Abstract
There is a pressing need to develop safe and effective radioprotector/radiomitigator agents for use in accidental or terroristic radiological emergencies. Naturally occurring vitamin E family constituents, termed tocols, include members, such as the tocotrienols, known to have radiation-protection properties. These agents, which work through multiple mechanisms, are promising radioprotectant agents having minimal toxicity. Although α-tocopherol (AT) is the most commonly studied form of vitamin E, the tocotrienols are more potent than AT in providing radioprotection and radiomitigation. Unfortunately, despite their very significant radioprotectant activity, tocotrienols have very short plasma half-lives and require dosing at very high levels to achieve necessary therapeutic benefits. Thus, it would be highly desirable to develop new vitamin E analogues with improved pharmacokinetic properties, specifically increased elimination half-life and increased area under the plasma level vs. time curve. The short elimination half-life of the tocotrienols is related to their low affinity for the α-tocopherol transfer protein (ATTP), the protein responsible for maintaining the plasma level of the tocols. Tocotrienols have less affinity for ATTP than does AT, and thus have a longer residence time in the liver, putting them at higher risk for metabolism and biliary excretion. We hypothesized that the low-binding affinity of tocotrienols to ATTP is due to the relatively more rigid tail structure of the tocotrienols in comparison to that of the tocopherols. Therefore, compounds with a more flexible tail would have better binding to ATTP and consequently would have longer elimination half-life and, consequently, an increased exposure to drug, as measured by area under the plasma drug level vs. time curve (AUC). This represents an enhanced residence of drug in the systemic circulation. Based on this hypothesis we developed of a new class of vitamin E analogues, the tocoflexols, which maintain the superior bioactivity of the tocotrienols with the potential to achieve the longer half-life and larger AUC of the tocopherols.
Keywords: radiation protection, tocoflexol, tocotrienol, countermeasures, molecular dynamics, bioavailability
INTRODUCTION
Protection from radiation hazards is an urgent necessity. In the event of a radiological/nuclear terrorist attack, three groups will require a radiation countermeasure to ameliorate the deleterious effects of ionizing radiation exposure: first responders, remediation workers, and the resident population. Thus, developing radiation countermeasures, applicable prior to or during exposure to prevent or limit injury, is a high-priority research area [Pellmar et al., 2005].
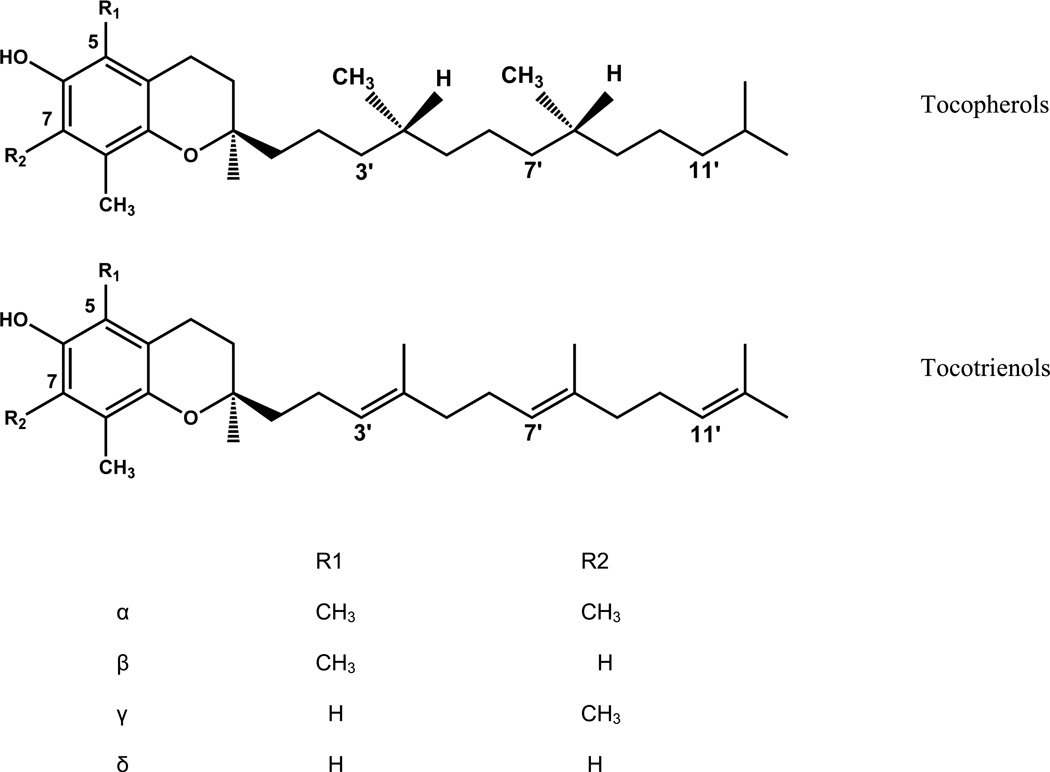
Vitamin E components have been reported to be radioprotective in various models [Berbeé et al., 2009; Ghosh et al., 2009; Kulkarni at al., 2010; Satyamitra et al., 2011; Berbeé and Hauer-Jensen, 2012]. Vitamin E comprises eight compounds, present in different ratios depending on the source [Müller et al., 2013]. These compounds are distributed in two chemical categories, the tocopherols and the tocotrienols. The tocopherols have a saturated side chain, phytyl, and the tocotrienols have three non-conjugated unstaturations on their side chain, farnesyl. All vitamin E components have the same chromanol head. Depending on the degree of methylation of the head, tocols can be categorized as α, β, γ, and δ [Fig. 1].
