Abstract
The equations for estimating kidney function have become very popular in the last decade. However, the clinical and prognostic meaning of these measures may be very different in older populations. Two cohorts of people aged 65–89 years (older sample) and 90 or more (oldest old sample) were used to investigate the prognostic significance of estimated glomerular filtration rate (eGFR). Additionally, we also investigated whether combining frailty and eGFR may improve the accuracy of frailty in predicting mortality. We found that lower eGFR values were significantly more frequent among frail subjects in both groups. eGFR < 30 was associated with increased risk for all-cause mortality either in subjects aged 65–89 years (HR = 3.71, 95% CI = 1.23–11.2) or in those aged 90 or more (HR = 1.53, 95% CI = 1.05–2.23). In the latter group, a not significant trend for increasing mortality was also observed among people with eGFR > 60 (HR = 1.28, 95% CI = 0.72–2.26). In addition, the oldest old subjects with eGFR > 60 and eGFR < 30 had the lowest hand-grip strength and ADL values. Combining eGFR and frailty status significantly improved the accuracy of frailty in predicting mortality only in the older sample. In conclusion, a U-shaped relationship exists between eGFR and mortality in the oldest old, but not in older individuals. Our findings suggest that eGFR needs to be adjusted for muscle mass/physical performance when estimating kidney function in people aged 90 or more. Nevertheless, in subjects aged 65–89 years, eGFR may improve the accuracy of frailty status in predicting prognosis, thus suggesting that eGFR may represent an additional dimension of frailty syndrome.
Keywords: Glomerular filtration rate, Chronic kidney disease, Frailty, Human aging
Introduction
Chronic kidney disease (CKD) is a major predictor of mortality in both the general population and selected diseased population (Anavekar et al. 2004; Go et al. 2004). For instance, the risk of death in a broad adult population over an average follow-up period of about 3 years dramatically increased for each 15-ml decrease in glomerular filtration rate (GFR) below the threshold of 60 ml min−1 m−2 (Go et al. 2004). In the elderly, CKD predicts mortality as well, besides being an important correlate of functional limitation(Odden et al. 2009; Lattanzio et al. 2012; Pedone et al. 2012), frailty (Bowling and Muntner 2012), and adverse reactions to hydrosoluble drugs(Corsonello et al. 2005, 2011).
Serum creatinine (Scr), the most universally used marker of renal function, is poorly reliable in elderly and disabled patients due to sarcopenia depleting the muscle content of creatine and, thus, serum creatinine. Furthermore, GFR more than Scr guarantees for a set of clinically meaningful intervals (Howard and Dunn 2002). Thus, formulas have been developed to estimate GFR on the basis of selected anthropometric and serum indicators. The Modification of Diet in Renal Disease (MDRD) formula, has been repeatedly validated against the gold standards (GFR obtained through radionuclide method or Iothalamate clearance), and found to lose accuracy in the upper range of GFR (Stevens et al. 2007). In an attempt to overcome this limitation, an equation has been developed in a large adult population and has been proved to gain in accuracy with respect to the MDRD (Levey et al. 2009), and successively updated in the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study (Inker et al. 2012). However, the MDRD and CKD-EPI study populations were almost completely devoid of subjects over 70 years, who are those with the highest prevalence of low GFR and the greatest burden of GFR-related negative outcomes (Levey et al. 2009). Additionally, a U-shaped relationship between creatinine-based eGFR and mortality has been reported (Cox et al. 2008; Tonelli et al. 2011; Shastri et al. 2012; Peters et al. 2013). Thus, the meaning of these measures may be very different in oldest populations. More recently, the Berlin Initiative Study (BIS) developed and validated a new creatinine-based equation for calculating eGFR in people aged 70 years or more (Schaeffner et al. 2012), and its prognostic significance is still worthy of testing in very old and frail subjects. Additionally, whether combining eGFR to frailty status may improve the ability of the latter to predict prognosis has not been fully elucidated.
Therefore, we aimed at investigating the relationship between eGFR, as calculated by BIS1 equation, and survival in two populations of older community-dwelling subjects, including a sample of people aged 90 years or more. Additionally, we also investigated whether frailty may improve the accuracy of eGFR in predicting mortality.
Materials and methods
Study population
The study was carried out on two samples of community-dwelling subjects representative of the general older population in the Calabria district.
The recruitment of subjects older than 90 years was carried out between 2002 and 2006, in the frame of two different European projects: the European Challenge for Healthy Aging (ECHA) (De Rango et al. 2008) and the GEnetics of Healthy Aging (GEHA) (Skytthe et al. 2011) projects. In both projects, old subjects were identified on the basis of demographic information obtained from local municipalities. During the ECHA project, all 409 Calabrian municipalities were initially contacted asking for the lists of all persons born before 1912 or earlier and with residence in those municipalities. From a population of 12,630 nonagenarians, 1,291 subjects were contacted. Six hundred eighty-one subjects were dead when we called, 81 subjects were excluded for dementia, 400 (clustered in 124 families) accepted to participate, while the others refused to enter the study. One hundred twenty-four subjects (one from each family) of this sample were included in older group analysed in the present study.
