Abstract
To determine the role of surfactant protein-A(SP-A) in antiviral host defense, mice lacking SP-A (SP-A–/–) were produced by targeted gene inactivation. SP-A–/– and control mice (SP-A+/+) were infected with respiratory syncytial virus (RSV) by intratracheal instillation. Pulmonary infiltration after infection was more severe in SP-A–/– than in SP-A+/+ mice and was associated with increased RSV plaque-forming units in lung homogenates. Pulmonary infiltration with polymorphonuclear leukocytes was greater in the SP-A–/– mice. Levels of proinflammatory cytokines tumor necrosis factor-α and interleukin-6 were enhanced in lungs of SP-A–/– mice. After RSV infection, superoxide and hydrogen peroxide generation was deficient in macrophages from SP-A–/– mice, demonstrating a critical role of SP-A in oxidant production associated with RSV infection. Coadministration of RSV with exogenous SP-A reduced viral titers and inflammatory cells in the lung of SP-A–/– mice. These findings demonstrate that SP-A plays an important host defense role against RSV in vivo.
Introduction
Surfactant protein-A (SP-A) is a 28- to 34-kDa member of the collectin subgroup of the mammalian C-type lectins that also includes surfactant protein-D (SP-D), mannose-binding protein, and conglutinin (1, 2). The collectins are thought to be involved in innate host defense against various bacterial and viral pathogens. For example, children deficient in mannose-binding proteins are more susceptible to bacterial infection (3). The collectins form multimeric structures resembling C1q (the first component of the complement cascade), consisting of multimeric collagenous NH2-terminal domains and globular COOH-terminal, carbohydrate-binding domains (2, 4). The C-type lectins bind carbohydrate surfaces of many microorganisms, mediating phagocytosis and killing by phagocytic cells (5–8).
SP-A is an abundant C-type lectin produced primarily by alveolar type II cells, nonciliated bronchiolar cells, and tracheobronchial gland cells in the lung. SP-A binds to specific cell-surface receptors on alveolar macrophages (9) and type II epithelial cells (10). In vitro, SP-A stimulates macrophage chemotaxis (11) and enhances the binding of serum-opsonized Staphylococcus aureus (12), non–serum-opsonized Escherichia coli, S. aureus, Hemophilus influenzae, and Mycobacterium tuberculosis to alveolar macrophages (13–15). SP-A also binds herpes simplex virus, herpes simplex virus–infected cells (16, 17), and influenza A virus (18, 19).
Respiratory syncytial virus (RSV) produces an upper respiratory tract disease that may progress to acute bronchiolitis or interstitial pneumonia. Approximately one in 100 children infected with RSV will require hospitalization (20); as many as 11% of hospitalized infants may require intensive care (21). Children with respiratory failure from RSV bronchiolitis have decreased concentrations of SP-A in bronchoalveolar lavage (BAL) fluid (22, 23). Conditions predisposing to severe RSV disease include prematurity, bronchopulmonary dysplasia, congenital heart disease, congenital or acquired immunodeficiency, and cystic fibrosis — conditions that may also be associated with decreased SP-A in the lung (24–26).
Specific as well as nonspecific immune mechanisms take part in RSV immunity and probably also RSV pathogenesis. The specific immune response includes cytotoxic T cells and antibody production. Nonspecific immune responses include natural killer cells, macrophages, neutrophils, eosinophils, and basophils (27). Alveolar macrophages are thought to play a critical role in host defense of the lung. Alveolar macrophages bind, phagocytose, and kill bacteria and viruses in association with cellular activation and release of intracellular proteases and reactive oxygen species. Reactive oxygen species are released by activated alveolar macrophages and may contribute to killing of viruses (28). In vitro, SP-A directly stimulates lucigenin-dependent chemiluminescence (12) and produces a dose-dependent enhancement of oxygen radical release from rat alveolar macrophages (29). In contrast, stimulated canine alveolar macrophages produce less superoxide anions in the presence of SP-A (30).
In spite of considerable in vitro evidence that SP-A is involved in host defense, its role in vivo has only recently been demonstrated. SP-A–deficient mice (SP-A–/–) produced by targeted gene inactivation are susceptible to group B streptococcal and Pseudomonas aeruginosa pneumonia after intratracheal administration of the organism (31, 32). Although there is compelling evidence that SP-A enhances host defense against viruses in vitro, its role in the clearance of viral pathogens in vivo has not been demonstrated. The present study tested whether SP-A–/– mice had increased susceptibility to RSV infection in vivo.
Methods
Animal production.
The murine SP-A gene was inactivated by gene targeting as described previously (33). Lungs of SP-A–/– mice do not contain detectable SP-A mRNA or protein (33). To limit variability related to strain differences, 129J wild-type (+/+) and SP-A–/– mice of the same strain were studied. Animals were housed and studied under Institutional Animal Care and Use Committee–approved protocols in the animal facility of the Children’s Hospital Research Foundation. Male and female mice weighing approximately 20–30 g (8–10 weeks old) were used.
