Abstract
As we age, there is an increased risk for the development of pulmonary diseases, including infections, but few studies have considered changes in lung surfactant and components of the innate immune system as contributing factors to the increased susceptibility of the elderly to succumb to infections. We and others have demonstrated that human alveolar lining fluid (ALF) components, such as surfactant protein (SP)-A, SP-D, complement protein C3, and alveolar hydrolases, play a significant innate immune role in controlling microbial infections. However, there is a lack of information regarding the effect of increasing age on the level and function of ALF components in the lung. Here we addressed this gap in knowledge by determining the levels of ALF components in the aging lung that are important in controlling infection. Our findings demonstrate that pro-inflammatory cytokines, surfactant proteins and lipids, and complement components are significantly altered in the aged lung in both mice and humans. Further, we show that the aging lung is a relatively oxidized environment. Our study provides new information on how the pulmonary environment in old age can potentially modify mucosal immune responses, thereby impacting pulmonary infections and other pulmonary diseases in the elderly population.
Keywords: Inflammation, Aging, Alveolar lining fluid, Surfactant, Complement
Introduction
Increasing age is associated with failing pulmonary health that includes the development of chronic obstructive pulmonary disease (COPD) (Ito and Barnes 2009) and an increased susceptibility to numerous pulmonary infections [influenza, pneumococcal pneumonia, tuberculosis (Cruz-Hervert et al. 2012; Olson et al. 2007; Wang et al. 2012; Wroe et al. 2012)]. Physical changes in pulmonary architecture and function are linked to these disease states (Dyer 2012; Fragoso and Lee 2012), but few studies have considered whether changes in components of pulmonary surfactant and/or the innate immune system can impact lung function sufficiently to increase the risk of developing age-associated pulmonary disorders or to increase susceptibility to infection in the elderly. We and others have previously demonstrated that some components of human surfactant [the collectins surfactant protein (SP)-A and SP-D, surfactant homeostatic hydrolytic enzymes (hydrolases), and lipids] are critical elements of the innate immune system during microbial infections (Arcos et al. 2011; Carlson et al. 2010; Ferguson et al. 1999, 2006; Gaynor et al. 1995). In addition, the complement system is active in the alveolar space and plays an important role in the microbe-macrophage encounter (Ferguson et al. 2004; Figueroa and Densen 1991; Mold 1999; Schlesinger et al. 1990; Tedesco 2008). Other investigators have shown that the aging process is associated with an increase in baseline pro-inflammatory cytokines in the systemic circulation, a process termed “inflammaging” (Baylis et al. 2013; Franceschi et al. 2000).
Alveolar lining fluid (ALF) is generated, secreted, and recycled by alveolar epithelial cells (AECs) and is essential for maintaining lung function (Notter 2000). Similarly, components of the complement system are produced by AECs (Strunk et al. 1988) and macrophages in the lung (Cole et al. 1983). ALF in elderly individuals has a slow turnover and degrades quickly because of inefficient regeneration by senescent AECs (Burton 2009). Therefore, slow ALF turnover plus the presence of inflammatory lung and parenchymal cells can drive baseline low-grade chronic inflammation in the lung during aging (Reynolds 1987). These events result in changes in alveolar surfactant and complement production and activity, the former as a result of a change in the local oxidation state (Schunemann et al. 1997). Moreover, alterations in surfactant lipid composition are predicted to negatively affect SP-A and SP-D function (Veldhuizen et al. 1998), and the latter proteins can be oxidized themselves (Umstead et al. 2009). Recent studies of ALF from cadaveric human lungs have shown that SP-A activity decreases with increasing age (from age 22–55) and that SP-A activity is lower than that estimated in other species (Rebello et al. 1996). This may indicate that the aged human lung is particularly vulnerable to ALF dysfunction and chronic inflammation. Since ALF dysfunction correlates with the defined function of SP-A in reducing complement activity in the lung (Watford et al. 2001), as well as in modulating inflammatory responses (Crowther et al. 2004; Henning et al. 2008; Nguyen et al. 2012), alterations in the balance of SP-A, other surfactant components, complement, and cytokines may contribute to chronic inflammation in old age. Moreover, SP-D deficiency has been associated with a chronic inflammatory state in which there is an increase in apoptotic and necrotic alveolar macrophages and intra-alveolar accumulation of surfactant lipids (Reid et al. 2005).
In this study, we examined components of ALF from both mice and humans, i.e., cytokines, surfactant, and complement in the basal pulmonary environment in adult and old age. We demonstrate that ALF components are altered in old age and that the pulmonary environment is relatively pro-inflammatory with an increase in oxidized components. These findings provide new information about the baseline immunologic state within the lung alveolus in old age. Such findings are critical in enabling future studies to elucidate the direct influence of these changes on the increased susceptibility of the elderly to infection and non-infectious pulmonary diseases.