During the GEHA project, Calabrian municipalities sent the lists of persons born in 1915 or earlier who resided in those municipalities. From a population of 12,551 nonagenarians included in these lists, potential siblings were identified by visiting local municipalities and performing genealogical reconstruction of families. Eligible persons were contacted by phone, and the aim of the GEHA project and procedure for participation was explained. If the person agreed to participate, an appointment for a home visit was made. In total, 425 families were contacted, of which 200 sib pairs agreed to participate. The remaining 225 families did not enter the project, 72 because of the death of at least one member in the period between the acquisition of the list and the contact for the visit (3 months on average), 81 because at least one member refused, 21 were untraceable, and 51 were excluded for dementia of one of the sibs. Two hundred subjects of this sample (one from each family) were included in older group analysed in the present study.
The remaining 286 subjects were sampled among families contacted in the frame of GEHA project, which could not enter the study one member of the sibship either could not or did not want participate.
Subjects in the age range of 65–85 years were recruited between 2007 and 2010 as part of a survey aimed at monitoring the health status of this population segment in Calabria. The subjects were invited to participate either through general physicians, who explained to them the aim of the project, or by means of the INRCA Hospital, which is a reference point for the care of the aging people in the Calabria region. The attendance rate was about 90 %.
Overall, 585 subjects aged 64–89 years (older) and 611 subjects aged 90 or more (oldest old) were enrolled in the study. Subjects with missing data for eGFR (4 subjects aged 64–89 and 9 subjects aged 90 or more) or frailty variables (48 subjects aged 64–89 and 97 subjects aged 90 or more) were excluded from the present study, leaving a final sample of 533 subjects in the older group (257 females and 276 males, median age 72 years) and 505 subjects in the oldest old group (296 females and 209 males, age range of 90–107 years, median age of 93 years). Written informed consent was obtained from all subjects at the time of enrollment. The study protocol was approved by the Ethics Committee of the University of Calabria.
Comprehensive geriatric assessment
Comprehensive geriatric assessment was carried out by a skilled geriatrician and included cognitive functioning (age-, sex-, and education-adjusted Mini-Mental State Examination) (Folstein et al. 1975), perceived health status (Self-Reported Health Status) (Idler and Benyamini 1997), self-reported functional status (Basic Activities of Daily Living) (Katz et al. 1963), and objectively measured physical performance (hand grip strength).
Hand grip (HG) strength was measured using a handheld dynamometer (SMEDLEY’s dynamometer TTM) while the subject was sitting with the arm close to his/her body. The test was repeated three times with the stronger hand, and the maximum value was used in the analyses. When a test was not carried out, it was specified if it was due to physical disabilities or because the subject refused to participate.
Using multiple regression models, we adjusted the crude HG scores for age, sex, and height; the crude MMSE scores were adjusted for age, sex, and education. Both fitted models were used to obtain adjusted mean values for the relevant variables (Howell 1997)
Definition of frailty
Enrolled subjects were classified as nonfrail, prefrail, or frail using a population-specific approach as recently reported (Montesanto et al. 2010). Briefly, Ward’s hierarchical cluster analysis with MMSE, HG, ADL, and SRHS as classification variables was carried out (Ward 1963). In the clustering algorithm, for MMSE and HG strength, standardized residuals were used after appropriate adjustment; ADL and SRHS were standardized to a mean of 0 and standard deviation of 1. The analysis was separately performed in older and oldest old subjects. The increase in the total within-cluster sum of squares against the number of clusters was plotted in order to choose the optimal number of clusters. We choose the optimal number of clusters where the largest drop in the total within-cluster sum of squares was observed.
Blood sampling and laboratory parameters
All enrolled subjects underwent a blood sampling after an overnight fasting for general laboratory screening and DNA extraction for molecular genetic studies at the time of enrollment. Serum samples were immediately stored at −80 °C until assays were performed. General laboratory panel included serum creatinine measured by standardized Jaffe method calibrated to isotope dilution mass spectrometry.
Estimated glomerular filtration rate
Estimated glomerular filtration rate (eGFR) was calculated using the Berlin Initiative Study 1 (BIS1) equation (Schaeffner et al. 2012):
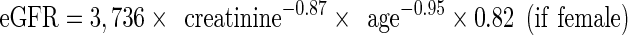 |
The BIS1 equation was chosen because it showed the lowest deviation from the measured glomerular filtration rate and the smallest misclassification rate among creatinine-based equations (Schaeffner et al. 2012). Additionally, a recent study comparing the accuracy of BIS1, MDRD, and CKD-EPI in older patients showed that BIS1 was the most reliable for assessing renal function in older white patients, especially in those with CKD stages 1–3 (Koppe et al. 2013). The eGFR values were categorized as >60, 45–60, 30–45, and <30 ml/min/1.73 m2 as recommended by the K/DOQI guidelines (National Kidney Foundation 2002).
Outcome
The main outcome of our study was survival of enrolled subjects during the study period. The follow-up duration from baseline enrollment visit was 48 months in the older subjects sample and 54 months in the oldest old subjects sample. For patients who died during the follow-up period, information about date, place, and cause of death were collected from death certificates provided by relatives or caregivers. City or town registers were consulted to retrieve information about death when neither relatives nor caregivers could be contacted.
Analytic approach
First, we compared older and oldest old subjects regarding their demographic, geriatric assessment scores, and eGFR. Testing for independence in contingency tables was carried out by Fisher’s exact test. Student’s t test was used to compare continuous normally distributed variables, while Mann–Whitney U test was used for comparison of ordinal variables.
