (P > 0.05) change in the reproductive performance of DCA-exposed groups as compared to control in terms of fertility.
Acute exposure of DCA to Oregon R+ flies caused increased ROS generation, oxidative stress, and expression of heat shock genes without propelling cell death
Assays for ROS generation, oxidative stress (OS) end points, and expression of hsps were performed in the flies exposed to different concentrations of DCA for 12–48 h along with their respective control. We observed a significant (P < 0.05) increase in ROS generation in 20.0 μg/ml DCA-treated group after 24 h (~40 %) and a nonsignificant change after 48 h in comparison to control. For the rest of the concentrations of DCA and exposure periods, the level of ROS generation in exposed flies was comparable to that in control (Fig. 2a).
Fig. 2.
Induction of ROS generation, oxidative stress end points, and hsps and noninduction of cell death in DCA-exposed D. melanogaster (Oregon R +). Graphs showing ROS generation (a), superoxide dismutase (SOD) and catalase (CAT) activities (b, c), malondialdehyde (MDA) content (d), glutathione S-transferase (GST) activity (e), glutathione (GSH) and protein carbonyl (PC) contents (f, g) in control and DCA-treated D. melanogaster for 12–48 h. qPCR data showing mRNA level (in terms of fold change against control) for hsp22, hsp27, and hsp70 in DCA-treated D. melanogaster for 12–48 h (h). Graph showing IETDase (caspase 9-like) and DEVDase (caspase 3-like) activities (i) in control and DCA-treated D. melanogaster for 24 h. Data represent mean ± SD of three identical experiments made in three replicates. Significance ascribed as *P < 0.05 vs. control
Figure 2b–g shows OS parameters assayed in control and DCA-exposed groups. Concomitant with the induction of ROS generation, we observed a significant (P < 0.05) increase in MDA and PC contents and GST activity in 20.0 μg/ml DCA-treated flies after 24 h (an increase of ~40, 52, and 47 % in MDA and PC contents and GST activity, respectively) while the same end points were found to be nonsignificantly changed after 48 h in comparison to control (Fig. 2d, g, e). Likewise, GSH content was found to be significantly decreased (~23 % decrease in comparison to control) in flies that were exposed to DCA for 24 h while the same after 48 h was nonsignificantly changed as compared to control (Fig. 2f). Interestingly, we observed a significant increase in the activities of antioxidant enzymes (maximum increase of ~59 and ~39 % in SOD and CAT activities, respectively, after 48 h) in 20.0 μg/ml DCA-treated flies after 24 and 48 h as compared to control (Fig. 2b, c). None of the lower concentrations (0.02, 0.2, 2.0 μg/ml) of DCA evoked any change in the above end points in exposed organism.
To examine the expression of hsps (hsp22, hsp23, hsp26, hsp27, hsp60, hsp70, and hsp83), qPCR assay was carried out in control and DCA-treated flies. We observed a significant (P < 0.05) increase in the expression of hsp22, hsp27, and hsp70 (maximum increase of ~1.9- and 2.1-fold in hsp22 and hsp70 expression, respectively, after 24 h and ~2.3-fold in hsp27 expression after 48 h of DCA exposure) in 20.0 μg/ml DCA-treated flies after 24 and 48 h (Fig. 2h). None of the tested concentrations of DCA evoked any significant increase in the expression of the other tested hsps in exposed organism during the entire exposure regimen (12–48 h) (data not shown).
To examine whether DCA (20.0 μg/ml) induced OS in exposed organism leads to cell death, we measured DEVD- and IETD-ase activities (caspase-3- and caspase-9-like activities) in control and treated flies after 24 h and observed a nonsignificant (P > 0.05) change in the enzyme activities in exposed organism as compared to control (Fig. 2i).
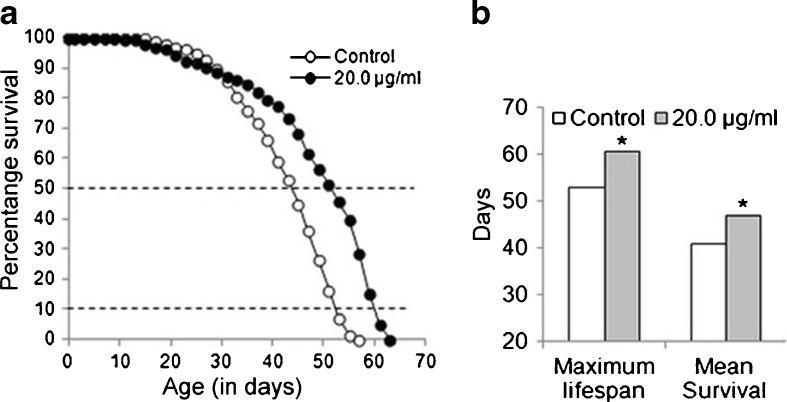
Prolonged exposure of DCA to Oregon R+ flies improved their survival
Figure 3a presents the survival of control and DCA-exposed flies. While we did not observe any significant (P > 0.05) change in the maximum life span and mean survival of flies that were exposed to the lower concentrations (0.02, 0.2, 2.0 μg/ml, data not shown) of DCA, a significant (P < 0.05) increase in the maximum life span (~14 %) and the mean survival (~15 %), respectively, was observed in 20.0 μg/ml DCA-treated flies (Fig. 3b).