Purification of human SP-A.
Human SP-A obtained from patients with alveolar proteinosis was purified by the 1-butanol extraction method of Haagsman et al. (34). Purified SP-A was dissolved (2 mg/ml) in NaHEPES (pH 7.2) and tested with the Limulus Amoebocyte Lysate assay (Sigma Chemical Co., St. Louis, Missouri, USA) for endotoxin. SP-A used in the present study had no detectable endotoxin (<0.06 EU/ml).
Preparation of virus.
HEp-2 cells were maintained in Eagle’s minimal essential media (EMEM) supplemented with glutamine, amphotericin, streptomycin, penicillin G, and 10% low-immunoglobulin FBS (10% EMEM). The A2 strain of RSV was kindly supplied by B.S. Graham (Vanderbilt University, Nashville, Tennessee, USA). RSV was plaque-purified three times under agarose. The third plaque was inoculated into a subconfluent HEp-2 cell monolayer. After adsorption for 1 h at room temperature, 10% EMEM was added, and the infection was allowed to proceed for 3 days at 37°C until the entire monolayer showed cytopathic effects. The contents of the flask were resuspended and distributed in 1-ml aliquots, quick-frozen with alcohol/dry ice, and stored at –80°C. Working stocks were derived from this master stock by infecting subconfluent HEp-2 monolayers at a multiplicity of infection of 0.1 and harvesting the monolayer when it appeared to be completely infected. The cells and media (50 ml) were sonicated (Ultrasonic homogenizer; Cole-Parmer Instrument Co., Chicago, Illinois, USA) on ice with eight 1-s bursts using an output of 50. The suspension was clarified by centrifugation at 1,800 g for 10 min. The supernatant was quick-frozen and stored at –80°C.
Intratracheal inoculation.
Administration of RSV into the respiratory tract of the mice was performed by intratracheal inoculation of 106 or 107 plaque-forming units (pfu) diluted in 10% EMEM. Intratracheal inoculation as described previously (31) was used to deliver RSV. Controls received sterile 10% EMEM.
Viral clearance.
Quantitative RSV cultures of lung homogenates were performed 3, 5, and 7 days after inoculation of the animals with RSV or RSV plus SP-A. Because previous studies demonstrated enhanced bacterial clearance in SP-A–/– mice with 100 μg of SP-A (35), this dose was chosen for the present study. The entire lung was removed as described (31), homogenized in 2 ml of sterile 10% EMEM, quick-frozen, weighed, and stored at –80°C. HEp-2 monolayers, 80% confluent in Costar 12-well plates, were used for the plaque assay.
Thawed tissues were clarified, serially diluted in 10% EMEM, plated on HEp-2 monolayer (50 μl), and adsorbed for 1 h, and 0.75% methylcellulose in 10% EMEM was added to each well. After 5 days at 37°C, the monolayers were fixed with 10% formalin phosphate, stained with hematoxylin and eosin, and viral plaques were counted under a dissecting microscope. The resulting titer was divided by the lung weight and reported as pfu per gram of lung. Experiments comparing viral titers in whole lung from fresh and frozen tissue revealed a twofold reduction in pfu with freezing. Viral titers in the lung of SP-A–treated mice were assessed in fresh lung homogenates.
Pulmonary pathology.
Lungs were inflated via a tracheal cannula at 20 cm of pressure with 4% paraformaldehyde and removed en bloc from the thorax. Lungs were dehydrated and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin.
Bronchoalveolar lavage.
Lung cells were recovered by bronchoalveolar lavage (BAL). Animals were sacrificed as described for viral clearance, and lungs were lavaged three times with 1 ml of sterile PBS. The fluid was centrifuged at 2,000 g for 10 min and resuspended in 600 μl of PBS, and total cells were stained with trypan blue and counted under light microscopy. Differential cell counts were performed on cytospin preparations stained with Diff-Quick (Scientific Products, McGaw Park, Indiana, USA).
Cytokine production.
Lung homogenates were centrifuged at 2,000 g, and the supernatants stored at –20°C. Tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and macrophage inflammatory protein-2 (MIP-2) were quantitated using murine sandwich ELISA kits (R&D Systems Inc., Minneapolis, Minnesota, USA) according to the manufacturer’s directions. All plates were read on a microplate reader (Molecular Devices, Menlo Park, California, USA) and analyzed with the use of a computer-assisted analysis program (Softmax; Molecular Devices). Only assays having standard curves with a calculated regression line value >0.95 were accepted for analysis.
Superoxide anion generation.