Second, using multiple regression models, we adjusted the crude HG scores for age, gender, and height; the crude MMSE scores were adjusted for age, gender, and education. Both fitted models were used to obtain adjusted mean values for the relevant variables.22
Third, a Ward’s hierarchical cluster analysis was separately performed in each study sample in order to obtain the above described frailty classification (Montesanto et al. 2010), and the distribution of eGFR values by group and frailty status was investigated.
Fourth, Kaplan–Meier survival curves with the Mantel–Cox log-rank test were used to compare crude survival of subjects with different values of eGFR. The time from enrollment visit through the day of death was used as the time to failure variable for the model. Survivors were censored on the day of the last follow-up visit. Spline evaluating the relationship between eGFR and mortality was also investigated after adjusting for age and gender.
Cox proportional hazard models (Cox 1972) were separately carried out in older and oldest old subjects to assess the independent contribution of eGFR on mortality risk. Age, gender, and frailty status were considered as potential confounders. Schoenfeld residuals (1982) were used to assess the proportional hazard assumption. In order to obtain a better understanding of the association between eGFR and mortality, we planned to investigate frailty variables across eGFR groups.
Finally, a receiver operating characteristic (ROC) curve was constructed to assess if the addition of eGFR to the frailty classification improved the mortality predictions. To this purpose, the predictive value of the frailty classification, alone or in combination with the eGFR values, was measured in terms of area under ROC curve (AUC). The differences between AUC values were tested using the method proposed by DeLong et al. (1988). By exploiting the mathematical equivalence of the AUC to the Mann–Whitney U statistic, this method allows the comparison of two correlated AUCs using the chi-square test.
All statistical analyses were performed with SPSS v.19.0 (IBM Corp., Armonk, NY, USA) except for survival analyses performed with the survival package Therneau T. A Package for Survival Analysis in S. R package version 2.37-4, http://CRAN.R-project.org/package=survival, 2013. of R (R Development Core Team 2009). A significance level of 0.05 was chosen in all the tests.
Results
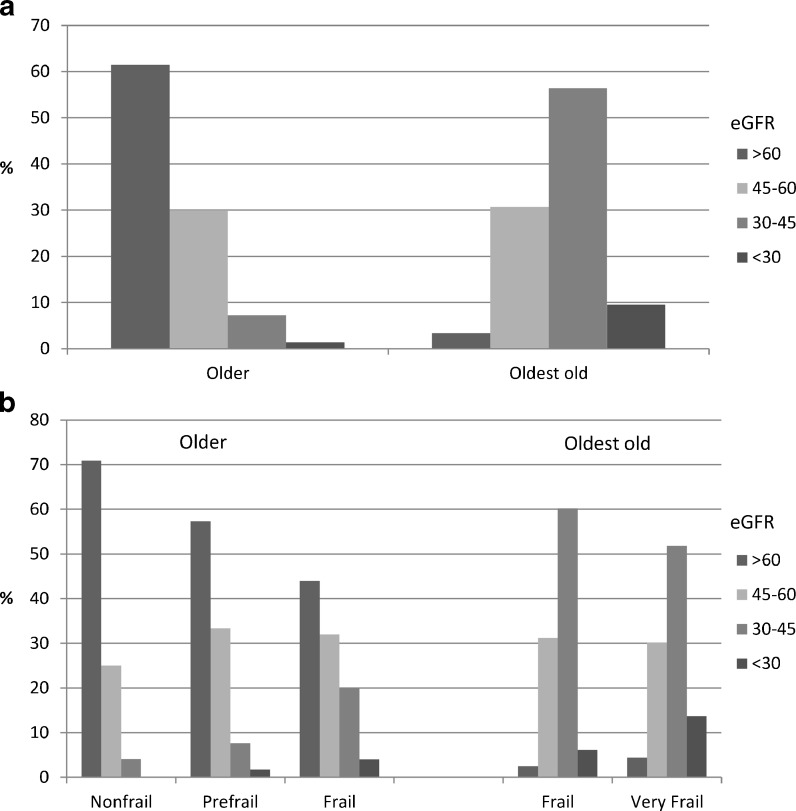
General characteristics of the groups studied are reported in Table 1. Subjects in the oldest old group were more frequently female and performed worse in all geriatric assessment tasks when compared with the older group. Nonagenarians also had lower eGFR values with respect to subjects aged 64–89 years. In particular, eGFR values <45 ml/min/1.73 m2 were more frequent among subjects in the oldest old group (Fig. 1a). About 1.0 % of the subjects belonging to older and about 10 % of the subjects belonging to the oldest old sample showed a severely reduced kidney function (eGFR < 30).
Table 1.
