Abstract
African dust storm events (ADE) travel across the Atlantic Ocean (ADEAO) and reach the Puerto Rican coast (ADEPRC), potentially impacting air quality and human health. To what extent seasonal variations in atmospheric particulate matter (PM) size fractions, composition and sources trigger respiratory-adverse effects to Puerto Ricans is still unclear. In the present study, we investigated the pro-inflammatory and cytotoxic effects of PM samples harvested during ADEAO (PM10), ADEPRC (PM2.5 and PM10) and Non-ADE (Preand Post-ADEAO and Non-ADEPRC), using BEAS-2B cells. Endotoxins (ENX) in PM2.5 and PM10 extracts and traces of metals (TMET) in PM2.5 extracts were also examined. IL-6 and IL-8 secretion and cytotoxicity were used as endpoints. ADEAO and ADEPRC extracts were found to be more cytotoxic than Non-ADE and ADEAO were more toxic than ADEPRC extracts. PM10 extracts from ADEAO and Post-ADEAO caused significant secretion of IL-8. IL-6 and IL-8 secretion was higher following treatment with PM10 and PM2.5 ADEPRC than with Non-ADEPRC extracts. ENX levels were found to be higher in PM10 ADEAO than in the rest of the samples tested. TMET levels were higher in PM2.5 ADEPRC than in Non-ADEPRC extracts. Deferoxamine significantly reduced cytotoxicity and IL-6 and IL-8 secretion whereas Polymyxin B did not. TMET in PM2.5 fractions is a major determinant in ADEPRC-induced toxicity and work in conjunction with ENX to cause toxicity to lung cells in vitro. ENX and TMET may be responsible, in part, for triggering PM-respiratory adverse responses in susceptible and predisposed individuals.
Keywords: dust storm, particulate matter, endotoxins, metals, BEAS-2B cells
1. INTRODUCTION
African dust storm events (ADE) have increased sharply since early 1970s. These environmental episodes have been attributed mainly to a drought period in the Saharan/Sahel region caused by changes in the global distribution of sea surface temperature [1-3]. Several hundred million tons of African dust is transported across the Atlantic Ocean (ADEAO) annually, leaving a trail of atmospheric pollutants throughout the Caribbean, Central and North America [4-7]. The seasonal influx of African dust, reaching the Northeastern coast of Puerto Rico (ADEPRC) during spring and summer, transports particulate matter (PM) capable of causing health-adverse effects [8-12]. However, to what degree ADEAO and ADEPRC fractions and constituents may contribute to the pathogenesis of respiratory and systemic illnesses observed in certain individuals during and after the ADEPRC, still remains unclear. More detailed (epidemiological, in vivo and in vitro) investigations are warranted to better understand the environmental factors and PM characteristics that play a critical role in the ADEPRCinduced respiratory related diseases.
Ambient PM is a complex mixture of solid and liquid particles of different sizes from various sources with distinct chemical compositions and constituents [13-18]. African dust carries large volumes of air masses characterized by a bimodal number distribution of particle sizes with a predominant mode near 0.6 μm but shifts slightly towards larger modes when it reaches the western Atlantic/Caribbean sites (1.2 - 2.5 μm in diameter) [19,20]. Airborne PMs with a median aerodynamic diameter of equal or less than 2.5 μm (PM2.5) and equal or less than 10 μm (PM10) are known as fine and inhalable coarse fractions, respectively. Airborne PM2.5 arises from combustion processes or atmospheric transformation of combustion emissions whereas PM10 contains mainly mineral particles of crustal materials. PM2.5 and PM10 fractions have shown to contain organic materials (e.g., bacterial endotoxins (ENX), fungi (spores), pollen fragments, polycyclic aromatic hydrocarbons (PAH), and carbonaceous elements), as well as inorganic materials (e.g., water soluble traces of metals-TMET), minerals (quartz, silicates), salts (ammonium-sulfates and nitrates), and soil dust particles [3,14,18,21-24]. Ambient PM induced health adverse effects can be triggered by the particle itself or the materials adsorbed to the particle [25-26]. When inhaled, this complex mixture may cause or exacerbate allergies, asthma, cardiovascular diseases and, in extreme cases, lung cancer and mortality [13-14, 19,24,27-36]. The extent of detrimental response to ambient PM exposure depends on different factors such as environmental (e.g., PM size and chemical constituents, demographics sources, climate and temperature changes, ozone and anthropogenic influences) and inter-individual variations (e.g., age, ethnicity, sex, health conditions and genetic predisposition). These multifactorial characteristics make difficult to underline the exact mechanism of PM-induced respiratory adverse effects. However, several in vitro studies have suggested different cell mechanisms associated to certain PM constituents such as: 1) bacterial ENX may be responsible for the inflammatory response due to the activation of Toll-like receptor-4 (TLR4) and transcription factors (e.g., NF-kB) and the release of pro-inflammatory cytokines (e.g., IL-6 and IL-8); 2) TMET [e.g., aluminum (Al), arsenic (As), iron (Fe), cadmium (Cd), copper (Cu), nickel (Ni), mercury (Hg), lead (Pb) and vanadium (V)] may cause cellular lipids, proteins and DNA damage, mutagenicity and carcinogenicity by generating reactive oxygen species (ROS); 3) PAH may cause oxidative stress, aberrant changes in cell cycling, gene expression and DNA functions by generating toxic metabolites due to the activation of aryl hydrocarbon (Ah) receptors and induction of Phase I and II metabolizing enzymes; and 4) air pollutants such as nitro-, sulfurand oxygen-elements (e.g., NO2, SO2, H2S, O3) may cause lung epithelial cell damage, oxidative cellular stress and carcinogenicity [3, 25-26,29,34,37-47]. Based on these and other published studies (Aust et al., 2002; Wang et al., 2013), inflammatory injury and oxidative damage are considered as common mechanisms of PM-induced health adverse effects.
Respiratory illnesses, such as allergy and asthma, in the Caribbean region have increased over the past 30 years, where the pediatric population has been particularly the most affected [12,48-51]. The association between respiratory illnesses in children and the presence of atmospheric contaminants as well as the mechanisms by which these adverse effects occur are not well understood. However, there is a growing body of evidence indicating that African dust storms transporting PM2.5 and PM10 fractions and contaminants, such as ENX and TMET, may play a major role in the pathogenesis of respiratory illness and contribute to the increase of emergency room (ER) visits and hospitalizations of children [48,52-54].
In the present study we investigated the pro-inflammatory and cytotoxic effects of ambient PM (PM2.5 and PM10) and its specific constituents (ENX and TMET) before, during and after dust storms (ADEAO and ADEPRC) using an in vitro model of BEAS-2B cells. The goal was to evaluate the hypothesis that ADE influx impacts the Puerto Rican coast air quality conditions and may be responsible, in part, for the respiratory-illnesses observed in susceptible and predisposed individuals.
2. MATERIALS AND METHODS
2.1. Sampling Selection
2.1.1. PM10 Collected Over the Atlantic Ocean (AO)
The ADEAO PM10 samples used in this study were collected in 2004 during the Aerosol and Oceanographic Science Expedition (AEROSE). Details of the AEROSE science plan, mission and sampling collection have already been reported elsewhere [10,20]. Briefly, the AEROSE project consisted of a series of intensive field experiments conducted aboard the U.S. National Oceanic and Atmospheric Administration (NOAA) ship Ronald H. Brown during the North Hemispheric spring (March 2004) and summer (June-July 2007) expedition. PM10 samples were collected during the AEROSE-I, which started on February 29, 2004 in Bridgetown, Barbados and ended in San Juan, Puerto Rico on March 27. March PM10 samples were selected for this investigation to correlate them with samples gathered during a severe dust storm event that reached the coast of Puerto Rico during this time period. In addition, airborne dust samples present before (Pre-ADEAO) and after (Post-ADEAO). ADEAO were also collected during the AEROSE-I expedition and analyzed for comparison purposes.
2.1.2. PM2.5 and PM10 Collected at Fajardo, Puerto Rican Coast (ADEPRC)
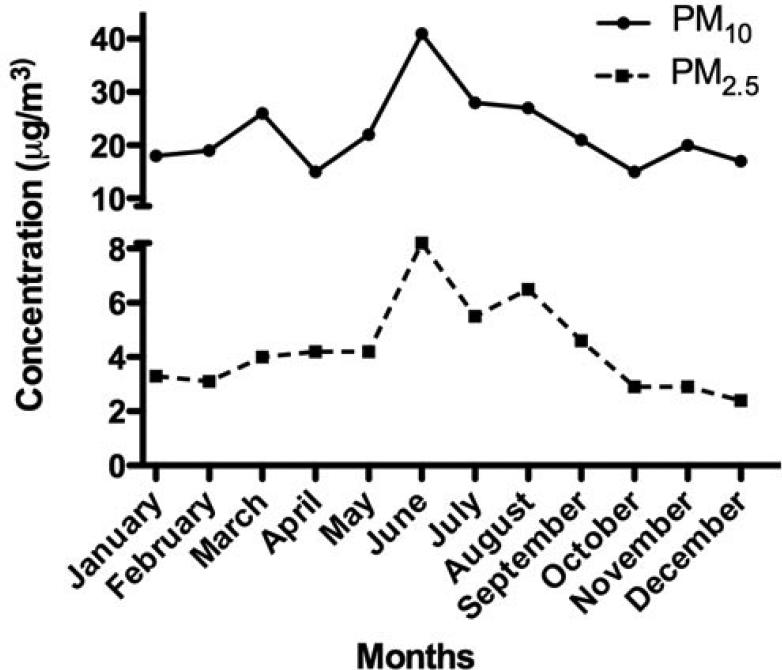
A total of 28 ADEPRC were monitored during 2004. The identification of 5 of these ADEPRC in March allowed the harvest and preparation of a composite sample mixture containing sufficient material to obtain extract for this research. During spring and summer of the year studied, the PM mass concentration data showed a Puerto Rico seasonal variation pattern with high levels of PM2.5 and PM10 fractions, which correlated with the temporal dust events pattern detected by the Total Ozone Mapping Spectrometer (TOMS). PM2.5 samples were collected on Teflon filters while PM10 samples on quartz filters by the PR Environmental Quality Board (PR-EQB) air monitoring program. The air samplers were identified as EQB station 22, located at the northeastern coast of PR, close to a lighthouse in the municipality of Fajardo. This station is situated at a remote site (natural reserve) that minimizes possible anthropogenic PM contributions. In order to designate the days containing the PM2.5 and PM10 events originating from the African continent, a combination of two data sources was used. One source was the daily satellite images gathered by TOMS [6] and the other was the onsite PM2.5 and PM10 concentrations obtained by the PR-EQB (Figure 1). A relative increase above the lowest PM2.5 and PM10 daily mean concentrations (2.8 mg/m3 and 18.3 mg/m3, respectively) at Fajardo location was established as criteria to suggest the possible occurrence of a dust event. Once it was identified by means of weight, the dates were evaluated further by the confirmation of clouds over the region using images from TOMS. The ADEPRC sample set included PM collected a day before and 1 - 2 days after the event since smaller particles from the event still remain in suspension after the main cloud front. The Non-ADE days were classified from the same period as the ADEAO. Samples harvested during the months of January, February, October, November and December of 2004, fulfilled the criteria of Non-ADE according to the guidelines mentioned above and were used as classification controls.