Results
ALF cytokines are altered in old age
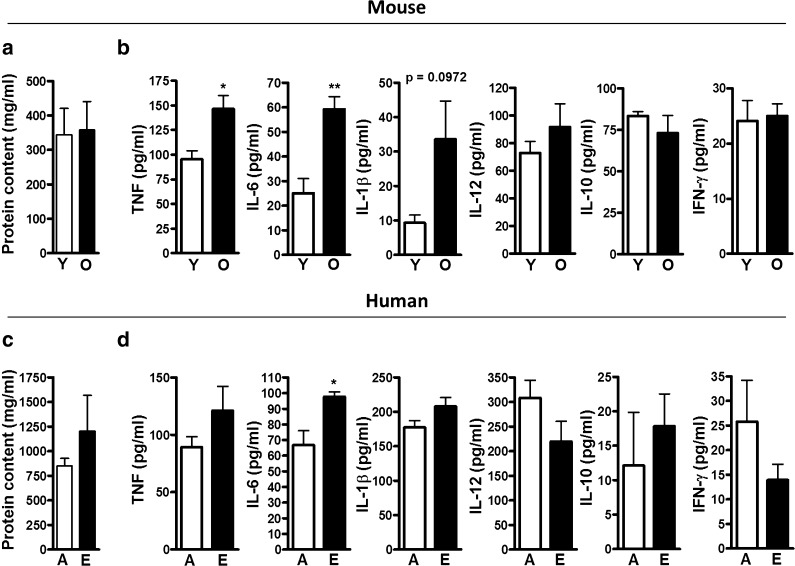
We hypothesized that the aging lung environment exists in a pro-inflammatory state similar to what has been observed in the systemic circulation (Baylis et al. 2013; Franceschi et al. 2000). Cytokines were determined in ALF depleted of lipids (ALF-L; see “Material and methods”) collected from naive old (18 months) and young (3 months) mice or human ALF-L from elderly (65+ years) and adult (25–44 years) subjects collected by bronchoalveolar lavage (BAL). ALF-L was normalized by protein content, which was equivalent in young and old mice (Fig. 1a) and adult and elderly humans (Fig. 1c). In mice, increasing age was associated with a significant increase in TNF and IL-6 in the ALF-L and a trend for increased IL-1β (Fig. 1b). IL-12, IL-10, and IFN-γ were equivalent to those measured in young mice. We observed a similar significant increase in the level of IL-6 in ALF-L samples from elderly humans and a trend for increased TNF and IL-1β (Fig. 1d). IL-12 and IFN-γ, while not significantly altered, trended toward decreased levels in the elderly. There was also a trend toward an increase in IL-10 that did not reach statistical significance. Overall, our results demonstrate that the aging lung (in mice and humans) contains a basal increase in pro-inflammatory cytokines.
Fig. 1.
ALF cytokine levels in the aging lung. ALF from young (Y) and old (O) mice or ALF from adult (A) and elderly (E) humans were obtained by BAL using 0.9 % NaCl. ALF total protein content was determined for a mouse ALF (n = 16/group) and c human ALF (n = 4/group). ALF was subsequently normalized for well loading by a protein concentration of 0.1 μg/μl (BCA) adding 100 μl/well. Cytokines were measured by ELISA following the manufacturer’s instructions. b Mouse (n = 5/group) and d human (n = 4/group) ALF cytokine levels for TNF, IL-6, IL-1β, IL-12, IL-10, and IFN-γ. Student’s t test of old mouse vs. young mouse ALF or elderly vs. adult human ALF. *p < 0.05; **p < 0.005
ALF SP-A and SP-D levels are elevated in old age
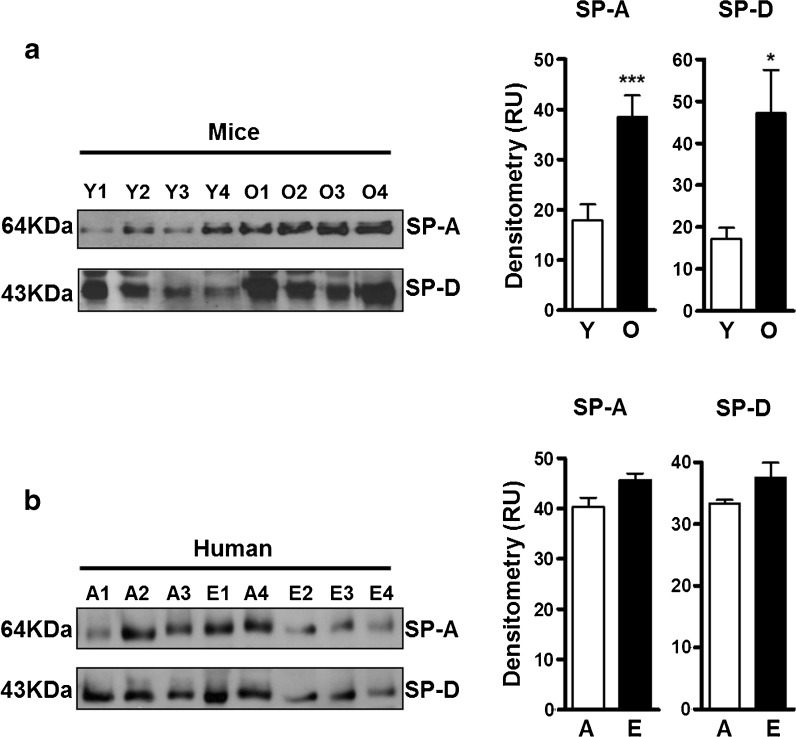
We hypothesized that SP-A and SP-D would be increased in the lungs of old mice and elderly subjects relative to their younger counterparts. This is in part thought to be a consequence of AEC dysfunction, resulting in higher amounts of serum-derived components in the lung which contribute to a relatively pro-inflammatory lung environment (King 1982). Levels of SP-A and SP-D were determined in ALF-L. Semiquantitative gel electrophoresis and Western blotting of individual samples showed that old mice had significantly higher levels of SP-A and SP-D in their ALF (Fig. 2a) compared to young mice. Similar trends were observed for SP-A and SP-D in humans (Fig. 2b), although these did not reach significance, which we interpret as a lack of power resulting from small sample size. The increased levels of SP-A and SP-D that we observed within the ALF-L in old age are in accordance with our hypothesis and supported by the fact that lung ALF recycling in old age is dysfunctional (Notter et al. 1980; Notter 2000) and increased ALF component levels are expected.
Fig. 2.
ALF SP-A and SP-D levels in the aging lung. ALF from young (Y) and old (O) mice were obtained by BAL using 0.9 % NaCl and normalized for gel loading by protein content (10 μg/well, BCA). a WB of SP-A and SP-D (shown are representative blots) in mouse ALF and densitometry analysis of the bands on the mouse ALF WB with their respective backgrounds subtracted (n = 17/group for SP-A and n = 15/group for SP-D). b WB of SP-A and SP-D in human ALF (n = 4/group) and densitometry analysis showing the levels of SP-A and SP-D in humans. A adult, E elderly, RU random units. Student’s t test of old mouse vs. young mouse ALF or elderly vs. adult ALF. *p < 0.05; ***p < 0.001
ALF complement levels are altered in old age
With the knowledge that ALF components such as SP-A and SP-D are elevated in old age, we further anticipated that complement components would also be altered. Complement protein levels were determined in ALF-L from old and young mice and adult and elderly humans by Western blotting. We focused primarily on the classical complement pathway as we and others have shown that components of the alternative complement pathway are at very low levels in ALF (Bolger et al. 2007; Ferguson et al. 2004). C2, C3, and C4 molecules were determined. For completeness, we also measured factor B (FB) of the alternative pathway and C5 and C7 molecules as indicators of an alteration in formation of the membrane attack complex (Bolger et al. 2007). Old mice had significantly higher levels of complement proteins C3β, C4α (not shown) and C4β (both products of C4 cleavage), and FBα and FBβ (Fig. 3a). C3α showed a trend for increased amounts, but this did not reach significance. C2 in the ALF-L of old mice was significantly lower than that measured in young mice. In human samples, we observed a trend for decreased C2 in the ALF-L of elderly subjects, but other complement components were unaltered (Fig. 3b).
