Abstract
This study compared the effects of low vs. high intensity training on tendon properties in an elderly population. Participants were pair-matched (gender, habitual physical activity, anthropometrics, and baseline knee extension strength) and then randomly assigned to low (LowR, i.e., ~40 % 1RM) or high (High R, i.e., ~80 % 1RM) intensity resistance training programmes for 12 weeks, 3× per week (LowR, n = 9, age 74 ± 5 years; HighR, n = 8, age 68 ± 6 years). Patellar tendon properties (stiffness [K], Young’s modulus [YM], cross-sectional area [TCSA], and tendon length [TL]) were measured pre and post training using a combination of magnetic resonance imaging (MRI), B-mode ultrasonography, dynamometry, electromyography and ramped isometric knee extensions. With training K showed no significant change in the LowR group while it incremented by 57.7 % in the HighR group (p < 0.05). The 51.1 % group difference was significant (p < 0.05). These differences were still apparent when the data was normalized for TCSA and TL, i.e., significant increase in YM post-intervention in HighR (p < 0.05), but no change in LowR. These findings suggest that when prescribing exercise for a mixed genders elderly population, exercise intensities of ≤40 % 1RM may not be sufficient to affect tendon properties.
Keywords: Elderly, Tendon properties, Resistance training, Load intensity
Introduction
Over the last 20 years, the number of people in the UK aged above 65 years has increased by 16 %, and this age bracket currently accounts for 17 % of the total population. These figures are set to rise with the percentage of the UK population aged over 65 years estimated to reach 21 % in 2026 (UK Office for National Statistics 2011). Aging is associated with a significant decline in neuromuscular function and performance, which can lead to the loss of functional mobility and independence, resulting in reliance on long-term care services (Comas-Herrera et al. 2006; Doherty 2003). The increase in the elderly population size is set to place a growing burden on these care services. Whilst life expectancy has increased, the number of years lived in good health has not risen at the same rate (Hebert 2004).
One of the major problems contributing to impaired mobility in aging adults is the increasing occurrence of serious falls (de Rekeneire et al. 2003). Approximately 28–35 % of people aged 65 years and older, fall each year, leading to injury and subsequent decreases in quality of life, and often death (World Health Organization 2007). The majority of falls occur due to a sudden loss of balance and/or inherent ageing-related decline in balance ability (Blake et al. 1988). The ability to maintain balance or stability has previously been associated with the structural and mechanical properties of tendons in the lower limbs, with stiffer tendon structures associated with increased balance ability (Onambele et al. 2006). A proposed explanation for this finding is related to the force transmission which is the primary function of tendons. Specifically, stiffer tendon structures enable more rapid, and ultimately higher, force transfers than compliant systems and thus, increase the efficiency of the muscle tendon complex in correcting the ‘catch and throw’ actions involved in maintaining postural balance (Loram and Lakie 2002). Indeed, stiffer tendon structures are shown to improve postural balance performance (Onambele et al. 2007a). Tendon properties also have the capacity to affect muscle force output and function, especially in the early stages of muscle contraction, with increased tendon compliance associated with detrimental muscle contractile characteristics (Morse et al. 2005; Onambele et al. 2006).
The previously reported decline in tendon stiffness with aging is a reversible process. Indeed, mechanical loading has been shown to influence tendon properties. It has been demonstrated previously that high intensity exercise training increases the stiffness of tendons (Burgess et al. 2007; Kubo et al. 2001; Reeves et al. 2003a). In an elderly population, 14 weeks of high load resistance training (at 80 % 5RM) has been shown to increase the stiffness of the patella tendon by 65 % (Reeves et al. 2003a). Similar findings have been reported by others including descriptions on how training modality and/or gender impact on the tendon responses (Onambele et al. 2008; Onambele-Pearson and Pearson 2012). In contrast, it has been observed that lower intensity exercise in the form of a 6-month progressive walking programme does not influence tendon properties in older adults (Kubo et al. 2008). In younger habitual distance runners (age <65 years), tendon stiffness is similar to that in non-running controls (Karamanidis and Arampatzis 2005; Rosager et al. 2002; Westh et al. 2008). Similarly, 6 months of training using body weight squats (50 repetitions (reps) daily) has been reported to have no significant effect on the stiffness of the vastus lateralis tendon (Kubo et al. 2003), thereby further indicating the possibility of loading threshold for tendon stiffness increments.