General characteristics of the two study populations
| Older group (64–89 years) | Oldest old group (≥90 years) | p value | |
|---|---|---|---|
| N (%) | 533 | 505 | |
| Age | <0.001 | ||
| Mean (SD) | 73.4 (6.4) | 94.0 (3.4) | |
| Range | 64-89 | 90–105 | |
| Female gender, N (%) | 257 (48.2) | 296 (58.6) | <0.001 |
| Frailty status variables | |||
| Hand grip strength (kg) | <0.001 | ||
| Mean (SD) | 23.4 (9.6) | 14.5 (6.8) | |
| Range | 4.0–55.0 | 1.0–42.0 | |
| Mini-Mental State Examination | <0.001 | ||
| Mean (SD) | 23.7 (4.5) | 15.3 (6.2) | |
| Range | 0–30 | 0–29 | |
| Self-Reported Health Status | 0.005 | ||
| Mean (SD) | 3.08 (0.94) | 3.19 (0.95) | |
| Range | 1–5 | 1–5 | |
| Activities of daily living | <0.001 | ||
| Mean (SD) | 4.77 (0.78) | 3.13 (1.98) | |
| Range | 0–5 | 0–5 | |
| Survival | |||
| Number of deaths, N (%) | 61 (11.5)a | 337 (66.7) | <0.001 |
| Survival time (months), mean (SE) | 34.0 (0.84) | 45.0 (0.41) | |
| eGFR (ml/min/1.73 m2) | <0.001 | ||
| Mean (SD) | 63.8 (14.5) | 41.7 (9.5) | |
| Range | 17.7-110.2 | 6.3–75.5 | |
aFour missing values for follow-up data
Fig. 1.
Distribution of eGFR values by sample (a) and frailty status (b)
Frailty status and relationship between frailty and eGFR
Using MMSE, HG, ADL, and SRHS as classification variables, subjects belonging to older and oldest old samples were separately classified in different frailty groups. On the basis of mean values of classification variables, within the different clusters of the older sample, we defined cluster 1 as nonfrail (the cluster with subjects showing the best scores for the classification variables), cluster 3 as frail (the clusters with subjects showing the worst scores for the classification variables), and cluster 2 as prefrail (the cluster with subjects showing intermediate scores for the classification variables). Similarly, on the basis of mean values of classification variables within the two clusters of the oldest old sample, we defined cluster 1 as frail (the cluster with subjects showing the best scores for the classification variables) and cluster 2 as very frail (the clusters with subjects showing the worst scores for the classification variables). The mean values of the classification variables are reported in Table 2.
Table 2.
Frailty classification: mean values (standard error in parenthesis) of MMSE, HG, ADL, and SRHS of the study populations respect to the cluster membership
| Cluster | N | HGa | MMSEa | ADL | SRHS |
|---|---|---|---|---|---|
| Older (64–89 years) | |||||
| 1 (Nonfrail) | 220 | 27.3 (0.40) | 24.4 (0.23) | 4.98 (0.01) | 3.87 (0.05) |
| 2 (Prefrail) | 288 | 20.8 (0.35) | 23.6 (0.20) | 4.88 (0.02) | 2.60 (0.03) |
| 3 (Frail) | 25 | 20.2 (1.23) | 18.5 (0.71) | 1.72 (0.23) | 1.64 (0.15) |
| Oldest old (≥90 years) | |||||
| 1 (Frail) | 279 | 16.5 (0.30) | 17.3 (0.29) | 4.78 (0.03) | 3.43 (0.05) |
| 2 (Very frail) | 226 | 11.9 (0.33) | 12.9 (0.33) | 1.09 (0.06) | 2.90 (0.06) |
aFor HG and MMSE, adjusted mean values were reported (see “Materials and methods”)
A significant relationship between frailty and eGFR was observed in both study groups. In the older group, lower eGFR values were observed among prefrail (eGFR 30–45, 7.6 %; eGFR < 30, 1.7 %) and frail subjects (eGFR 30–45, 20.0 %; eGFR < 30, 4.0 %) with respect to nonfrail ones (eGFR 30–45, 4.1 %; eGFR < 30, 0.0 %; p < 0.001). In the oldest old group, eGFR < 30 was more prevalent among very frail compared to frail subjects (13.7 % vs 6.1 %, p = 0.014) (Fig. 1b).
eGFR and survival
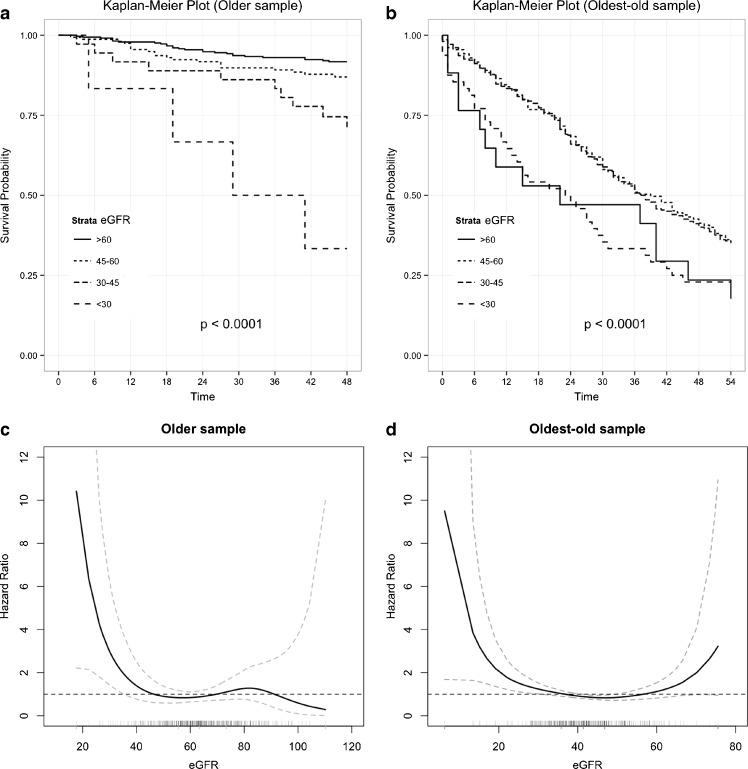
Kaplan–Meier survival curves across eGFR classes are reported in Fig. 2a. In the older group, an increased mortality in subjects with lower values of eGFR was observed. The relationship between eGFR and mortality was clearly different among subjects belonging to the oldest old group where the highest mortality rates were observed among subjects with highest (>60) and lowest (<30) eGFR values. The age- and gender-adjusted splines for eGFR revealed an evident U-shaped relationship with mortality in the oldest old, but not in the older group (Fig. 2b). Given the above findings, Cox regression analysis was carried out by considering eGFR = 45–60 ml/min/1.73 m2 as a reference category, and it showed that severely reduced kidney function (eGFR < 30 ml/min/1.73 m2) was significantly associated with increased mortality in both older and oldest old group. Such associations remained significant even after controlling for frailty status (Table 3).