Figure 1.
PM2.5 and PM10 monthly mean concentrations reported in 2004 by PREQB for the rural coastal region of Fajardo, Puerto Rico.
2.2. PM2.5 and PM10 Extractions
All glassware used was acid washed using a modified cleanup procedure as described by Molinelli et al., 2006 [22]. Briefly, all filters were extracted for a 48 h period in 150 - 200 ml of hexane/acetone (Fisher, H303SK4 and A9294) 1:1 using a Soxhlet extraction apparatus. The resulting extracts were reduced to a volume of 2 - 8 ml using a rotor evaporator under vacuum, subsequently dried under a gentle stream of pure nitrogen and their masses determined gravimetrically. The organic extracts were dissolved in dimethylsulfoxide (DMSO, Sigma, Cat No D2650) to a concentration of 100 mg/ml. All extracts were stored at −20°C until further use. Blank filters were also extracted using the previous methodology for quality control purposes.
2.3. PM2.5 and PM10 Endotoxin Quantification
2.3.1. Filters
ENX in PM10 filters collected at sea during AEROSE were determined with the Kinetic Limulus Amebocyte Lysate assay (Pyrochrome, Associates of Cape Cod, East Falmouth, MA, USA) following the manufacturer's instructions.
2.3.2. Extracts
ENX concentrations in PM10 and PM2.5 organic extracts were determined in duplicate using the Kinetic Limulus Amebocyte Lysate Assay (Kinetic-QCL, Lonza, Walkersville, MD, USA) as described by the manufacturer. A 1/1000 organic extract dilution was used to quantify ENX in the extracts. A Lipopolysaccharide (LPS, Sigma, Cat No L2630) positive control was included for each sample in order to guarantee the specificity of the assay. Absorbance readings at 37°C were recorded on an ELx 808 micro-plate reader (Biotek Instruments Inc., Winooski, VT, USA) and data analysis was performed with the WinKQCL Software (Lonza, Walkersville, MD, USA).
2.4. PM2.5 Metal Analyses
PM2.5 organic extracts were diluted in 1 ml of 2% nitric acid and metals quantified by inductive coupled plasma mass spectrometry using an Agilent 7500 (ICPMS). A modified Environmental Protection Agency 200.8 method was employed. The modification consisted of using helium or hydrogen to remove isobaric interferences through a reaction cell. Each sample was run in triplicate and mean concentration determined for: aluminum (Al), cadmium (Cd), copper (Cu), iron (Fe), lead (Pb) and vanadium (V).
2.5. Cell Culture and Treatments
BEAS-2B cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA; Cat No CRL-9609). Cells were cultured according to ATCC protocols, maintained in keratinocyte basal medium (KBM-2, Lonza, Walkersville, MD, USA Cat No CC 3103) and supplemented with keratinocyte growth medium (KGM-2 SingleQuots; Lonza, Walkersville, MD, USA; Cat No CC4152). Cells were used at passages 44 - 59 and maintained at 37°C in a humidified atmosphere of 5% CO2 . Cells were seeded at a density of 5 × 104 cells/well onto 96-well plates and incubated for 24 h. Extracts were diluted in cell media at final concentration of 0.1 % DMSO and used to expose cells to PM10 (ADEAO and ADEPRC) and PM2.5 (ADEPRC) at concentrations ranging from 10 to 100 mg/ml for 24 h. Based on diesel exhaust particles studies [55], this extract concentration range is biologically relevant. Using this range it is possible to induce biologic effects and can be achieved in vivo after the appropriate adjustments (e.g. breathing patterns, airway anatomy, diseased airways, particle size and deposition). Three independent exposure experiments were performed. Two additional set of cells (n = 3 independent dishes) were also used to concurrently be treated for a 24 h period with: 1) ADEPRC PM10 organic extract (10 and 25 mg/ml) pre-incubated with polymyxin B sulfate (PMB, Sigma Cat No P0972), an ENX inhibitor, at a final concentration 10 mg/ml; and 2) ADEPRC PM2.5 organic extract (50, 75 and 100 mg/ml) pre-incubated with deferoxamine mesylate (DF, Sigma, Cat No D9533), a metal chelator, at a final concentration of 50 mM. Appropriate positive (LPS at a final concentration of 10 ug/ml and LPS pre-treated with PMB) and negative controls (medium, DMSO and media with DF and PMB) were run simultaneously in each experiment. Cell supernatants were collected after each exposure and stored at −80°C until further cytokine analyses.
2.6. Cell Viability
The Neutral Red bioassay was employed to assess cell viability. Following 24 h treatment with PM extracts and inhibitors, BEAS-2B cells were incubated with Neutral Red dye (Sigma, Cat No N2889) at a final concentration of 100 mg/ml for 3 h. The dye was then removed; cells were fixed in 1% calcium chloride, 0.5% formaldehyde, rinsed with phosphate buffered saline (PBS) and lysed using 1% acetic acid and 50% ethanol. Cell viability was spectrophotometrically determined at 540 nm using an Ultramark microplate reader (Bio Rad, Richmond, CA, USA). Three independent cytotoxicity experiments were performed. Triton-X (50 mg/ml) was used as a positive control for cell toxicity. Negative controls exhibited 90% or greater cell viability. A viability of less than 80% was considered cytotoxic, leaving a range of 20% for normal cell death.
2.7. Cytokine Secretion Measurements
The levels of IL-6 and IL-8, as well as other pro-inflammatory cytokines and proteins such as TNF-a, interleukin-1 beta (IL-1b), monocyte chemotactic protein-1 (MCP-1) and granulocyte-macrophage colony-stimulating factor (GMCSF) were analyzed using a multiplex bead assay (MAP Kit from Millipore, Billerica, MA, USA or Fluorokine Multi-analyte Profiling Kit from R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Cytokine concentrations were determined using the dual laser flow analyzer Luminex 100 (Luminex Corp, Austin, TX, USA). Standard curves for each cytokine were plotted employing a 5-paremeter logistic fit (5-PL). Three independent cytokine secretion experiments were performed.
2.8. Statistical Analyses
To assess differences among groups ANOVA statistical assays were performed followed by the non-parametric Tukey test for multiple comparisons. To analyze differences between individual groups the unpaired Student's t Test was employed. The criterion for statistical significance was set at p ≤ 0.05. Statistical analyses were performed using the GraphPad InStat 3 software. Analyses were based on 3 independent experiments per cell response.
3. RESULTS
3.1. PM10 Fractions
3.1.1. ENX Levels and Cytotoxicity of PM10 ADEAO and ADEPRC Extracts
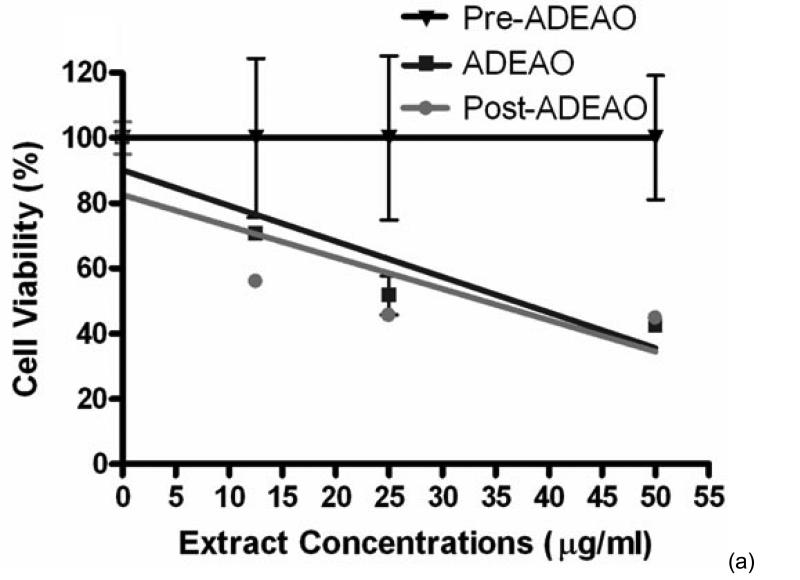
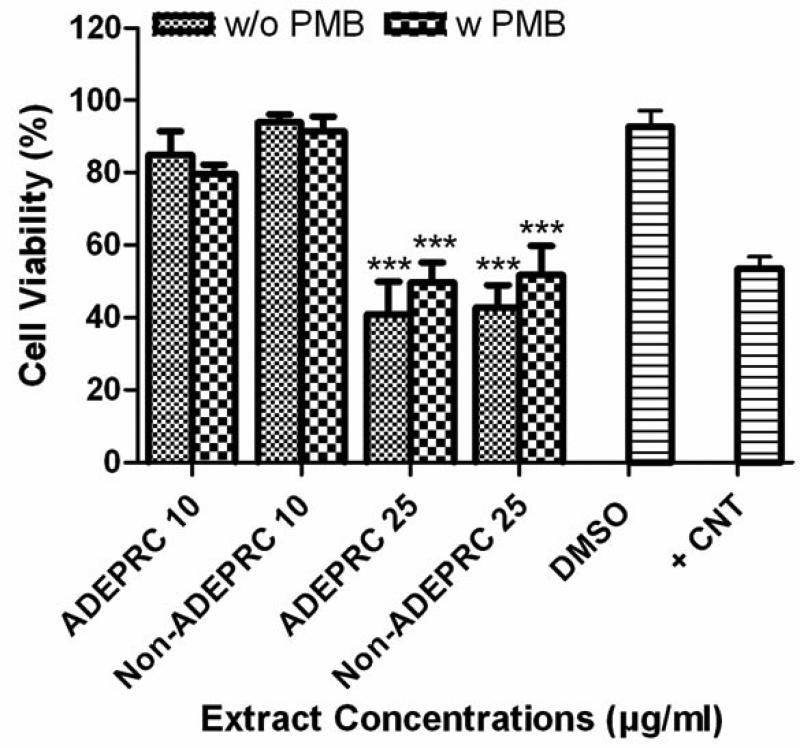
ADEAO extracts were found to be more cytotoxic to BEAS-2B cells than extracts from Pre-ADEAO, but as toxic as Post-ADEAO (Figure 2(a)). Pre-ADEAO samples were not toxic to BEAS-2B cells at all. ADEAO samples were found to be as toxic as ADEPRC samples (LC50 = 37 mg/ml and 38 mg/ml, respectively). ADEPRC extracts showed a dose-response cytotoxic effect and were highly toxic at 25mg/ml (Figure 2(b)). The ADEPRC and Non-ADEPRC extracts tested showed a non-significant increase in cell viability with the addition of PMB (Figure 3) at either dose of 10 or 25 mg/ml.
