Abstract
The contribution of DNA damage to the pathogenesis of age-related macular degeneration (AMD) has been reported. Recently, a genomewide association study detected the association of a single-nucleotide polymorphism (SNP) in RAD51B (rs8017304 A>G) with AMD. RAD51B is involved in recombinational repair of DNA double-strand breaks. We analyzed RAD51B influence on AMD using two cohorts from Caucasian and Han Chinese populations. The Caucasian set replicated the rs8017304 A>G association and revealed two novel AMD-associated SNPs in RAD51B, rs17105278 T>C and rs4902566 C>T. Under the dominant model, these two SNPs exhibit highly significant disease risk. SNP–SNP interaction analysis on rs17105278 T>C and rs4902566 C>T homozygous demonstrated a synergistic effect on AMD risk, reaching an odds ratio multifold higher than well-established AMD susceptibility loci in genes such as CFH, HTRA1, and ARMS2. Functional study revealed lower RAD51B mRNA expression in cultured primary human fetal retinal pigment epithelium (hfRPE) carrying rs17105278 T>C variants than in hfRPE carrying rs17105278 wild type. We concluded that the risk of developing AMD exhibits dose dependency as well as an epistatic combined effect in rs17105278 T>C and rs4902566 C>T carriers and that the elevated risk for rs17105278 T>C carriers may be due to decreased transcription of RAD51B. This study further confirms the role of DNA damage/DNA repair in AMD pathogenesis.
Keywords: Age-related macular degeneration, RAD51B, DNA repair, Single-nucleotide polymorphism, Functional genomicsl, Gene expression
Introduction
Age-related macular degeneration (AMD) is currently the leading cause of irreversible central vision loss in the elderly (Chou et al. 2013), and its incidence is only expected to increase with the aging population (Clemons et al. 2005a). AMD is a complex and multifactorial disease that involves significant interplay between genetic and environmental factors. Smoking, diet, and age have all been linked to AMD (Coleman et al. 2008). Twin studies and family studies have revealed an important genetic component to AMD. Multiple AMD susceptibility loci, such as CFH, ARMS2/HTRA1, CFB, C2, and C3, have been reported through genomewide association studies (GWAS) and have demonstrated strong size effect and highly repeatable results (Ding et al. 2009; Dewan et al. 2006; Chen et al. 2006).
Oxidative stress has been linked to various types of DNA damage that play a significant role in aging and age-related disorders (Singh et al. 2009; Wilson and Bohr 2007). The retina, particularly the macula, is an environment with elevated oxygen levels, prolonged exposure to irradiation, and the continual phagocytosis of photoreceptors (Beatty et al. 2000). Previous studies have demonstrated DNA damage arising from oxidative stress to be a significant contributor to AMD (Szaflik et al. 2009; Wozniak et al. 2009).
Recently, a large-scale GWAS conducted by the International AMD Genomics Consortium reported several new susceptibility loci associated with AMD including rs8017304 A>G in the gene RAD51B (Fritsche et al. 2013). RAD51B is a known member of the RAD51 paralogs and is involved in homologous recombinational repair of DNA double-strand breaks by promoting the activity of the central recombinase (Suwaki et al. 2011). Absence of the RAD51B protein is thought to disrupt formation of the RAD51 nucleoprotein filament, the initial stage of homologous recombinational repair (Takata et al. 2000). Considering the demonstrated link between DNA damage and AMD, the critical role of RAD51B in DNA repair, and the recent report of rs8017304 A>G association with AMD, we chose RAD51B as a candidate gene for our study. We used two independent AMD sample sets to analyze genotype frequencies in tag-nucleotide polymorphisms (SNPs) spanning RAD51B including the reported rs8017304 A>G. RAD51B associations with AMD were extensively analyzed using independent cohorts, phenotypic stratification, and SNP–SNP interaction. In addition, we conducted a functional assay to uncover any correlation between RAD51B genotypes and their corresponding mRNA expression levels in cultured primary human fetal retinal pigment epithelium (hfRPE).
Materials and methods
Study subjects
This research followed the tenets of the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board of the National Eye Institute, USA and the Zhongshan Ophthalmic Center (ZOC), China. All participants gave informed consent. The study group demographics are summarized in Table 1. Methods for participant selection and clinical evaluation of subjects have been previously defined and are briefly described below (Age-Related Eye Disease Study Research 2000; Clemons et al. 2005b; Davis et al. 2005).
Table 1.