The majority of exercises prescribed for an elderly population is of a lower intensity than that which has produced beneficial adaptations in tendon properties. These recommendations are due to age-related co-morbidities such as osteoarthritis, in order to prevent older adults lifting heavy weights (Breen and Phillips 2011). However, it is possible that lower intensity exercise does not produce the required stimulus for tendon adaptation. Given the aging population demographics, and the barriers that exist in this population in terms of exercise participation, determining the level of exercise intensity required to induce tendon adaptations is of paramount importance in this population. Therefore the purpose of this study was to: (1) determine whether a low intensity exercise training programme (resistance ~40 % 1RM) affects tendon mechanical properties in an elderly population, and (2) compare these effects to those of a higher intensity exercise training protocol (resistance ~80 % 1RM).
Methods
Participants
Twenty elderly adults participated in the present study. All participants gave their written informed consent to take part in the study. The participants were healthy, non-obese (BMI < 28), did not take any prescription medication, had no known joint, muscle or tendon pathology, and no known history of cardiovascular, inflammatory, or myopathic disease, and were community-dwelling, habitually active individuals (as determined using an abbreviated version of the Allied Dunbar Activity Survey questionnaire, which helps to quantify habitual activity in min/week), with no recent history of structured resistance training (i.e., had partaken in ≤1.0 h/week of resistance training in the 12 months prior to participating in this study). The local Human Ethics Committee approved all experimental procedures.
Of the 20 participants who started the study, 17 completed the 12-week exercise intervention (see Table 1). Participants were pair-matched in terms of: gender, habitual physical activity level, anthropometrics, and baseline strength. Subjects were assigned to either a low (LowR) or a high (HighR) resistance training group. In the LowR group, nine individuals completed the intervention (five females, four males) while eight individuals completed the intervention (five females, three males) in the HighR group.
Table 1.
Anthropometric data and physical activity of the investigated population in LowR and HighR groups
| LowR group n = 9 (5 ♀) | HighR group n = 8 (5 ♀) | |
|---|---|---|
| Age (years) | 74 ± 5 | 68 ± 6 |
| Height (m) | 1.64 ± 0.08 | 1.64 ± 0.07 |
| Body mass (kg) | 71.3 ± 10.9 | 73.5 ± 12.1 |
| Habitual physical activity (min/week) | 339.8 ± 42.7 | 300.0 ± 56.7 |
Data are mean ± SD
Assessment of Tendon Mechanical Properties
Prior to (no more than 14 days; range 4–14 days) and following (no more than 7 days; range 4–7 days) the 12-week exercise intervention, the patellar tendon mechanical properties were determined using the following procedures.
Measurement of patellar tendon forces
Torque output during isometric knee extension was determined using a dynamometer (Cybex NORM, New York, USA) with data sampled at 2,000 Hz. The knee was fixed at 90° flexion (full extension = 0°), and hip at 85° (supine = 0°). The centre of rotation of the dynamometer lever arm was aligned with the knee joint centre, and straps were fixed across the chest, hip, and thigh of the test limb to prevent any extraneous movement. A lever attachment cuff was placed on the lower leg at 3 cm above the medial malleolus. Three maximal isometric knee extension efforts were carried out to ensure tendon pre-conditioning prior to the test. Participants were instructed to perform ramped isometric knee extensions to maximum over a 4 s time period. Two trials of the test were performed with 3-min rest between the contractions. Tendon force was calculated as Ftend = (P + Pantag)/Tarm, where Ftend is the force in the patellar tendon, P is the observed knee extensor torque output, Pantag is the antagonistic (hamstring) co-contraction torque, and Tarm is the patellar tendon moment arm. In order to measure Tarm participants laid on their side with the knee joint of their dominant leg held straight in a 0.2-Tesla magnetic resonance imaging (MRI) scanner (E-scan, Esaote Biomedica, Genoa, Italy). MRI scans were taken in the sagittal plane using a spin–echo TI (time to inversion or delay time) half Fourier (HF) sequence with a slice thickness of 8 mm, inter-slice gap of 0.6 mm, and the parameters time to repetition/echo time/number of excitations (TR/TE/NEX), 420/18/1; field of view, 160 × 160 mm; matrix, 256 × 256 pixels.