Figure 2.
Cytotoxic effect of PM10 extracts in BEAS-2B Cells. Cells were treated for 24 h with PM10 extracts collected from the Atlantic Ocean (A: ADEAO) and Puerto Rican Coast (B: ADEPRC). LC50 (lethal concentration of the PM extracts that kills 50% of the cells in 24 h) values were determined. ADEAO (LC50=37 mg/ml; Post-ADEAO LC50=34 mg/ml) and ADEPRC (LC50=38 mg/ml; Non-ADEPRC LC50=55 mg/ml) extracts were cytotoxic; however pre-ADEAO extracts were not. All extract concentrations were tested in 3 independent experiments.
Figure 3.
Effect of Polymyxin B (PMB, an ENX inhibitor) and PM10 ADEPRC extracts on BEAS-2B cell viability. Cells were treated with PM10 ADEPRC extracts in the presence (w PMB) and absence of PMB (w/o PMB) at 10 mg/ml for 24 h and cell viability was evaluated. Triton-X (+CNT) at 25 mg/ml was used as positive control. Bars represent mean cell viability ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. All samples were tested in three independent experiments; ***p < 0.001
PM10 ADEAO extracts contained approximately 5 times the amount of ENX found in the Pre-ADEAO or Post-ADEAO extracts (Table 1). ENX levels present in the PM10 oceanic quartz filters were found to be as high as 6.41 EU/m3 during ADEAO as compared to 0.50 in Pre-ADEAO and 0.80 EU/m3 in Post-ADEAO. PM10 ADEAO ENX levels were higher when compared to those found in PM10 ADEPRC extracts, 511 and 114 EU/mg, respectively. The amount of ENX present in PM10 organic extracts was also higher than that found in PM2.5 samples, <50 EU/mg (Table 1).
Table 1.
Endotoxin concentration in PM2.5 and PM10 extracts.
| Particulate Matter | Sample | Endotoxins (EUa/mg) |
|---|---|---|
| PM10 | Pre-ADEAO | 155.0 |
| ADEAO | 511.0 | |
| Post-ADEAO | 75.9 | |
| ADEPRC | 114 | |
| Non-ADEPRC | 111 | |
| PM25 | ADEPRC | <50 |
| Non-ADEPRC | <50 |
3.1.2. IL-6 and IL-8 Release by PM10 ADEAO and ADEPRC Extracts
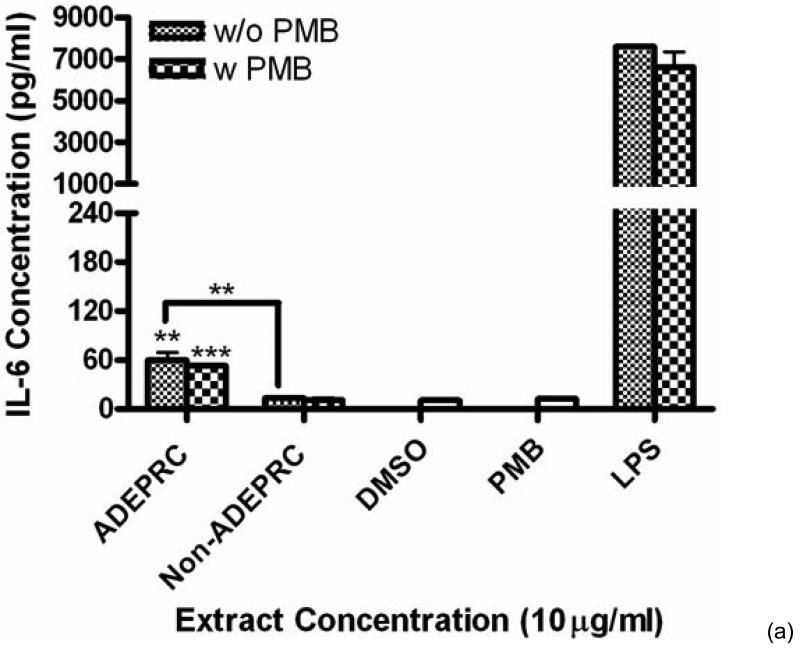
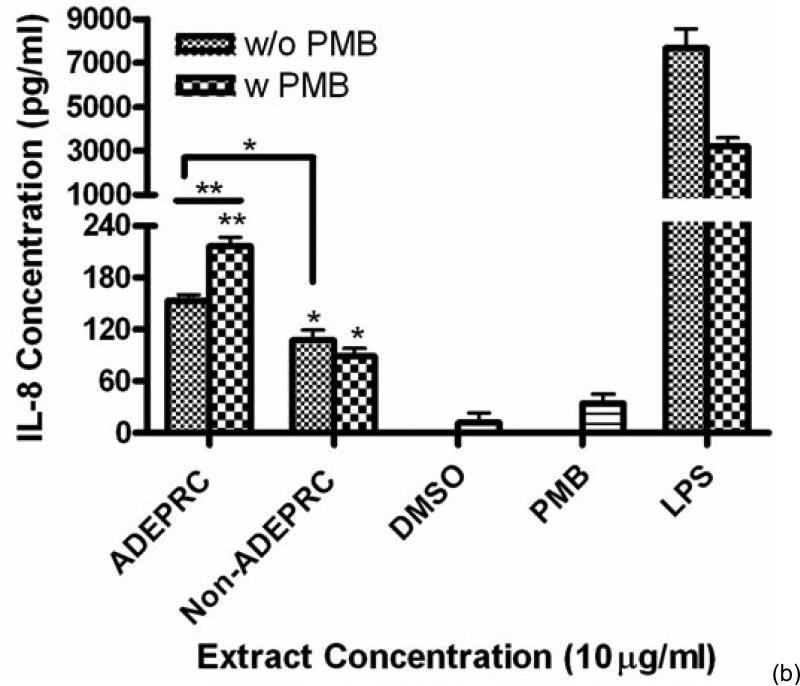
ADEAO and Post-ADEAO extracts at 25 mg/ml induced significantly IL-8 secretion (Figure 4) while PreADEAO extracts did not. The IL-6 and IL-8 secretion was significantly higher in ADEPRC extracts than in Non-ADEPRC (Figures 5(a) and (b)). The use of PMB on ADEPRC and Non-ADEPRC extracts decreased the levels of IL-6, but not significantly. Conversely, PMB was found to increase significantly the secretion of IL-8 in the presence of the ADEPRC extracts at 10 mg/ml (Figure 5(b)). PMB and DMSO alone did not cause any changes in IL-6 or IL-8 levels. Furthermore, extracts did not provoke secretion of any of the other pro-inflammatory mediators tested such as TNF-a, IL-1b, GMCSF and MCP-1 (data not shown).
Figure 4.
Induction of IL-8 in BEAS-2B exposed to PM10 extracts collected before, during and after the Atlantic Ocean dust storm (ADEAO). LPS (10 mg/ml) was used as a positive control. Bars represent mean IL-8 concentrations ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. Stars over the line indicate a comparison between two treatments. All samples were tested in three independent experiments; *p < 0.05.
Figure 5.
Effect of Polymyxin B (PMB, an ENX inhibitor) at 10 mg/ml on the induction IL-6 (A) and IL-8 (B) in BEAS-2B exposed to PM10 (ADEPRC) extracts for 24 h. Cells were treated with PM10 ADEPRC extracts in the presence (w PMB) and absence of PMB (w/o PMB) and cytokine induction was measured. LPS (10 mg/ml) was used as a positive control. Bars represent mean IL-8 concentration ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. Stars over the line indicate a comparison between two treatments. All samples were tested in three independent experiments; ***p < 0.001, **p < 0.01, *p < 0.05.
3.2. PM2.5 Fractions
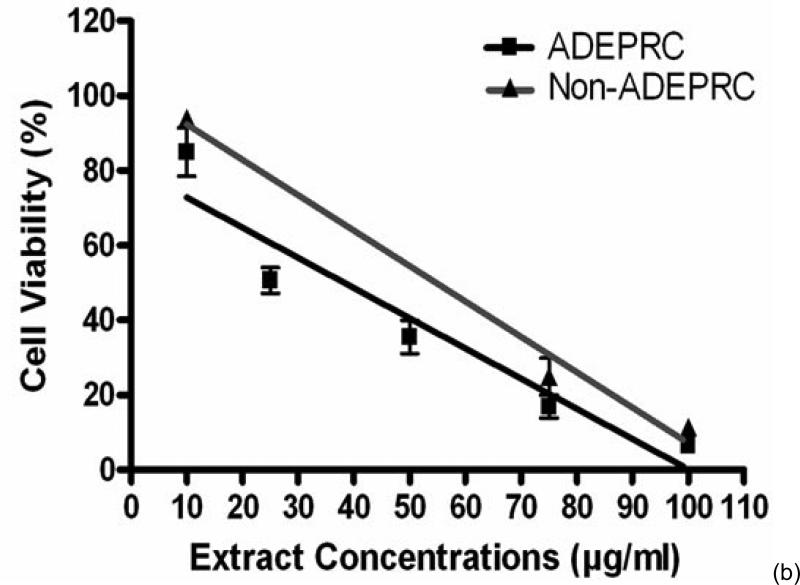
3.2.1. Cytotoxicity of PM2.5 ADEPRC Extracts
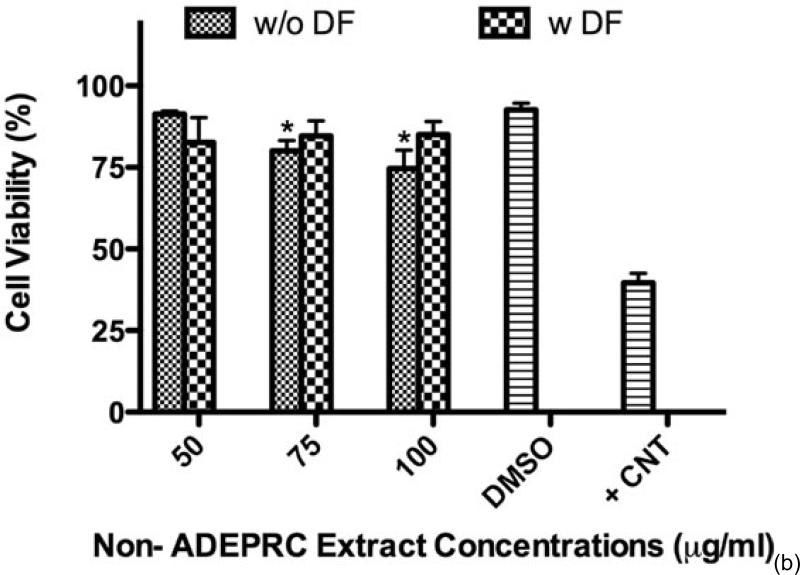
The dose-response exhibited an inverse relationship between cell viability and extract concentration (ADEPRC R2 = 0.96 and Non-ADEPRC R2 = 0.81). ADEPRC extracts were more toxic than Non-ADEPRC to BEAS-2B cells (Figure 6). ADEPRC extracts were concurrently tested with DF at various concentrations. DF increased cell viability at all concentrations tested and a significant effect was observed at 75 mg/ml (Figure 7(a)). Non-ADEPRC (Figure 7(b)) incubated with DF did not significantly increase cell viability at 75 and 100 mg/ml. Cytotoxicity caused by ADEPRC extracts correlated with the presence of high levels of metals, including Cu, Pb and V (Table 2).