Fig. 3.
Improved survival of D. melanogaster (Oregon R +) flies after prolonged exposure to DCA. Graphs showing survivorship (a) and maximum life span and mean survival (b) in control and 20.0 μg/ml DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 vs. control
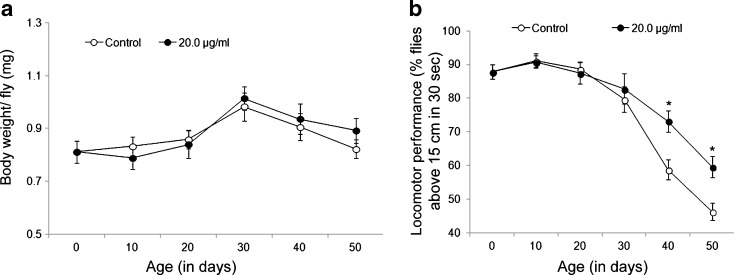
Prolonged exposure of DCA to Oregon R+ flies attenuated their age-dependent decline in locomotor performance without compromising organismal growth
To examine whether increased survival in DCA-exposed flies compromises with their healthspan, we measured growth and fitness in terms of their body weight and locomotor performance, respectively, at different days of life (0, 10, 20, 30, 40, 50 days) parallel to the age-matched control. Although we did not observe any apparent change in the body weight of exposed flies in comparison to control (Fig. 4a), a significant (P < 0.05) increase in locomotor performance was observed in 40 and 50-day-old DCA-exposed flies in comparison to their age-matched controls (~24 and 28 % increased locomotor performance was observed in 40- and 50-day DCA-exposed flies, respectively, in comparison to unexposed control flies of similar age) (Fig. 4b).
Fig. 4.
Prolonged exposure of DCA to D. melanogaster (Oregon R +) flies attenuated an age-dependent locomotor decline without compromising growth. Graphs depicting body weight (a) and locomotor performance (b) at different days of age (0, 10, 20, 30, 40, 50 days) in control and 20.0 μg/ml DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 as compared to control of similar age
Prolonged exposure of DCA to Oregon R+ flies rescued them from age-dependent ROS generation and GSH depletion
To corroborate with the increased life span of 20.0 μg/ml DCA-exposed flies, ROS generation and GSH content were also evaluated in exposed flies at the above stated days of age. We observed a significant reduction in ROS generation in 40 and 50-day-old exposed flies (~32 and ~30 % lower ROS generation in 40- and 50-day-old exposed flies, respectively, as compared to unexposed flies of similar age) (Fig. 5a). Parallel to the lowered ROS levels in these flies, we observed a significant increase (P < 0.05) in the GSH content in exposed flies with an advance in age (~42 and 39 % higher GSH content in 40- and 50-day-old DCA-treated flies, respectively, as compared to unexposed flies of similar age) (Fig. 5b).
Fig. 5.
Rescued ROS generation and GSH depletion in aged D. melanogaster (Oregon R +) flies after their prolonged exposure to DCA. Graphs depicting ROS generation (a) and GSH content (b) at different days of age (0, 10, 20, 30, 40, 50 days) in control and 20.0 μg/ml DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 as compared to control of similar age
Enhanced expression of gclc and gclm in aged Oregon R+ flies after their prolonged exposure to DCA
To substantiate the increased GSH content, expression of gclc and gclm (genes coding for GCLc and GCLm, respectively) was examined in control and 20.0 μg/ml DCA-exposed flies at different days of their age (0, 10, 20, 30, 40, 50 days) by qPCR assay. We observed a significant (P < 0.05) increase in the expression of gclc and gclm in 40- and 50-day-old exposed flies (~1.8- and 1.7-fold increased expression of gclc and ~1.9- and 1.6-fold increase in gclm expression in 40- and 50-day-old exposed flies, respectively, as compared to unexposed control flies) (Fig. 6a, b).
Fig. 6.
