The past decade has witnessed major advances in our understanding of the molecular mechanisms that regulate lymphocyte activation. The observation that cytoplasmic protein tyrosine kinases (PTKs) are activated rapidly following ligation of lymphocyte antigen receptors provided a convenient starting point for investigators to begin to identify signaling intermediates in lymphocytes. It is now known that both conserved, ubiquitously expressed molecules and novel lymphocyte-specific proteins are substrates of these PTKs and that they regulate various signal transduction cascades. Among these substrates, the class of molecules known collectively as adapter proteins has emerged as an important component of several signaling cascades linking apical tyrosine kinases with more distal signaling pathways. This Perspective will focus on recent advances in our understanding of the role adapter proteins play in the integration of signals initiated by engagement of the T-cell receptor (TCR) for antigen.
Two families of PTK have been implicated in initiating antigen receptor signaling (Figure 1a). Ligation of the TCR results first in activation of members of the Src family, including Lck and Fyn. These kinases are targeted constitutively to the inner face of the plasma membrane as a result of their NH2-terminal myristylation and are further localized to the activated TCR complex via noncovalent associations with the coreceptors CD4 or CD8 (1). Substrates of the Src PTKs include conserved immunoreceptor tyrosine-based activation motifs (ITAMs) found within the intracellular domains of the CD3 (δ, ε, γ) and TCRζ chains (2). Once phosphorylated, these motifs bind to additional proteins, thus providing an inducible mechanism for recruitment of cytosolic signaling molecules. The Syk family PTK, ZAP-70, is one such cytosolic protein recruited in this manner (3). However, recruitment of ZAP-70 alone is not sufficient to initiate signaling, as Syk family PTKs require further modification for activity. In this capacity, the Src family PTKs can phosphorylate ZAP-70, which promotes its subsequent activation (4). Following the realization that PTK activation is an early and required event for all downstream signaling events following TCR ligation, identification of Src and Syk family PTK substrates became the focus of intense investigation in an effort to link these enzymes with more distal signaling pathways.
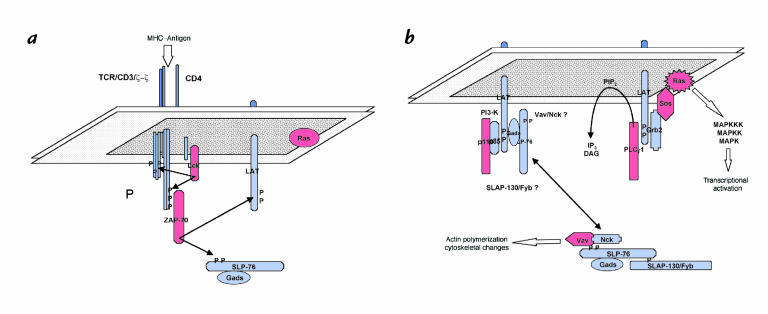
Figure 1.
Proximal activation of tyrosine kinases and the assembly of adapter protein signaling complexes following TCR ligation. (a) Engagement of the TCR by MHC–antigen complexes activates membrane-localized Src kinases (Lck), which then phosphorylate (P) a number of substrates, including ITAMs found in the cytoplasmic domains of the CD3 complex and the TCR-associated ζ chains. Phosphorylated ITAMs recruit the Syk family tyrosine kinase ZAP-70, which then phosphorylates additional substrates, including the adapter proteins LAT and SLP-76. (b) Once phosphorylated, SLP-76 and LAT recruit a number of proteins, including various effector molecules (red) as well as additional adapter proteins (blue). In the case of SLP-76 and Gads, the association is thought to be constitutive. Although Gads and SLP-76 have been found in a complex with LAT, it is not clear if additional SLP-76–associated molecules, such as Vav and Nck, are also recruited to LAT. DAG, diacylglycerol; IP3, inositol tris-phosphate; MAPK, mitogen-activated protein kinase; MHC, major histocompatibility complex; P, phosphotyrosine; PIP2, phosphatidylinositol bis-phosphate.