Figure 6.
Cytotoxic effect of PM2.5 extracts in BEAS-2B Cells. Cells were treated for 24 h with PM2.5 extracts collected in the Puerto Rican coast during the African storm (ADEPRC) and non-storm (Non-ADEPRC) season. LC50 (lethal concentration of the PM extracts that kills 50% of the cells in 24 h) values were determined. ADEPRC (LC50 = 122 mg/ml (extrapolated) and Non-ADEPRC (LC50 = 191 mg/ml (extrapolated) were cytotoxic. All extract concentrations were tested in 3 independent experiments
Figure 7.
Effect of Deferoxamine (DF, a metal chelator) and PM2.5 ADEPRC extracts on BEAS-2B cell viability. Cells were treated with PM2.5 extracts collected during the dust storm (ADEPRC) and non-storm (Non-ADEPRC) season in the presence (w DF) and absence of DF (w/o DF) at 50 μM for 24 h and cell viability was evaluated. Triton-X (+CNT) at 25 mg/ml was used as positive control. Bars represent mean cell viability ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. Stars over the line indicate a comparison between two treatments. All extract concentrations were tested in 3 independent experiments; ***p < 0.001, *p < 0.05.
Table 2.
Metal Content in Organic Extracts vs Total Composition. Trace elements values for aqueous and organic extraction were combined and expressed as total % composition. Shaded in gray are crustal elements and Non-shaded are anthropogenic elements.
| % Composition | ||||
|---|---|---|---|---|
| Trace Element | Organic Extracts | Total | ||
| ADEPRC | Non-ADEPRC | ADEPRC | Non-ADEPRC | |
| A1 | 16.4 | 33.3 | 63.5 | 54.7 |
| Fe | 39.9 | 40.4 | 16.8 | 13.3 |
| Cd | 0.4 | 0.3 | 0.1 | 0.1 |
| Cu | 30.7 | 14,2 | 5.1 | 3.9 |
| Pb | 9.3 | 7.3 | 2.3 | 1.5 |
| V | 3.4 | 1.1 | 0.8 | 11 |
3.2.2. IL-6 and IL-8 release by PM2.5 ADEPRC Extracts
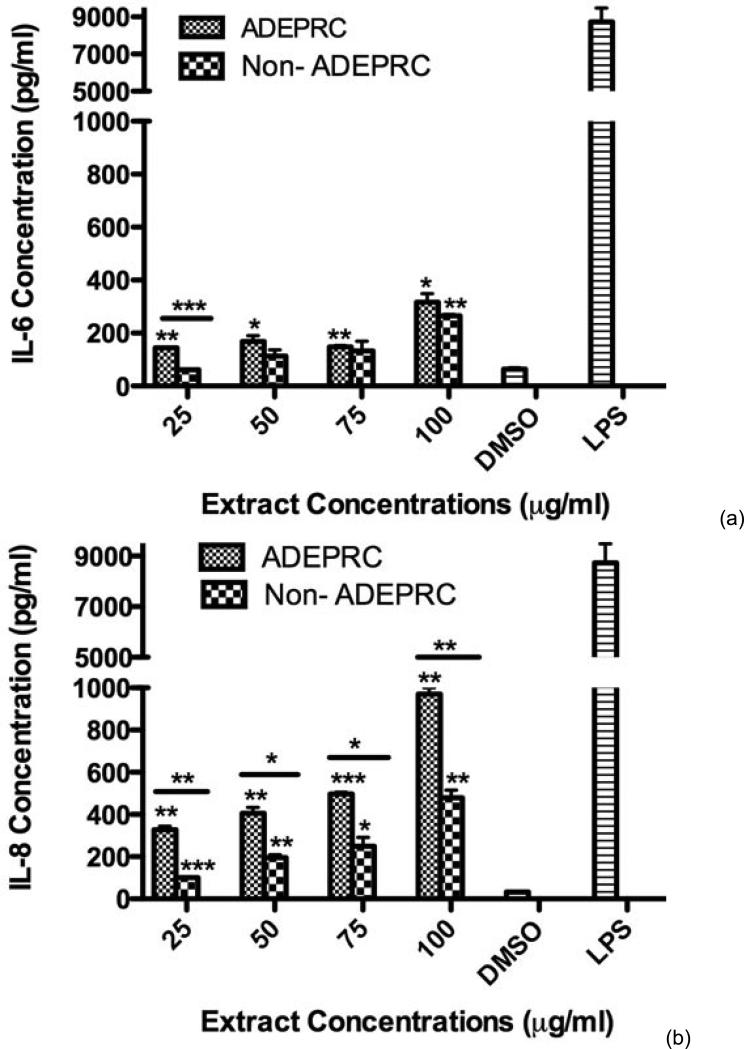
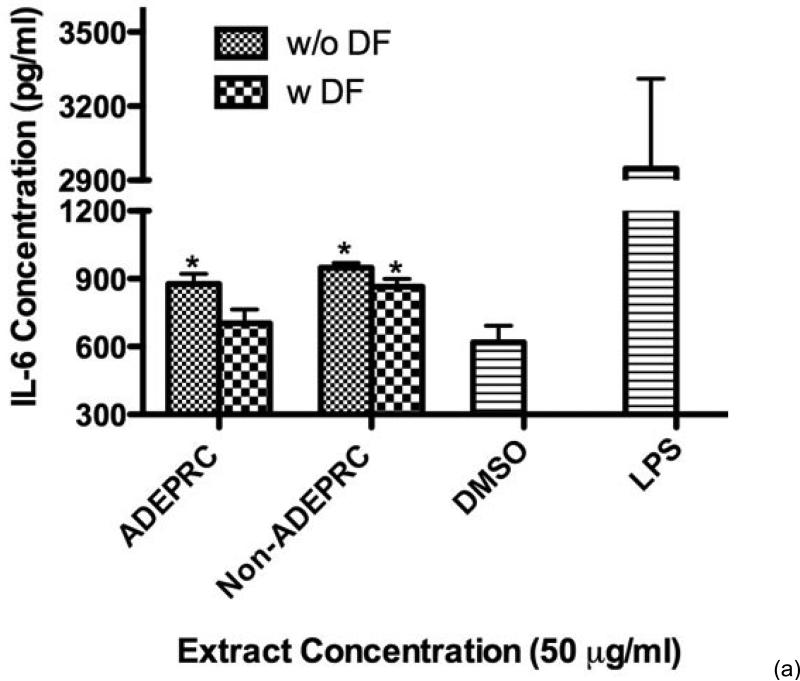
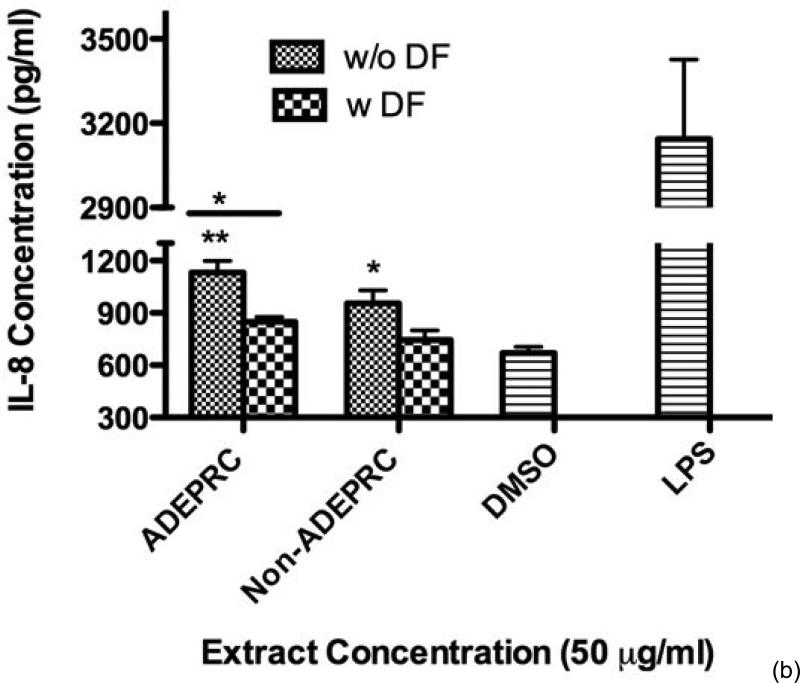
Significant differences were observed on immunological responses between ADEPRC and Non-ADEPRC particularly on the induction of IL-6 and IL-8 secretion by BEAS-2B (Figures 8(a) and (b)). This effect follows that of a dose-response type. This induction was distinct at the non-toxic concentrations of PM extracts tested at 25 and 50 μg/ml. It is evident that the effect of ADEPRC extracts on cytokine secretion by BEAS-2B was far more prominent than in the Non-ADEPRC extracts. Cells simultaneously treated with ADEPRC extracts at 50 mg/ml and DF at 50 mM reduced IL-6 (Figure 9(a)) and significantly IL-8 (Figure 9(b)) secretions. This effect was also observed with the Non-ADEPRC extracts, but not significantly. PM2.5 extracts significantly decreased the secretion of MCP-1. No changes were observed with the use of DF (data not shown). PM2.5 extracts did not cause any change in other pro-inflammatory mediators such as TNF-a, IL-1b and GM-CSF (data not shown).
Figure 8.
Induction of IL-6 (a) and IL-8 (b) in BEAS-2B exposed to Puerto Rican coast (ADEPRC) PM2.5 extracts for 24 h. LPS (10 mg/ml) was used as a positive control. Bars represent mean IL-6 and IL-8 concentrations ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. Stars over the line indicate a comparison between two treatments. All samples were tested in three independent experiments; ***p < 0.001, **p < 0.01, *p < 0.05.
Figure 9.