Estimation of hamstring co-contraction during knee extension — using electromyographic activity
The electromyographic activity (EMG) of the long head of the biceps femoris muscle (BF) was measured in order to ascertain the level of antagonistic muscle co-contraction during the isometric knee extension performances (Pearson and Onambele 2006) in order to accurately quantify quadriceps muscle forces. Assumptions were that BF was representative of its constituent muscle group (Carolan and Cafarelli 1992), and the BF EMG relationship with knee flexors torque was linear (Lippold 1952). Two self-adhesive Ag–AgCl electrodes 10 mm in diameter (Medicotest, Rugmarken, Denmark), were placed in a bipolar configuration with a constant inter-electrode distance of 20 mm, at a site corresponding to the distal one-third of the length, in the mid-line of the belly of the BF. Prior to electrode attachment the skin was prepared by shaving, abrading, and cleaning with an alcohol-based solution in order to minimise its resistance. The reference electrode was placed on the lateral tibial condyle of the test limb. The raw EMG signal was sampled at 2,000 Hz, preamplified, and filtered using high- and low-pass filters set at 10 and 500 Hz, respectively (Biopac Systems Inc., California, USA). All EMG and torque signals were displayed in real time in Acknowledge software (Biopac systems, Inc.) via a computer (Macintosh G4). Two maximal isometric knee flexion contractions were carried out to obtain the EMG at maximal flexion torque. The root mean square (RMS) EMG activity corresponding to the peak torque period was analysed over 50-ms epochs and averaged for a 1-s period during the plateau of peak torque. This has previously been suggested to be acceptable in terms of signal to noise ratio (Hermens et al. 2000). EMG activity of the BF during knee extension was divided by the maximal BF flexor EMG, and the maximal flexor torque was then multiplied by this value to determine co-contraction torque (Onambele et al. 2007b).
Measurement of patella tendon elongation
Elongation of the patella tendon was assessed during the graded isometric knee extensions using a 7.5-MHz, 40-mm linear array, B-mode ultrasound probe (AU5; Esaote Biomedica, Italy) with a depth resolution of 49.3 mm. The probe was positioned in the sagittal plane over the patella tendon at the apex of the patella. Three efforts graded to maximum were recorded. An echo-absorptive marker was placed between the probe and the skin to act as a fixed reference from which measures of elongation could be made.
Ultrasound images were recorded in real time onto mini DV via S-video output and captured onto PC at 25 Hz using Quintic Biomechanics (9.03 v 11). The ultrasound output was synchronized (using an electronic square-wave signal generator) with the force and EMG records to allow temporal alignment. Tendon displacements were determined at intervals of 10 % of the maximal force (from 0 % to 100 %) using image J (National Institute of Health, Bethesda, MD, USA).
Calculation of tendon properties
The tendon force–elongation relationships were fitted with second-order polynomial functions forced through zero. Tendon stiffness (K) measures (in N mm−1) were calculated from the slopes of the tangents at 10 % force intervals. In addition, K was also computed from the gradient of a linear fit between 60 % and 100 % MVC to allow comparison with previous studies that have used this method (Pearson and Onambele 2012). Patella tendon resting length (TL) and cross-sectional area (TCSA) were also assessed with the knee joint at 90°. TL was determined from sagittal-plane ultrasound images and measured form the inferior pole of the patellar to the superior aspect of the tibial tuberosity. TCSA was measured as the average from transverse-plane ultrasound images taken at 25 %, 50 %, and 75 % TL. Young’s modulus (YM) was calculated as the product of stiffness and the ratio between TL and TCSA. Tendon strain (%) was calculated as the ratio of tendon elongation to the TL. Tendon stress was calculated by dividing force in the tendon by TCSA.
Exercise intervention
Determination of one repetition maximum (1RM)
During a familiarisation session 2–7 days prior to the 12-week intervention, the 1RM of the participants were determined for all exercises included in the training programme: the seated leg press (to load the quadriceps, hamstrings, gluteus maximus and minimus muscle groups), leg extension (to load the quadriceps muscle group), calf rotator (to load the triceps surae muscle group), and gluteal conditioner (to load both the gluteal and hamstring muscles) machines (Technogym, Gambettola, Italy). The participants first performed a standardised warm-up on the leg press (6 × 50 % perceived 1RM; 4 × 70 % perceived 1RM with 3-min recovery). The perceived sub maximal efforts (as determined through both individual heart/pulse rate monitors and a 10-point Borg RPE scale) were used to ensure fatigue was not induced in the participants and to reduce the risk of injury by asking them to perform a maximal contraction on a ‘cold’ muscle. After warming up, the load was set at 90 % of the initially estimated 1RM and increased after each successful lift by 5 kg until failure. Each participant was given six lifting attempts in order to achieve their 1RM, and a maximum of two attempts to lift the weight, once it had been established. The greatest amount of weight lifted successfully was recorded to determine the training load. Between successive attempts, 3-min rest periods were allowed. A repetition was valid if the participant used correct form and was able to complete the entire lift in a controlled manner without assistance up to full extension or flexion depending on the exercise. The 1RM of the participants in each of the four exercises performed in the training programme were reviewed every second week during training. If 1RM had increased, the training load was adjusted accordingly. Additionally, if any participants felt that in-between 1RM assessments the training load was not providing adequate resistance, the load was increased so they were always lifting at the desired percentage of their maximum.