In other cell types, it had been demonstrated that the adapter protein Grb2, which associates constitutively with the guanine nucleotide exchange factor (GEF) Sos, is recruited to newly phosphorylated tyrosine residues in the cytoplasmic domains of growth factor receptors after ligand binding (5). Translocation of the Grb2–Sos complex to the activated receptor places Sos in proximity to membrane-bound Ras, resulting in exchange of GDP for GTP on Ras and the subsequent initiation of a signaling cascade, which culminates in the transcriptional activation of numerous genes (6). Grb2 also associates with Sos in T lymphocytes, although it remains unclear how the Grb2–Sos complex may regulate Ras activation following TCR engagement. Adding to the complexity, T lymphocytes express several Grb2-like molecules with similar SH3–SH2–SH3 domain architectures in addition to Grb2. The adapter proteins Grap and Gads are expressed predominantly in lymphoid tissues and, like Grb2, associate inducibly with the adapter protein Shc following TCR ligation (7–9). However, whereas TCR ligation promotes the association between Grap and Sos, Gads does not bind to Sos (9). Thus, Grb2, Grap, and Gads may mediate both overlapping and distinct signaling pathways in lymphocytes. The lymphoid-restricted expression of Grap and Gads suggests a more specialized function for these two adapter proteins. The exact role of each of these molecules in regulating lymphocyte signaling remains to be determined and will require a generation of experimental models in which the expression of one or more of these proteins can be manipulated.
Given the potential role for Grb2 and Grb2-like proteins in mediating TCR-dependent signaling events, several laboratories focused attention on the identification and characterization of additional Grb2-associated signaling intermediates in lymphocytes. The linker of activated T cells, or LAT, was originally viewed as a predominant 36-kDa Grb2-associated tyrosine phosphoprotein isolated from stimulated T-cell lysates. LAT contains a transmembrane domain and several intracellular tyrosine residues that are phosphorylated following TCR ligation (10). These phosphorylated tyrosine residues provide a scaffold for the recruitment of Grb2, the p85 subunit of phosphatidylinositol 3′-kinase (PI3-K), and PLCγ1 (Figure 1b). A number of additional signaling molecules, including SLP-76 and Cbl (see below), are also recruited to phosphorylated LAT, most probably as a consequence of association with Grb2, Gads, or PLCγ1. Sos can also be found in LAT immunoprecipitates from stimulated T-cell extracts, suggesting that LAT is a strong candidate for recruiting a Grb2–Sos complex to the membrane and initiating Ras activation after TCR engagement (10).
The important contribution of LAT to T-lymphocyte signaling has been demonstrated by several studies. Expression of a mutant form of LAT in which two of the cytosolic tyrosines have been substituted with phenylalanine diminishes TCR-dependent transcriptional activation (10). This LAT mutant also fails to recruit PLCγ1, p85 PI3-K, Grb2, and Sos following TCR ligation. A mutant T-cell line that does not express LAT has been described (11). These cells fail to generate soluble inositol phosphates, to flux intracellular calcium, or to activate transcriptional components of the interleukin-2 (IL-2) gene following TCR ligation. Inducible tyrosine phosphorylation of PLCγ1 is not observed in the LAT-deficient cells. However, global activation of tyrosine kinase activity remains intact. Taken together, these data implicate LAT as a key intermediate in coupling the TCR-activated PTKs with more distal signaling events.
Immunofluorescent studies have confirmed the membrane localization of LAT (10). However, membrane localization alone is not sufficient for the inducible phosphorylation of LAT. LAT is further targeted to glycolipid-enriched microdomains (GEMs) as a consequence of posttranslational palmitoylation, and the majority of tyrosine-phosphorylated LAT is found in the GEM fraction following TCR ligation (12). A number of signaling molecules, including members of the Src PTK family, heterotrimeric G proteins, and Ras, have been found to partition in the GEMs (13). After TCR ligation, phosphorylated TCR (ZAP-70, Shc, and PLCγ1 also localize to the GEM fraction (14). Disruption of GEMs, either with polyene antifungal agents or by promoting internalization of GEM components, impairs intracellular calcium release after TCR ligation (15). Together, these data provide compelling evidence that GEMs represent concentrated signaling microenvironments and that the inducible assembly of signaling complexes within these microdomains is a prerequisite for efficient TCR signaling.