Demographic information of participants in the two sample sets used in this study
| Sample set | Cohort | N | Age (mean ± SD) | Ever smokers N (%) | Female N (%) | Advanced AMD N (%) | GA N (%) | nAMD N (%) |
|---|---|---|---|---|---|---|---|---|
| NEI | Control | 177 | 73.9 ± 10.8 | 24 (15.0) | 92 (57.5) | 0 | 0 | 0 |
| AMD | 249 | 78.8 ± 8.3 | 130 (52.2) | 136 (54.6) | 208 (83.5) | 46 (18.5) | 162 (65.1) | |
| China | Control | 46 | 71.7 ± 8.3 | 27 (58.7) | 0 | 0 | 0 | |
| AMD | 117 | 73.6 ± 9.2 | 38 (32.5) | 117 (100) | 24 (20.5) | 96 (79.5) |
AMD patients and control subjects from the National Eye Institute (NEI) sample set were evaluated by NEI ophthalmologists (CBM and EYC in author list) using the age-related eye disease study (AREDS) criteria (Age-Related Eye Disease Study Research 2001; Davis et al. 2005). AMD cases were confirmed by independent grading of fundus photographs. All controls presented either no drusen or fewer than five small drusen (<63 μm in diameter) and no signs of other retinal diseases including, but not limited to, high myopia, retinal dystrophies, central serous retinopathy, vein occlusion, diabetic retinopathy, or uveitis. Geographic atrophy was diagnosed when a patient’s retina was affected in a discrete area greater than 175 μm in diameter and was characterized by a sharp border as well as the presence of visible choroidal vessels. Neovascular AMD was defined as serous or hemorrhagic detachment of the sensory retina or RPE, the presence of choriodal neovascular vessels, subretinal or sub-RPE hemorrhages, or subretinal fibrous scarring or the history of treatment for neovascular AMD. The patients and controls in the NEI cohort were all self-identified as Caucasian and non-Hispanic and reside in the local area. The recruited controls were all older than 50 years of age. Smoking status was simply recorded as nonsmoker, current smoker or past smoker. Considering the long-term effect of smoking on AMD, we combined “current smoker” and “past smoker” into one “ever smoker” status.
The ZOC sample set was collected from a hospital-based study. The enrollment of cases and controls followed the criteria described above with race limited to Han Chinese.
RAD51B (refseq NG_023267) SNP selection and genotyping
We selected rs8017304 A>G because of its reported association with AMD. Six additional tag-SNPs across Rad51b were then selected using SNPinfo Web server (http://snpinfo.niehs.nih.gov/) by searching its alien gene name RAD51L1 in default settings, e.g., minor allele frequency cut-off to 0.01, R2 linkage disequilibrium (LD) larger than 0.8 and maximum distance to calculate LD as 250,000 bp. SNP genotyping was performed using the Taqman SNP Genotyping Assay from ABI (Applied Biosystems, Foster City, CA). All the assays were inventory stocks. The characteristics of the selected SNPs and their assay information are listed in Table 2.
Table 2.
Characteristics of selected RAD51B SNPs and their assay information
| RSID | Chromosome position | Type | Taqman SNP assay ID | MAF CEU/CHB |
|---|---|---|---|---|
| rs34583846 G>A | 67360038 | Missense (Val>Met) at AA 9 | C__63254941_10 | NS |
| rs33929366 T>G | 67423666 | Missense (Ser>Ala) at AA 250 | C__25967258_20 | NS |
| rs3784099 G>A | 67819680 | Intron between exons 6 and 7 | C__27481679_10 | NS |
| rs17105278 T>C | 67798232 | Intron between exons 6 and 7 | C__29282952_10 | 0.325/0.078 |
| rs8017304 A>G | 67854830 | Intron between exons 7 and 8 | C__31753961_20 | 0.371 |
| rs12878858 A>G | 67856550 | Intron between exons 7 and 8 | C__31753963_10 | 0.373 |
| rs4902566 C>T | 67863307 | Intron between exons 8 and 9 | C__34019695_20 | 0.277 |
CEU Utah residents with ancestry from northern and western Europe, CHB Han Chinese in Beijing, China
Transcript/mRNA expression of RAD51B in hfRPE
Fetal eyes were obtained from Advanced Bioscience Resources (Alameda, CA) at 16 to 18 weeks of gestation. The separation and culture of primary hfRPE was reported previously (Maminishkis et al. 2006). This study included hfRPE from 28 donors. Genomic DNA was extracted from the hfRPE of each donor to identify genotypes at rs17105278 T>C and rs4902566 C>T. Total RNA from hfRPE was extracted using Trizol (Invitrogen, Carlsbad, CA). cDNA was synthesized by reverse transcriptase (Taqman reverse transcription reagents, Applied Biosystems). The primers/probes for RAD51B spanning exons 4–5 (assay ID: Hs00172522_m1) and exons 9–10 (assay ID: Hs01568767_m1) were purchased from Life Technologies as inventoried TaqMan gene expression reagents. The primers/probes for RAD51B spanning exons 8–9, in which rs17025278 is located, were custom-designed with forward primer 5′-AGGCATCCTCCTTGAAGTATTTGG-3′, reverse primer 5′-GCTCCACTCAGATGGGTTGTAATC-3′, and probe 5′-CTGGGATTGAAAACTC-3′. Relative quantitative real-time polymerase chain reaction (qRT-PCR) was performed to determine the fold changes by the 2−ΔΔCt analysis method using actin as an endogenous control. The relative expression of RAD51B in rs17105278 T>C major allele homozygotes, heterozygotes and minor allele homozygotes (TT, TC, and CC, respectively) were compared. Each sample was analyzed in duplicate.