Training programme
The training programme was 12 weeks in duration and consisted of one supervised gym-based class, and two home-based sessions per week in LowR. In HighR, the programme was two supervised gym based classes, and one home-based session per week. All exercise sessions were 1 h in duration. Briefly, the supervised exercise classes consisted of a 10- to 15-min warm-up (stretching, aerobic, and coordination work), and resistance exercises using cable weight machines for the lower limb muscles of interest (detailed above). Progression was from 8–11 reps in 2–4 sets at 40 % (LowR) or 80 % (HighR) 1RM). A cool-down (i.e., static stretches, Pilates, Tai Chi) was also incorporated at the end of each training session (~5 min).
The unsupervised home-based exercises were similar in design to the supervised classes, with the exception that all the resistance work was carried out using resistance bands (Thera-band, The Hygenic Corporation, Akron, OH, USA), and a 20-min brisk walk was also included. The home-based training lasted from ~1 h to 1 h and 20 min. A custom-made exercise booklet illustrated, using photographic and/or cartoons, all the home exercises in detail, with a demonstration of each exercise carried out at the onset of the study. The exercise band protocol used in the home-based programmes loaded the same muscle groups as those used for the gym equipment, though at much lower intensities (repetitions and numbers of sets were matched for the gym and home-based sessions). Home-based exercise was not to be performed the day preceding or following the supervised class exercise.
Statistical analyses
SigmaPlot 11.0 (Systat Software, San Jose, CA, USA) was used to run statistical analyses. The data obeyed the assumptions of parametricity (as determined using the Shapiro–Wilk and Levene’s tests). Hence, independent samples Student’s t-tests were used to test for differences between the LowR and HighR groups at baseline. For all reported variables, differences in time (pre- and post-intervention, the within factor) and group (LowR and HighR, the between factor) were tested using a factorial mixed-design analysis of variance (2 × 2 ANOVA), with post hoc Tukey tests applied where a significant main effect was identified. Data are presented as mean ± SEM (unless otherwise stated). Statistical significance was set at p ≤ 0.05.
Results
The mean anthropometric and habitual physical activity data for the two groups are reported in Table 1. The two intervention groups did not differ significantly in age, habitual physical activity levels, height, mass, 1RM measures, or any of the measured patella tendon properties (Table 2) at the onset of the study (p > 0.05).
Table 2.
Patella tendon mechanical and structural properties for LowR and HighR, pre- and post-intervention
| LowR group | HighR group | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| K 60–100% MVC (N/mm) | 851 ± 110 | 891 ± 120 | 828 ± 83 | 1295 ± 177 *† |
| YM60–100% MVC (GPa) | 0.48 ± 0.04 | 0.48 ± 0.04 | 0.56 ± 0.07 | 0.84 ± 0.09 *† |
| Kmean10–100%MVC (N/mm) | 700 ± 95 | 780 ± 120 | 680 ± 63 | 1068 ± 137*† |
| T CSA (mm2) | 78.5 ± 3.5 | 80.5 ± 4.0 | 71.8 ± 5.3 | 72.1 ± 5.8 |
| T L (mm) | 45.9 ± 1.7 | 45.0 ± 1.5 | 47.3 ± 1.3 | 47.0 ± 1.3 |
Data are mean ± SEM
K 60–100% MVC is Patella tendon stiffness calculated as the gradient of a linear fit between 60 % and 100%MVC, YM60–100% MVC is Young’s modulus calculated as the gradient of a linear fit between 60 % and 100 % MVC, K mean10–100%MVC (N/mm) is the mean patella tendon stiffness calculated as the gradient of a linear fit between 10 % and 100%MVC, T CSA is tendon cross-sectional area, and T L is tendon resting length
*Significant difference between pre and post values
†Significant differences between LowR and HighR groups regarding post-intervention data
At baseline, the maximal patella tendon force was also not significantly different between the two groups (p > 0.05). With training, maximal tendon force was significantly increased in HighR group by 13.6 ± 6.9 % (2,428.8 ± 293.7 and 2,725.6 ± 330.4 N, respectively, before and after intervention) (p < 0.05), while the increase was 17.8 ± 5.5 % in the LowR group (2,346.7 ± 265.8 and 2,751.6 ± 330.4 N, respectively, before and after intervention) (p < 0.05).
As shown in Table 2, with training, anatomical characteristics of the Patella tendon (TL and TCSA) were neither significantly altered in the LowR group nor in the HighR group (p > 0.05).