Fig. 3.
ALF complement levels in the aging lung. ALF from mice and humans from both age groups were normalized by protein content at 10 μg/well in a loading gel, and WB against complement components was performed. a Representative blots for mouse ALF and densitometry (lower panel) analysis of bands on WB (n = 12/group for C2 and C4β and n = 9/group for the rest). b Levels of C2, C5, C7, and C3-β in humans. Densitometry analysis of bands on WB (n = 4/group). Student’s t test of old mouse vs. young mouse ALF or elderly vs. adult ALF. *p < 0.05; **p < 0.005; ***p < 0.001. RU random units
ALF lung hydrolase activity is decreased in old age
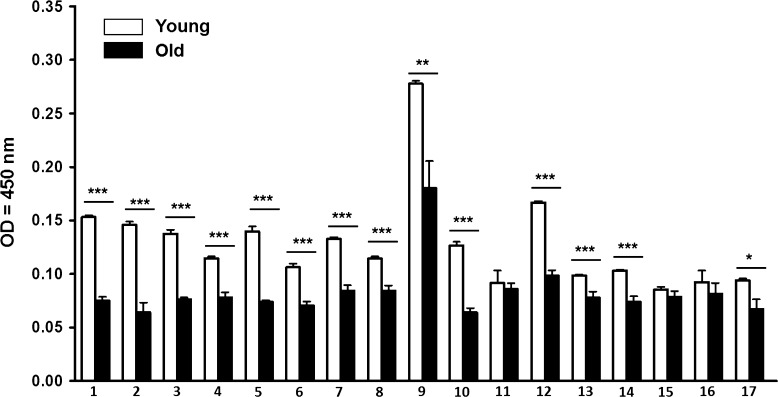
We recently showed that human and mouse ALF contains abundant homeostatic and antimicrobial enzymes (hydrolases) (Hawgood and Poulain 2001; Mason 2006; van Golde 1985; Williams 2003) that are capable of altering the cell envelope of Mycobacterium tuberculosis (Arcos et al. 2011), thus identifying additional modulators of lung function that can be modified with increasing age. We determined the hydrolase activity of ALF-L from old and young mice. Our results indicate that old mice have an overall decrease in lung hydrolase activity (Fig. 4). This decrease could also result from poor ALF recycling by dysfunctional AECs as well as lung physiological dysfunction which is associated with chronic inflammation (Notter et al. 1980; Notter 2000).
Fig. 4.
Basal expression of ALF hydrolase activities in young and old mice. Hydrolase activities present in concentrated ALF of mouse (n = 8) were quantified using a colorimetric method. Hydrolytic activities: 1 acid phosphatase, 2 α-mannosidase, 3 α-galactosidase, 4 β-galactosidase, 5 α-glucosidase, 6 β-glucosidase, 7 α-xylosidase, 8 α-fucosidase, 9 arylsulfatase, 10 fatty acid esterase-I, 11 non-specific esterase, 12 alkaline phosphatase, 13 alkaline phosphodiesterase, 14 phospholipase C, 15 peroxidase, 16 α-rhamnosidase, 17 fatty acid esterase-II. Student’s t tests of old mouse (solid bars) vs. young mouse (open bars) ALF. *p < 0.05; **p < 0.005; ***p < 0.001
ALF in old age contains increased myeloperoxidase and oxidized components
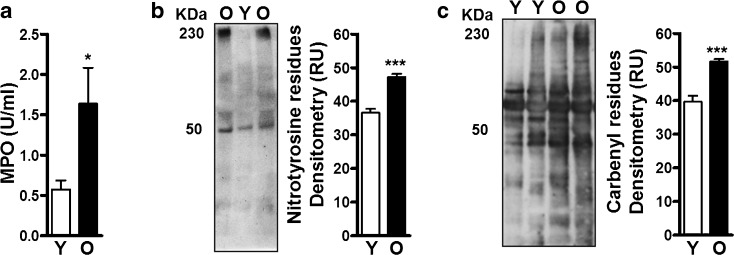
Increasing age is highly associated with increased oxidative stress (Romano et al. 2010; Schunemann et al. 1997). Therefore, we anticipated that the aged lung would represent a relatively oxidized environment. Although ALF from young and old mice was found to contain similar protein content (Fig. 1a), levels of myeloperoxidase (MPO) (Fig. 5a) and carbonyl and nitrotyrosine residues [both reliable and stable markers of oxidative damage in proteins (Dalle-Donne et al. 2005; Ho et al. 2013)] (Fig. 5b, c) were significantly elevated in old mice suggesting that during aging there is an increase in oxidative damage in the alveolar environment.
Fig. 5.