Statistics and bioinformatics analysis
The power to detect effects of SNP variants on AMD was calculated using binomial distribution. Association analyses were performed using SNP & Variation Suite (SVS) software (version 7.4.1; HelixTree Genetics Analysis Software, Golden Helix, Bozeman, MT, htpp://www.goldenhelix.com/SNP_Variation/HelixTree/index.html). SNP allelic association, genotypic association under a dominant model (carriers of at least one minor allele versus those with two major alleles) or under a recessive model (carriers of two minor alleles versus those with at least one major allele), and SNP–SNP interactions were analyzed using logistic regression in which case-versus-control status was designated as the outcome and was adjusted for age, gender, and smoking status. P values, odds ratio (OR) of association, and Hardy–Weinberg equilibrium (HWE) P value were computed using a Chi-square test. Multiple testing was corrected by false discovery rate (FDR; Dudbridge and Koeleman 2004).
The means for RAD51B mRNA expression were compared using Student’s t test. Statistical significance was set at P < 0.05.
SNPinfo Web server (http://snpinfo.niehs.nih.gov/) was used to make functional predictions such as altered transcriptional binding sites (TFBS), splicing sites, and the involvement of microRNA sequences. The LD analysis on SNPs of interest was conducted using SVS software by adopting public data from the “Affy 6.0 HapMap 1258 Samples Genotypes.”
Results
Statistical power analysis
We used published rs8017304 A>G-AMD association data as a reference for power analysis (Fritsche et al. 2013). Based on a predefined two-sided alpha of 0.05, there was greater than 95 % power to detect a ±6 % departure from the rs8017304 A>G allele frequency of 38.0 % in the NEI sample set. Study power remained greater than 80 % after stratifying the cases to neovascular AMD in the NEI set. The ZOC set was underpowered but was only used for supplementary replication purposes.
rs17105278 T>C and rs4902566 C>T are associated with AMD
The seven SNPs across RAD51B were genotyped and produced call rates larger than 98.8 % for each SNP in the NEI sample set. Two of the seven SNPs (rs34583846 G>A and rs33929366 T>G) were in the coding regions of RAD51B (Table 2). Because the genotype results of these two SNPs in the coding regions were all wild-type in the NEI sample set, we excluded them from the statistical analysis. The allelic associations of the remaining five SNPs, located in RAD51B noncoding regions, in the NEI sample set is shown in Table 3. RAD51B rs17105278 T>C, rs4902566 C>T and the previously reported rs8017304 A>G reached statistical significance for AMD association at initial analysis (P < 0.05). rs8017304 A>G showed a moderate association with AMD, meeting a P < 0.05 criterion for significance before any adjustment (Table 3, P = 0.0432, OR = 0.74). rs17105278 T>C showed an extremely significant association with AMD (Table 3, P = 3.63 × 10−4, OR = 1.67) as well as rs4902566 C>T (Table 3, P = 8.11 × 10−7, OR = 2.12).
Table 3.
Allele distribution of RAD51B SNPs and their association with AMD in the NEI sample set
| SNP | Control | AMD | P | OR (95 % CI) | FDR | HWE P of control | ||
|---|---|---|---|---|---|---|---|---|
| Minor/major (%) | Call rate (%) | Minor/major (%) | Call rate (%) | |||||
| rs17105278 T>C | 114/232 (32.9) | 97.7 | 225/273 (45.2) | 100 | 3.63 × 10−4 | 1.67 (1.26–2.23) | 7.3 × 10−4 | 0.445 |
| rs3784099 G>A | 114/238 (32.4) | 99.4 | 132/364 (26.6) | 99.6 | 0.0679 | 0.75 (0.56–1.02) | 0.077 | 0.874 |
| rs8017304 A>G | 133/217 (38.0) | 98.9 | 154/338 (31.3) | 98.8 | 0.0432 | 0.74 (0.56–0.99) | 0.058 | 0.579 |
| rs12878858 A>G | 128/226 (36.2) | 100 | 156/340 (31.5) | 99.6 | 0.1515 | 0.81 (0.61–1.08) | 0.151 | 0.352 |
| rs4902566 C>T | 86/268 (24.3) | 100 | 201/295 (40.5) | 99.6 | 8.11 × 10−7 | 2.12 (1.57–2.87) | 3.2 × 10−6 | 0.821 |
We did more extensive analysis on the influence of rs17105278 T>C and rs4902566 C>T because of their high degree of association with AMD. Adjusting for confounding factors including smoking, age, and gender, rs17105278 T>C adhered to the dominant model (Table 4, P/Pa = 9.53 × 10−3, OR = 1.69) and rs4902566 C>T also showed an association with AMD under the dominant model (Table 4, P/Pa = 5.84 × 10−4, OR = 1.98). Recessive model analysis maintained a significant rs17105278 T>C association with AMD (Table 4, P/Pa = 2.16 × 10−3, OR = 2.30) as well as a rs4902566 C>T association with AMD (Table 4, P/Pa = 1.12 × 10−5, OR = 4.20). The rs17105278 T>C association with AMD under the dominant and recessive models remained significant after multiple testing correction (FDR = 0.02 and 4.32 × 10−3, respectively) as well as the rs4902566 C>T association with AMD (FDR = 1.56 × 10−3 and 8.93 × 10−5, respectively). The ZOC sample set was used to replicate results for the rs17105278 T>C association with AMD. Although P < 0.05 was not reached, the data was consistent in terms of the OR indicated by the NEI sample set and suggested that rs17105278 T>C minor allele could possibly be a risk allele for AMD in a non-Caucasian population (Table 5, P = 0.11, OR = 3.19). In both the NEI and ZOC sample sets, the allelic frequencies of all SNPs genotyped in their control groups were within the boundaries of HWE (P > 0.05). We attempted phenotypic stratification of the NEI sample set and did not observe any departure in the association odds of geographic atrophy or neovascular AMD subgroups from AMD as whole in the carriers of rs17105278 T>C or rs4902566 C>T (data not shown).