Superoxide anion and hydrogen peroxide production by alveolar macrophages was determined as described (36). Three days after intratracheal inoculation of RSV (106 pfu), alveolar macrophages were collected by BAL with 3 ml of dye-free RPMI media (GIBCO BRL, Grand Island, New York, USA). BAL fluid from six mice was pooled to provide sufficient numbers of macrophages for analysis. The lavage was centrifuged at 1,200 g for 10 min, and the pellet resuspended in 200 μl of PBS. One hundred thousand cells were placed in wells of a 96-well plate with 1.2 mg/ml (∼100 μmol/l) cytochrome c, with or without 20 μg/ml superoxide dismutase or catalase (200 U/105 cells), in a final volume of 200 μl of HBSS. Superoxide anion production was determined after activation with 100 ng/ml phorbol myristate acetate (PMA). Optical density at 550 nm was determined using a THERMOmax microplate reader (Molecular Devices) linked to a laboratory computer. Measurements were made initially and at 5, 10, and 15 min, then every 15 min until 2 h at 37°C. Optical density was converted to nanomoles of cytochrome c reduced using a molar extinction coefficient of 21.1/mM·cm. Each measurement was the mean of at least two replicates with six determinations at each time. Data were expressed as nanomoles cytochrome c reduced per 105 cells. Superoxide production was assessed by subtracting activity in the presence of superoxide dismutase and hydrogen peroxide by subtracting activity in the presence of catalase from total oxygen radical production. Hydrogen peroxide production by macrophages was also measured using a commercially available assay (Bioxytech H2O2 560 assay; OXIS International, Portland, Oregon, USA) based on the oxidation of ferrous ions (Fe2+) to ferric ions (Fe3+) by hydrogen peroxide under acidic conditions. Methods followed the manufacturer’s recommendations.
Statistical methods.
Lung viral titers, total cell counts, and differential analysis were compared using the nonparametric Van der Waerden rank rest. Superoxide and hydrogen peroxide peak values were compared using the median scores nonparametric test. Comparisons among SP-A–/– and wild-type mice treated with SP-A were performed with the least squared means model analysis. Findings were considered statistically significant at probability levels <0.05.
Results
Pulmonary pathology after RSV administration.
The viral dose used in the current study was 106 or 107 pfu. This dose range has been shown to generate inflammation in the lungs of mice over a seven-day period without mortality (37). Intratracheal administration of RSV was well tolerated, and all animals survived the study period at the 106 and 107 pfu dose. No alterations in activity or physical appearance of the animals were detected throughout the study.
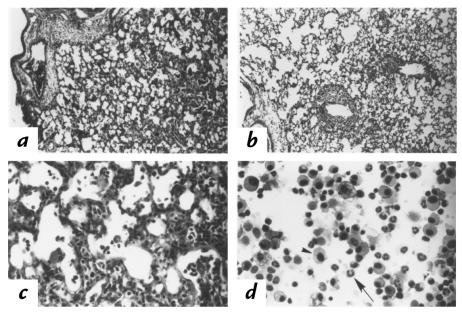
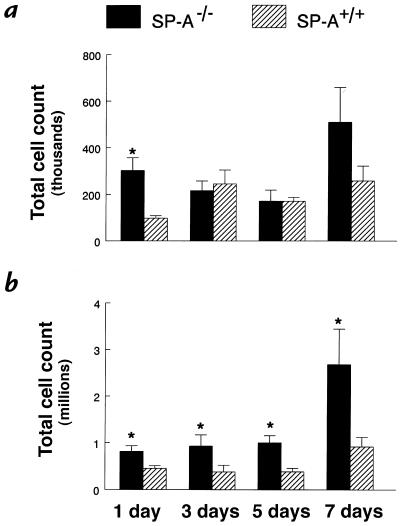
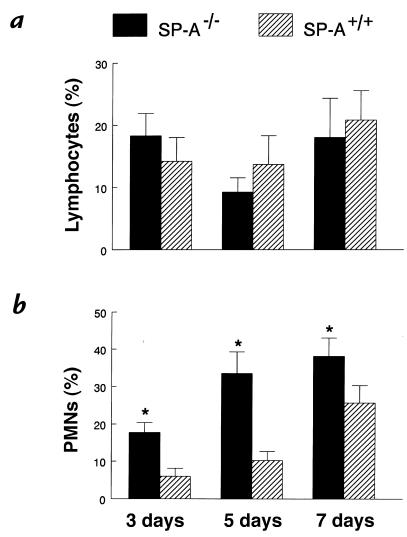
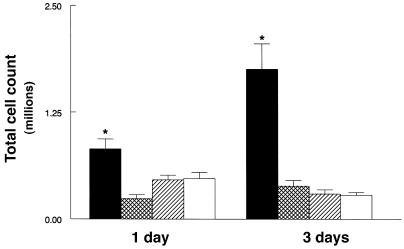
Pulmonary infiltrates were observed in SP-A–/– and SP-A+/+ mice five days after administration of 107 pfu RSV. The infiltrates consisted primarily of macrophages. In SP-A–/– and SP-A+/+ mice, peribronchiolar, perivascular, and alveolar infiltrates consisting of macrophages and polymorphonuclear leukocytes were observed at seven days (Figure 1). Although the extent of pulmonary inflammation appeared similar by histological examination, three, five, and seven days after 107 pfu RSV, SP-A–/– mice had increased total cells counts in BAL fluid (Figure 2). Baseline total cell counts in BAL fluid from control (SP-A+/+) mice inoculated with sterile 10% EMEM were 1.4 × 105 ± 1.4 × 104 (mean ± SEM). A significantly greater percentage of polymorphonuclear leukocytes (PMNs) were detected in BAL fluid from SP-A–/– compared with SP-A+/+ mice at all time points. No differences were detected in percentages of lymphocytes (Figure 3). Pulmonary infiltrates were not observed in SP-A+/+ mice inoculated with sterile 10% EMEM (n = 5).