Oxidative and nitrosative stress in the aging lung. ALF from young (Y) and old (O) mice were normalized for ELISA or gel loading by protein content at 10 μg/well. Myeloperoxidase (MPO) was directly detected in ALF by ELISA and nitrotyrosine and carbonyl residues by WB. a A significant amount of MPO (2.85-fold or 65 % increase, n = 16/group) and b nitrotyrosine (indicator of NOS oxidation, n = 5/group) and c carbenyl (indicator of ROS oxidation, n = 5/group) residues in ALF proteins were found as detected by densitometry of the WB (representative blots shown). Student’s t test of old mouse vs. young mouse ALF. *p < 0.05; ***p < 0.001. RU random units
POPC, a marker of inflammation, is elevated in ALF in old age
1-Palmitoyl,2-oleoyl-sn-glycero-3-phosphocholine (POPC or PC16:0/18:1, mass (M) = m/z 760, shown in Fig. 6 as the m/z 782 [M − H + Na]+ sodiated form) is a marker of inflammation and is thought to originate from necrosis and membrane shedding from activated inflammatory cells within the lung. Thus, we examined the level of POPC by extracting surfactant lipids from ALF from old and young mice and directly analyzing the samples by electrospray ionization tandem mass spectrometry (ESI/MS/MS) in a positive ion mode (Postle 2008). Our data show that young mice accumulate 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC or PC16:0/16:0, M = m/z 734, shown in Fig. 6 as the m/z 752 [M − H + Na]+ sodiated form) as expected. In contrast, old mice accumulate POPC rather than DPPC (Fig. 6). Our ESI+ results together with the other data provide evidence for the existence of a pro-inflammatory environment within the aging lung.
Fig. 6.
ESI-MS of mouse surfactant phospholipids in a positive mode. ESI+ spectra shown mainly contain sodium adducts for DPPC [M − H + Na]+ at m/z 756 and for POPC [M − H + Na]+ at m/z 782. An increase in POPC in the aging lung surfactant is indicative of an oxidative environment. A representative experiment of n = 3/group is shown (top panel old mice, lower panel young mice). PC phosphatidylcholine, POPC 1-palmitoyl,2-oleoyl-sn-glycero-3-phosphocholine, DPPC 1,2-dipalmitoyl-sn-glycero-3-phosphocholine
Discussion
In this study, we analyzed selected components of ALF in aged mice and humans. We show that ALF from old mice have elevated levels of pro-inflammatory cytokines, SP-A and SP-D, and several complement components. We further demonstrate that the ALF of mice contains an increase in oxidized molecules consistent with oxidative damage and an imbalance in DPPC and POPC, consistent with basal pulmonary inflammation. A comparative analysis of ALF-L from human samples showed that ALF-L in the elderly also contained increased amounts of pro-inflammatory cytokines and of SP-A and SP-D. High levels of pro-inflammatory cytokines such as IL-6 in ALF from elderly individuals were reported in a previous study (Meyer et al. 1996). For cytokines, we observed species disparity between IL-10 and IL-12, two interrelated cytokines (Retini et al. 2001), in mice and humans. IL-10 acts as an immunomodulator of IL-12 secretion via the ERK1/2 molecular switch (Saraiva and O’Garra 2010) and it is plausible that this biological switch works differently as we age and/or its functionality varies among species, but these results could also be simply explained by the lack of power in some of our analyses due to the limited human ALF-L sample size in both groups. Overall, our studies demonstrate that the pulmonary environment in old age is significantly altered resulting in an enhanced inflammatory state, supporting the concept of inflammaging in old age that extends beyond the systemic circulation to the lung alveolar environment. The impact of local inflammation on the development of common pulmonary diseases of the elderly, including COPD and the increased susceptibility to infection, has received little attention. Our studies lay the foundation for future mechanistic studies to establish a causal link between pulmonary inflammaging and lung disease.
Enhanced inflammatory signatures are suggestive of altered inflammatory cell numbers within the lung with increasing age. Betsuyaku et al. (1999) reported an increase in neutrophils in human ALF as a marker of acute and/or chronic inflammation, i.e., from 2 % total neutrophils in ALF from healthy adults (mean age 28) to 8 % in ALF from idiopathic pulmonary fibrosis patients (mean age 58). However, similar studies from the same authors showed that healthy young adults have more neutrophils in their ALF than elderly subjects (1.1 vs. 0.3 %, respectively) (Betsuyaku et al. 2004). Although we were unable to acquire the cytological data from our human samples, our mouse cytological data showed a similar trend with a relatively low number of neutrophils in ALF from young and old mice (4.41 and 3.1 %, respectively), indicating that the basal lung inflammation observed in aging may not be driven solely by an influx of neutrophils into the alveolar space in the absence of other stimuli.
As the lung ages, it loses alveolar complexity and surface area (Mauderly and Hahn 1982), and changes in the amount and function of surfactant and other innate immune determinants occur. Information about the composition of the lung innate immune determinants in humans as we age is relevant from a clinical perspective to understand respiratory infections. Following infection of the lower respiratory track, a given microbe resides within the alveoli where ALF is located. Thus, ALF components such as SP-A, SP-D, hydrolases, lipids, and complement are likely to modulate and define the interaction between the microbe and AMs. Determinants that promote microbe survival and an aberrant host response in this context will be detrimental to the host, whereas factors that promote effective microbial clearance with an appropriately focused and localized host response will be protective. In this regard, increased levels of SP-D drive susceptibility to fungal infections (Geunes-Boyer et al. 2009), whereas high levels of SP-A indirectly drive susceptibility to M. tuberculosis infection (Beharka et al. 2002; Kang et al. 2005) and modulate the lung inflammatory response by regulating several macrophage activities (Crowther et al. 2004; Henning et al. 2008; Nguyen et al. 2012). Our results using mouse ALF showed an increase in SP-A and SP-D in old mice. This trend was also observed in human ALF. We opted to normalize by total protein content based on our results in Fig. 1a which show similar protein content in ALF from young and old mice and from adult and elderly humans. Other studies have normalized by urine, by albumin, or by recovered ALF volume; thus, the process of normalization generates some discrepancies in the levels of SP-A and SP-D reported. This is a challenge when comparing ALF-soluble components between age groups. Human studies comparing adults vs. middle-aged/elderly subjects that normalized by recovered ALF volume showed a slight decrease in SP-A and no differences in SP-D with age (Betsuyaku et al. 2004). However, in this study (Betsuyaku et al. 2004), the ALF recovery among subjects varied, and thus, as pointed out by the authors, this may affect the comparisons made between age groups. This problem does not exist in our study since we normalized samples by protein amount.