Table 4.
Model analysis of rs17105278 T>C and rs4902566 C>T genotypes in the NEI sample set
| Genotype | Control | AMD | P/P a | OR (95 % CI) | FDR P | ||
|---|---|---|---|---|---|---|---|
| N (%) | |||||||
| Dominant model | rs17105278 T>C | TT | 80 (46.2) | 84 (33.7) | 9.53 × 10−3 | 1.69 (1.135–2.516) | 0.02 |
| TC+CC | 93 (53.8) | 165 (66.3) | |||||
| rs4902566 C>T | CC | 102 (57.6) | 101 (40.7) | 5.84 × 10−4 | 1.98 (1.339–2.927) | 1.56 × 10−3 | |
| CT+TT | 75 (42.4) | 147 (59.3) | |||||
| Recessive model | rs17105278 T>C | TT+TC | 152 (87.9) | 189 (75.9) | 2.16 × 10−3 | 2.30 (1.35–3.95) | 4.32 × 10−3 |
| CC | 21 (12.1) | 60 (24.1) | |||||
| rs4902566 C>T | CC+CT | 166 (93.8) | 194 (78.2) | 1.12 × 10−5 | 4.20 (2.13–8.26) | 8.93 × 10−5 | |
| TT | 11 (6.2) | 54 (21.8) | |||||
P values are adjusted for age, gender, and smoking status (P a); FDR false discovery rate to adjust for multiple testing
Table 5.
Allele and genotype distribution of RAD51B rs17105278 T>C and association with AMD in the China sample set
| SNP | Control | AMD | P values | OR (95 % CI) | HWE P of Control |
|---|---|---|---|---|---|
| Minor/major | Minor/major (%) | ||||
| rs17105278 T>C | |||||
| C/T | 2/82 (2.4) | 14/180 (7.2) | 0.11 | 3.19 (0.71–14.36) | 0.445 |
| rs17105278 T>C | |||||
| (CT + CC)/TT | 2/40 (4.8) | 14/83 (14.4) | 0.10 | 3.37 (0.73–15.56) | |
The call rate of the China set is 85.3 %
Interaction between rs17105278 T>C and rs4902566 C>T in AMD susceptibility
Categorizing the genotypes of these two SNPs in control subjects and AMD cases in the NEI sample set revealed a strong combination effect of minor C allele homozygosity at rs17105278 T>C and minor T allele homozygosity at rs4902566 C>T toward AMD susceptibility (Table 6). Out of 172 individuals, two (1.2 %) in the control group were homozygous carriers of both rs17105278 T>C and rs4902566 C>T minor alleles as compared to 44 out of 246 cases (17.9 %) in the AMD group, demonstrating that individuals homozygous for the two SNPs had substantially increased disease risk compared with all other genotypes (Table 6, OR = 16.97, 95 % CI 4.05–71.10). The odds increased further when compared to individuals homozygous for the wild-type alleles of the two SNPs (Table 6, OR = 19.22, 95 % CI 4.34–80.65). The OR of 19.22 is more than fivefold greater than the product of the OR of the homozygous rs17105278 T>C risk allele multiplied by the OR of the homozygous rs4902566 C>T risk allele (1.69 × 1.98 = 3.35) under the dominant model.
Table 6.