Figure 1.
Pulmonary pathology after RSV infection. Photomicrographs of lung parenchyma in (a) SP-A–/– and (b) SP-A+/+ mice were prepared 7 days after intratracheal administration of 107 pfu RSV. Histological sections were stained with hematoxylin and eosin. Infiltration was observed surrounding the terminal bronchi and pulmonary vessels in the SP-A–/– and SP-A+/+ mice. Higher magnification demonstrates infiltration with mononuclear cells and polymorphonuclear leukocytes in SP-A–/– mice (c). Cells from BAL consisted of mononuclear cells (arrowhead) and polymorphonuclear leukocytes (arrow) (d). a and b: ×10; c and d: ×40. BAL, bronchoalveolar lavage; pfu, plaque-forming units; RSV, respiratory syncytial virus; SP-A, surfactant protein-A.
Figure 2.
Increased total cell counts in BAL fluid from SP-A–/– mice. Lung cells were recovered by BAL, stained with trypan blue, and counted under light microscopy. (a) The low dose of RSV (106 pfu) caused less migration of inflammatory cells in the lungs from both genotypes; however, an earlier influx of cells was found in SP-A–/– (filled bars) BAL fluid than in SP-A+/+ (hatched bars) mice. (b) SP-A–/– mice infected with 107 pfu RSV had significantly greater total cell counts in BAL fluid at all time points. Data are mean ± SEM with n = 5 mice per group (a) and n = 9 mice per group (b). *P < 0.05 compared with SP-A+/+ mice.
Figure 3.
Increased neutrophils in BAL fluid from SP-A–/– mice after RSV infection. Cytospin preparations of BAL fluid were stained with Diff-Quik to identify macrophages, lymphocytes, and polymorphonuclear leukocytes. (a) The percentage of neutrophils in BAL fluid was significantly greater 3, 5, and 7 days after administration of 107 pfu RSV to SP-A–/– (filled bars) compared with SP-A+/+ (hatched bars) mice. (b) Percentages of lymphocytes in BAL fluid were not different between the two groups. Data are mean ± SEM with n = 9 mice per group. *P < 0.05 compared with SP-A+/+ mice. PMNs, polymorphonuclear leukocytes.
Decreased viral clearance in SP-A–/– mice.
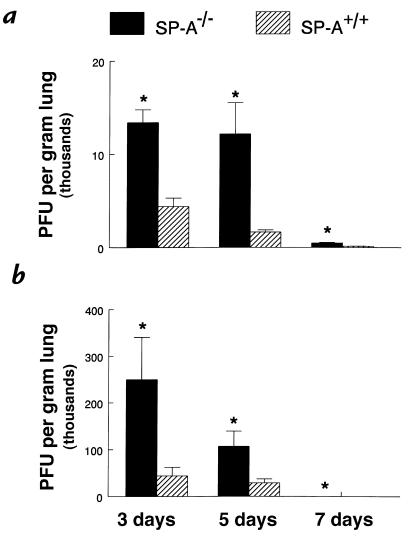
Viral titers in lung homogenates were increased in SP-A–/– mice three, five, and seven days after infection (Figure 4). The difference in viral titers between SP-A–/– and SP-A+/+ mice was found with both the 106 and 107 pfu inoculum of RSV and was most evident at three and five days, indicating that RSV was cleared from the lungs of SP-A–/– mice at a slower rate than from the lungs of SP-A+/+ mice.
Figure 4.
Increased RSV in lung homogenates from SP-A–/– mice. RSV titers were determined by quantitative plaque assays of lung homogenates. Viral titers were significantly greater 3, 5, and 7 days after administration of both 106 (a) and 107 (b) pfu RSV in SP-A–/– (filled bars) compared with SP-A+/+ (hatched bars) mice. Data are mean ± SEM with n = 6 mice per group (a) and n = 10 mice per group (b). *P < 0.05 compared with SP-A+/+ mice.
Cytokine levels in lung homogenates.
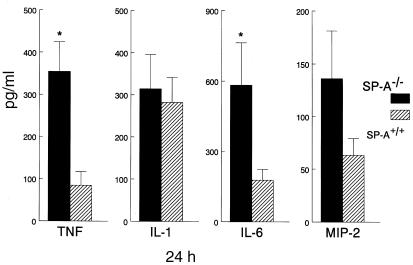
Twenty-four hours after RSV infection, proinflammatory cytokines TNF-α and IL-6 were significantly increased in lung homogenates from SP-A–/– compared with SP-A+/+ mice (Figure 5). Three days after RSV infection, TNF-α, IL-6, and MIP-2 levels were similar between SP-A–/– and SP-A+/+ mice (data not shown). IL-1β levels were similar at one day in SP-A–/– and SP-A+/+ mice (Figure 5) but were significantly greater three days after RSV infection in the SP-A–/– mice (data not shown).