Enhanced expression of gclc and gclm in D. melanogaster (Oregon R +) flies after their prolonged exposure to DCA. Graphs showing mRNA level by qPCR assay (in terms of fold change against control of 0 days) for gclc (a) and gclm (b) at different days of age (0, 10, 20, 30, 40, 50 days) in control and 20.0 μg/ml DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 as compared to control of similar age
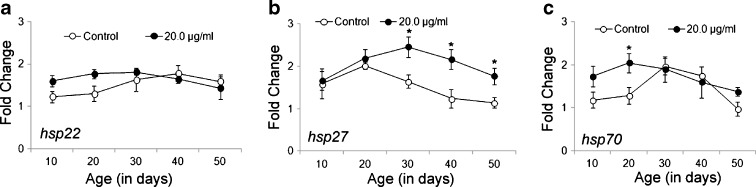
Enhanced expression of hsp27 in Oregon R+ flies after their prolonged exposure to DCA
To examine whether increased life span of DCA-exposed flies is causally linked to increased expression of hsps, hsp22, hsp27, and hsp70 expressions in the exposed organism at different days of their age (0, 10, 20, 30, 40, 50 days) along with the age-matched control were assayed by qPCR. We observed a nonsignificant (P < 0.05) change in the expression of hsp22 in exposed flies from 0–50 days in comparison to their respective control (Fig. 7a). A similar trend was observed for the expression of hsp70 except that a significant (P < 0.05) increase in hsp70 expression was observed in 10- and 20-day-old exposed flies in comparison to their corresponding control (~1.5- and 1.6-fold increase in hsp70 expression in 10- and 20-day DCA-exposed flies, respectively) (Fig. 7c). On the other, hsp27 expression was observed to be significantly increased in 30–50-day-old exposed flies (maximum increase of ~1.9-fold in 40-day-old flies) in comparison to the age-matched control (Fig. 7b).
Fig. 7.
Prolonged exposure of DCA to D. melanogaster (Oregon R +) flies enhanced expression of hsp27. Graphs showing mRNA level by qPCR assay (in terms of fold change against control of 0 days) for hsp22 (a), hsp27 (b), and hsp70 (c) at different days of age (0, 10, 20, 30, 40, 50 days) in control and 20.0 μg/ml DCA-exposed D. melanogaster. Data represent mean ± SD. Significance ascribed as *P < 0.05 as compared to control of similar age
hsp27 knockdown flies exhibited reduced survival along with an age-dependent increase in GSH depletion, ROS generation, and locomotor insufficiency after their prolonged exposure to DCA
To examine the effect of DCA on hsp27 expression in exposed organism, we generated strains wherein hsp27 was either knocked down (Act-Gal4 > UAS-hsp27RNAi) or overexpressed (Act-Gal4 > UAS-hsp27). We observed a significant (P < 0.05) reduction (~19 %) in the mean survival of Act-Gal4 > UAS-hsp27RNAi flies and a nonsignificant (P > 0.05) increase (~8 %) in the mean survival of Act-Gal4 > UAS-hsp27 flies that were exposed to 20.0 μg/ml DCA as compared to unexposed flies. In comparison to exposed Act-Gal4 > w1118 flies (genetic control), a significant (P < 0.05) reduction (~43 %) in the mean survival of exposed Act-Gal4 > UAS-hsp27RNAi flies and a nonsignificant (P > 0.05) increase (~5 %) in the mean survival of exposed Act-Gal4 > UAS-hsp27 flies was observed (Fig. 8a, b). To investigate whether reduced GSH content in hsp27 knockdown flies leads to the reduced survival of DCA-exposed organism, GSH content was measured in control and 20.0 μg/ml DCA-treated Act-Gal4 > UAS-hsp27RNAi flies at different days of their age as mentioned above. We observed a significant (P < 0.05) decline in the GSH content in 20- and 30-day-old exposed Act-Gal4 > UAS-hsp27RNAi flies in comparison to unexposed flies of similar age (~28 and 32 % decline in the GSH content in 20- and 30-day-old flies as against control, respectively). However, a decline in the GSH content was more apparent when a comparison was made between exposed Act-Gal4 > UAS-hsp27RNAi and Act-Gal4 > w1118 flies (~35 and 48 % decline in the GSH content in 20- and 30-day-old exposed Act-Gal4 > UAS-hsp27RNAi flies, respectively) (Fig. 8c). Further, levels of ROS generation were examined in control and 20.0 μg/ml DCA-treated hsp27 knockdown flies at different days of age. Concomitant to a decrease in the GSH content, we observed a significant (P < 0.05) increase in the level of ROS generation in 20.0 μg/ml DCA-treated Act-Gal4 > UAS-hsp27RNAi flies as compared to the age-matched control and genetic control of similar age (~21 and 15 % increase in ROS generation 20- and 30-day-old Act-Gal4 > UAS-hsp27RNAi flies as compared to unexposed flies, respectively, while a ~26 and 20 % increase in the ROS generation was observed in hsp27 knockdown flies as against exposed Act-Gal4 > w1118 flies) (Fig. 8d). To examine the effect of hsp27 knockdown on the fitness of DCA-exposed flies, we monitored locomotor performance of Act-Gal4 > UAS-hsp27RNAi flies and observed a significant (P < 0.05) decline in the locomotor performance of Act-Gal4 > UAS-hsp27RNAi flies that were exposed to 20.0 μg/ml DCA (~21 and 27 % poor performance in 30-day-old flies as compared to the age-matched unexposed flies and Act-Gal4 > w1118 flies, respectively) (Fig. 8e).