Effect of Deferoxamine (DF, a metal chelator) at 50 μM on the induction IL-6 (a) and IL-8 (b) in BEAS-2B exposed to PM2.5 (ADEPRC) extracts for 24 h. Cells were treated with PM2.5 extracts collected during the dust storm (ADEPRC) and non-storm (Non-ADEPRC) season in the presence (w DF) and absence of DF (w/o DF) cytokine induction was measured. LPS (10 mg/ml) was used as a positive control. Bars represent mean IL-6 and IL-8 concentrations ± SEM. Stars over the bars indicate comparison of a treatment with DMSO. Stars over the line indicate a comparison between two treatments. All samples were tested in three independent experiments; **p < 0.01, *p < 0.05.
3.2.3. TMET Levels in PM2.5 ADEPRC Samples
Al, Cd, Cu, Fe, Pb, and V were measured to determine their contribution to the toxicity and pro-inflammatory characteristics on ADEPRC extracts (Table 2). The ADEPRC extracts contained relative amounts of TMET and were ranked as follows: Cd < V Table 2), which include both the organic and aqueous phase, shows the relative abundance of Fe and Al and the contribution to local sources of these two heavy metals.
4. DISCUSSION
African dust transports persistent organic pollutants, traces of metals and viable microorganisms across the Atlantic Ocean to the Caribbean, affecting the air quality and impacting human health in the region. However, due to seasonal variations, global climate changes, human activity, PM complexity, and individual characteristics, the health impact and the local environmental factors responsible for triggering PM-adverse effects are difficult to predict and identify. Here, we provide data suggesting that ENX and TMET constituents in PMADEAO/-ADEPRC are responsible for triggering the secretion of pro-inflammatory cytokines and inducing cytotoxicity in human bronchial epithelial cells.
The presence of gram-negative bacteria, containing lipopolysaccharides (LPS) in their cell walls, has been previously reported in African dust clouds crossing the Atlantic Ocean [3]. LPS is an endotoxin (ENX) capable of causing toxicity and immunogenicity due to its lipid and polysaccharide components. LPS elicits a variety of inflammatory responses by activating Toll-like receptor-4 (TLR4) and triggering the signaling cascade mediated by transcription factors such as NF-κB to secrete pro-inflammatory cytokines (e.g., IL-6, IL-8, TNF-α); these cytokines at high concentrations may initiate health adverse conditions such as asthma [27,37-38,56-59]. In the present study, ENX was detected in all PM2.5 as well as in PM10 extracts from the Atlantic Ocean and the Puerto Rican coast. The secretion of IL-6 and IL-8 was found to increase considerably following PM treatment to BEAS- 2B cells. TNF-α, IL-1β, GM-CSF, and MCP-1 were not induced by the PM extracts. More detailed investigations are needed to determine the mechanisms associated with this cellular response using BEAS-2B cells. PM2.5 and PM10 extracts were significantly cytotoxic to BEAS-2B cells; however, the degree of cytotoxicity was not found to be directly dependent on the levels of ENX present in the samples (See Table 1 and Figures 3 and 7). The levels of ENX found in the PM10 extracts collected during Pre-ADEAO (155 EU/mg) were comparable to those PM10 samples collected during ADEPRC (114 EU/mg) and Non-ADEPRC (111 EU/mg); but Pre-ADEAO samples were not cytotoxic to BEAS-2B cells. In addition, PM10 Post-ADEAO samples contained low levels of ENX (75.9 EU/mg) as compared to the rest of the samples collected but were as toxic as PM10 ADEAO samples which contained the highest ENX concentration (511 EU/mg). In this study, we also found that the levels of ENX present in the PM10 samples collected in the Atlantic Ocean and in the Puerto Rican coast were higher and more cytotoxic than those present in the PM2.5 fractions, which presented the lowest ENX concentrations (<50 EU/mg). These findings are in agreement with previous studies indicating that ENX content in fine PM fractions (2.5), reaching the Caribbean and Puerto Rican coast, is usually lower or negligible when compared to the content in the coarse (10) fractions [10,37,59]. Interestingly, PMB, a known LPS inhibitor, did not reduce cell damage caused by the PM10 extracts. In summary, these findings suggest that: 1) ENX were initially deposited onto PM fractions (e.g., PM10) during the dust storm originated in the African Continent and gradually lost during its course trajectory through the Caribbean and Puerto Rican coast; 2) ENX content in African dust plays a minor role in the cytotoxicity caused by PM10; and 3) IL-6 and IL-8 were the predominant induced cytokines in this in vitro system, as compared to the rest of the cytokines tested (e.g., TNF-α, IL-1β, GM-CSF, and MCP-1).
In contrast, the administration of DF (metal chelator) to BEAS-2B cells, treated with ADEPRC-PM2.5 extracts, significantly reduced the secretion of cytokines and cytotoxicity; supporting the hypothesis that TMET play a major role in causing lung epithelial cell injury in vitro [60]. Previous studies have demonstrated that human airway epithelial cells exposed to metals found in air particles release significant amounts of pro-inflammatory mediators and are toxic in vitro [61,62]. In the present study, the concentration of some metals such as Al, Cd, Cu, Fe, Pb and V were detected in the PM2.5 fractions during the Non-ADE season but most of these metals increased when ADEAO approached the Puerto Rican coast (Table 2); however, these findings are in disagreement with previous studies which suggested that the levels of TMET present in the ADEAO-PM2.5 fraction are expected to be lower [10].
The presence of TMET in the atmospheric air, as well as other atmospheric contaminants such as ENX, fluctuates and is dependent on the source, the frequency and magnitude of each particular dust storm. Studies conducted by the Center for Environmental and Toxicological Research (CETR) at the University of Puerto Rico, Medical Sciences Campus (UPR-MSC) have shown that the influx of African dust affects considerably the air quality conditions in the Northeastern areas of Puerto Rico [12,22,62-67]. These studies have demonstrated that PM10 fractions collected from Puerto Rican urban areas (e.g., Guaynabo, Salinas) contained higher concentrations of ENX and TMET, respectively (e.g., Cd, Cu, Fe, Ni, Hg, Pb and V) than extracts collected from rural coastal regions (e.g., Fajardo) and were significantly toxic to in vitro systems [12,22,62]. Subsequent work also demonstrated that PM2.5 fractions collected from indoor areas contained higher concentrations of these metals than PM samples obtained from outdoor areas, causing significant release of pro-inflammatory cytokines in vitro [64-68]. These results suggest that PM2.5 and PM10 fractions and some of their constituents (e.g., ENX and TMET) present at different Puerto Rican locations (urban and rural areas) may play a key role in the exacerbation of certain respiratory diseases (e.g. asthma) in predisposed individuals exposed to African dust clouds [8,10,12]. Epidemiological studies by the U.S. CDC and others have reported a high prevalence of asthma, particularly in children [49,69,70]. Similar results have also been reported by other investigations performed in the Caribbean region [46,50,51,54,59,71- 74].
Respiratory illnesses such as allergies and asthma are a major concern in Puerto Rico, which has one of the highest asthma prevalence rates in the world between other races/ethnicities, including other Hispanics [69,70, 75]. According to CDC, Puerto Ricans are more likely to have respiratory illness, specifically asthma than white non-Hispanics and total Hispanics. Furthermore, epidemiological studies conducted by the island's health department during 2007 and 2010, indicated that an average of 25,000 cases were filed each year and more than 90,000 adults could not work or perform regular activeties due to suffering of respiratory diseases. Despite of decades of research in this area, it is still uncertain why Puerto Ricans suffer so much from asthma; however health officials in the island suspect that environmental and individual characteristics are responsible for triggering respiratory diseases. PM2.5 and PM10 are a major threat to children because of their higher exposure to ambient PM, mostly when involved in outdoor activities as compared to adults and because of their immature state of the lung and immune system function [72,76]. In addition to ENX and TMET, other particle pollutants such as PAH, NO2, SO2, and O3 have also been associated with the pathogenesis of asthma and the increase of ER visits and hospitalizations of children; inflammation and oxidative stress are considered the major factors responsible for such adverse conditions [48,53,77-78]. A recent report has suggested an epigenetic and genetic variant in ADCYAP1R1 associated with asthma in Puerto Rican children [79]. ADCYAP1R1, also known as PAC1, is a gene encoding for adenylate cyclase activating polypeptide 1 (pituitary) receptor type I. PAC1 is a membrane-associated protein that shares significant homology with members of the glucagon/secretin receptor family. This receptor is highly expressed in the central nervous system (CNS), but it has been found in considerable levels in other tissues (e.g., spinal cord, digestive system, liver, adrenal medulla, and smooth muscle). ADCYA P1R1 mediates diverse biological actions of adenylate cyclase. For example, it regulates stress response across species [80] and also has been linked to post-traumatic stress disorder in adult and anxiety in children.
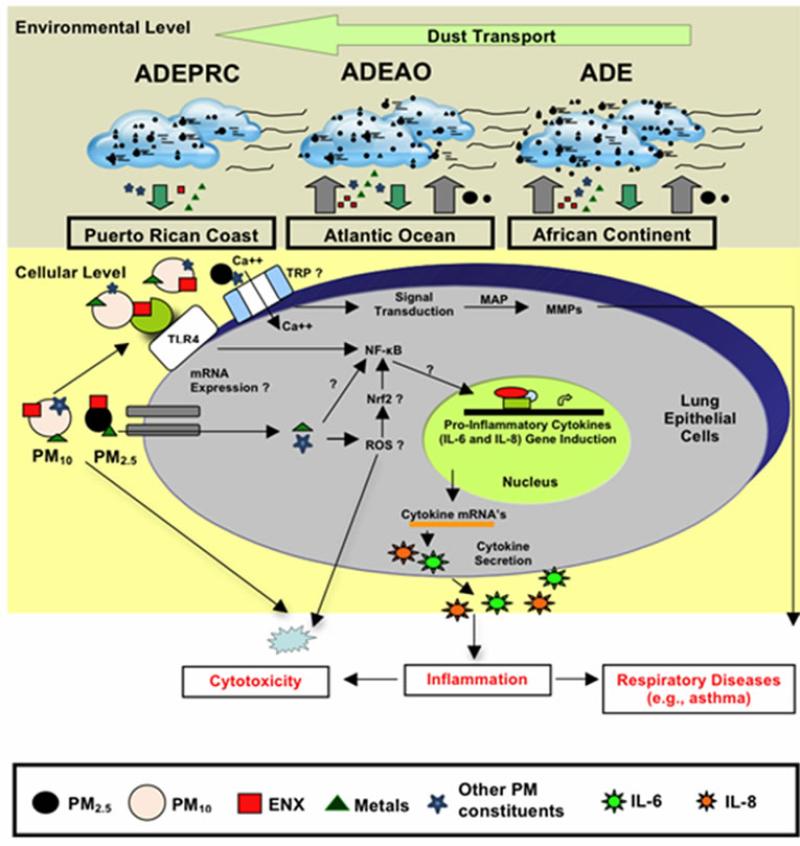
Another area of intense investigation in recent years is the participation of transient receptor potential (TRP) cation channels and calcium transporters in regulating the acute toxicological effects of ambient PM. The mammalian TRP was initially identified as particle sensors, whose primary function was to regulate the cell membrane permeability to a variety of ions. Subsequently, it was demonstrated that, some of the TRP subfamilies were linked to many pathophysiological and specific diseases; and also capable of mediating airway injury, hyper-reactivity and cardiovascular toxicity of inhaled pollutants [81-88]. The mammalian TRP family consists of six main subfamilies referred to as TRPC, TRPV, TRPM, TRPP, TRPML and TRPA groups. TRPV1, TRPV4, TRPA1, and TRPM8 have been proposed to mediate health adverse effect following exposure to urban air pollution [88-90]. It has been hypothesized that these four TRP subfamily members trigger acute adverse respiratory and cardiovascular effects by mediating calcium influx, activating signal transduction pathways and matrix-metalloproteinases (MMP) [91]. The increased MMP secretion by lung epithelial cells exposed to ambient PM has been linked to respiratory diseases [92-94], including pediatric acute lung injury [95]. Recent work has also demonstrated that TRPV4 polymorphism is associated with COPD [96] and cough in individuals without asthma [97]; and that TRPVI and TRV4 loss of function by a genetic variant lower the risk of active childhood asthma [98]. In addition to TRPs and calcium transporters, humic-like substances (HLS) and cell iron equilibrium have also been associated with lung injury following exposure to ambient PM pollutants [99,100]. Studies analyzing function and expression of TRP cellular channels in the lung may enable us to better understand the development of diseases caused by ambient PM and develop new therapeutic strategies for respiratory illnesses in the near future. We have briefly discussed the possible contributions and effects of PM constituents in human lung epithelial cells. A summary of the transport of dust storms across the Atlantic Ocean and the possible pathways of PM constituents that may lead to cytokine induction, cytotoxicity and respiratory disease are shown in Figure 10.