The SH2 domain leukocyte protein of 76 kDa, or SLP-76, is another predominant substrate of the TCR-coupled PTKs (16). Like LAT, SLP-76 contains no enzymatic activity but harbors a number of domains that dictate interactions with other proteins. These include a COOH-terminal SH2 domain, a proline-rich central domain, and an acidic NH2-terminal region that contains several tyrosines that are phosphorylated by ZAP-70 after TCR ligation (16–18). Unlike LAT, SLP-76 contains no transmembrane domain and is located predominantly in the cytosol. SLP-76 expression is restricted to cells of hematopoietic lineage, including T cells, natural killer (NK) lymphocytes, macrophages, and platelets (19, 20). SLP-76 expression is regulated during T-cell development, with highest expression found at early and late stages of thymic maturation. Mice made deficient for SLP-76 expression by targeted disruption of the SLP-76 locus fail to develop a peripheral T-cell population because of an early and complete block in thymocyte development (21, 22). This developmental arrest occurs at a stage of thymic development where SLP-76 is normally expressed at high levels and pre-TCR expression is required to signal further maturation. Interestingly, SLP-76 levels fall as developing thymocytes transit to the next stage of development and remain low as these cells are subjected to a TCR-dependent selection process. The relatively low levels of SLP-76 expressed during thymocyte selection may effectively raise the threshold for TCR-signaling strength needed to promote selection and maintain the stringent nature of thymocyte selection.
Characterization of a mutant Jurkat T-cell line that has lost SLP-76 expression has confirmed the important contribution of SLP-76 to TCR signaling. These cells manifest a severe defect in the generation of an intracellular calcium flux and Erk activation after TCR ligation (23). Although TCR-dependent tyrosine phosphorylation of Syk family tyrosine kinases is intact, the inducible phosphorylation of PLCγ1 is lost after TCR ligation. A similar uncoupling of TCR-activated PTKs from more distal signaling pathways is also observed in SLP-76–deficient platelets (20). In vitro studies have demonstrated that SLP-76–deficient platelets fail to aggregate or release granule contents after exposure to collagen. Although collagen-induced Syk phosphorylation is detectable, tyrosine phosphorylation of PLCγ2 is markedly reduced in the absence of SLP-76. Collectively, studies utilizing SLP-76–deficient mice and T cells have helped define a function for SLP-76 in coupling Syk family tyrosine kinases with more distal signaling events in developing thymocytes, mature T cells, and platelets. Although experimental evidence gathered to date supports the notion that SLP-76 and LAT regulate similar signal transduction pathways, it is not known if these two important adapter proteins function in series or in parallel. It is clear that SLP-76 and LAT do not merely serve redundant functions, as neither can compensate for the loss of the other.
SLP-76 has been demonstrated to associate with a number of molecules, including SLAP-130/Fyb (22, 25), Vav (26), and, most recently, the adapter proteins Gads and Nck (27, 28) (Figure 1b). However, the contribution of these molecules to SLP-76 function is not completely understood. Although SLP-76 was originally viewed and isolated as a result of an in vitro association between the proline-rich domain of SLP-76 and the SH3 domains of Grb2, it has been difficult to recapitulate this observation in vivo. This is likely due to competition for SLP-76 binding by Gads, which binds to the same proline-rich domain of SLP-76 as Grb2 (27). Coexpression of SLP-76 and Gads has a synergistic effect on TCR-dependent transcriptional activation, although it is not known if the two need to interact for this effect to be seen. The SH2 domain of Gads binds to tyrosine-phosphorylated LAT, suggesting that Gads may physically link LAT and SLP-76 after TCR ligation. A mutation in the SH2 domain of Gads that prevents LAT binding confers a dominant–negative effect after transfection of the Gads mutant, highlighting the importance of the Gads–LAT association (27).