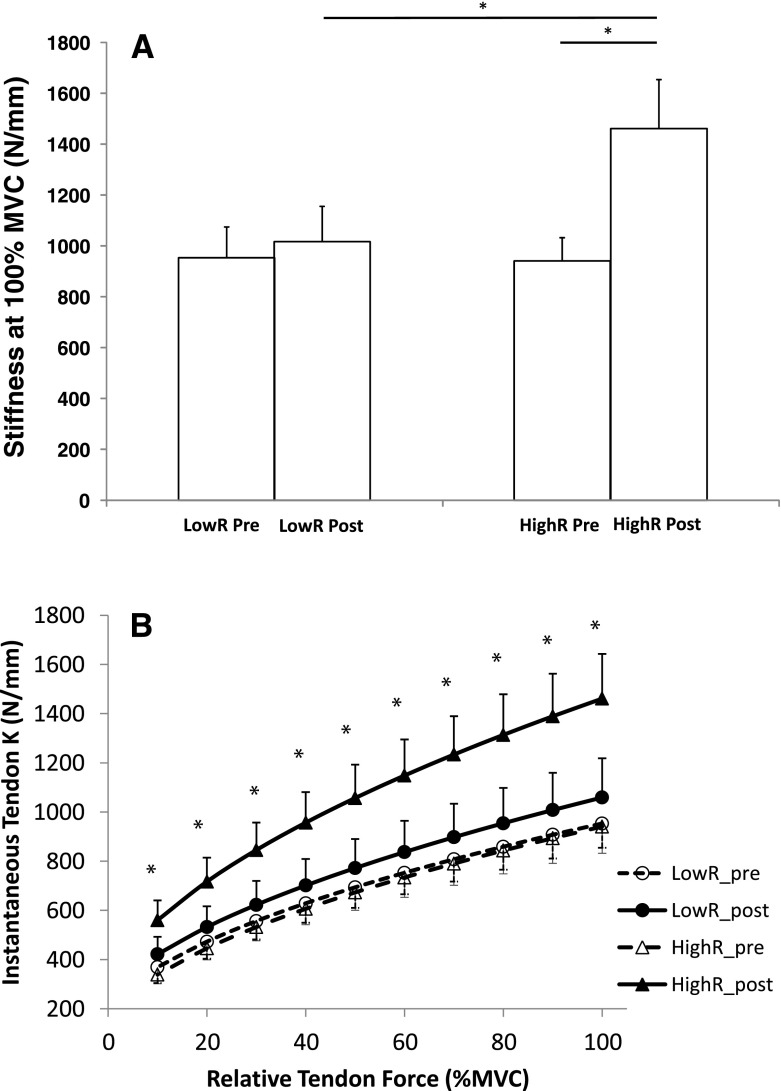
Following the completion of the training programme a two-way ANOVA for tendon stiffness (K) at 100 % MVC revealed a significant 57.7 ± 15.7 % increase in the HighR group (940.7 ± 91.4 N mm−1 pre intervention vs. 1461.0 ± 192.7 N mm−1 post intervention, p < 0.05). However, no significant change was observed in the LowR group (953.3 ± 121.0 N mm−1 pre-intervention vs. 1,016.4 ± 139.4 N mm−1, p > 0.05) (Fig. 1a). Where all the force levels at 10 % intervals of MVC were taken into account to compute an average K value over the entire force–elongation relationship, significantly higher mean K values were reported post-intervention in the HighR group (p < 0.05), but not in the LowR group (p > 0.05) (Fig. 1b).
Fig. 1.
Patella tendon stiffness pre and post training intervention for the LowR and HighR groups. a Maximal stiffness. b Stiffness every 10 % MVC. *Significant pre- to post-training differences as well as significant differences between groups at the post training phase. Data are mean ± SEM
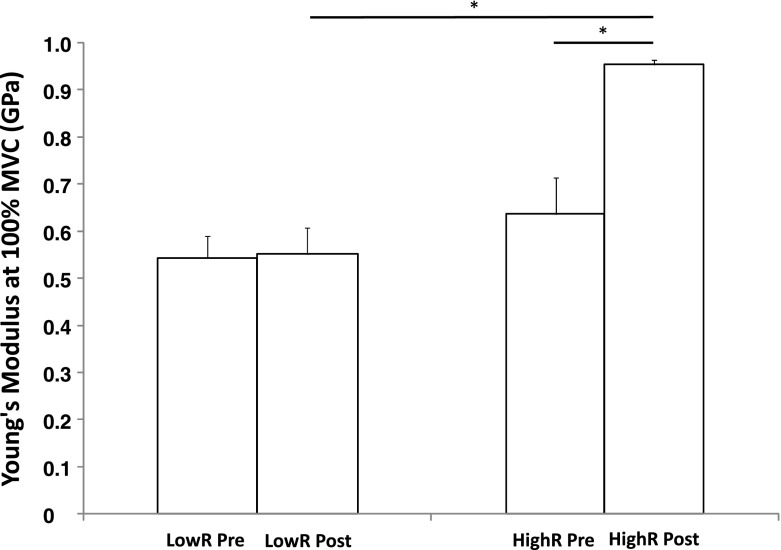
When the maximal tendon stiffness data was normalised for TL and TCSA by converting to YM, a two-way ANOVA revealed a significant 57.9 ± 17.8 % increase in YM at 100 % MVC in the HighR group after training as compared with pre-intervention (p < 0.01), while no change was found in the LowR group (2.0 ± 8.6 %; p > 0.05) (Fig. 2). The analysis of variance also highlight a significantly higher YM at every 10 % relative force (10–100 % MVC) post-intervention in the HighR than in the LowR group (p < 0.01).