Figure 5.
Increased tumor necrosis factor-α (TNF) and interleukin-6 (IL-6) in lung homogenates from SP-A–/– mice after RSV infection. Concentrations of TNF-α, interleukin-1β (IL-1), IL-6, and macrophage inflammatory protein-2 (MIP-2) were assessed in lung homogenates from SP-A–/– (filled bars) and SP-A+/+ (hatched bars) mice. Increased concentrations of TNF-α and IL-6 were found in lung homogenates from the SP-A–/– mice at 24 h. Although macrophage inflammatory MIP-2 was increased in SP-A–/– at 24 h, differences did not reach statistical significance. Data are expressed as picograms per milliliter and represent mean ± SEM with n = 10 mice per group. *P < 0.05 compared with SP-A+/+ mice.
Decreased superoxide and hydrogen peroxide production by alveolar macrophages from SP-A–/– mice after RSV infection.
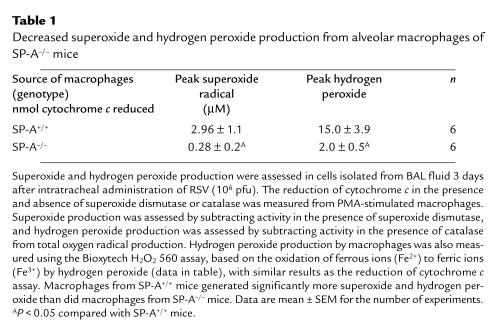
Superoxide and hydrogen peroxide production was assessed in macrophages isolated from BAL fluid three days after intratracheal administration of RSV (106 pfu). After stimulation with PMA, superoxide radical and hydrogen peroxide production by alveolar macrophages was significantly decreased in SP-A–/– compared with SP-A+/+ mice (Table 1).
Table 1.
Decreased superoxide and hydrogen peroxide production from alveolar macrophages of SP-A–/– mice
Exogenous SP-A increases viral clearance and decreases lung inflammation in SP-A–/– mice.
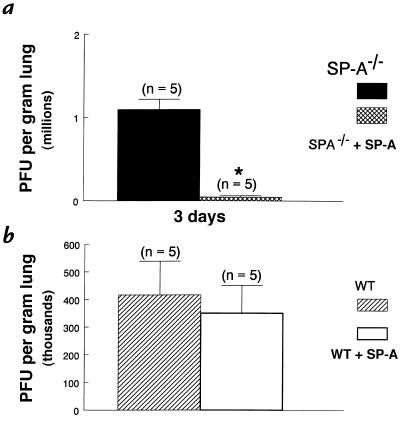
Three days after infection, the clearance of RSV in the SP-A–/– mice was significantly enhanced when RSV was coadministered with SP-A (100 μg) (Figure 6). RSV clearance in SP-A–treated SP-A–/– mice was similar to that in SP-A+/+ mice. Treatment of SP-A+/+ mice with a single dose of SP-A did not enhance viral clearance at three days (Figure 6). Total cell counts in BAL fluid one and three days after RSV infection were significantly reduced in the SP-A–/– mice treated with SP-A compared with untreated SP-A–/– mice. SP-A+/+ mice treated with SP-A had similar cell counts in BAL fluid as did untreated SP-A+/+ mice (Figure 7).
Figure 6.
SP-A enhanced RSV clearance from the lung of SP-A–/– mice. RSV titers were determined by quantitative plaque assays of lung homogenates 3 days after infection with 107 pfu of RSV. Viral titers in the lung were significantly reduced in SP-A–/– mice treated with SP-A (100 μg) (cross-hatched bar) compared with untreated SP-A–/– mice (filled bar) (a). Treatment of SP-A–/– mice with SP-A reduced viral titers to the wild-type level. Viral titers were similar in SP-A–treated (open bar) and untreated wild-type mice (hatched bar) (b). Data are mean ± SEM. *P < 0.05 compared with SP-A–/– mice. WT, wild-type.
Figure 7.
SP-A decreased inflammatory cells in BAL fluid from SP-A–/– mice. After RSV infection (107 pfu), lung cells were recovered by BAL, stained with trypan blue, and counted under light microscopy. One and 3 days after RSV infection, total cell counts in BAL fluid were significantly reduced in SP-A–/– mice treated with SP-A (100 μg) (cross-hatched bars) compared with untreated SP-A–/– mice (filled bars). Treatment of SP-A–/– mice reduced BAL cell counts to the wild-type level (hatched bars). Total cell counts in BAL fluid were similar in SP-A–treated (open bars) and untreated wild-type mice (hatched bars). Data are mean ± SEM. *P < 0.05 compared with SP-A+/+ mice.