The contribution of SLAP-130/Fyb to T-lymphocyte activation remains controversial. SLAP-130/Fyb also has characteristics of an adapter protein in that this molecule has no demonstrable enzymatic activity but contains several domains that dictate protein interactions (24, 25). These include a proline-rich NH2-terminus, a tyrosine-rich domain, and an SH3 domain–like motif in the COOH-terminus. Although overexpression of SLAP-130/Fyb in the Jurkat T-cell line has been reported to inhibit SLP-76–dependent augmentation of TCR signaling (24), expression of SLAP-130/Fyb in a murine T-cell hybridoma has been found to augment TCR-dependent IL-2 production (25). These differences may be attributable to the different assays of function or cell types used. It is also possible that, although SLAP-130/Fyb that is physically bound to SLP-76 may negatively regulate SLP-76 function, free SLAP-130/Fyb may positively impact signaling pathways in an SLP-76–independent manner. Differential expression of molecules that associate with SLAP-130/Fyb may also influence the function of SLP-76/SLAP-130/Fyb complexes and the outcome of TCR ligation in developing thymocytes, mature T cells, and different T-cell lines.
TCR engagement induces a number of cytoskeletal changes, including actin polymerization and internalization of receptor complexes. The inducible interaction between the SH2 domain of Vav and the phosphorylated tyrosines of SLP-76 suggests a potential role for this complex in the regulation of cytoskeletal changes that are initiated by TCR ligation. Vav contains a guanine nucleotide exchange factor domain and catalyzes the exchange of GDP for GTP on the Rho family of small GTPases. Substitution of the tyrosine residues at either position 113 or 128 within the NH2-terminal domain of SLP-76 with phenylalanine abolishes Vav binding while having a minimal effect on the ability of transiently overexpressed SLP-76 mutant to augment TCR-dependent transcriptional activation (17). Whereas these data suggest that SLP-76 and Vav need not interact for at least this signaling pathway, a more direct role for the SLP-76/Vav complex in the regulation of cytoskeletal organization has been reported recently. After TCR ligation, SLP-76 associates inducibly with the SH2 domain of Nck (28), an adapter protein that also associates with the p21-activated kinase (Pak) and the Wiskott-Aldrich syndrome protein (WASP) (29, 30). Both WASP and Pak bind to activated Rho-GTPases, implicating SLP-76 in coupling Vav with potential substrates that are brought into the complex with Nck. Indeed, the in vivo assembly of a trimolecular complex containing SLP-76, Vav, and Nck has been demonstrated (28). Mutations in SLP-76, Nck, or Vav that prevent formation of the trimolecular complex abrogate TCR-dependent Pak activation. Furthermore, these same mutations profoundly inhibit polarization of F-actin after TCR stimulation, implicating the SLP-76/Nck/Vav complex in the regulation of cytoskeletal reorganization in response to TCR ligation. Actin polymerization and cytoskeletal reorganization are prerequisite events for efficient signaling, desensitization (receptor internalization), and cell motility. It will now be of interest to determine the role of SLP-76 and its associated proteins in the regulation of these process in lymphocytes as well as additional immune cell populations.
Although SLP-76 is not expressed in B lymphocytes, several potential B cell–specific SLP-76–like adapter proteins have been described. The B-cell linker protein, or BLNK, was originally identified as a Grb2-associated tyrosine phosphoprotein in BCR-stimulated cell extracts (31). BLNK was also cloned independently in another laboratory and named SLP-65 (32). The domain structure of BLNK/SLP-65 is similar to that of SLP-76, although the two proteins demonstrate only 33% homology overall. BLNK is phosphorylated by Syk in B-cell lines and, like SLP-76, tyrosine-phosphorylated BLNK associates with PLCγ1, Vav, and Nck (31). A mutant BLNK where four NH2-terminal tyrosine residues that are present in similar motifs to tyrosine 113, 128, and 145 of SLP-76 are mutated to phenylalanine fails to augment tyrosine phosphorylation of PLCγ1/2, intracellular calcium flux, or activation of the NF-AT transcription factor after BCR ligation, supporting the notion that BLNK functions in a manner analogous to SLP-76 (31). A SLP-76–like protein has also been identified in the chicken bursa of Fabricius. Unlike BLNK, the B-cell adapter–containing SH2 domain protein (BASH) is expressed predominantly in immature B cells but not in mature B-cell lines (33). Despite the overall structural similarity to SLP-76 and the high degree of homology with BLNK, BASH appears to play a negative regulatory role in receptor-mediated signaling. It is not clear why B and T lymphocytes, which share a common early lymphocyte progenitor, would evolve separate but homologous adapter proteins for the purpose of transducing antigen receptor–dependent signals. It may be necessary for these cell types to express custom adapter intermediates that are capable of integrating signals derived from the diverse array of surface receptors and signaling intermediates found in T and B lymphocytes and their progenitors.