Fig. 8.
Prolonged exposure of DCA to hsp27 knockdown flies caused reduced survival along with increased GSH depletion, ROS generation, and poor locomotor performance with an advancement of age. Graphs showing survivorship (a) and maximum life span and mean survival (b) in control and 20.0 μg/ml DCA-exposed Act-Gal4/w 1118, Act-Gal4 > UAS-hsp27, and Act-Gal4 > UAS-hsp27 RNAi flies. Graphs depicting GSH content (c), ROS generation (d), and locomotor performance (e) at different days of age (0, 10, 20, 30, 40, 50 days) in control and 20.0 μg/ml DCA-exposed Act-Gal4/w 1118 and Act-Gal4 > UAS-hsp27 RNAi flies. Data represent mean ± SD. Significance ascribed as *P < 0.05 as compared to unexposed control and #P < 0.05 as compared to exposed Act-Gal4/w 1118
Correlation among different parameters
A correlation was drawn for ROS generation, GSH content, expression of hsp27, and locomotor performance with respect to age in DCA-exposed flies (Table 2). A significant negative correlation (r = −0.966, P = 0.0017) was observed between ROS generation and GSH content and between locomotor performance and ROS generation (r = −0.949, P = 0.0038). We observed a positive correlation (r = 0.996, P < 0.0001) between locomotor performance and GSH content. Further, a significant positive correlation (r = 0.847, P = 0.033) was observed between hsp27 and GSH content and a negative correlation (r = −0.894, P = 0.016) between hsp27 and ROS generation.
Table 2.
Correlation among different parameters
| hsp27 | LP | ROS | GSH | |
|---|---|---|---|---|
| hsp27 | 1 | |||
| LP | 0.835* (P = 0.038) | 1 | ||
| ROS | −0.894* (P = 0.016) | −0.949* (P = 0.0038) | 1 | |
| GSH | 0.847* (P = 0.033) | 0.996* (P < 0.0001) | −0.966* (P = 0.0017) | 1 |
LP locomotor performance
*P < 0.05 (significant ascribed)
Discussion
The in vivo study presented here examined the cellular and organismal effects of DCA in exposed D. melanogaster. The study revealed attenuated an age-related cellular (in terms of GSH and ROS levels) and functional (in terms of locomotor performance) decline and increased longevity after prolonged DCA exposure to D. melanogaster.
Environmentally relevant concentrations of DCA, used in this study, showed no adverse effect in terms of cellular injury (oxidative stress, cell death, etc.), indicating that exposure in such limits is not disparaging. However, the highest tested concentration of DCA, i.e., 20.0 μg/ml (lower than that suggested for clinical use), evoked cellular as well as organismal responses in exposed Drosophila.
Xenobiotic exposures to organisms leading to their developmental and reproductive changes are well reported (Oakes et al. 2004; Singh et al. 2009). Earlier reports have shown that DCA causes developmental and reproductive adversities in exposed organisms (rat and zebrafish) wherein very high concentrations of DCA (~400–4,000 mg/kg) were used (Hassoun et al. 2005; Linder et al. 1997; Smith et al. 1992). However, none of the tested DCA concentrations were found to induce developmental and reproductive adversities in exposed Drosophila as evidenced by nonapparent change in the emergence pattern and reproductive performance of exposed flies. Although species-specific sensitivity cannot be ruled out, the importance of dose paradigm as one of the determinants of any observed response against xenobiotic exposure remains a distinct possibility.
Adaptive responses (e.g., increased longevity, resistance to stress) as a consequence of repetitive exposure to mild stress, towards biological benefit have been well reported in mammals and insects (Hercus et al. 2003; Minois 2000; Rattan 1998). In the same context, prolonged exposure of low concentrations of DCA to Drosophila improved the survival of exposed organism as evidenced by a significant increase in maximum life span and mean survival of exposed flies. However, acute exposure of DCA to Drosophila evoked their cellular defense system as evident by enhanced antioxidant enzyme activities along with a moderate increase in the ROS generation without induction of cell death. Thus, increased survival of exposed flies as a consequence of repetitive stimulation to antioxidant defense system can be considered as an adaptive response against prolonged exposure to DCA.