Fig. 2.
Young’s modulus of the patella tendon pre and post training intervention for the LowR and HighR groups. *Significant pre- to post-training differences as well as significant differences between groups at the post training phase. Data are mean ± SEM
When tendon stiffness and YM were calculated over the 60–100 % MVC linear fit, similar findings were observed. The two-way ANOVA shows that following the intervention period, K60–100 significantly increased by 59.0 ± 16.3 % (p < 0.05), and YM significantly increased by 58.9 ± 18.1 % (p < 0.05) in the HighR group, whereas the LowR group showed non-significant changes in both K and YM60-100 (5.5 ± 9.7 % and 0.7 ± 8.8 %, respectively) (p > 0.05) (Table 2).
Discussion
The purpose of this study was to: (1) determine whether a low intensity exercise training programme (resistance ~40 % 1RM; LowR) would affect tendon mechanical properties in an elderly population, and 2) compare these effects to those of a higher intensity exercise training protocol (resistance ~80 % 1RM; HighR). The present results show that 12 weeks of LowR had no significant effect on the mechanical properties of the patella tendon in the elderly (p > 0.05). As expected, however, HighR did produce significant increases in tendon stiffness and YM. Consequently, HighR produced significantly greater changes in patella tendon stiffness and YM than LowR.
The results reported in the present study are in line with the previous studies in terms of the magnitude of increase in tendon stiffness observed following ‘high’ intensity resistance training protocols. Specifically, the 57.7 % increase in patella tendon stiffness at 100 % MVC, and 59.0 % at 60–100 % MVC are congruent with previous data from Reeves et al., (Reeves et al. 2003a, b) and Onambélé et al.(2008). These previous studies demonstrated an increase in patella tendon stiffness in the range of 54–65 % when measured at 60–100 % MVC following prolonged resistance training programmes (≥12 weeks) at 80 % 1RM. The congruency between findings was echoed in the reported changes in YM, 57.9 % reported here at 100 % MVC vs. 69–70 % reported previously (Onambele-Pearson and Pearson 2012; Reeves et al. 2003a).
The novelty of the present study’s findings lies in the observation that no significant difference in patella tendon stiffness and YM were found when the intensity of the resistance training was low (~40 % 1RM). Although not previously observed, in elderly humans this finding is in line with the theory that there is a threshold value for the exercise-induced increase in mechanical properties of tendon tissue (Lavagnino and Arnoczky 2005). This is related to the changes in collagen synthesis which are observed following mechanical loading. Thus, the current data has important implications for older persons seeking to offset aging-related mal-adaptations, given that LowR does not appear to promote similar benefits as HighR, insofar as tendon mechanical properties are concerned.
When tendon is subjected to repeated mechanical loading, a number of signals at the extracellular matrix may be induced (Arampatzis et al. 2007). In order to regulate specific alterations in the extracellular matrix’s composition, tenocytes sense force-induced deformations in their extracellular matrix (Chiquet 1999; Chiquet et al. 2003), triggering specific anabolic and catabolic pathways in response to loading (Arampatzis et al. 2007). It is thought that there is a ‘set point’ beyond which the induction of these signals/pathways occurs (which can be regulated by the cells), and when loading is below this, the biological response is not initiated (Lavagnino and Arnoczky 2005). Interestingly, it is thought that once the stimulus is beyond this current threshold, the upregulation in collagen synthesis appears to be minimally affected by the magnitude of exercise (Kjaer et al. 2009). Given the current findings, it could be speculated that LowR was insufficient to meet (or indeed surpass) the threshold required to initiate the signaling response required for altering collagen synthesis.
Findings from previous studies (Kongsgaard et al. 2007; Kubo et al. 2008) support the idea that the mechanical loading threshold for tendon adaptation was not met with LowR. Kubo et al. (2008) demonstrated that the tendon/apponeurosis properties of the vastus lateralis and medial gastrocnemius muscles in an elderly population were unchanged following a 6-month progressive walking program. Kongsgaard et al. (2007) confirmed that in a young male population, high intensity, single leg resistance training (70 % 1RM) increased patella tendon stiffness in the high intensity resistance trained limb. However, in addition these authors also found that performing low load resistance training with the contralateral leg, produced no significant changes in the mechanical properties of the patella tendon in that limb. Not only does this fit with the ‘threshold’ concept, it also highlights the need for the mechanical loading to be site specific. In the current study the training performed would have induced loading in the patella tendon leading to changes in its mechanical properties in the HighR group.