The interaction of rs17105278 T>C and rs4902566 C>T on AMD susceptibility
| rs17105278 T>C | rs4902566 C>T, N (%) | |||
|---|---|---|---|---|
| CC | CT | TT | ||
| Control | TT | 58 (33.7) | 17 (9.8) | 5 (2.9) |
| TC | 32 (18.6) | 36 (20.9) | 3 (1.7) | |
| CC | 9 (5.2) | 10 (5.8) | 2 (1.2) | |
| AMD | TT | 67 (27.2) | 16 (6.5) | 1 (0.4) |
| TC | 32 (13.0) | 63 (25.6) | 9 (3.7) | |
| CC | 1 (0.4) | 13 (5.3) | 44 (17.9)* | |
* OR = 16.97 (95 % CI 4.05–71.10), rs17105278 T>C and rs4902566 C>T homozygotes versus all other genotypes; OR = 19.22 (95 % CI 4.34–80.65), rs17105278 T>C and rs4902566 C>T homozygotes versus noncarrier homozygotes
We also attempted SNP–SNP interaction analysis between the RAD51B and well-known AMD SNPs such as CFH, HTRA1, and ARMS2, for which we have data published on our sample set (Tuo et al. 2008; Ross et al. 2007; Chan et al. 2007). The results did not reveal any interactions between rs17105278 T>C or rs4902566 C>T and these previously identified SNPs (data not shown).
rs17105278 T>C homozygous C carriers express lower RAD51B in RPE
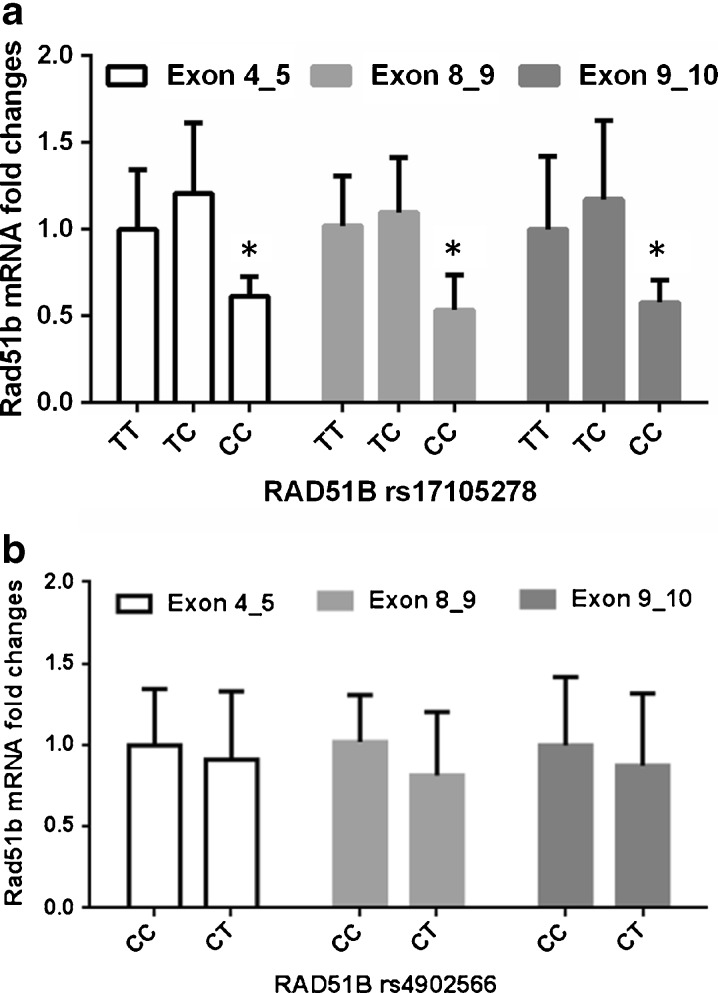
We collected hfRPE from 28 donors for our functional study. Because none of the 28 hfRPE donors were homozygous carriers of rs4902566 C>T, we focused on rs17105278 T>C for this analysis. We hypothesized that the SNP, being located in a noncoding region, might possess regulatory function, particularly on adjacent exons. Therefore, using the cultured hfRPE from 28 donors, we measured RAD51B expression in three portions of the transcript, spanning exons 4–5, exons 8–9, and exons 9–10 (rs17025278 is located between exons 8 and 9). The distribution of rs17105278 T>C genotypes among the 28 donors consisted of ten samples homozygous for TT, 15 samples heterozygous for TC, and three samples homozygous for CC. We selected six TTs (all major allele homozygous CC for rs49025266), three TCs (all heterozygous CT for rs4902566), and then three CCs (all heterozygous CT for rs4902566) to compare the relative mRNA expression of RAD51B. We found consistently decreased RAD51B expression in rs17105278 T>C homozygotes (CC) relative to both heterozygotes (TC) and major allele homozygotes (TT) using primers/probes spanning exons 4–5, exons 8–9, and exons 9–10 (Fig. 1a). RAD51B expression corresponding to rs4902566 genotypes is summarized in Fig. 1b.
Fig. 1.
a RAD51B mRNA expression in hfRPE as a function of rs17105278 T>C genotype. Error bars indicate SD. *P < 0.05 in comparison with TT or TC group. b RAD51B mRNA expression in hfRPE as a function of rs4902566 C>T genotype
We attempted to uncover a functional role of the noncoding rs17105278 T>C using bioinformatics analysis, but did not find predicted function in TFBS, splicing sites, or microRNA.