Figure 10.
Schematic model explaining the movement of African dust through the Atlantic Ocean (ADEAO) reaching the Puerto Rican coast (ADEPRC) and the possible relationship between its constituents (e.g., PM2.5-10, ENX, TMET, other PM constituents). Briefly, as African dust crosses the Atlantic Ocean, PM2.5 and PM10 distribution along with their constituents are modified. Upon arrival to the Puerto Rican coast, ENX levels are reduced whereas the levels of TMET are increased. ENX and TMET are cytotoxic to human bronchial epithelial cells, and can lead to inflammation and oxidative stress. This detrimental response may be caused due to the release of pro-inflammatory cytokines (e.g., IL-6 and IL-8), activation of nuclear receptor pathways (e.g., NF-kB, Nrf2), and the formation of reactive oxygen species (ROS). Interrogative symbols (?) represent factors involved in the signal transduction mediating cytokine secretion that are currently under study. Transient receptor potential (TRP) has also been associated with health adverse effects of ambient PM. TRP mediate calcium influx, activate signal transduction pathways (e.g., MAP kinases) and modulate MMP (matrix metalloproteases) causing lung injury. Small circles represent PM2.5 and large circles PM10; Endotoxins (ENX) are represented as red squares, traces of metals (TMET) as green triangles and other potential PM constituents as light blue stars. Cytokines are represented as dark blue and green stars.
Therefore, it is of considerable importance to conduct more defined epidemiological and in vivo and in vitro studies to 1) better understand the precise environmental factors that play a critical role in the ADEPRC-induced respiratory related diseases; 2) identify the biological mechanisms that render one population being more susceptible than another to PM health adverse effects; and 3) develop new strategies, educational programs and treatments for the patients and ultimately reduce asthma hospitalization and morbidity rate on the island. Taken together, the present study indicates that ENX and TMET can cause toxic effects to lung cells and TMET is the main responsible for the toxicity seen in this in vitro study. We hypothesize that ENX triggers the release of pro-inflammatory cytokines, such as IL-6 and IL-8, and render the cells more susceptible to PM contaminants such as metals, causing epithelial lung injury. Our findings support previous studies indicating that PM2.5 and PM10 may have the potential to trigger allergic and asthma episodes in susceptible and predisposed individuals, especially in children during ADEPRC season. This research also recognizes the importance of PM size fractions, composition and origin in causing potential lung adverse effects.
ACKNOWLEDGEMENTS
The authors thank the Puerto Rico Environmental Quality Board for providing PM10 and PM2.5 materials used in this research. Also, we like to thank Dr. Wilfredo Delgado from the University of Puerto Rico, Biochemistry Department, for his help and access to equipment; Dr. Felix Román-Velázquez and graduate student, Diana Sánchez from the University of Puerto Rico Mayagüez Campus, Chemistry Department for the ICP-MS metal analysis and Drs. Roy Amstrong, Yasmin Detrés and Vernon Morris for their involvement in the Atlantic Ocean trip coordination and PM sample collection. The research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R25GM061838. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Infrastructure support was provided in part by grant from the National Institute on Minority Health and Health Disparities (8G12MD007600).
REFERENCES
- 1.Prospero JM, Nees RT. Dust concentration in the atmosphere of the equatorial north Atlantic: Possible relationship to the Sahelian drought. Science. 1977;196:1196–1198. doi: 10.1126/science.196.4295.1196. http://dx.doi.org/10.1126/science.196.4295.1196. [DOI] [PubMed] [Google Scholar]
- 2.Wittig R, König K, Schmidt M, Szarzynski J. A study of climate change and anthropogenic impacts in West Africa. Environmental Science and Pollution Research. 2007;14:182–189. doi: 10.1065/espr2007.02.388. http://dx.doi.org/10.1065/espr2007.02.388. [DOI] [PubMed] [Google Scholar]
- 3.Griffin DW. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clinical Microbiology Reviews. 2007;20:459–477. doi: 10.1128/CMR.00039-06. http://dx.doi.org/10.1128/CMR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prospero JM, Lamb PJ. African droughts and dust transport to the Caribbean: Climate change implications. Science. 2003;302:1024–1027. doi: 10.1126/science.1089915. http://dx.doi.org/10.1126/science.1089915. [DOI] [PubMed] [Google Scholar]
- 5.Griffin DW, Seba DB. Atmospheric transport of mold spores in clouds of desert dust. Archives of Environmental Health. 2003;58:498–504. [PubMed] [Google Scholar]
- 6.Chiapello I, Moulin C, Prospero JM. Understanding the long-term variability of African dust transport across the Atlantic as recorded in both Barbados surface concentrations and large-scale Total Ozone Mapping Spectrometer (TOMS) optical thickness. Journal of Geophysical Research. 2005;110 D18S10. http://dx.doi.org/10.1029/2004JD005132. [Google Scholar]
- 7.Monteil MA. Saharan dust clouds and human health in the English-speaking Caribbean: What we know and don't know. Environmental Geochemistry and Health. 2008;30:339–343. doi: 10.1007/s10653-008-9162-0. http://dx.doi.org/10.1007/s10653-008-9162-0. [DOI] [PubMed] [Google Scholar]
- 8.Montealegre F, Chardon D, Tarrats H. Environmental factors precipitating bronchial asthma exacerbations in southern Puerto Rico: A pilot study. Journal of Asthma. 1993;30:219–227. doi: 10.3109/02770909309054520. http://dx.doi.org/10.3109/02770909309054520. [DOI] [PubMed] [Google Scholar]
- 9.Reid JS, Kinney JE, Wastphal DL, Holben BN, Welton EJ, Tsay S-C, et al. Analysis of measurements of Saharan dust by airborne and ground-based remote sensing methods during the Puerto Rico Dust Experiment (PRIDE). Journal of Geophysical Research. 2003;108:8586. http://dx.doi.org/10.1029/2002JD002493. [Google Scholar]
- 10.Jiménez-Vélez B, Detrés Y, Armstrong RA, Gioda A. Characterization of African Dust (PM2.5) across the Atlantic Ocean during AEROSE 2004. Atmospheric Environment. 2009;43:2659–2664. http://dx.doi.org/10.1016/j.atmosenv.2009.01.045. [Google Scholar]
- 11.Trapp JM, Millero FJ, Prospero JM. Temporal variability of the elemental composition of African dust measured in trade wind aerosols at Barbados and Miami. Atmospheric Environment. 2010;120:71–82. http://dx.doi.org/10.1016/j.marchem.2008.10.004. [Google Scholar]
- 12.Ortiz-Martinez M, Rivera-Ramirez E, Mendez-Torres L, Jiménez-Vélez BD. Biodiversity Science for Humanity. Athens Institute for Educations & Research; 2010. Role of chemical and biological constituents of PM10 from Saharan dust in the exacerbation of asthma in Puerto Rico. pp. 101–118. [Google Scholar]
- 13.Aust AE, Ball JC, Hu AA, Lighty JS, Smith KR, Straccia AM, et al. Particle characteristics responsible for effects on human lung epithelial cells. Research Report (Health Effects Institute) 2002;110:1–65. http://www.ncbi.nlm.nih.gov/pubmed/12578113. [PubMed] [Google Scholar]
- 14.Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M. The health relevance of ambient particulate matter characteristics: Coherence of toxicological and epidemiological inferences. Inhalation Toxicology. 2006;18:95–125. doi: 10.1080/08958370500306016. http://dx.doi.org/10.1080/08958370500306016. [DOI] [PubMed] [Google Scholar]
- 15.Schwarze PE, Øvrevik J, Hetland RB, Becher R, Cassee FR, Låg M, et al. Importance of size and composition of particles for effects on cells in vitro. Inhalation Toxicology. 2007;19:17–22. doi: 10.1080/08958370701490445. http://dx.doi.org/10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]
- 16.Duzgoren-Aydin NS. Health effects of atmospheric particulates: A medical geology perspective. Journal of Environmental Science and Health Part C: Environmental Carcinogenesis & Ecotoxicology Reviews. 2008;26:1–39. doi: 10.1080/10590500801907340. http://dx.doi.org/10.1080/10590500801907340. [DOI] [PubMed] [Google Scholar]
- 17.Demerjian KL, Mohnen VA. Synopsis of the temporal variation of particulate matter composition and size. Journal of the Air & Waste Management Association. 2008;58:216–233. doi: 10.3155/1047-3289.58.2.216. http://dx.doi.org/10.3155/1047-3289.58.2.216. [DOI] [PubMed] [Google Scholar]
- 18.Bell ML, HEI Health Review Committee Assessment of the health impacts of particulate matter characteristics. Research Report (Health Effects Institute) 2012;161:5–38. http://www.ncbi.nlm.nih.gov/pubmed/22393584. [PubMed] [Google Scholar]
- 19.Garrison VH, Foreman WT, Genualdi SD, Griffin W, Kellogg CA, Majewski, et al. Saharan dusta carrier of persistent organic pollutants, metals and microbes to the Caribbean? International Journal of Tropical Biology. 2006;54:9–21. [Google Scholar]
- 20.Morris V. Personal Communication. Howard University; Washington DC.: 2010. [Google Scholar]
- 21.Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environmental Health Perspectives. 1997;105:1053–1060. doi: 10.1289/ehp.97105s51053. http://dx.doi.org/10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molinelli AR, Santacana GE, Madden MC, Jiménez BD. Toxicity and metal content of organic solvent extracts from airborne particulate matter in Puerto Rico. Environmental Research. 2006;102:314–325. doi: 10.1016/j.envres.2006.04.010. http://dx.doi.org/10.1016/j.envres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhalation Toxicology. 2009;21:1–31. doi: 10.1080/08958370802105405. http://dx.doi.org/10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- 24.Lippmann M, Chen LC. Health effects of concentrated ambient air particulate matter (CAPs) and its components. Critical Reviews in Toxicology. 2009;39:865–913. doi: 10.3109/10408440903300080. http://dx.doi.org/10.3109/10408440903300080. [DOI] [PubMed] [Google Scholar]
- 25.Ma JY, Ma JK. The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. Journal of Environmental Science and Health Part C: Environmental Carcinogenesis & Ecotoxicology Reviews. 2002;20:117–147. doi: 10.1081/GNC-120016202. http://dx.doi.org/10.1081/GNC-120016202. [DOI] [PubMed] [Google Scholar]