These findings have implications when prescribing exercise interventions in an elderly population. Previous studies and guidelines have advocated the use of progressive resistance in an elderly population at moderate-to-high intensity levels (Nelson et al. 2007; Weening-Dijksterhuis et al. 2011), with exercises performed using 50–80 % 1RM producing more credible results than those using lighter weights or elastic bands (Hautier and Bonnefoy 2007). These recommendations would also be appropriate to gain beneficial changes in the mechanical properties of tendon. However, although this ‘moderate-to-high’ intensity resistance training is advocated in the literature, very few elderly individuals are engaged in this level of exercise. Data from the USA’s Centers for Disease Control and Prevention indicate that few older persons engage in regular physical activity, with only 31 % of individuals aged 65–74 years reporting participating in 20 min of moderate physical activity 3 or more days per week. Even with those who were more physically active, few would meet the intensity requirements for tendon adaptation. Anecdotally, when looking at the provision of exercise classes and exercise regimes for the elderly, the majority of these involve little resistance exercise or low-load resistance/theraband exercises (similar to the LowR group in this study). In order to get the maximum benefits there needs to be alignment between the recommendations and what occurs in practice. However, there are many barriers and difficulties in obtaining engagement in exercise in an older population, and these might be partially responsible for the disparity between recommendations and practice (for reviews, see Bunn et al. 2008 and Schutzer and Graves 2004).
Further to this point, recent evidence in young adults suggests that low-load resistance exercise (30 % 1RM) performed to volitional failure, promoted an equivalent rise in acute rates of myofibrillar protein synthesis to that seen with traditional high-load resistance exercise loads lifted to failure (Burd et al. 2010). Furthermore, when practiced as part of a regular exercise programme, low-load-to-failure lifting, promoted equivalent muscle hypertrophy to high-load lifting (Mitchell et al. 2012). It has been suggested that this form of low-load, high-volume resistance exercise is an attractive alternative to traditional high-load lifting for older adults, insofar as muscle hypertrophy is concerned, due to the reduction in force loading about compromised older joints (Breen and Phillips 2011). Based on the current data, it cannot be said whether a greater number of repetitions in the LowR group to promote near-fatigue, would have modulated tendon properties similar to HighR. However, this seems unlikely given that tendon collagen adaptations are induced through mechanical force which is defined by the load lifted, whereas muscle adaptation is brought about through the orderly and maximal recruitment of muscle fibres, a process that can be achieved through a wider range of loading stimuli. To date, the impact of low-load resistance exercise to failure on acute and chronic muscle/tendon adaptive responses in the elderly has not been investigated, but clearly warrants further study given the practical relevance of lifting lighter loads for geriatric populations.
Future work should also determine whether this effect may be gender specific since previous work (Onambele-Pearson and Pearson 2012) hints to older females responding to resistance training at loads <40 % 1RM, and male counterparts at loads >40 %. Similarly, the mixed gender in the current study may have masked such an effect. Indeed the small population size in the current study did not allow for a gender effect to be investigated. Moreover, future work could address the interesting finding of similar degree of maximal force at the tendon increases in the presence of both high and low intensity resistance exercise. It may be that differences in habitual physical activity in the two training populations (which was not controlled in our study), or a negative impact of high intensity exercise induced cytokines elevation, in an already inflamed endocrine milieu (as seen in normal ageing), may have modulated the response we have observed in this study.
Conclusion
Low intensity resistance exercise does not result in beneficial tendon adaptations in an elderly population. Increases in tendon stiffness and YM are possible in an elderly population with high intensity resistance training, and this increased stiffness has been associated with increased balance ability, and hence lower fall risk. In order to gain these beneficial adaptations in this population high intensity resistance training would therefore need to be recommended. Further work needs to be conducted to assist with the implementation of this recommendation in an elderly population.