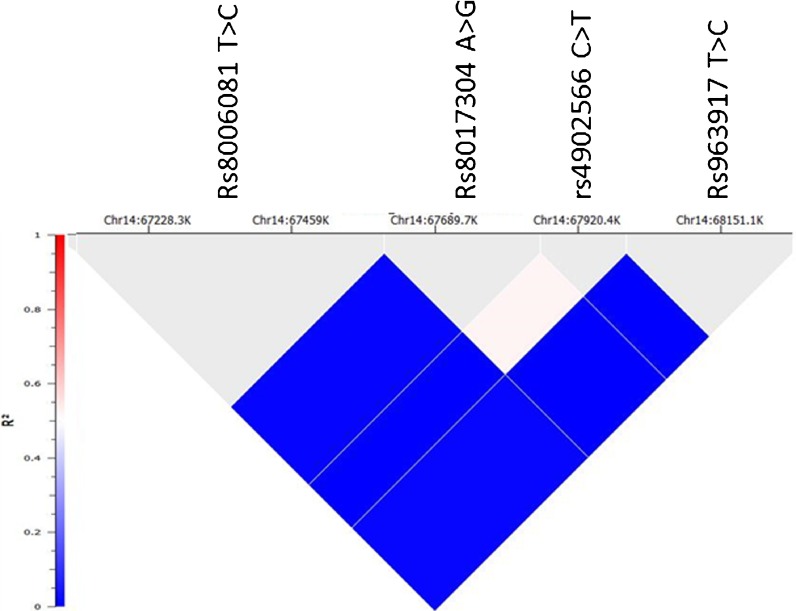
Moderate LD exists between rs4902566 C>T and rs8017304 A>G
We searched our SNPs of interest in “Affymetrix 6.0 HapMap 1258 Samples Genotypes,” a database which we found to contain rs8017304 A>G and rs4902566 C>T, but not rs17105278 T>C. We selected rs8017304 A>G and rs4902566 C>T in addition to two nearby SNPs (rs8006081 T>C and rs963917 T>C) as references for LD analysis. The rs8017304 A>G and rs4902566 C>T SNPs are 8 kb apart with 0.52 of R2 and 0.99 of D′, indicating a moderate LD relationship (Fig. 2).
Fig. 2.
LD analysis of four SNPs across RAD51B
Discussion
Growing evidence of the involvement of oxidative stress and DNA repair in AMD pathogenesis alongside recent report of SNP rs8017304 A>G association with AMD by the International AMD Genomics Consortium led us to analyze the influence of RAD51B on AMD in the well-established NEI sample set (Tuo et al. 2006, 2008; Ross et al. 2007; Ardeljan et al. 2013). Looking at allelic frequencies in the reported rs8017304 A>G and six additional tag-SNPs across the RAD51B gene, we found significant AMD-association with the reported rs8017304 A>G and two novel RAD51B SNPs, rs17105278 T>C and rs4902566 C>T. Our data indicate substantial risk association with the minor alleles of rs17105278 T>C and rs4902566 C>T under the dominant model. In addition to the independent risk association identified with either the rs17105278 T>C or the rs4902566 C>T minor alleles, the combined risk is strikingly amplified; the OR of individuals homozygous for both rs17105278 T>C and rs4902566 C>T is nearly 20 times greater than the OR of individuals not carrying any risk alleles of the two SNPs. rs17105278 T>C and rs4902566 C>T are located 65 kb apart, suggesting an epistatic relationship between the two SNPs.
The novel RAD51B SNP AMD associations reported here appear to be comparable to several well-established susceptibility loci including CFH, HTRA1, and ARMS2 that have been analyzed and reported within this sample set. Notably, rs17105278 T>C and rs4902566 C>T, in combination, have a multifold greater odds risk than those previously identified in this sample set and in other studies with reasonable sample size (Table 7). Additionally, our replication of rs17105278 T>C risk association in the ZOC sample set suggests that the association might cross races and exists in both the Caucasian/European ancestry population and the Chinese population.
Table 7.