- 26.Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicology and Applied Pharmacology. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. http://dx.doi.org/10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz DA. Does inhalation of endotoxin cause asthma? American Journal of Respiratory and Critical Care Medicine. 2001;163:305–313. doi: 10.1164/ajrccm.163.2.ed2000a. http://dx.doi.org/10.1164/ajrccm.163.2.ed2000a. [DOI] [PubMed] [Google Scholar]
- 28.de Kok TM, Driece HA, Hogervorst JG, Briedé JJ. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutation Research. 2006;613:103–122. doi: 10.1016/j.mrrev.2006.07.001. http://dx.doi.org/10.1016/j.mrrev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. Journal of Environmental Science and Health Part C: Environmental Carcinogenesis & Ecotoxicology Reviews. 2008;26:339–362. doi: 10.1080/10590500802494538. http://dx.doi.org/10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 30.D'Amato G, Cecchi L. Effects of climate change on environmental factors in respiratory allergic diseases. Clinical & Experimental Allergy. 2008;38:1264–1274. doi: 10.1111/j.1365-2222.2008.03033.x. http://dx.doi.org/10.1111/j.1365-2222.2008.03033.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang YC, Li Z, Carter JD, Soukup JM, Schwartz DA, Yang IV. Fine ambient particles induce oxidative stress and metal binding genes in human alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology. 2009;41:544–552. doi: 10.1165/rcmb.2008-0064OC. http://dx.doi.org/10.1165/rcmb.2008-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook RD, Rajagopalan SC, Pope III A, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010:2331–2377. doi: 10.1161/CIR.0b013e3181dbece1. http://dx.doi.org/10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed]
- 33.Jie Y, Ismail NH, Jie X, Isa ZM. Do indoor environments influence asthma and asthma-related symptoms among adults in homes? A review of the literature. Toxicology. 2011;299:125–132. doi: 10.1016/j.jfma.2011.07.003. http://dx.doi.org.proxy.library.emory.edu/10.1016/j.jfma.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.de Longueville F, Ozer P, Doumbia S, Henry S. Desert dust impacts on human health: An alarming worldwide reality and a need for studies in West Africa. International Journal of Biometeorology. 2012;3:1–19. doi: 10.1007/s00484-012-0541-y. [DOI] [PubMed] [Google Scholar]
- 35.Van Berlo D, Hullmann M, Schins RP. Toxicology of ambient particulate matter. Molecular, Clinical and Environmental Toxicology: Experientia Supplementum. 2012;101:165–217. doi: 10.1007/978-3-7643-8340-4_7. http://dx.doi.org/10.1007/978-3-7643-8340-4_7. [DOI] [PubMed] [Google Scholar]
- 36.Weichenthal S. Selected physiological effects of ultrafine particles in acute cardiovascular morbidity. Environmental Research. 2012;115:26–36. doi: 10.1016/j.envres.2012.03.001. http://dx.doi.org/10.1016/j.envres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Hetland RB, Cassee FR, Refsnes M, Schwarze PE, Låg M, Boere AJ, et al. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicology in Vitro. 2004;18:203–212. doi: 10.1016/s0887-2333(03)00142-5. http://dx.doi.org/10.1016/S0887-2333(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 38.Hetland R, Cassee F, Låg M, Refsnes M, Dybing E, Schwarze PE. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Particle and Fibre Toxicology. 2005;2:1–15. doi: 10.1186/1743-8977-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbas I, Saint-Georges F, Billet S, Verdin A, Mulliez P, Shirali P, et al. Air pollution particulate matter (PM2.5)-induced gene expression of volatile organic compound and/or polycyclic aromatic hydrocarbon-metabolizing enzymes in an in vitro coculture lung model. Toxicology in Vitro. 2008;23:37–46. doi: 10.1016/j.tiv.2008.09.020. http://dx.doi.org/10.1016/j.tiv.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Maier KL, Alessandrini F, Beck-Speier I, Hofer TP, Diabaté S, Bitterle E, et al. Health effects of ambient particulate matter-biological mechanisms and inflammatory responses to in vitro and in vivo particle exposures. Inhalation Toxicology. 2008;20:319–337. doi: 10.1080/08958370701866313. [DOI] [PubMed] [Google Scholar]
- 41.Billet S, Abbas I, Le Goff J, Verdin A, André V, Lafargue P-E, et al. Genotoxic potential of polycyclic aromatic hydrocarbons-coated onto airborne particulate matter (PM2.5) in human lung epithelial A549 cells. Cancer Letters. 2008;270:144–155. doi: 10.1016/j.canlet.2008.04.044. http://dx.doi.org/10.1016/j.canlet.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 42.Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, et al. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. Journal of Leukocyte Biology. 2009;86:303–312. doi: 10.1189/jlb.1008587. http://dx.doi.org/10.1189/jlb.1008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, et al. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicological Sciences. 2010;118:574–585. doi: 10.1093/toxsci/kfq290. http://dx.doi.org/10.1093/toxsci/kfq290. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Mohan C. Toll-like receptor signaling pathways-therapeutic opportunities. Mediators of Inflammation, 2010. 2010:78123. doi: 10.1155/2010/781235. Article ID. http://dx.doi.org/10.1155/2010/781235. [DOI] [PMC free article] [PubMed]
- 45.Aust AE, Cook PM, Dodson RF. Morphological and chemical mechanisms of elongated mineral particle toxicities. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2011;14:40–75. doi: 10.1080/10937404.2011.556046. http://dx.doi.org/10.1080/10937404.2011.556046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dergham M, Lepers C, Verdin A, Billet S, Cazier F, Courcot D, et al. Prooxidant and proinflammatory potency of air pollution particulate matter (PM2.5-0.3) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B). Chemical Research in Toxicology. 2012;25:904–919. doi: 10.1021/tx200529v. http://dx.doi.org/10.1021/tx200529v. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Tighe RM, Feng F, Ledford JG, Hollingsworth JW. Genes of innate immunity and the biological response to inhaled ozone. Journal of Biochemical and Molecular Toxicology. 2013;27:3–16. doi: 10.1002/jbt.21453. http://dx.doi.org/10.1002/jbt.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi OV, Kinnula VL, Tienari J, Huhti E. Association of severe asthma attacks with weather, pollen, and air pollutants. Thorax. 1993;48:244–248. doi: 10.1136/thx.48.3.244. http://dx.doi.org/10.1136/thx.48.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ledogar RJ, Penchaszadeh A, Garden CC, Iglesias G. Asthma and Latino cultures: Different prevalence reported among groups sharing same environment. American Journal of Public Health. 2000;90:929–935. doi: 10.2105/ajph.90.6.929. http://dx.doi.org/10.2105/AJPH.90.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gyan K, Henry W, Lacaille S, Laloo A, LamseeEbanks C, McKay S, et al. African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. International Journal of Biometeorology. 2004;49:371–376. doi: 10.1007/s00484-005-0257-3. http://dx.doi.org/10.1007/s00484-005-0257-3. [DOI] [PubMed] [Google Scholar]
- 51.Prospero JM, Blades E, Naidu R, Mathison G, Thani H, Lavoie MC. Relationship between African dust carried in the Atlantic trade winds and surges in pediatric asthma attendances in the Caribbean. International Journal of Biometeorology. 2008;52:823–832. doi: 10.1007/s00484-008-0176-1. http://dx.doi.org/10.1007/s00484-008-0176-1. [DOI] [PubMed] [Google Scholar]
- 52.Karakatsani A, Analitis A, Perifanou D, Ayres JG, Harrison RM, Kotronarou A, et al. Particulate matter and air pollution and respiratory symptoms in individuals having either asthma or chronic obstructive pulmonary disease: A European multicentre panel study. Environmental Health. 2012;11:75. doi: 10.1186/1476-069X-11-75. http://dx.doi.org/10.1186/1476-069X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G, Jiang R, Zhao Z, Song W. Effects of ozone and the particulate matter (PM2.5) on rat system inflammation and cardiac function. Toxicology Letters. 2013;217:23–33. doi: 10.1016/j.toxlet.2012.11.009. http://dx.doi.org/10.1016/j.toxlet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Zora JE, Sarnat SE, Raysoni AU, Johnson BA, Li WW, Greenwald R, et al. Associations between urban air pollution and pediatric asthma control in El Paso, Texas. Science of the Total Environment. 2013;448:56–65. doi: 10.1016/j.scitotenv.2012.11.067. http://dx.doi.org/10.1016/j.scitotenv.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 55.Li N, Hao M, Phalen R, Hinds W, Nel A. Particulate air pollutants and asthma A paradigm for the role of oxidative stressing PM-induced adverse health affects. Clinical Immunology. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. http://dx.doi.org/10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicology and Applied Pharmacology. 2001;171:20–26. doi: 10.1006/taap.2000.9096. http://dx.doi.org/10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- 57.Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, et al. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicological Sciences. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. http://dx.doi.org/10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;11:1161–1208. doi: 10.2174/0929867053764635. http://dx.doi.org/10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 59.Gualtieri M, Øvrevik J, Holme JA, Grazia-Perrone M, Bolzacchini E, Schwarze PE, et al. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicology in Vitro. 2010;24:29–39. doi: 10.1016/j.tiv.2009.09.013. http://dx.doi.org/10.1016/j.tiv.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Riley MR, Boesewetter DE, Kim AM, Sirvent FP. Effects of metals Cu, Fe, Ni, V, and Zn on rat lung epithelial cells. Toxicology. 2003;190:171–184. doi: 10.1016/s0300-483x(03)00162-8. [61] http://dx.doi.org/10.1016/S0300-483X(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 61.Carter JD, Ghio A, Samet J, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal dependent. Toxicology and Applied Pharmacology. 1997;146:180–188. doi: 10.1006/taap.1997.8254. [62] http://dx.doi.org/10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- 62.Reyes D, Rosario O, Rodríguez JF, Jiménez BD. Toxic evaluation of organic extracts from airborne particulate matter in Puerto Rico. Environmental Health Perspectives. 2010;108:635–640. doi: 10.1289/ehp.00108635. [63] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acevedo-Figueroa D, Rodríguez-Sierra CJ, Jimé- nez-Vélez BD. Concentrations of Ni and V, other heavy metals, arsenic, elemental and organic carbon in atmospheric fine particles (PM2.5) from Puerto Rico. Toxicology & Industrial Health. 2006;22:87–99. doi: 10.1191/0748233706th247oa. [64] http://dx.doi.org/10.1191/0748233706th247oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gioda A, Pérez U, Rosa Z, Jiménez-Vélez BD. Concentrations of trace elements in airborne PM10 from Jobos Bay National Estuary, Puerto Rico. Water, Air, and Soil Pollution. 2006;174:141–159. [65] http://dx.doi.org/10.1007/s11270-005-9069-7. [Google Scholar]