Increased ROS generation is casually linked to aging vis-à-vis decreased life span. When we examined the level of ROS generation in DCA-exposed flies at different days of their life, we observed that prolonged exposure of DCA to Drosophila reduced the level of ROS generation with an advancement of their age. This is in agreement with a previous study wherein reduced ROS level was evident in DCA-exposed C. elegans (Schaffer et al. 2011). Concurrent with reduced ROS level, a significantly higher GSH content was observed in DCA-exposed aged flies as evident by a negative correlation drawn between ROS and GSH content (YROS = −0.848GSH + 1.881, r = −0.966, P = 0.0017) with respect to age. Synthesis of GSH is catalyzed by the rate-limiting enzyme GCL. Parallel to the increased GSH content, we observed enhanced expression of gclc and gclm in DCA-exposed flies with respect to age as evident by a significant positive correlation between GSH and gclc (YGSH = 0.563gclc + 0.396, r = 0.889, P < 0.018) and gclm (YGSH = 0.519gclm + 0.471, r = −0.902, P < 0.014). Thus, a higher GSH content in DCA-exposed flies might be a consequence of its de novo synthesis stimulated by the elevated expression of gclc and gclm, which is in agreement with earlier studies wherein DCA-induced upregulation of GSH via induction of GCL was observed in mice (Theodoratos et al. 2012). Parallel to the above discussion, overexpression of gclc and gclm was reported to extend life span of Drosophila (Orr et al. 2005) and de novo synthesis of GSH was also reported against long-term low-dose γ-irradiation in mice (Lee et al. 2013).
Increased antioxidant levels and decreased ROS generation can play important roles towards better healthspan (Gruber et al. 2008, 2009). Our observation of increased healthspan in Drosophila after their prolonged exposure to DCA as evidenced by their improved locomotor performance with an advancement of age supports the above discussion. In this context, a significant positive correlation drawn between GSH content and locomotor performance (YLA = 0.610GSH + 0.406, r = −0.996, P < 0.0001) and a significant negative correlation between locomotor performance and ROS generation (YLA = −0.663ROS + 1.706, r = −0.949, P = 0.0038) also suggest that prolonged exposure of DCA to Drosophila helps them to counter the ROS-mediated organismal adversities with respect to age.
Increased survival is casually linked with modulation of hsps. In Drosophila, increased copies of hsps and their overexpression are reported to have beneficial effect on the organism. Increased copy number of hsp70 has been reported to reduce mortality and improve overall survival of organism (Tatar et al. 1997). Overexpression of hsp27 (Liao et al. 2008) and hsp22 (Kim et al. 2010) were shown to provide increased longevity to flies. We observed a significant increase in hsp22, hsp27, and hsp70 expression in the flies that were exposed to DCA up to 48 h. However, in flies that were exposed to DCA for a prolonged period, hsp27 expression was significantly upregulated in 30–50-day-old organism. hsp27 is reported to maintain cellular redox by upholding intracellular glutathione (Arrigo et al. 2005; Concannon et al. 2003; McCollum et al. 2006) and by suppressing ROS generation (Liu et al. 2007; Wyttenbach et al. 2002). In agreement with the above discussion, we observed decreased ROS generation and GSH depletion concurrent with increased hsp27 expression in DCA-exposed aged flies which was further evident by a significant positive correlation drawn between hsp27 expression and GSH content (YGSH = 0.592hsp27 + 0.341, r = 0.847, P = 0.033) and a negative correlation between hsp27 expression and ROS generation (YROS = −0.549hsp27 + 1.653, r = −0.894, P = 0.016). We therefore hypothesized that modulation hsp27 expression might benefit the DCA-exposed organism. When hsp27 was knocked down in Drosophila (Act-Gal4 > UAS-hsp27RNAi), survival of these organisms significantly went down as compared to control (Act-Gal4 > w1118) along with a significant increase in the ROS generation and GSH depletion and poorer locomotor activities, suggesting an age-dependent decline in the fitness of fly against DCA exposure. To rule out the general effect of knockdown of hsps, we further analyzed the survival of flies wherein another small hsp, viz., hsp22, was knocked down (Act-Gal4 > UAS-hsp22RNAi). After DCA exposure to these flies, we observed a nonsignificant change in their survival as compared to unexposed flies (data not shown). Thus, longevity and better healthspan of aged flies after their prolonged exposure to DCA may be a consequence of increased hsp27 and increased GSH levels (Fig. 9).