Reported susceptibility loci associated with AMD
| Cohort | Gene | RSID | Odds ratio (OR) | Probability | References |
|---|---|---|---|---|---|
| NEI sample set | RAD51B | rs17105278 T>C | 1.69 (1.14–2.52) | 9.53 × 10−3 | Current study |
| rs4902566 C>T | 1.98 (1.34–2.93) | 5.84 × 10−4 | |||
| CFH | rs380390 G>C | 2.74 (2.18–3.44) | <0.0001 | (Tuo et al. 2006) | |
| Htra1 | rs11200638 G>A | 2.12 (1.37–3.29) | 7.9 × 10−4 | (Tuo et al. 2008) | |
| ARMS2 | rs10490924 T>G | 2.61 (1.89–3.61) | 1.42 × 10−9 | (Ross et al. 2007)* | |
| TIMP3/SYN3 | rs9621532 C>A | 0.32 (0.11–0.89) | 0.02 | (Ardeljan et al. 2013) | |
| Columbia Univ. (European descent) | CFH | rs1061170 T>C (Y402H) | 2.25 (1.79–2.75) | 1.64 × 10−13 | (Hageman et al. 2005) |
| Moran Eye Center, Univ. of Utah | Htra1 | rs11200638 G>A | 1.86 (1.35–2.56) | 1 × 10−9 | (Yang et al. 2006) |
| Kellogg Eye Center, Univ. of Michigan | ARMS2 | rs10490924 T>G | 2.66 | 5.3 × 10−30 | (Kanda et al. 2007) |
* Includes subset of age-related eye diseases study (AREDS) cohort
Using RAD51B primers/probes spanning exons 4–5, 8–9, and 9–10, we demonstrated rs17105278 T>C influence on RAD51B expression. The results show that rs17105278 T>C, located between exons 8 and 9, does not change exon expression locally but rather has an overall global effect on RAD51B expression. We attempted a bioinformatics analysis to uncover potential functional sites altered; however, no connotation was found to be associated with TFBS, splicing sites, or microRNA. Further study will be required to understand exactly how rs17105278 T>C influences RAD51B transcript expression.
Interestingly, our study identified novel SNP associations in a gene recently linked to AMD susceptibility (Fritsche et al. 2013). Searching for the three SNPs that reached significance in the NEI sample set in “Affymetrix 6.0 HapMap 1258 Samples Genotypes” (available in the data sets installed in HelixTree Genetics Analysis Software), we found that rs8017304 A>G and rs4902566 C>T were present, but not rs17105278 T>C. While the potential outcomes produced by shared data for meta-analysis are substantial, differences in platforms can pose considerable limitations on the detectability of associations. The absence of rs17105278 T>C from Affymetrix HapMap may account for the discrepancy in our findings against those of the Consortium, while rs4902556, present in Affymetrix 6.0, may have been missed because a considerable number of samples were analyzed using Illumina or other platforms (Fritsche et al. 2013).
The link between DNA repair and AMD is currently understood through the established role of oxidative stress in AMD pathophysiology. Elevated reactive oxygen species levels in the retina, particularly in the macula, as well the involvement of oxidative stress in aging (Beatty et al. 2000), suggest a critical role for efficient and robust DNA repair in macular environment (Blasiak et al. 2012). Associations with AMD in DNA repair genes, such as XRCC1, XPD, ERCC6, hOGG, MUTYH, SMUG1, and UNG, have been identified (Blasiak et al. 2012, 2013), implicating the various mechanisms of DNA repair, but to date, few studies have been able to demonstrate reduced DNA repair efficacy as a direct result of polymorphic variation.
RAD51B has been confirmed to play a role in the DNA repair of double-strand breaks through the characterized RAD51 central recombinase. Impairment of RAD51B has been linked to lower homologous recombinational repair frequency and poor response to abiotic stress (Yao et al. 2013). The expression of the RAD1 gene, the product of which plays a crucial role in the repair of DNA double-strand breaks by homologous recombination, was shown to be downregulated in response to oxidative stimulus in AMD patients (Strunnikova et al. 2005). The coordinated involvement of Rad51 and Rad1 in recombinational repair of DNA damage implicates the potential role of RAD51-dependent DNA repair in circumstances of elevated oxidative reaction species (Keller-Seitz et al. 2004).
In summary, our data indicate that minor allele carriers of rs17105278 T>C and rs4902566 C>T carriers are at a higher risk of developing AMD and that the risk for rs17105278 T>C carriers may be due to decreased transcription of RAD51B. This study also provided further evidence of DNA damage/DNA repair mechanism in AMD pathogenesis.
Acknowledgments
The authors thank Angel Garced, R.N., Katherine Shimel, R.N., and Sun–min Ro, R.N. for their assistance in contacting study participants and collecting blood samples; Arvydas Maminishkis, Ph.D. for providing fhRPE cells. We also thank the study participants and their families for enrolling in this study. This research was supported by “The Intramural Research Program of the National Eye Institute, NIH,” the “Specialized Research Foundation for Doctoral Program of Higher Education in China (20120171110086),” and the “Science and Technology Planning Project of Guangzhou City (11C22060787) of China.”