Fig. 2.
Kaplan–Meier curves of survival across eGFR groups in older (a) and oldest old (b) groups, and age- and gender-adjusted spline of the relationship between eGFR and all-cause mortality in the two study cohorts (c, d)
Table 3.
Cox regression analysis of eGFR to all-cause mortality separately performed in older (upper panel) and oldest old (lower panel) subjects
| Variable | Adjusted for age and gender | Adjusted for age, gender, and frailty status | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p value | HR | 95 % CI | p value | |
| Older | ||||||
| >60 | 1.0 | 0.54–1.85 | 0.988 | 1.0 | 0.55–1.86 | 0.979 |
| 45–60 | Ref. | – | – | Ref. | – | – |
| 30–45 | 1.76 | 0.81–3.83 | 0.151 | 1.59 | 0.72–3.51 | 0.245 |
| <30 | 4.60 | 1.54–13.8 | 0.006 | 3.71 | 1.23–11.2 | 0.020 |
| Oldest old | ||||||
| >60 | 1.46 | 0.82–2.58 | 0.195 | 1.28 | 0.72–2.27 | 0.402 |
| 45–60 | Ref. | – | – | Ref. | – | – |
| 30–45 | 0.98 | 0.77–1.25 | 0.866 | 0.99 | 0.78–1.27 | 0.979 |
| <30 | 1.63 | 1.11–2.37 | 0.012 | 1.53 | 1.05–2.23 | 0.028 |
The analysis of frailty variables across eGFR groups revealed that oldest old subjects with eGFR > 60 and eGFR < 30 had the lowest hand grip strength and ADL values (p = 0.020 and p = 0.006, respectively). No significant difference across eGFR groups was observed for MMSE and SRHS. Among older subjects, declining eGFR was characterized by a significant worsening of ADL and SRHS (p < 0.001 in both cases, Table 4).
Table 4.
Frailty variables (standard error in parenthesis) across the eGFR groups in both samples
| Older | ||||
| eGFR | HG* | MMSE* | ADL** | SRHS** |
| >60 | 23.3 (0.36) | 23.7 (0.20) | 4.9 (0.04) | 3.3 (0.22) |
| 45–60 | 23.5 (0.51) | 23.8 (0.29) | 4.7 (0.06) | 2.9 (0.08) |
| 30–45 | 22.6 (1.10) | 23.2 (0.62) | 4.3 (0.24) | 2.6 (0.06) |
| <30 | 19.5 (2.61) | 23.4 (1.48) | 3.8 (0.48) | 2.0 (0.16) |
| Oldest old | ||||
| eGFR | HG*** | MMSE* | ADL**** | SRHS* |
| <60 | 11.6 (1.31) | 13.1 (1.29) | 2.4 (0.49) | 3.3 (0.92) |
| 45–60 | 14.3 (0.43) | 15.4 (0.42) | 3.2 (0.16) | 3.2 (0.96) |
| 30–45 | 14.9 (0.32) | 15.6 (0.31) | 3.3 (0.11) | 3.2 (0.92) |
| <30 | 13.2 (0.77) | 14.3 (0.76) | 2.3 (0.09) | 3.1 (1.09) |
For HG and MMSE, adjusted mean values were reported (see “Materials and methods”)
*p > 0.05; **p < 0.001; ***p = 0.020; ****p = 0.006
Impact of eGFR on predictive accuracy of frailty classification
In order to assess if the addition of eGFR to the frailty classification improved the prediction of mortality, the predictive value of this classification alone and in combination with the eGFR was evaluated in terms of AUC. A significant improvement in the prediction of mortality was observed when frailty classification was used in combination with eGFR classification (0.715 vs 0.659, p = 0.0021) in the older, but not in the oldest old sample (0.623 vs 0.617, p = 0.6174).