Complement proteins (and their cleavage products) have been detected in human ALF obtained from healthy hosts, where complement levels differed from levels in human serum (Bolger et al. 2007). Complement is a complex, highly regulated protein system with an essential role in host defense through bacterial lysis, stimulation of phagocytosis, recruitment of immune cells to infected/damaged tissue, and promotion of the inflammatory response. The relative changes in complement components observed in the ALF of old mice (decreased C2, increased C3 and C4) indicate that increasing age may lead to problems associated with MAC formation or antibody complex clearance associated with the classical arm of the complement cascade. This is supported by the reported reduced function of antibodies in elderly donors and may contribute to their increased susceptibility to infections (Busse and Mathur 2010; Doria et al. 1978; Eaton et al. 2004). Similarly, our data indicate that the low levels of C2 and high levels of factor B observed in the ALF of old mice may favor the alternative pathway in lower respiratory tract secretions (Robertson et al. 1976). This is in contrast to what we have seen in the healthy human adult lung (Ferguson et al. 2004). We also failed to observe similar changes in complement components between adult and elderly human donors, although the sample size precludes making any firm conclusions about these data. The significance of different complement components between mouse and human requires further investigation.
A variety of hydrolases have been related to ALF recycling and degradation (Hook 1978), many of which have lysosomal-type degradative functions. Some of these hydrolases are highly active (i.e., alkaline and acid phosphatases and non-specific esterases) while others much less so (i.e., α-mannosidase and arylsulfatase) (Arcos et al. 2011). The presence of low hydrolase activities in the lungs of old mice implies that ALF has not been efficiently recycled by type 2 AEC (de Vries et al. 1985; DiAugustine 1974; Edelson et al. 1988; Gilder et al. 1981; Hook and Gilmore 1982; Young et al. 1993), probably due to cell dysfunction. This may result in two potential outcomes: hydrolase-induced tissue destruction, and promotion of inflammation and oxidation (Laskin and Pendino 1995). Although little is known about the exact role of ALF hydrolases in stabilizing the healthy lung environment or during microbial infection, we recently showed that ALF hydrolases can alter the cell envelope of M. tuberculosis during infection promoting pro-inflammation (Arcos et al. 2011; Torrelles 2012).
The aged lung is an oxidative environment as indicated by large amounts of MPO and carbonyl and nitrotyrosine residues in ALF proteins and by the presence of oxidized lipids such as POPC. POPC is a marker of inflammation. It is thought to originate from necrosis and membrane shedding from activated inflammatory cells within the lung. POPC accumulation has been reported in lung surfactant from cystic fibrosis patients as well as in neutrophils (Postle 2008), which are major contributors to inflammation. Lung surfactant is composed of ~90 % lipids. In all species, phosphatidylcholine (PC) comprises ~80 % of the total surfactant lipid, about half of which is di-palmitoyl-PC (DPPC) (Rebello et al. 1996). The rest of the lipids are charged phospholipids, cholesterol, acylglycerols, and free fatty acids (Veldhuizen et al. 1998). Surfactant lipids modulate the release of oxidative and inflammatory mediators from inflammatory cells (Hayakawa et al. 1992; Tonks et al. 2001; Walti et al. 1997), and alterations in their composition lead to lung surfactant dysfunction (Reid et al. 2005) including a decrease in SP-A function (Yu and Possmayer 1996). Alterations in surfactant phospholipid profiles have been directly related to cases of chronic obstructive pulmonary disease (COPD) (Lusuardi et al. 1992), a disease prevalent in older people (Taffet et al. 2014).
A plausible explanation for the changes we observed in the ALF may be AEC dysfunction in old age which slows down ALF recycling and allows for accumulating ALF components in the lung. This buildup would trigger a series of events including surfactant lipid oxidation, leading to surfactant dysfunction that contributes to chronic inflammation in conjunction with increased complement levels with poorly regulated activity. In this regard, activated macrophages in the setting of chronic inflammation may also secrete more complement locally (Cole et al. 1983). Surfactant lipid accumulation and oxidation would likely also affect SP-A function. In this regard, our results show that the aging lung contains an altered DPPC:POPC ratio. SP-A binds to DPPC but not to POPC (Gupta et al. 2012), suggesting that soluble SP-A may be particularly susceptible to oxidation in the ALF of elderly individuals. In this context, just the mere presence of POPC directly interferes with SP-A-DPPC complex formation (Gupta et al. 2012). Non-functional oxidized SP-A would not effectively control complement activation, thereby further enhancing inflammation and lung injury in aging. Moreover, nitrated SP-A has decreased ability to aggregate surfactant lipids (Gupta et al. 2012). Reduced SP-A and/or SP-D activity is also expected to contribute to increased lipid oxidation in the lungs of old individuals, since both have been shown to be potent inhibitors of surfactant lipid peroxidation (Bridges et al. 2000).
The relevance of ALF in the elderly during microbial infection or the development of chronic pulmonary diseases is essentially unknown. Thus, the importance of this study is in the characterization of baseline levels of selected components that are likely to play a major role in the local alveolar immune response. This characterization now enables us to move forward with mechanistic studies in vitro and in vivo in the future. Of particular significance is our observation that several ALF components are similar in both mice and humans, making the study of long-term in vivo phenotypic and functional changes possible using the mouse model. Furthermore, the changes observed in specific-pathogen-free (SPF) housed old mice are representative of a natural aging phenotype without the confounding factors that are associated with environmental challenges seen in humans, and therefore, the similarities we observed between mouse and human indicate that those changes we found in human samples are indeed related to increasing age. In the end, comparative studies in mice and humans will provide the most accurate and comprehensive analysis of the aging process. In summary, we predict that the inflammatory/oxidative environment of the lung in old age will impact innate and adaptive immune responses with marked consequences on the ability of the elderly to handle respiratory infections and manage chronic inflammatory diseases.
Material and methods
Chemical reagents
All chemical reagents were of the highest grade from Sigma-Aldrich-Fluka unless otherwise specified.