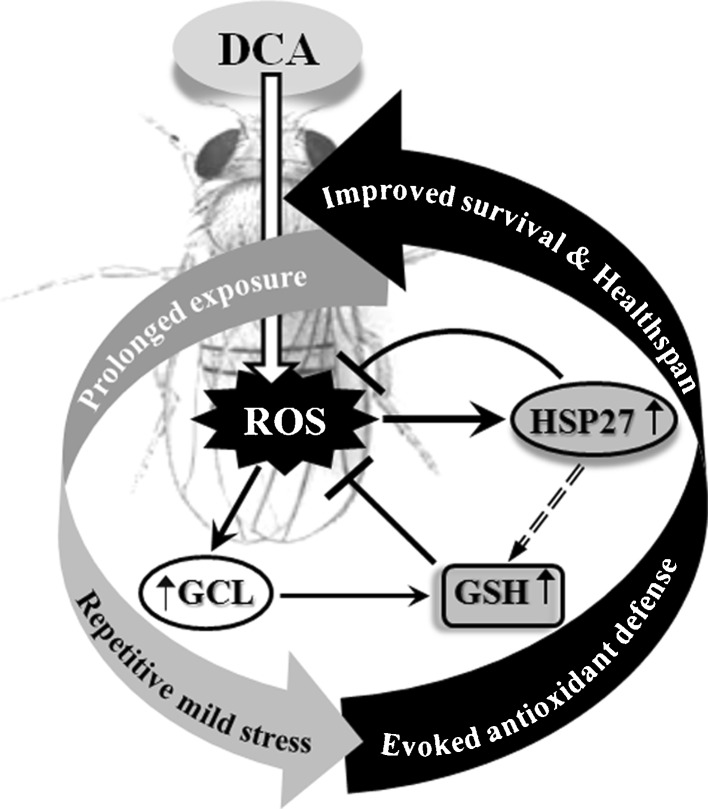
Fig. 9.
Schematic representation of cellular and organismal response of Drosophila against prolonged exposure to DCA. The model shows improved survival and healthspan of flies as a consequence of stimulation to antioxidant defense system and hsps induction (enhanced GSH synthesis and hsp27 expression) along with mild ROS generation which can be attributed as an adaptive response of the organism after prolonged exposure to DCA. ROS reactive oxygen species, GSH glutathione, GCL glutamate cysteine ligase, DCA dichloroacetic acid
Taken together, we show here that attenuation of age-dependent cellular (ROS generation and GSH depletion) and functional (survival and organismal fitness) declines along with involvement of hsp27 modulation in flies that were exposed to DCA for a prolonged period. To the best of our knowledge, this is the first report on the attenuation of age-dependent insufficiencies by DCA exposure using a well-accepted in vivo model Drosophila. However, further studies are warranted to understand the possible role of hsp27 modulation vis-à-vis age-dependent benefit in DCA-exposed organisms towards its therapeutic benefit.
Acknowledgments
The authors are grateful to the director of the CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow for the support and to Dr. M. K. R. Mudiam, senior scientist, and Mr. Ratnashekhar Ch, CSIR-SRF, Analytical Chemistry Section, CSIR-IITR for the GC-ECD analysis of DCA. We thank the Drosophila Stock Centre, Bloomington (Oregon R+, Act-Gal4/Cyo); William Ja, The Scripps Research Institute, Florida (w 1118); H. D. Wang, California Institute of Technology, California (UAS-hsp27); and Vienna Drosophila RNAi Center, Austria (UAS-hsp27 RNAi, UAS-hsp22 RNAi) for the fly strains. Financial assistance to AP as SRF [20-6/2008(ii)EU-IV], SC as SRF [20-12/2009(ii)EU-IV] from the University Grants Commission (UGC), New Delhi, DV as JRF [19-12/2010(i)EU-IV] from the Council of Scientific and Industrial Research (CSIR), SS as DBT-Project Fellow (grant no. BT/PR14716/BRB/10/876/2010), and DKC from the CSIR, New Delhi (NWP-BSC0103, UNDO) and DBT (BT/PR14716/BRB/10/876/2010) is thankfully acknowledged. IITR communication number is 3193.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2005;7:414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Ayyanathan K, Kesaraju S, Dawson-Scully K, Weissbach H. Combination of sulindac and dichloroacetate kills cancer cells via oxidative damage. PLoS One. 2012;7:e39949. doi: 10.1371/journal.pone.0039949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benford DJ, Hanley AB, Bottrill K, Oehlschlager S, Balls M, Branca F, Castegnaro JJ, Descotes J, Hemminiki K, Lindsay D, Schilter B. Biomarkers as predictive tools in toxicity testing. ATLA. 2000;28:119–131. [Google Scholar]
- Cai P, Boor PJ, Khan MF, Kaphalia BS, Ansari GA, Konig R. Immuno- and hepato-toxicity of dichloroacetic acid in MRL(+/+) and B(6)C(3)F(1) mice. J Immunotoxicol. 2007;4:107–115. doi: 10.1080/15476910701337225. [DOI] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/A:1021601103096. [DOI] [PubMed] [Google Scholar]
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, Badrinath A, Levine RL, Bradley TJ, Tavare S, Tower J. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Bichem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Gayathri MV, Krishnamurthy NB. Studies on the toxicity of mercurial fungicide Agallol3 in Drosophila melanogaster. Environ Res. 1981;24:89–95. doi: 10.1016/0013-9351(81)90135-3. [DOI] [PubMed] [Google Scholar]