Discussion
Pulmonary clearance of intratracheally administered RSV was impaired in SP-A–/– compared with SP-A+/+ mice. Lung inflammation and viral titers were increased in the SP-A–/– mice, with decreased inflammation and enhanced clearance of the virus in SP-A–/– mice treated with exogenous SP-A. After RSV infection, superoxide radical and hydrogen peroxide production was markedly decreased in alveolar macrophages from SP-A–/– mice. These findings support the concept that SP-A plays an important role in pulmonary host defense against RSV infection, mediated in part by increased alveolar macrophage oxygen radical production and viral killing.
Impaired clearance of RSV from the lung of SP-A–/– mice supports the importance of SP-A in host defense. SP-A is a member of the C-type lectin family of polypeptides that includes mannose-binding protein, conglutinin, and SP-D. These proteins share structural features including collagenous NH2-terminal and “globular” COOH-terminal, carbohydrate recognition domains, and all function as opsonins in vitro. In the presence of calcium, SP-A binds a variety of monosaccharides including D-mannose, L-fucose, D-glucose, and D-galactose (2). Adherence and phagocytosis of RSV may be mediated through the heavily glycosylated proteins on the viral surface, e.g., F protein and G protein, which may be recognized by the carbohydrate recognition domain of SP-A. Carbohydrate recognition by SP-A and opsonization of viruses may play an important role in the early clearance of viruses from the lungs. SP-A is expressed primarily in the respiratory epithelial cells and is hypothesized to play a role in protecting the lung from bacterial, viral, and fungal infections (1, 2). In vitro studies with herpes simplex virus type 1 as a model for virus phagocytosis revealed increased phagocytosis by alveolar macrophages in the presence of SP-A. When antibodies to SP-A were added to this in vitro system, the phagocytosis of herpes simplex virus by macrophages was abolished (16). The addition of SP-A to cell cultures infected with influenza A virus resulted in a reduction of viral infected cells (18), and SP-A enhanced the association of influenza A virus with alveolar macrophage in a dose-dependent manner (19). Deglycosylated SP-A did not bind viral infected cells, suggesting that the carbohydrate moiety of SP-A is involved in the recognition of viruses by SP-A (17, 18). The current study demonstrates impaired clearance of RSV from the lung in the absence of SP-A in vivo.
Infiltration of neutrophils after RSV administration was more intense in SP-A–/– than in SP-A+/+ mice. Enhanced neutrophil chemotaxis may have resulted from the increased cytokine production in the lungs of the SP-A–/– mice. MIP-2, a neutrophil chemoattractant, was elevated consistent with increased neutrophils in SP-A–/– BAL fluid. PMNs are able to adhere to RSV-infected epithelial cells (38). SP-A–/– mice had greater viral titers in the lung, which may provide increased binding sites for PMNs. In vitro, macrophages infected with RSV have increased TNF-α and increased IL-6 mRNA and protein production (39). TNF-α has been shown to have antiviral activity in vitro (40); however, in the present in vivo model, viral clearance was impaired in SP-A–/– mice despite increased TNF-α. RSV is known to be a poor inducer of IL-1 compared with viruses like parainfluenza and influenza (41). These data are consistent with the present study, demonstrating similar IL-1 levels in SP-A–/– and SP-A+/+ mice despite increased viral titers in SP-A–/– mice. The role of SP-A and cytokine production in vitro is controversial. SP-A stimulated TNF-α, IL-1, and IL-6 production by mononuclear cells (42). In contrast, McIntosh et al. (43) reported that SP-A blunted TNF-α release from lipopolysaccharide-stimulated macrophages. The present finding that cytokine production was more modest in SP-A+/+ than in SP-A–/– mice in vivo supports the McIntosh et al. study, suggesting that SP-A decreases the release of cytokines in response to viral infection. It is unclear from the present study whether the alveolar macrophages and/or respiratory epithelium have enhanced production of inflammatory cytokines in the absence of SP-A.
Phagocytic cells generate reactive oxygen species that are involved in viral killing. Phagocytosis of invading microorganisms leads to increased oxygen consumption and to reduction of oxygen to superoxide, which is then secreted into the phagosome, where it dismutates to hydrogen peroxide. Production of superoxide and hydrogen peroxide contributes to microbial killing. In the current study, stimulated macrophages from SP-A–/– mice generated less superoxide and hydrogen peroxide than did SP-A+/+ macrophages, demonstrating a critical role of SP-A in oxidant production. Differences in oxygen radical production may not, however, be directly related to RSV infection, as the macrophages were stimulated with PMA. Decreased production of superoxide by macrophages from SP-A–/– mice was also demonstrated previously during bacterial infection (35). In vitro, SP-A enhanced lucigenin-dependent chemiluminescence of rat alveolar macrophages (12), indicating that SP-A may stimulate the release of superoxide radicals to enhance bacterial killing. Other studies, however, failed to demonstrate effects of SP-A on intracellular killing of bacteria by monocytes; and SP-A–opsonized S. aureus did not induce the production of reactive oxygen intermediates (44). Superoxide and hydrogen peroxide production by alveolar macrophages from SP-A–/– mice was decreased compared with SP-A+/+ mice. Influenza viruses, paramyxoviruses, and the hepatitis B virus have been shown to activate monocytes and polymorphonuclear leukocytes in vitro to generate reactive oxygen species (28). The current study suggests that SP-A may enhance RSV killing through macrophage activation and production of reactive oxygen species.