Animals
Specific-pathogen-free, female, C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) at 3 months of age (young) or at 18 months of age (old) through a contract with the National Institute on Aging. Mice were housed in a standard vivarium in micro-isolator cages and were acclimated to the facility for at least 1 week prior to manipulation. Mice were examined at necropsy and those with gross lesions were excluded from the study. All procedures were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Human samples
This study was carried out in strict accordance with the United States Code of Federal Regulations, Local Regulations (institutional review board (IRB)), and Good Clinical Practice as approved by the National Institutes of Health (National Institute on Aging and National Institute of Allergy and Infectious Diseases). A total of four adults (two healthy and two lung resection patients) and five elderly (all lung resection patients) were enrolled. For the elderly group, the average age was 77 years (range 71–87), 100 % white, with two females and three males. For the adult group, the average age was 31 years (range 25–42), 100 % white, with two females and two males. Both genders were included without discrimination of race or ethnicity. For the lung resection patients, the pre-operative diagnoses were lung nodule (two adults and two elderly), lung nodule and mass (one elderly), and abnormal parathyroid and thymus (one adult and one elderly). Two elderly and two adult subjects had pulmonary function test (PFT) performed prior to surgery. One elderly had moderate to severe obstructive impairment, and the other had normal spirometry with the exception of suboptimal inspiratory flow-volume loop. One young subject had restrictive disease with low diffusion capacity, and the other had normal spirometry and lung volume with reduced diffusion capacity but normalized when corrected to volume. Donors who were smokers, injection and non-injection drug users, or excessive alcohol users or those with acute pneumonia were excluded from this study. For lung resection patients, individuals with the following comorbidities were excluded: heart disease, diabetes, asthma, COPD, renal failure, liver failure, hepatitis, thyroid disease, rheumatoid arthritis, immunosuppression, HIV/AIDS, cancer requiring chemotherapy, leukemia/lymphoma, and tuberculosis. BAL was performed on the healthy lobe of the subject’s lung.
Collection of ALF
Old and young mice were euthanized by CO2 asphyxiation following The Ohio State University (OSU) ILACUC-approved protocols. Upon euthanasia, ALF was obtained by bronchoalveolar lavage (BAL) in mice by washing the lungs ten times using 0.5 ml of sterile saline (0.9 % NaCl) each time. ALF protein and phospholipid contents were determined, and aliquots were quickly frozen and stored at −80 °C until use.
Human ALF was obtained by BAL from healthy donors and lung resection patients (adults and elderly) following our OSU IRB human subject-approved protocols and as we previously described in detail (Arcos et al. 2011). For lung resection patients, BAL was performed on healthy-appearing lung segments (as determined by the surgeon and pre-operative X-rays) of live human subjects (no samples taken from isolated tissues). Protein content was determined using the Micro BCA Assay (Pierce, Rockford, IL). After protein determination, crude ALF was concentrated 20-fold by using a 10-kDa molecular mass cutoff membrane Centricon Plus (Amicon Bioseparations) device at 4 °C to achieve the ALF volume present within the lung as we previously described (Arcos et al. 2011). Concentrated ALF was then separated into two different fractions: ALF-L defined as ALF depleted of surfactant lipids leaving functional SP-A, SP-D, complement, and hydrolases; and SL defined as the surfactant lipid fraction as we previously described (Arcos et al. 2011) and stored at −80 °C. No differences in ALF components from healthy or lung resection patients were observed.
Cytokine and MPO ELISAs
The cytokine content of ALF (mouse or human) was measured by ELISA following the manufacturer’s instructions. All samples were normalized by total protein content and volume (0.1 μg/μl) adding 100 μl/well (i.e., total 10 μg protein/well). For mice, we used BD Biosciences TNF (555268), IL-6 (555240), IL-1β (559603), IL-12p40 (555165), IL-10 (555252), and IFN-γ (555138). For humans, we used R&D Systems TNF (DY210), IL-6 (DY206), IL-1β (DY201), IL-12p40 (DY1240), IL-10 (DY217B), and BD Biosciences IFN-γ (555142). ELISA to detect mouse and human cytokines was performed following the manufacturer’s recommendations and established methods (Arcos et al. 2011). For mouse cytokine studies, the number of samples was n = 5/group. For human cytokine studies, the number of samples was n = 4/group. MPO was detected using a flurometric detection kit (Enzo Life Sciences, ADI-907-029) as per the manufacturer’s instructions. The number of mouse samples studied for MPO detection was n = 16/group.
Western blotting
All samples were normalized by protein content (10 μg protein/lane). For mouse ALF, the following antibodies (ABS) from Santa Cruz Biotechnology, Inc. (www.scbt.com) were used: SP-A (sc-166914), SP-D (sc-59695), C2 (sc-134639), C3 (sc-70474 or sc-28294), C4b (sc-74524), donkey anti-mouse IgG-HRP (sc-2314), factor B (sc-67141), goat anti-rabbit IgG-HRP (sc-2004), OxiSelect Nitrotyrosine Immunoblot Kit (BioCell Labs), and OxiSelect Carbonyl Immunoblot Kit (BioCell Labs). For human ALF, the following ABS were used: SP-A (sc-166914, Santa Cruz Biotechnology, Inc.), SP-D (sc-59695, Santa Cruz Biotechnology, Inc.), C2 (sc-134639, Santa Cruz Biotechnology, Inc.), C3 (sc-28294/B9, Santa Cruz Biotechnology, Inc.), C5 (A220, Complement Technology, Inc., www.complementtech.com), and C7 (sc-160195, Santa Cruz Biotechnology, Inc.). Western blotting (WB) to detect SP-A, SP-D, and complement components and to determine the presence of nitrotyrosine and carbonyl residues were performed following the manufacturer’s recommendations and established methods (Bolger et al. 2007; Clay et al. 2008). The carbonyl and nitrotyrosine presence was determined assessing the band density of all bands in the lane. Control lanes without protein in all Western blot experiments were used to subtract the background due to non-specific binding of antibody or artifacts during the membrane development process. All densitometry results are plotted after background subtraction. For mouse SP-A/SP-D studies, the number of samples was n = 17/group for SP-A and n = 15/group for SP-D. For human SP-A and SP-D studies, the number of samples was n = 4/group. For mouse complement studies, the number of samples was n = 12/group for C2 and C4β and n = 9/group for the rest. For human complement studies, the number of samples was n = 4/group. For mouse nitrotyrosine and carbonyl studies, the number of samples was n = 5/group.