- Geist S, Lesemann C, Schutz C, Seif P, Frank E. Determination of chloroacetic acids in surface water. In: Angeletti G, Bjorseth A, editors. Organic micropollutants in the aquatic environment. Dordrecht: Kluwer; 1991. pp. 393–397. [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of aging—where do we stand? Front Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- Gruber J, Ng LF, Poovathingal SK. Deceptively simple but simply deceptive—Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett. 2009;583:3377–3387. doi: 10.1016/j.febslet.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Siddique HR, Saxena DK, Chowdhuri DK. Hazardous effect of organophosphate compound, dichlorvos in transgenic Drosophila melanogaster (hsp70-lacZ): induction of hsp70, anti-oxidant enzymes and inhibition of acetylcholinesterase. Biochim Biophys Acta. 2005;1725:81–92. doi: 10.1016/j.bbagen.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Ha KN, Chen Y, Cai J, Sternberg PJ. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: implication for protection against oxidative stress. Invest Ophthalmol Vis Sci. 2006;47:2709–2715. doi: 10.1167/iovs.05-1322. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hassoun EA, Dey S. Dichloroacetate- and trichloroacetate-induced phagocytic activation and production of oxidative stress in the hepatic tissues of mice after acute exposure. J Biochem Mol Toxicol. 2008;22:27–34. doi: 10.1002/jbt.20210. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Ray S. The induction of oxidative stress and cellular death by the drinking water disinfection by-products, dichloroacetate and trichloroacetate in J774.A1 cells. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135:119–128. doi: 10.1016/S1532-0456(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Hassoun E, Kariya C, Williams FE. Dichloroacetate-induced developmental toxicity and production of reactive oxygen species in zebrafish embryos. J Biochem Mol Toxicol. 2005;19:52–58. doi: 10.1002/jbt.20051. [DOI] [PubMed] [Google Scholar]
- Hercus MJ, Loeschcke V, Rattan SI. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi: 10.1023/A:1024197806855. [DOI] [PubMed] [Google Scholar]
- IARC monographs on the evaluation of carcinogenic risk to humans, vol. 84, Some drinking-water disinfectants and contaminants. Lyon: IARC; 2004. p. 359. [PMC free article] [PubMed] [Google Scholar]
- Jeibmann A, Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci. 2009;10:407–440. doi: 10.3390/ijms10020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Sheng Y, Ji L. The age-related change of glutathione antioxidant system in mice liver. Toxicol Mech Methods. 2013;23:396–401. doi: 10.3109/15376516.2013.769655. [DOI] [PubMed] [Google Scholar]
- Jones MA, Grotewiel M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp Gerontol. 2011;46:320–325. doi: 10.1016/j.exger.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Morrow G, Westwood JT, Michaud S, Tanguay RM. Gene expression profiling implicates OXPHOS complexes in lifespan extension of flies over-expressing a small mitochondrial chaperone, Hsp22. Exp Gerontol. 2010;45:611–620. doi: 10.1016/j.exger.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging. 2012;4:768–789. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Kim JA, Kim JS, Park SJ, Heo K, Yang KM, Son TG. Activation of de novo GSH synthesis pathway in mouse spleen after long term low-dose γ-ray irradiation. Free Radic Res. 2013;47:89–94. doi: 10.3109/10715762.2012.747678. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Liao PC, Lin HY, Yuh CH, Yu LK, Wang HD. The effect of neuronal expression of heat shock proteins 26 and 27 on lifespan, neurodegeneration, and apoptosis in Drosophila. Biochem Biophys Res Commun. 2008;376:637–641. doi: 10.1016/j.bbrc.2008.08.161. [DOI] [PubMed] [Google Scholar]
- Linder RE, Klinefelter GR, Strader LF, Suarez JD, Roberts NL. Spermatotoxicity of dichloroacetic acid. Reprod Toxicol. 1997;11:681–688. doi: 10.1016/S0890-6238(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang X, Qian B, Min X, Gao X, Li C, Cheng Y, Huang J. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:762–769. doi: 10.1016/j.ejheart.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lliadi KG, Knight D, Boulianne GL. Healthy aging—insights from Drosophila. Front Physiol. 2012;3:106. doi: 10.3389/fphys.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum AK, Teneyck CJ, Sauer BM, Toft DO, Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res. 2006;66:10967–10975. doi: 10.1158/0008-5472.CAN-06-1629. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- Minois N. Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology. 