Discussion
The relationship between CKD and frailty
Our study showed that CKD was highly prevalent in two cohorts of older individuals. Additionally, CKD was more prevalent among subjects aged 90 or more compared to those aged 64–89 years. Finally, a significant relationship between eGFR and frailty status was observed in both groups. Studies have shown individuals with end-stage renal disease have significantly lower physical functioning compared to the general population (Singer et al. 1999), as well as in comparison to those with other common chronic diseases, such as diastolic heart failure or chronic obstructive pulmonary disease, or at high risk for cardiovascular disease (Hartmann et al. 2009). There may be several mechanisms explaining the CKD-related impairment in physical performance of older patients. Sarcopenia, defined as a loss of muscle mass with limited mobility (Morley et al. 2011), is a common finding in older adults, and its prevalence rises sharply with declining kidney function in adults (Foley et al. 2007). Furthermore, it is widely known that inflammatory markers are elevated during CKD (Shlipak et al. 2004; Fried et al. 2006), and higher levels of interleukin-6 (IL-6) have been associated with incident mobility disability in older adults, perhaps due to a decline in muscle strength. Indeed, it has been suggested that inflammation mediates the association between CKD and functional limitation in older community dwelling adults (Fried et al. 2006). Finally, comorbidities frequently observed in subjects affected by CKD, such as cardiovascular and respiratory diseases (Rifkin and Winkelmayer 2010), osteoporosis (Urena-Torres et al. 2011), and cognitive decline (Helmer et al. 2011), may further contribute to such an association.
CKD and mortality
Our study showed a U-shaped relationship between eGFR and mortality in the oldest old, but not in the older group. A similar U-shaped association between creatinine-based eGFR and mortality was observed in at least four other studies. Using MDRD equation, Cox et al. (2008) showed for the first time that all-cause mortality risk increased in subjects with eGFR higher than 89 ml/min/1.73 m2 enrolled in the Hull and East Yorkshire renal and diabetes registers. An increased risk of cardiovascular events, and a near significant increase in total and cardiovascular mortality was observed in octogenarians with CKD-EPI-based eGFR values of 75 ml/min/1.73 m2 or more enrolled in the HYVET trial (Peters et al. 2013). All-cause mortality, but not mortality from myocardial infarction or stroke, was significantly increased in patients with MDRD-based eGFR greater or equal to 90 ml/min/1.73 m2 in the large population study of the Alberta Kidney Disease Network repository (Tonelli et al. 2011). Finally, a similar U-shaped relationship between eGFR and mortality was observed in the Cardiovascular Health Study with creatinine-based CKD-EPI, but not with cystatin-C-based CKD-EPI equation (Shastri et al. 2012). Interestingly, our observation was done using the only creatinine-based eGFR equation specifically validated in a population of subjects aged 70 years or more (BIS), which proved to be less biased compared to those used in former studies(Cox et al. 2008; Tonelli et al. 2011; Shastri et al. 2012; Peters et al. 2013). The observed U-shaped relationship between eGFR and mortality suggests the hypothesis that sarcopenic patients might clusterize in the group with higher eGFR values. However, former studies did not provide data on muscle mass or strength, and thus, the mechanisms underlying this finding remained to be elucidated (Shastri and Sarnak 2011). Our observation add to the current knowledge by demonstrating that oldest old group subjects with eGFR > 60 and eGFR < 30 had lowest muscle strength, as measured by hand grip, and lowest ADL score. Accordingly, higher eGFR partly reflects subjects with lower muscle strength (i.e., a proxy of sarcopenia) and those with greater physical dependency, thus explaining the observed increased mortality. We did not find a similar reduction of cognitive function and overall health status score across eGFR groups, confirming that only physical dimension of frailty contributes to the observed U-shaped relationship between eGFR and mortality in oldest old subjects. Finally, frailty indexes worsened at reducing eGFR in subjects aged 64–89 years, demonstrating that the clustering of sarcopenic subjects in the high eGFR group is specific of oldest old group. Thus, our study definitely confirms that creatinine-based eGFR equations should be adjusted for muscle mass/strength and/or physical performance when used to estimate kidney function in oldest old people.
Impact of eGFR on predictive accuracy of frailty
The above considerations also help to explain why our study showed that adding eGFR to frailty status may not improve mortality prediction in oldest old people, where creatinine-based eGFR is more likely to be biased because of sarcopenia. We also showed that adding eGFR to the frailty model could significantly improve the predictive ability of frailty in older people, and such finding is in keeping with the recent demonstration that combining eGFR and geriatric assessment may improve predictive accuracy (Pilotto et al. 2012). It is widely known that inflammatory markers increase together with decreasing eGFR (Shlipak et al. 2004; Fried et al. 2006), and higher levels of inflammatory markers have been associated with frailty in older adults(Fulop et al. 2010). Indeed, it has been suggested that inflammation mediates the association between reduced kidney function, as measured by cystatin C or eGFR and functional limitation in older community dwelling adults (Fried et al. 2006). However, it is conceivable that eGFR may be able to capture an additional dimension of frailty not included in the proposed model and then improve the prediction of mortality. For example, selected nutrient deficits might underlie the relationship between kidney function and frailty. Indeed, vitamin D deficiency, which is highly prevalent among elders, may predispose to osteoporosis and related complications, such as vertebral fractures (Cesari et al. 2011). Interestingly, it has been recently demonstrated that vitamin D supplementation improved muscle strength, functional ability, and balance in patients with reduced eGFR and vitamin D deficit undergoing conservative or peritoneal dialysis treatment (Taskapan et al. 2011). Additionally, vitamin B12 and folic acid deficiency, whose prevalence increases with decreasing eGFR, could also contribute to bone loss (Golbahar et al. 2004), peripheral neuropathy, and subcortical encephalopathy (Krishnan and Kiernan 2009). Finally, anemia, which frequently occurs in relation to reducing eGFR (Babitt and Lin 2012), was also associated with lower knee extensor and handgrip strength in community-dwelling older subjects (Penninx et al. 2004).