- 65.Gioda A, Pérez U, Rosa Z, Jiménez-Vélez BD. Particulate matter (PM10 and PM2.5) from different areas of Puerto Rico. Fresenius Environmental Bulletin. 2007;16:861–868. [66] [Google Scholar]
- 66.Gioda A, Fuentes-Mattei E, Jiménez-Vélez B. Evaluation of cytokine expression in BEAS cells exposed to fine particulate matter (PM2.5) from specialized indoor environments. International Journal of Environmental Health Research. 2011;21:106–119. doi: 10.1080/09603123.2010.515668. [67] http://dx.doi.org/10.1080/09603123.2010.515668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiménez-Vélez BD, Gioda A, Fuentes-Mattei E. Organic and aqueous extracts from particulate matter (PM2.5) and their effects on the immunological response of BEAS cells. In: Collery P, editor. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris: 2006. pp. 267–272. [68] [Google Scholar]
- 68.Fuentes-Mattei E, Rivera E, Gioda A, Sanchez-Rivera D, Roman-Velazquez FR, Jiménez-Vélez BD. Use of human bronchial epithelial cells (BEAS-2B) to study immunological markers resulting from exposure to PM2.5 organic extract from Puerto Rico. Toxicology and Applied Pharmacology. 2010;243:381–389. doi: 10.1016/j.taap.2009.12.009. [69] http://dx.doi.org/10.1016/j.taap.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention, National Center for Health Statistics Asthma prevalence, health care use, and mortality: United States, 2005-2009. National Health Statistics Reports. 2011 [70] http://www.cdc.gov/nchs/data/nhsr/nhsr032.pdf. [PubMed]
- 70.Centers for Disease Control and Prevention, National Center for Health Statistics National surveillance of Asthma: United States, 2001-2010. Vital and Health Statistics. Series 3. 2012 [71] http://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. [PubMed]
- 71.Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, Ramsdell JW. Desert dust exposure is associated with increased risk of asthma hospitalization in children. American Journal of Respiratory and Critical Care Medicine. 2010;182:1475–1481. doi: 10.1164/rccm.201002-0296OC. [72] http://dx.doi.org/10.1164/rccm.201002-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li S, Williams G, Jalaludin B, Baker P. Panel studies of air pollution on children's lung function and respiratory symptoms: A literature review. Journal of Asthma. 2012;49:895–910. doi: 10.3109/02770903.2012.724129. [73] http://dx.doi.org/10.3109/02770903.2012.724129. [DOI] [PubMed] [Google Scholar]
- 73.Tam WW, Wong TW, Wong AH, Hui DS. Effect of dust storm events on daily emergency admission for respiratory diseases. Respirology. 2012;17:143–148. doi: 10.1111/j.1440-1843.2011.02056.x. [74] http://dx.doi.org/10.1111/j.1440-1843.2011.02056.x. [DOI] [PubMed] [Google Scholar]
- 74.Manzano-Leon N, Quintana R, Sanchez B, Serrano J, Vega E, Vazquez-Lopez, et al. Variation in the composition and in vitro pro-inflammatory effect of urban particulate matter from different sites. Journal of Biochemical and Molecular Toxicology. 2013;27:87–97. doi: 10.1002/jbt.21471. [75] http://dx.doi.org/10.1002/jbt.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asthma in Puerto Rico: BRFSS analysis for the year 2000 Asthma Statistical Report. 2002 [76] http://www.rcm.upr.edu/publichealth/diabetes/Asthma_Statistical_Report_ Vol1.pdf.
- 76.Heinrich J, Slama R. Fine particles, a major threat to children. International Journal of Hygiene and Environmental Health. 2007;210:617–622. doi: 10.1016/j.ijheh.2007.07.012. [77] http://dx.doi.org/10.1016/j.ijheh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: Evidence of association and effect modification. Environmental Research. 2011;11:418–424. doi: 10.1016/j.envres.2011.01.014. [78] http://dx.doi.org/10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Saravia J, Lee GI, Lomnicki S, Dellinger B, Cormier SA. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: A review. Journal of Biochemical and Molecular Toxicology. 2013;27:56–68. doi: 10.1002/jbt.21465. [79] http://dx.doi.org/10.1002/jbt.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W, Boutaoui N, Brehm JM, Han Y-Y, Schmitz C, Cressley A, et al. ADCYAP1R1 and asthma in Puerto Rican children. American Journal of Respiratory and Critical Care Medicine. 2013;187:584–588. doi: 10.1164/rccm.201210-1789OC. [80] http://dx.doi.org/10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Molecular Psychiatry. 2012;18:742–743. doi: 10.1038/mp.2012.98. [81] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramsey S, Delling M, Clapham DE. An introduction to TRP channels. Annual Review of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [82] http://dx.doi.org/10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 82.Nilius B. TRP channels in disease. Biochimica et Biophysica Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [83] http://dx.doi.org/10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor cation channels in disease. Physiological Review. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [84] http://dx.doi.org/10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 84.Ghelfi E, Rhoden CR, Wellenius GA, Lawurence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicological Sciences. 2008;102:328–336. doi: 10.1093/toxsci/kfn005. [85] http://dx.doi.org/10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- 85.Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, et al. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): A probable mechanism of cute pulmonary toxicity for DEP. Chemical Research in Toxicology. 2011;24:950–959. doi: 10.1021/tx200123z. [86] http://dx.doi.org/10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deering-Rice CE, Johansen ME, Roberts JK, Thomas KC, Romero EG, Lee J, et al. Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material. Molecular Pharmacology. 2012;81:411–419. doi: 10.1124/mol.111.076067. [87] http://dx.doi.org/10.1124/mol.111.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. The Journal of Physiology. 2011;589:1527–1534. doi: 10.1113/jphysiol.2010.202077. [88] http://dx.doi.org/10.1113/jphysiol.2010.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fariss MW, Gilmour MI, Reilly CA, Liedtke W, Ghio A. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicological Sciences. 2013;132:253–267. doi: 10.1093/toxsci/kft001. [89] http://dx.doi.org/10.1093/toxsci/kft001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agopyan N, Bhatti T, Yu S, Simon SA. Vanilloid receptor activation by 2- and 10-μm particles induces responses leading to apoptosis in human airway epithelial cells. Toxicology and Applied Pharmacology. 2003;192:21–35. doi: 10.1016/s0041-008x(03)00259-x. [90] http://dx.doi.org/10.1016/S0041-008X(03)00259-X. [DOI] [PubMed] [Google Scholar]
- 90.Agopyan N, Li L, Yu S, Simon SA. Negatively charged 2- and 10-μM particles activate vanilloid receptors, increase cAMP, and induce cytokine release. Toxicology and Applied Pharmacology. 2003;186:63–76. doi: 10.1016/s0041-008x(02)00013-3. [91] http://dx.doi.org/10.1016/S0041-008X(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 91.Inoue R, Jensen JL, Shi J, Morita H, Nishida M, Honda A, et al. Transient receptor potential channels in cardiovascular function and disease. Circulation Research. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [92] http://dx.doi.org/10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 92.Segura-Valdez L, Pardo A, Caxiola M, Uhal BD, Becerril C, Selma M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–694. doi: 10.1378/chest.117.3.684. [93] http://dx.doi.org/10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 93.Joos L, He JQ, Shepherdson MB, Connett JE, Anthonisen NR, Pare PD, et al. The role of matrix metalloproteinase polymorphysms in the rate of decline in lung function. Human Molecular Genetics. 2002;11:569– 576. doi: 10.1093/hmg/11.5.569. [94] http://dx.doi.org/10.1093/hmg/11.5.569. [DOI] [PubMed] [Google Scholar]
- 94.Atkinson J, Senior RM. Matrix metalloproteinase-9 in lung remodeling. American Journal of Respiratory Cell and Molecular Biology. 2003;8:12–24. doi: 10.1165/rcmb.2002-0166TR. [95] http://dx.doi.org/10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 95.Kong MYF, Gaggar A, Li Y, Winkler M, Blalock JE, Clancy J. Matrix metalloproteinase activity in pediatric acute lung injury. International Journal of Medical Sciences. 2009;61:9–17. doi: 10.7150/ijms.6.9. [96] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2610341/#!po=73.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu G, Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, et al. Associaiton of TRPV4 gene polymorphism with chronic obstructive pulmonary disease. Human Molecular Genetics. 2009;18:2053–2062. doi: 10.1093/hmg/ddp111. [97] (ICGN Investigators) http://dx.doi.org/10.1093/hmg/ddp111. [DOI] [PubMed] [Google Scholar]
- 97.Smit LAM, Kogevinas M, Anto JM, Bouzigon E, Gonzalez JR, Moual NL, et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respiratory Research. 2012;13:26. doi: 10.1186/1465-9921-13-26. [98] http://dx.doi.org/10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantero-Recasens G, Gonzalez JR, Fandos C, DuranTauleria E, Smit LAM, Kauffmann F, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. The Journal of Biological Chemistry. 2010;285:27532–27535. doi: 10.1074/jbc.C110.159491. [99] http://dx.doi.org/10.1074/jbc.C110.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghio AJ, Stonehuerner J, Pritchard RJ, Piantadosi CA, Quigley DR, Dreher KL, et al. Humiclike substances in air pollution particles correlate with concentration of transition metals and oxidant generation. Inhalation Toxicology. 1996;8:479–494. [100] http://dx.doi.org/10.3109/08958379609005441. [Google Scholar]
- 101.Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. http://dx.doi.org/10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]