2000;1:15–29. doi: 10.1023/A:1010085823990. [DOI] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martinez-Palma L, Bolatto C, Trias E, Gandelman M, Radi R, Barbeito L, Cassina P. Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis. PLoS One. 2012;7:e34776. doi: 10.1371/journal.pone.0034776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudiam MKR, Jain R, Varshney M, Ch R, Chauhan A, Goyal SK, Khan HA, Murthy RC. In matrix derivatization of trichloroethylene metabolites in human plasma with methyl chloroformate and their determination by solid-phase microextraction-gas chromatography-electron capture detector. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;925:63–9. doi: 10.1016/j.jchromb.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Nazir A, Mukhopadhyay I, Saxena DK, Kar Chowdhuri D. Chlorpyrifos-induced hsp70 expression and effect on reproductive performance in transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Arch Environ Contam Toxicol. 2001;41:443–449. doi: 10.1007/s002440010270. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Oakes KD, Sibley PK, Solomon KR, Mabury SA, Van der Kraak GJ. Impact of perfluorooctanoic acid on fathead minnow (Pimephales promelas) fatty acyl-CoA oxidase activity, circulating steroids, and reproduction in outdoor microcosms. Environ Toxicol Chem. 2004;23:1912–1919. doi: 10.1897/03-190. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem Mol Biol Int. 1998;45:753–759. doi: 10.1080/15216549800203162. [DOI] [PubMed] [Google Scholar]
- Salway KD, Gallagher EJ, Page MM, Stuart JA. Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev. 2011;132:287–297. doi: 10.1016/j.mad.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Gruber J, Ng LF, Fong S, Wong YT, Tang SY, Halliwell B. The effect of dichloroacetate on health- and lifespan in C. elegans. Biogerontology. 2011;12:195–209. doi: 10.1007/s10522-010-9310-7. [DOI] [PubMed] [Google Scholar]
- Singh MP, Reddy MM, Mathur N, Saxena DK, Chowdhuri DK. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: role of ROS generation. Toxicol Appl Pharmacol. 2009;235:226–243. doi: 10.1016/j.taap.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Smith MK, Randall JL, Read EJ, Stober JA. Developmental toxicity of dichloroacetate in the rat. Teratology. 1992;46:217–223. doi: 10.1002/tera.1420460305. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW. The dichloroacetate dilemma: environmental hazard versus therapeutic goldmine—both or neither? Environ Health Perspect. 2011;119:155–158. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, James MO. Clinical pharmacology and toxicology of dichloroacetate. Environ Health Perspect. 1998;106:989–994. doi: 10.1289/ehp.98106s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Kurtz TL, Han Z, Langaee T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev. 2008;60:1478–1487. doi: 10.1016/j.addr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Wang HD, Liu RM, Liu JK, Hagen TM. (R)-α-Lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- Theodoratos A, Blackburn AC, Cappello J, Tummala P, Dahlstrom JE, Board PG. Dichloroacetic acid up-regulates hepatic glutathione synthesis via the induction of glutamate-cysteine ligase. Biochem Pharmacol. 2012;83:427–433. doi: 10.1016/j.bcp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Thompson JA, White CC, Cox DP, Chan JY, Kavanagh TJ, Fausto N, Franklin CC. Distinct Nrf1/2-independent mechanisms mediate As 3 + -induced glutamate-cysteine ligase subunit gene expression in murine hepatocytes. Free Radic Biol Med. 2009;46:1614–1625. doi: 10.1016/j.freeradbiomed.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Hsps and aging. Trends Endocrinol Metab. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46:355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol. 2008;109:394–402. doi: 10.1016/j.ygyno.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- Zhao HW, Zhou D, Haddad GG. Antimicrobial peptides increase tolerance to oxidant stress in Drosophila melanogaster. J Biol Chem. 2010;286:6211–6218. doi: 10.1074/jbc.M110.181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007;43:444–453. doi: 10.1016/j.freeradbiomed.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]