References
- Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol. 2007;210(Pt 15):2743–2753. doi: 10.1242/jeb.003814. [DOI] [PubMed] [Google Scholar]
- Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: prevalence and associated factors. Age Ageing. 1988;17(6):365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab (Lond) 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn F, Dickinson A, Barnett-Page E, McInnes E, Horton K. A systematic review of older people’s perceptions of facilitators and barriers to participation in falls-prevention interventions. Ageing Soc. 2008;28:449–472. doi: 10.1017/S0144686X07006861. [DOI] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5(8):e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess KE, Connick MJ, Graham-Smith P, Pearson SJ. Plyometric vs. isometric training influences on tendon properties and muscle output. J Strength Cond Res. 2007;21(3):986–989. doi: 10.1519/R-20235.1. [DOI] [PubMed] [Google Scholar]
- Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. J Appl Physiol. 1992;73(3):911–917. doi: 10.1152/jappl.1992.73.3.911. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18(5):417–426. doi: 10.1016/S0945-053X(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22(1):73–80. doi: 10.1016/S0945-053X(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Comas-Herrera A, Wittenberg R, Costa-Font J, Gori C, Dimaio A, Patxot C, Pickard L, Pozzi A, Rothgang H. Future long-term care expenditure in Germany, Spain, Italy and the United Kingdom. Ageing Soc. 2006;26:285–302. doi: 10.1017/S0144686X05004289. [DOI] [Google Scholar]
- de Rekeneire N, Visser M, Peila R, Nevitt MC, Cauley JA, Tylavsky FA, Simonsick EM, Harris TB. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(6):841–846. doi: 10.1046/j.1365-2389.2003.51267.x. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Hautier C, Bonnefoy M. Training for older adults. Ann Readapt Med Phys. 2007;50(6):475–479. doi: 10.1016/j.annrmp.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Hebert K. Life expectancy in Great Britain rises—but later years are still spent in poor health. BMJ. 2004;329(7460):250. doi: 10.1136/bmj.329.7460.250-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol. 2005;208(Pt 20):3907–3923. doi: 10.1242/jeb.01830. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 2007;191(2):111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol. 2001;91(1):26–32. doi: 10.1152/jappl.2001.91.1.26. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Miyatani M, Tachi M, Fukunaga T. Effect of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand. 2003;178(1):25–32. doi: 10.1046/j.1365-201X.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ishida Y, Suzuki S, Komuro T, Shirasawa H, Ishiguro N, Shukutani Y, Tsunoda N, Kanehisa H, Fukunaga T. Effects of 6 months of walking training on lower limb muscle and tendon in elderly. Scand J Med Sci Sports. 2008;18(1):31–39. doi: 10.1111/j.1600-0838.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005;23(5):1211–1218. doi: 10.1016/j.orthres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lippold OC. The relation between integrated action potentials in a human muscle and its isometric tension. J Physiol. 1952;117(4):492–499. doi: 10.1113/jphysiol.1952.sp004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiol. 2002;540(Pt 3):1111–1124. doi: 10.1113/jphysiol.2001.013077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113(1):71–77. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Birch KM, Narici MV. Tendon elongation influences the amplitude of interpolated doublets in the assessment of activation in elderly men. J Appl Physiol. 2005;98(1):221–226. doi: 10.1152/japplphysiol.00774.2004. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100(6):2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Rejc E, Maganaris CN. Contribution of calf muscle-tendon properties to single-leg stance ability in the absence of visual feedback in relation to ageing. Gait Posture. 2007;26(3):343–348. doi: 10.1016/j.gaitpost.2006.09.081. [DOI] [PubMed] [Google Scholar]
- Onambele GN, Burgess K, Pearson SJ. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J Orthop Res. 2007;25(12):1635–1642. doi: 10.1002/jor.20404. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Maganaris CN, Mian OS, Tam E, Rejc E, McEwan IM, Narici MV. Neuromuscular and balance responses to flywheel inertial versus weight training in older persons. J Biomech. 2008;41(15):3133–3138. doi: 10.1016/j.jbiomech.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Onambele-Pearson GL, Pearson SJ. The magnitude and character of resistance-training-induced increase in tendon stiffness at old age is gender specific. Age (Dordr) 2012;34(2):427–438. doi: 10.1007/s11357-011-9248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson SJ, Onambele GN. Influence of time of day on tendon compliance and estimations of voluntary activation levels. Muscle Nerve. 2006;33(6):792–800. doi: 10.1002/mus.20529. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Onambele GL. Computation methods affect the reported values of in vivo human tendon stiffness. J Mech Behav Biomed Mater. 2012;5(1):291–297. doi: 10.1016/j.jmbbm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548(Pt 3):971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003;28(1):74–81. doi: 10.1002/mus.10392. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12(2):90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- UK Office for National Statistics (2011) 2011 census. Retrieved from http://www.ons.gov.uk/ons/guide-method/census/2011/census-data/index.html
- Weening-Dijksterhuis E, de Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: a comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90(2):156–168. doi: 10.1097/PHM.0b013e3181f703ef. [DOI] [PubMed] [Google Scholar]
- Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports. 2008;18(1):23–30. doi: 10.1111/j.1600-0838.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2007) Global report on falls in older age. Retrieved from http://www.who.int/ageing/publications/Falls_prevention7March.pdf