Viral titers of RSV in the lung were greater in the SP-A–/– mice; however, coadministration of RSV with SP-A enhanced clearance and decreased inflammation from lungs of SP-A–/– mice to the level of SP-A+/+ mice. The total pool of SP-A in the lung is found in the macrophage, tissue compartment, and alveolar fluid. Administration of exogenous SP-A may increase the free SP-A available to enhance viral clearance. Treatment of wild-type mice with SP-A did not enhance clearance or decrease inflammation in the lung, suggesting that wild-type levels of SP-A are sufficient to clear the injected dose of RSV. During lung injury, changes in the concentration of surfactant proteins may be caused by changes in SP-A synthesis or degradation. SP-A levels are reduced in BAL fluid from children with respiratory failure due to RSV (22). The present study supports the concept that low levels of SP-A reduce RSV clearance, leading to enhanced inflammation.
In summary, the present study demonstrates a role of SP-A in pulmonary clearance of RSV in vivo. RSV clearance was associated with an enhanced respiratory burst by the alveolar macrophage. Because the airway is usually the portal of entry for RSV and other respiratory pathogens, local production of SP-A is likely to play a role in innate defenses preventing pneumonia. SP-A concentrations are decreased in preterm infants (25) and in primates with bronchopulmonary dysplasia (24), conditions that may influence susceptibility to RSV infection and inflammation. Exogenous administration of SP-A enhanced clearance of RSV and decreased inflammation in the SP-A–/– mice and, therefore, may represent a strategy to prevent or treat RSV pulmonary infections.
Acknowledgments
We thank Barney Graham for providing the RSV stocks, Jaymi Semona for assistance with animal production, and Peter Gartside for assistance with statistical methods. Supported by National Institutes of Health grants HL 28623, HL 58795, and HL 03905 and by The Cystic Fibrosis Foundation.
References
- 1.Thiel S, Reid K. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989;250:78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- 2.Sastry K, Ezekowitz RA. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993;5:59–66. doi: 10.1016/0952-7915(93)90082-4. [DOI] [PubMed] [Google Scholar]
- 3.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra R, Thiel S, Reid KB, Sim RB. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990;172:955–959. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friis-Christiansen P, et al. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990;31:453–460. doi: 10.1111/j.1365-3083.1990.tb02792.x. [DOI] [PubMed] [Google Scholar]
- 6.Ezekowitz RAB, Kuhlman M, Groopman JE, Byrn RA. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeely TB, Coonrod JD. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 8.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pison U, Wright JR, Hawgood S. Specific binding of surfactant apoprotein SP-A to rat alveolar macrophages. Am J Physiol. 1992;262:L412–L417. doi: 10.1152/ajplung.1992.262.4.L412. [DOI] [PubMed] [Google Scholar]
- 10.Wright JR, Borchelt JD, Hawgood S. Lung surfactant apoprotein SP-A (26–36 kDa) binds with high affinity to isolated alveolar type II cells. Proc Natl Acad Sci USA. 1989;86:5410–5414. doi: 10.1073/pnas.86.14.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JR, Youmans DC. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophages. Am J Physiol. 1993;264:L338–L344. doi: 10.1152/ajplung.1993.264.4.L338. [DOI] [PubMed] [Google Scholar]
- 12.van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LMG. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Manz-Keinke H, Plattner H, Schlepper-Schafer J. Lung surfactant protein A (SP-A) enhances serum-independent phagocytosis of bacteria by alveolar macrophages. Eur J Cell Biol. 1992;57:95–100. [PubMed] [Google Scholar]
- 14.McNeely TB, Coonrod JD. Aggregation and opsonization if type A but not type B Hemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol. 1994;11:114–122. doi: 10.1165/ajrcmb.11.1.8018334. [DOI] [PubMed] [Google Scholar]
- 15.Pascula R, et al. Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:209–217. doi: 10.1165/ajrcmb.17.2.2469. [DOI] [PubMed] [Google Scholar]
- 16.van Iwaarden JF, et al. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am J Physiol. 1991;261:L204–L209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]
- 17.van Iwaarden JF, et al. Binding of surfactant protein A (SP-A) to herpes simplex virus type 1–infected cells is mediated by the carbohydrate moiety of SP-A. J Biol Chem. 1992;267:25039–25043. [PubMed] [Google Scholar]
- 18.Benne CA, et al. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. J Infect Dis. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- 19.Benne CA, Benaissa-Trouw B, van Strijp JAG, Kraaijeveld CA, van Iwaarden JFF. Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur J Immunol. 1997;27:886–890. doi: 10.1002/eji.1830270413. [DOI] [PubMed] [Google Scholar]