Hydrolase activity in ALF
Hydrolase activities present in ALF were measured using a colorimetric method based on the release of p-nitrophenol upon specific substrate cleavage as we previously described (Arcos et al. 2011). Samples were normalized by ALF phospholipid content (1 mg phospholipid/ml of ALF) mimicking the physiological conditions described within the healthy lung as we previously reported (Arcos et al. 2011). For these studies, the number of samples was n = 8/group.
ALF lipid content
ALF lipids were extracted from ALF samples (normalized by protein content, 100 μg) from young and old mice using organic solvents as previously described (Postle 2008). Samples were directly analyzed by ESI/MS/MS in a positive ion mode as previously described (Postle 2008). For these studies, the number of samples was n = 3/group.
Statistical analysis
Data were plotted and analyzed using the GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA). Unpaired two-tailed Student’s t tests were used to determine statistical significance comparing mouse data among young vs. old and human data among adult vs. elderly.
Acknowledgements
This work was supported by a The Ohio State University Public Health Preparedness for Infectious Diseases (PHPID) pilot award to JT, JBT, LSS, WL, SW, J(X)P, and PR Jr, and a Julie Martin Mid-Career American Federation of Aging Research (AFAR) award to JT, and partially by a NIH/NIAD R01 [AI-093570] to JBT. JIM was partially supported by a NIH/NIGMS T32-GM068412. We thank Dr. Patsy Skabla for assisting with patient enrollment. We also thank the Campus Chemical Instrument Center (CCIC) at The Ohio State University for their services.
Footnotes
Juan I. Moliva and Murugesan V. S. Rajaram contributed equally to this work.
Contributor Information
Joanne Turner, Phone: +1-614-2926724, FAX: +1-614-2929616, Email: joanne.turner@osumc.edu.
Jordi B. Torrelles, Phone: +1-614-2920777, FAX: +1-614-2929616, Email: jordi.torrelles@osumc.edu
References
- Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis D, Bartlett D, Patel H, Roberts H. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:1–8. doi: 10.1186/2046-2395-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159:1985–1991. doi: 10.1164/ajrccm.159.6.9809043. [DOI] [PubMed] [Google Scholar]
- Betsuyaku T, Kuroki Y, Nagai K, Nasuhara Y, Nishimura M. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J. 2004;24:964–970. doi: 10.1183/09031936.04.00064004. [DOI] [PubMed] [Google Scholar]
- Bolger MS, Ross DS, Jiang H, Frank MM, Ghio AJ, Schwartz DA, et al. Complement levels and activity in the normal and LPS-injured lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L748–L759. doi: 10.1152/ajplung.00127.2006. [DOI] [PubMed] [Google Scholar]
- Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, et al. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem. 2000;275:38848–38855. doi: 10.1074/jbc.M005322200. [DOI] [PubMed] [Google Scholar]
- Burton DGA. Cellular senescence, ageing and disease. Age (Dordr) 2009;31:1–9. doi: 10.1007/s11357-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010;126:690–699. doi: 10.1016/j.jaci.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TK, Brooks M, Meyer D, Henning L, Murugesan V, et al. Pulmonary innnate immunity: soluble and cellular host defenses of the lung. In: Marsh C, Tridandapani S, Piper M, et al., editors. Regulation of innate immune function. Kerala: Transworld Research Network; 2010. pp. 165–211. [Google Scholar]
- Clay CD, Soni S, Gunn JS, Schlesinger LS. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol. 2008;181:5568–5578. doi: 10.4049/jimmunol.181.8.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole FS, Matthews WJ, Jr, Rossing TH, Gash DJ, Lichtenberg NA, Pennington JE. Complement biosynthesis by human bronchoalveolar macrophages. Clin Immunol Immunopathol. 1983;27:153–159. doi: 10.1016/0090-1229(83)90065-X. [DOI] [PubMed] [Google Scholar]
- Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, et al. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- Cruz-Hervert LP, Garcia-Garcia L, Ferreyra-Reyes L, Bobadilla-del-Valle M, Cano-Arellano B, Canizales-Quintero S, et al. Tuberculosis in ageing: high rates, complex diagnosis and poor clinical outcomes. Age Ageing. 2012;41:488–495. doi: 10.1093/ageing/afs028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, et al. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom Rev. 2005;24:55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- de Vries AC, Schram AW, Tager JM, Batenburg JJ, van Golde LM. A specific acid alpha-glucosidase in lamellar bodies of the human lung. Biochim Biophys Acta. 1985;837:230–238. doi: 10.1016/0005-2760(85)90046-3. [DOI] [PubMed] [Google Scholar]
- DiAugustine RP. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974;249:584–593. [PubMed] [Google Scholar]
- Doria G, D’Agostaro G, Poretti A. Age-dependent variations of antibody avidity. Immunology. 1978;35:601–611. [PMC free article] [PubMed] [Google Scholar]
- Dyer C. The interaction of ageing and lung disease. Chron Respir Dis. 2012;9:63–67. doi: 10.1177/1479972311433766. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson JD, Shannon JM, Mason RJ. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am Rev Respir Dis. 1988;138:1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun. 2004;72:2564–2573. doi: 10.1128/IAI.72.5.2564-2573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, et al. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso CAV, Lee PJ. The aging lung. J Gerontol. 2012;67A:233–235. doi: 10.1093/gerona/glr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- Geunes-Boyer S, Oliver TN, Janbon G, Lodge JK, Heitman J, Perfect JR, et al. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun. 2009;77:2783–2794. doi: 10.1128/IAI.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder H, Haschemeyer RH, Fairclough GF, Jr, Mynarcik DC. Isolation and characterization of lamellar body material from rat lung homogenates by continuous linear sucrose gradients. J Lipid Res. 1981;22:1277–1285. [PubMed] [Google Scholar]
- Gupta GS, Gupta A, Gupta RK. Animal lectins: form, functions and clinical applications. London: Springer; 2012. [Google Scholar]
- Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol. 2001;63:495–519. doi: 10.1146/annurev.physiol.63.1.495. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Giridhar G, Myrvik QN, Kucera L. Pulmonary surfactant phospholipids modulate priming of rabbit alveolar macrophages for oxidative responses. J Leukoc Biol. 1992;51:379. doi: 10.1002/jlb.51.4.379. [DOI] [PubMed] [Google Scholar]
- Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E, Karimi GK, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook GE. Extracellular hydrolases of the lung. Biochemistry. 1978;17:520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Hook GE, Gilmore LB. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982;257:9211–9220. [PubMed] [Google Scholar]
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RJ. Pulmonary surfactant. J Appl Physiol. 1982;53:1–8. doi: 10.1063/1.331592. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- Lusuardi M, Capelli A, Carli S, Tacconi MT, Salmona M, Donner CF. Role of surfactant in chronic obstructive pulmonary disease: therapeutic implications. Respiration. 1992;59(Suppl 1):28–32. doi: 10.1159/000196100. [DOI] [PubMed] [Google Scholar]
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, Hahn FF. The effects of age on lung function and structure of adult animals. Adv Vet Sci Comp Med. 1982;26:35–77. [PubMed] [Google Scholar]
- Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153:1072–1079. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- Mold C. Role of complement in host defense against bacterial infection. Microbes Infect. 1999;1:633–638. doi: 10.1016/S1286-4579(99)80063-X. [DOI] [PubMed] [Google Scholar]
- Nguyen HA, Rajaram MV, Meyer DA, Schlesinger LS. Pulmonary surfactant protein A and surfactant lipids upregulate IRAK-M, a negative regulator of TLR-mediated inflammation in human macrophages. Am J Physiol Lung Cell Mol Physiol. 2012;303:L608–L616. doi: 10.1152/ajplung.00067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter RH. Lung surfactants: basic science and clinical applications. New York: Marcel Dekker; 2000. pp. 1–444. [Google Scholar]
- Notter RH, Tabak SA, Mavis RD. Surface properties of binary mixtures of some pulmonary surfactant components. J Lipid Res. 1980;21:10–22. [PubMed] [Google Scholar]
- Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4:e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle AD. Phospholipid profiling. In: Griffiths W, editor. Metabolomics, metabonomics, and metabolite profiling. Cambridge: The Royal Society of Chemistry; 2008. pp. 116–133. [Google Scholar]
- Rebello CM, Jobe AH, Eisele JW, Ikegami M. Alveolar and tissue surfactant pool sizes in humans. Am J Respir Crit Care Med. 1996;154:625–628. doi: 10.1164/ajrccm.154.3.8810596. [DOI] [PubMed] [Google Scholar]
- Reid KB, Clark H, Palaniyar N. Surfactant and lung inflammation. Thorax. 2005;60:620–622. doi: 10.1136/thx.2004.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retini C, Kozel TR, Pietrella D, Monari C, Bistoni F, Vecchiarelli A. Interdependency of interleukin-10 and interleukin-12 in regulation of T-cell differentiation and effector function of monocytes in response to stimulation with Cryptococcus neoformans. Infect Immun. 2001;69:6064–6073. doi: 10.1128/IAI.69.10.6064-6073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HY. Lung inflammation: normal host defense or a complication of some diseases? Annu Rev Med. 1987;38:295–323. doi: 10.1146/annurev.me.38.020187.001455. [DOI] [PubMed] [Google Scholar]
- Robertson J, Caldwell JR, Castle JR, Waldman RH. Evidence for the presence of components of the alternative (properdin) pathway of complement activation in respiratory secretions. J Immunol. 1976;117:900–903. [PubMed] [Google Scholar]
- Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23(Suppl 15):S29–S36. [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- Schunemann HJ, Muti P, Freudenheim JL, Armstrong D, Browne R, Klocke RA, et al. Oxidative stress and lung function. Am J Epidemiol. 1997;146:939–948. doi: 10.1093/oxfordjournals.aje.a009220. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Eidlen DM, Mason RJ. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. 1988;81:1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi: 10.2147/CIA.S52999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco F. Inherited complement deficiencies and bacterial infections. Vaccine. 2008;26(Suppl 8):I3–I8. doi: 10.1016/j.vaccine.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Tonks A, Morris RH, Price AJ, Thomas AW, Jones KP, Jackson SK. Dipalmitoylphosphatidylcholine modulates inflammatory functions of monocytic cells independently of mitogen activated protein kinases. Clin Exp Immunol. 2001;124:86–94. doi: 10.1046/j.1365-2249.2001.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: a battle for survival. In: Cardona PJ, editor. Understanding tuberculosis—analyzing the origin of Mycobacterium tuberculosis pathogenicity. Rijeka: Intech; 2012. pp. 1–46. [Google Scholar]
- Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am J Physiol Lung Cell Mol Physiol. 2009;296:L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- van Golde LM. Synthesis of surfactant lipids in the adult lung. Annu Rev Physiol. 1985;47:765–774. doi: 10.1146/annurev.ph.47.030185.004001. [DOI] [PubMed] [Google Scholar]
- Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/S0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Walti H, Polla BS, Bachelet M. Modified natural porcine surfactant inhibits superoxide anions and proinflammatory mediators released by resting and stimulated human monocytes. Pediatr Res. 1997;41:114–119. doi: 10.1203/00006450-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Wang SH, Carruthers B, Turner J. The influence of increasing age on susceptibility of the elderly to tuberculosis. Open Longetivity Science. 2012;6:73–82. doi: 10.2174/1876326X01206010073. [DOI] [Google Scholar]
- Watford WT, Wright JR, Hester CG, Jiang H, Frank MM. Surfactant protein A regulates complement activation. J Immunol. 2001;167:6593–6600. doi: 10.4049/jimmunol.167.11.6593. [DOI] [PubMed] [Google Scholar]
- Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205:1589–1592. doi: 10.1093/infdis/jis240. [DOI] [PubMed] [Google Scholar]
- Young SL, Fram EK, Larson E, Wright JR. Recycling of surfactant lipid and apoprotein-A studied by electron microscopic autoradiography. Am J Physiol Lung Cell Mol Physiol. 1993;265:L19–L26. doi: 10.1152/ajplung.1993.265.1.L19. [DOI] [PubMed] [Google Scholar]
- Yu SH, Possmayer F. Effect of pulmonary surfactant protein A and neutral lipid on accretion and organization of dipalmitoylphosphatidylcholine in surface films. J Lipid Res. 1996;37:1278–1288. [PubMed] [Google Scholar]