In addition to adapter proteins serving as positive regulators of TCR-dependent signaling pathways, there is increasing evidence that adapter proteins may also interfere with TCR signaling and function in some cases to mediate signals that promote anergy. The 116-kDa Grb2-associated tyrosine phosphoprotein viewed after TCR ligation has been identified as the product of the proto-oncogene c-cbl (34). Cbl contains an NH2-terminal phosphotyrosine binding (PTB) domain, a proline-rich region, and several tyrosine residues that are phosphorylated after TCR ligation (34, 35). Overexpression of Cbl in a T-cell line diminishes TCR-dependent AP-1 activation (36), consistent with the observation that the Caenorhabditis elegans homologue of Cbl (Sli-1) prevents Ras activation after Let-23 ligation (37). Cbl binds to the NH2-terminal SH3 domain of Grb2 in a constitutive manner and may effectively compete with Sos for Grb2 binding in resting T cells (38). TCR ligation and subsequent tyrosine phosphorylation of Cbl promotes dissociation from Grb2 and the recruitment of additional molecules, including the p85 subunit of PI-3K and the adapter protein CrkL (38, 39). In anergic T cells, CrkL associates preferentially with Cbl and C3G, a guanine nucleotide exchange factor for the Ras family member Rap-1 (40). One current model suggests that the preferential formation of a CrkL–Cbl–C3G complex and the subsequent activation of Rap-1 would sequester Raf-1, the kinase immediately distal to Ras. This would presumably short circuit Ras-dependent signaling and ultimately promote an anergic state. In support of this notion, several studies have demonstrated that TCR ligation in tolerized T-cell clones fails to catalyze Ras activation and promote Ras-dependent distal signaling pathways.
It is clear that adapter proteins can mediate both positive and negative signaling cascades, depending on which effector molecules are recruited to the signaling complex after receptor ligation. Adapter proteins may also function to regulate lymphocyte signaling simply by interfering with the recruitment of signaling intermediates. SAP is a recently identified T cell–restricted protein that contains a single SH2 domain followed by a short COOH-terminal tail (41). After SLAM (Cdw150) ligation, SAP is recruited to phosphorylated tyrosine residues in the cytoplasmic tail of SLAM. These same residues also display affinity for the SH2 domain of SHP-2, a tyrosine phosphatase implicated in downregulating lymphocyte activation. Mutations in the SH2 domain of SAP abrogate the ability of SLAM to recruit SAP. Expression of SAP in a heterologous cell system prevents binding of SHP-2 to SLAM, suggesting that SAP can block SHP-2 recruitment to the plasma membrane and maintain the cell in an activated state (41). In support of this notion, transfected SAP promotes transcriptional activation of the IL-2 gene following coligation of the TCR and SLAM. The biological significance of SAP function has been highlighted by the demonstration that mutations in the SAP gene, including those that interfere with the ability of SAP to bind SLAM, are responsible for X-linked lymphoproliferative (XLP) disorder (41), a disease characterized by the expansion of B-cell populations infected with Epstein-Barr virus.
In the past few years, much progress has been made in identifying molecules that couple antigen receptors with more distal signaling events. Whereas future discoveries of additional signaling intermediates will undoubtedly contribute to our understanding of how lymphocyte signaling is regulated, the direct visualization of macromolecular signaling complexes within the intact cell will be required to determine which adapter protein complexes function in the regulation of receptor-dependent signaling cascades. To this end, several elegant studies have provided intriguing evidence for the focal assembly of signaling intermediates at the interface between a T cell and an antigen-presenting cell (APC). By means of computer-assisted imaging techniques, the receptor complexes that are localized at the contact point between a T cell and an APC have been viewed directly (42). This approach has also facilitated the identification of several signaling intermediates that are recruited to the interface and has provided direct evidence that PKCγ, Fyn, and Lck localize to the same region as the TCR–CD3 complex following major histocompatibility complex (MHC) and antigen engagement (42, 43).