Limitations deserve to be considered. First, our results were obtained using a creatinine-based measure of eGFR, and cystatin-C was not available in our dataset. The relationship between cystatin C-based eGFR and mortality may be very different from that observed with creatinine-based eGFR (Shastri et al. 2012). However, even cystatin-C should be used cautiously in older people. Indeed, fat-free mass, a parameter inversely related to age, affects cystatin C level, and in older patients with chronic kidney disease, GFR estimation improves when fat-free mass is taken into account (Macdonald et al. 2006). Second, since we used a population-specific approach to define frailty status in our study (Montesanto et al. 2010), the impact of eGFR on predictive ability of other frailty models (Fried et al. 2001) is worthy of testing. Finally, a measure of muscle mass was not available in our dataset. However, hand grip was found to be strongly related to muscle mass in similar populations of older subjects (Hairi et al. 2010). Thus, hand grip measure can be considered a good proxy of muscle mass. Even with the above limitations, our study adds to the present knowledge by investigating contemporarily frailty status and eGFR in a population of older people including nonagenarians. Additionally, the long duration of follow-up and the related mortality allowed an almost optimal exploration of the prognostic impact of study variables.
In conclusion, low eGFR, as estimated by BIS1 creatinine-based equation, is highly prevalent in older and oldest old individuals, and a significant relationship exists between eGFR and frailty status. Our study showed a U-shaped relationship between eGFR and mortality in the oldest old, but not in the older group. Oldest old subjects with higher and lower eGFR values also have lowest muscle strength and ADL score. It is worth noting that the BIS1 equation was originally developed in an older population and showed greater accuracy in the estimation of GFR compared to MDRD and CKD-EPI equations in the clinical setting (Schaeffner et al. 2012; Koppe et al. 2013). Nevertheless, our results suggest that even BIS1 equation likely need to be adjusted for muscle mass/strength and/or physical performance when used in oldest old subjects. While adjusting eGFR for frailty symptoms may be difficult to apply in clinical practice, searching for proxies of muscle or physical function to be included in eGFR equations could provide an easier way to resolve this important issue in the future. Additionally, the accuracy and cost effectiveness of filtration markers independent of muscle mass, such as cystatin C, β-trace protein, and β2-microglobulin are worth of testing in older populations (Foster et al. 2013). Finally, eGFR may improve the accuracy of frailty status in predicting prognosis in subjects aged 65–89 years. The possibility that low levels of eGFR may represent an additional dimension of frailty syndrome warrants further investigations.
Acknowledgments
This work was supported by INRCA funds and by the European Union’s Seventh Framework Programme (FP7/2007-2011) (grant number 259679). The funding organization had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci. 2012;67:1379–1386. doi: 10.1093/gerona/gls173. [DOI] [PubMed] [Google Scholar]
- Cesari M, Incalzi RA, Zamboni V, Pahor M. Vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int. 2011;11:133–142. doi: 10.1111/j.1447-0594.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsonello A, Pedone C, Corica F, Mussi C, Carbonin P, Antonelli Incalzi R. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med. 2005;165:790–795. doi: 10.1001/archinte.165.7.790. [DOI] [PubMed] [Google Scholar]
- Corsonello A, Pedone C, Lattanzio F, Onder G, Antonelli Incalzi R. Association between glomerular filtration rate and adverse drug reactions in elderly hospitalized patients: the role of the estimating equation. Drugs Aging. 2011;28:379–390. doi: 10.2165/11588280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cox DR (1972) Regression models and life tables. J Roy Stat Soc 34:187--220
- Cox HJ, Bhandari S, Rigby AS, Kilpatrick ES. Mortality at low and high estimated glomerular filtration rate values: a ‘U’ shaped curve. Nephron Clin Pract. 2008;110:c67–c72. doi: 10.1159/000151720. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- De Rango F, Dato S, Bellizzi D, Rose G, Marzi E, Cavallone L, Franceschi C, Skytthe A, Jeune B, Cournil A, Robine JM, Gampe J, Vaupel JW, Mari V, Feraco E, Passarino G, Novelletto A, De Benedictis G. (2008) A novel sampling design to explore gene-longevity associations: the ECHA study. Eur J Hum Genet 16:236--242 [DOI] [PubMed]
- Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster MC, Inker LA, Levey AS, Selvin E, Eckfeldt J, Juraschek SP, Coresh J. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis. 2013;62:42–51. doi: 10.1053/j.ajkd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, Harris T, Newman AB. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Golbahar J, Hamidi A, Aminzadeh MA, Omrani GR. Association of plasma folate, plasma total homocysteine, but not methylenetetrahydrofolate reductase C667T polymorphism, with bone mineral density in postmenopausal Iranian women: a cross-sectional study. Bone. 2004;35:760–765. doi: 10.1016/j.bone.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ, Sambrook PN. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- Hartmann EL, Kitzman D, Rocco M, Leng X, Klepin H, Gordon M, Rejeski J, Berry M, Kritchevsky S. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer C, Stengel B, Metzger M, Froissart M, Massy ZA, Tzourio C, Berr C, Dartigues JF. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77:2043–2051. doi: 10.1212/WNL.0b013e31823b4765. [DOI] [PubMed] [Google Scholar]