Conflict of interest
None
References
- Age-Related Eye Disease Study Research G. Risk factors associated with age-related macular degeneration. A case–control study in the age-related eye disease study: age-related eye disease study report number 3. Ophthalmology. 2000;107(12):2224–2232. doi: 10.1016/S0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research G A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D, Meyerle CB, Agron E, Jin Wang J, Mitchell P, Chew EY, Zhao J, Maminishkis A, Chan CC, Tuo J. Influence of TIMP3/SYN3 polymorphisms on the phenotypic presentation of age-related macular degeneration. Eur J Hum Gen: EJHG. 2013;21(10):1152–1157. doi: 10.1038/ejhg.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/S0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Blasiak J, Glowacki S, Kauppinen A, Kaarniranta K. Mitochondrial and nuclear DNA damage and repair in age-related macular degeneration. Int J Mol Sci. 2013;14(2):2996–3010. doi: 10.3390/ijms14022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Synowiec E, Salminen A, Kaarniranta K. Genetic variability in DNA repair proteins in age-related macular degeneration. Int J Mol Sci. 2012;13(10):13378–13397. doi: 10.3390/ijms131013378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Shen D, Zhou M, Ross RJ, Ding X, Zhang K, Green WR, Tuo J. Human HtrA1 in the archived eyes with age-related macular degeneration. TAm Ophthalm Soc. 2007;105:92–97. [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Liu DT, Tam PO, Chan WM, Liu K, Chong KK, Lam DS, Pang CP. Association of complement factor H polymorphisms with exudative age-related macular degeneration. Mol Vis. 2006;12:1536–1542. [PubMed] [Google Scholar]
- Chou CF, Frances Cotch M, Vitale S, Zhang X, Klein R, Friedman DS, Klein BE, Saaddine JB. Age-related eye diseases and visual impairment among U.S. adults. Am J Prev Med. 2013;45(1):29–35. doi: 10.1016/j.amepre.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL, 3rd, Age-Related Eye Disease Study Research G. Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL., III Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris FL, III, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC. The age-related eye disease study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F, Koeleman BP. Efficient computation of significance levels for multiple associations in large studies of correlated data, including genomewide association studies. Am J Hum Genet. 2004;75(3):424–435. doi: 10.1086/423738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP, Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, NK C, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR, Consortium AMDG Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–439. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104(41):16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Seitz MU, Certa U, Sengstag C, Wurgler FE, Sun M, Fasullo M. Transcriptional response of yeast to aflatoxin B1: recombinational repair involving RAD51 and RAD1. Mol Biol Cell. 2004;15(9):4321–4336. doi: 10.1091/mbc.E04-05-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47(8):3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RJ, Bojanowski CM, Wang JJ, Chew EY, Rochtchina E, Ferris FL, III, Mitchell P, Chan CC, Tuo J. The LOC387715 polymorphism and age-related macular degeneration: replication in three case–control samples. Invest Ophthalmol Vis Sci. 2007;48(3):1128–1132. doi: 10.1167/iovs.06-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Ahn B, Bohr VA. Roles of RECQ helicases in recombination based DNA repair, genomic stability and aging. Biogerontology. 2009;10(3):235–252. doi: 10.1007/s10522-008-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikova N, Hilmer S, Flippin J, Robinson M, Hoffman E, Csaky KG. Differences in gene expression profiles in dermal fibroblasts from control and patients with age-related macular degeneration elicited by oxidative injury. Free Radic Biol Med. 2005;39(6):781–796. doi: 10.1016/j.freeradbiomed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Suwaki N, Klare K, Tarsounas M. RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol. 2011;22(8):898–905. doi: 10.1016/j.semcdb.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Szaflik JP, Janik-Papis K, Synowiec E, Ksiazek D, Zaras M, Wozniak K, Szaflik J, Blasiak J. DNA damage and repair in age-related macular degeneration. Mutat Res. 2009;669(1–2):169–176. doi: 10.1016/j.mrfmmm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Takata M, Sasaki MS, Sonoda E, Fukushima T, Morrison C, Albala JS, Swagemakers SM, Kanaar R, Thompson LH, Takeda S. The Rad51 paralog Rad51B promotes homologous recombinational repair. Mol Cell Biol. 2000;20(17):6476–6482. doi: 10.1128/MCB.20.17.6476-6482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Ning B, Bojanowski CM, Lin ZN, Ross RJ, Reed GF, Shen D, Jiao X, Zhou M, Chew EY, Kadlubar FF, Chan CC. Synergic effect of polymorphisms in ERCC6 5′ flanking region and complement factor H on age-related macular degeneration predisposition. Proc Natl Acad Sci U S A. 2006;103(24):9256–9261. doi: 10.1073/pnas.0603485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Ross RJ, Reed GF, Yan Q, Wang JJ, Bojanowski CM, Chew EY, Feng X, Olsen TW, Ferris FL, 3rd, Mitchell P, Chan CC. The HtrA1 promoter polymorphism, smoking, and age-related macular degeneration in multiple case-control samples. Ophthalmology. 2008;115(11):1891–1898. doi: 10.1016/j.ophtha.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair. 2007;6(4):544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Wozniak K, Szaflik JP, Zaras M, Sklodowska A, Janik-Papis K, Poplawski TR, Blasiak J, Szaflik J. DNA damage/repair and polymorphism of the hOGG1 gene in lymphocytes of AMD patients. J Biomed Biotechnol. 2009;2009:827562. doi: 10.1155/2009/827562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, DeWan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- Yao Y, Bilichak A, Titov V, Golubov A, Kovalchuk I. Genome Stability of Arabidopsis atm, ku80 and rad51b Mutants: Somatic and Transgenerational Responses to Stress. Plant Cell Physiol. 2013;54(6):982–989. doi: 10.1093/pcp/pct051. [DOI] [PubMed] [Google Scholar]