- 20.Kim HW, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. Am J Epidemiol. 1973;98:216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 21.Green M, Brayer AF, Schenkman KA, Wald ER. Duration of hospitalization in previously well infants with respiratory syncytial virus infection. Pediatr Infect Dis J. 1989;8:601–605. doi: 10.1097/00006454-198909000-00007. [DOI] [PubMed] [Google Scholar]
- 22.LeVine AM, et al. Surfactant content in children with inflammatory lung disease. Crit Care Med. 1996;24:1062–1067. doi: 10.1097/00003246-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Dargaville PA, South M, McDougall PN. Surfactant abnormalities in infants with severe viral bronchiolitis. Arch Dis Child. 1996;75:133–136. doi: 10.1136/adc.75.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coalson JJ, et al. SP-A deficiency in a primate model of bronchopulmonary dysplasia with infection. Am J Respir Crit Care Med. 1995;151:854–866. doi: 10.1164/ajrccm/151.3_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 25.Gerdes JS, Abbasi S, Karp K, Hull W, Whitsett JA. Surfactant protein-A in bronchoalveolar lavage fluid from neonates with RDS on conventional and high-frequency oscillatory ventilation. Pediatr Pulmonol. 1990;9:166–169. doi: 10.1002/ppul.1950090308. [DOI] [PubMed] [Google Scholar]
- 26.Griese M, Birrer P, Demirsoy A. Pulmonary surfactant in cystic fibrosis. Eur Respir J. 1997;10:1983–1988. doi: 10.1183/09031936.97.10091983. [DOI] [PubMed] [Google Scholar]
- 27.Kimpen JLL, Heymans HSA. Respiratory syncytial virus: immunity and immune injury. Immunol Infect Dis. 1993;3:281–288. [Google Scholar]
- 28.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med. 1996;21:641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 29.Weissbach S, Neuendank A, Pettersson M, Schaberg T, Pison U. Surfactant protein A modulates release of reactive oxygen species from alveolar macrophages. Am J Physiol. 1994;267:L660–L666. doi: 10.1152/ajplung.1994.267.6.L660. [DOI] [PubMed] [Google Scholar]
- 30.Weber H, Heilmann P, Meyer B, Maier KL. Effect of canine surfactant protein (SP-A) on the respiratory burst of phagocytic cells. FEBS Lett. 1990;270:90–94. doi: 10.1016/0014-5793(90)81241-f. [DOI] [PubMed] [Google Scholar]
- 31.LeVine AM, et al. Surfactant protein A–deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 32.LeVine AM, et al. Surfactant protein-A deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 33.Korfhagen TR, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haagsman HP, et al. The major surfactant protein, SP-A 28–36, is a calcium-dependent carbohydrate-binding protein. J Biol Chem. 1987;262:13877–13880. [PubMed] [Google Scholar]
- 35.LeVine AM, et al. Surfactant protein-A (SP-A) binds group B streptococcus, enhancing phagocytosis and clearance from lungs of SP-A deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 36.Sedgwick JB, Vrtis RF, Gourley MF, Busse WW. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J Allergy Clin Immunol. 1988;81:876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- 37.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 38.Faden H, Hong JJ, Ogra PL. Interaction of polymorphonuclear leukocytes and viruses in humans: adherence of polymorphonuclear leukocytes to respiratory syncytial virus-infected cells. J Virol. 1984;52:16–23. doi: 10.1128/jvi.52.1.16-23.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6 and IL-8) production by respiratory syncytial virus–infected human alveolar macrophages. J Immunol. 1991;147:4307–4312. [PubMed] [Google Scholar]
- 40.Merolla R, Rebert NA, Tsiviste PT, Hoffmann SP, Panuska JR. Respiratory syncytial virus replication in human lung epithelial cells: inhibition by tumor necrosis factor α and interferon β. Am J Respir Crit Care Med. 1995;152:1358–1366. doi: 10.1164/ajrccm.152.4.7551395. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy DO, Domurat FM, Nichols JE, Roberts NJ., Jr Interleukin-1 inhibitor production by human mononuclear leukocytes and leukocyte subpopulations exposed to respiratory syncytial virus: analysis and comparison with the response to influenza virus. J Leukoc Biol. 1989;46:189–198. doi: 10.1002/jlb.46.3.189. [DOI] [PubMed] [Google Scholar]
- 42.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol. 1994;267:L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-α activity from LPS-stimulated macrophages. Am J Physiol. 1996;271:L310–L319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 44.Geertsma MF, Nibbering PH, Haagsman HP, Daha MR, Van Furth R. Binding of surfactant protein A to C1q receptors mediates phagocytosis of Staphylococcus aureus by monocytes. Am J Physiol. 1994;267:L578–L584. doi: 10.1152/ajplung.1994.267.5.L578. [DOI] [PubMed] [Google Scholar]