- Howard PA, Dunn MI. Severe heart failure in the elderly: potential benefits of high-dose and continuous infusion diuretics. Drugs Aging. 2002;19:249–256. doi: 10.2165/00002512-200219040-00001. [DOI] [PubMed] [Google Scholar]
- Howell DC (1997) Statistical methods for psychology. 4th edn. Belmont, CA; London: Duxbury Press
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. doi: 10.2307/2955359. [DOI] [PubMed] [Google Scholar]
- Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Koppe L, Klich A, Dubourg L, Ecochard R, Hadj-Aissa A. Performance of creatinine-based equations compared in older patients. J Nephrol. 2013;26:716–723. doi: 10.5301/jn.5000297. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol. 2009;5:542–551. doi: 10.1038/nrneurol.2009.138. [DOI] [PubMed] [Google Scholar]
- Lattanzio F, Corsonello A, Abbatecola AM, Volpato S, Pedone C, Pranno L, Laino I, Garasto S, Corica F, Passarino G, Antonelli Incalzi R. Relationship between renal function and physical performance in elderly hospitalized patients. Rejuvenation Res. 2012;15:545–552. doi: 10.1089/rej.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48:712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Montesanto A, Lagani V, Martino C, Dato S, De Rango F, Berardelli M, Corsonello A, Mazzei B, Mari V, Lattanzio F, Conforti D, Passarino G. A novel, population-specific approach to define frailty. Age (Dordr) 2010;32:385–395. doi: 10.1007/s11357-010-9136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- Odden MC, Shlipak MG, Tager IB. Serum creatinine and functional limitation in elderly persons. J Gerontol A Biol Sci Med Sci. 2009;64:370–376. doi: 10.1093/gerona/gln037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedone C, Corsonello A, Bandinelli S, Pizzarelli F, Ferrucci L, Incalzi RA. Relationship between renal function and functional decline: role of the estimating Equation. J Am Med Dir Assoc. 2012;13(84):e11–e84. doi: 10.1016/j.jamda.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, Guralnik JM, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Poulter R, Burch L, Narkiewicz K, Fagard R, Nitsch D, Wang N, Li M, Fletcher A, Bulpitt C. Kidney function in the very elderly with hypertension: data from the hypertension in the very elderly (HYVET) trial. Age Ageing. 2013;42:253–258. doi: 10.1093/ageing/afs109. [DOI] [PubMed] [Google Scholar]
- Pilotto A, Sancarlo D, Aucella F, Fontana A, Addante F, Copetti M, Panza F, Strippoli GF, Ferrucci L. Addition of the multidimensional prognostic index to the estimated glomerular filtration rate improves prediction of long-term all-cause mortality in older patients with chronic kidney disease. Rejuvenation Res. 2012;15:82–88. doi: 10.1089/rej.2011.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin DE, Winkelmayer WC. Medication issues in older individuals with CKD. Adv Chron Kidney Dis. 2010;17:320–328. doi: 10.1053/j.ackd.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, Kuhlmann MK, Schuchardt M, Tolle M, Ziebig R, van der Giet M, Martus P. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- Shastri S, Sarnak MJ. Chronic kidney disease: High eGFR and mortality: high true GFR or a marker of frailty? Nat Rev Nephrol. 2011;7:680–682. doi: 10.1038/nrneph.2011.153. [DOI] [PubMed] [Google Scholar]
- Shastri S, Katz R, Rifkin DE, Fried LF, Odden MC, Peralta CA, Chonchol M, Siscovick D, Shlipak MG, Newman AB, Sarnak MJ. Kidney function and mortality in octogenarians: Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2012;60:1201–1207. doi: 10.1111/j.1532-5415.2012.04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Singer MA, Hopman WM, MacKenzie TA. Physical functioning and mental health in patients with chronic medical conditions. Qual Life Res. 1999;8:687–691. doi: 10.1023/A:1008917016998. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Valensin S, Jeune B, Cevenini E, Balard F, Beekman M, Bezrukov V,Blanche H, Bolund L, Broczek K, Carru C, Christensen K, Christiansen L, Collerton JC, Cotichini R, de Craen AJ, Dato S, Davies K, De Benedictis G, Deiana L, Flachsbart F, Gampe J, Gilbault C, Gonos ES, Haimes E, Hervonen A, Hurme MA, Janiszewska D, Jylhä M, Kirkwood TB, Kristensen P, Laiho P, Leon A, Marchisio A, Masciulli R, Nebel A, Passarino G, Pelicci G, Peltonen L, Perola M, Poulain M, Rea IM, Remacle J, Robine JM, Schreiber S, Scurti M, Sevini F, Sikora E, Skouteri A, Slagboom PE, Spazzafumo L, Stazi MA, Toccaceli V, Toussaint O, Törnwall O, Vaupel JW, Voutetakis K, Franceschi C; GEHA consortium (2011) Design, recruitment, logistics, and data management of the GEHA (Genetics of Healthy Ageing) project. Exp Gerontol 46:934–945 [DOI] [PMC free article] [PubMed]
- Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol. 2011;76:110–116. doi: 10.5414/CN107160. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, Hemmelgarn BR. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80:1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- Urena-Torres P, Metzger M, Haymann JP, Karras A, Boffa JJ, Flamant M, Vrtovsnik F, Gauci C, Froissart M, Houillier P, Stengel B. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. 2011;58:544–553. doi: 10.1053/j.ajkd.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236--244