It is now evident that adapter proteins play a crucial and required role in the process of T-cell development and activation. These molecules carry out these functions by acting as molecular scaffolds upon which signaling complexes are assembled and localized to distinct cellular domains. Now that several critical adapter protein complexes have been characterized, it will be of great interest to determine the temporal formation and spatial localization of these molecules in intact, resting, and activated lymphocytes. These experiments will require further development of sophisticated imaging techniques but will facilitate the determination of how different adapter proteins direct the assembly of signaling complexes, which surface receptors employ adapter protein signaling complexes to transduce signals, and where these complexes are localized after receptor ligation.
References
- 1.Turner JM, et al. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 2.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 3.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 4.Chan AC, et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenstein EJ, et al. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 6.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng GS, et al. Grap is a novel SH3-SH2-SH3 adaptor protein that couples tyrosine kinases to the Ras pathway. J Biol Chem. 1996;271:12129–12132. doi: 10.1074/jbc.271.21.12129. [DOI] [PubMed] [Google Scholar]
- 8.Trub T, Frantz JD, Miyazaki M, Band H, Shoelson SE. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signaling. J Biol Chem. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 9.Liu SK, McGlade CJ. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene. 1998;17:3073–3082. doi: 10.1038/sj.onc.1202337. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 11.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 14.Montixi C, et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 16.Jackman J, et al. Molecular cloning of SLP-76, a 76kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 17.Fang N, Motto DG, Ross SE, Koretzky GA. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- 18.Wardenburg JB, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 19.Clements JL, Ross-Barta SE, Tygrett LT, Waldschmidt TJ, Koretzky GA. SLP-76 expression is restricted to hematopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J Immunol. 1998;161:3880–3889. [PubMed] [Google Scholar]
- 20.Clements JL, et al. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements JL, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 22.Pivniouk V, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 23.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76 deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 24.Musci M, et al. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J Biol Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 25.da Silva A, et al. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 27.Liu SK, Fang N, Koretzky GA. The hematopoietic-specific adaptor protein, Gads functions in T cell signaling via interactions with SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–65. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 28.Bubeck Wardenburg J, et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 29.Rivero-Lezcano OM, Marcilla A, Sameshima JH, Robbins KC. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol Cell Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 31.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 32.Wienands J, et al. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goitsuka R, et al. BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J Immunol. 1998;161:5804–5808. [PubMed] [Google Scholar]
- 34.Donovan JA, Wange RL, Langdon WY, Samelson LE. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 35.Lupher ML, Jr, Reedquist KA, Miyake S, Langdon WY, Band H. A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- 36.Rellahan BL, Graham LJ, Stoica B, DeBell KE, Bonvini E. Cbl-mediated regulation of T cell receptor-induced AP1 activation. Implications for activation via the Ras signaling pathway. J Biol Chem. 1997;272:30806–30811. doi: 10.1074/jbc.272.49.30806. [DOI] [PubMed] [Google Scholar]
- 37.Jongeward GD, Clandinin TR, Sternberg PW. sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics. 1995;139:1553–1566. doi: 10.1093/genetics/139.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meisner H, Conway BR, Hartley D, Czech MP. Interactions of Cbl with Grb2 and phosphatidylinositol 3′-kinase in activated Jurkat cells. Mol Cell Biol. 1995;15:3571–3578. doi: 10.1128/mcb.15.7.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buday L, Khwaja A, Sipeki S, Farago A, Downward J. Interactions of Cbl with two adapter proteins, Grb2 and Crk, upon T cell activation. J Biol Chem. 1996;271:6159–6163. doi: 10.1074/jbc.271.11.6159. [DOI] [PubMed] [Google Scholar]
- 40.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 41.Sayos J, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 42.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 43.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]