Fig. 1.
Chemical structures of vitamin E components
Studies on radioprotection provided by vitamin E components have focused mainly on AT and its acetate and hemisuccinate esters. One of the earliest indications that AT provided radioprotection was reported by Prince et al. [1973] who showed that AT protects erythrocytes from radiation-induced hemolysis. AT subcutaneously administered (400 IU/kg) to mice, 24 h before cobalt-60 irradiation (10.5 Gy), demonstrated significant survival benefit compared to AT administration at 12 h pre-irradiation [Kumar et al., 2002].
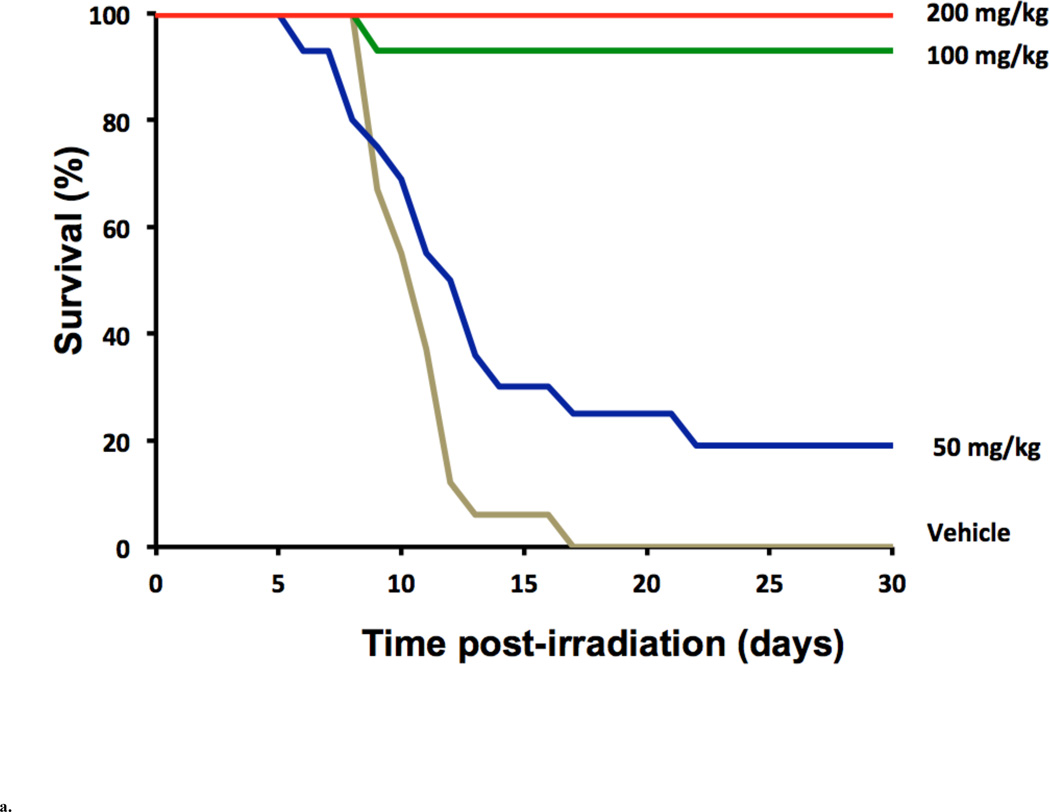
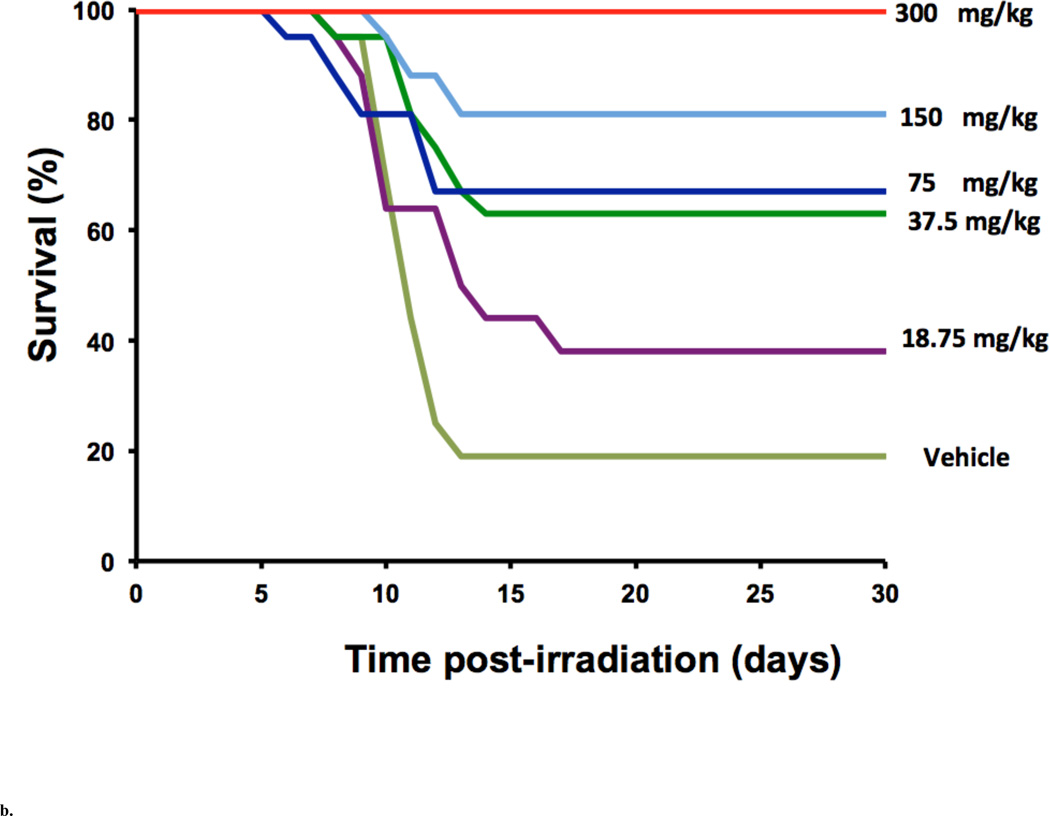
Compared to the vast number of studies done with AT, radioprotective studies done with tocotrienols are limited. Recent research at the Armed Forces Radiobiology Research Institute (AFRRI) and the University of Arkansas for Medical Sciences (UAMS) has focused on γ-tocotrienol (GT3) and δ-tocotrienol (DT3). When tested in the CD2F1 mouse model, AT or GT3 were administered subcutaneously (400 mg/kg) 24 h before 11 Gy Cobalt-60 gamma radiation. The GT3 treated group showed a 40% higher survival than the AT treated or vehicle groups. Systematic dose optimization, time optimization, and DRF studies were conducted at AFRRI for both the tocotrienols, GT3 and DT3. Ghosh et al. [2009] reported the highest efficacy (100% survival at 11 Gy compared to vehicle) by a single dose of 200 mg/kg of GT3 in CD2F1 mice when administered 24 h before radiation as compared to three other doses used (50 and 100 mg/kg). See Fig. 2a. A dose-dependent (up to 300 mg/kg) survival enhancement was observed in CD2F1 mice dosed subcutaneously with either DT3 or vehicle (PEG-400/5% Tween-80) 24 h before 9 Gy radiation (Fig. 2b). When administered 24 h prior to total body irradiation, 200 mg/kg of GT3 and 300 mg/kg of DT3 exhibited comparable dose-reduction factors (DRF) of 1.29 and 1.26, respectively. Both compounds produced enhanced hematopoietic recovery over 30 days after whole body radiation at sub-lethal doses. Both GT3 and DT3 also protected gastrointestinal crypt cells and prevented bacterial translocation after exposure to a lethal dose of radiation [Gosh et al., 2009; Satyamitra et al., 2011].
Fig. 2.
a. Radioprotection provided by GT3. Thirty-day survival of mice (n = 16 per group) treated 24 h before receiving 11 Gy of cobalt-60 gamma radiation, with a single s.c. injection of vehicle (5% Tween 80) or GT3 at doses of 50, 100 or 200 mg/kg body weight (Adapted from Ghosh et al. [2009])
b. Radioprotection provided by DT3. Thirty-day survival of mice (n = 16 per group) treated 24 h before receiving 9 Gy of cobalt-60 gamma radiation, with a single s.c. injection of vehicle (PEG-400/5% Tween-80) or DT3 at doses of between 18.75 and 300 mg/kg body weight (Adapted from Satyamitra et al. [2011])
Preliminary data from AFRRI indicate that GT3 administered orally was only minimally effective to protect from lethal radiation doses, in contrast to the efficacy of GT3 given subcutaneously, which protected against even a supra-lethal dose of radiation [Ghosh et al., 2009]. This suggests that the limited residence of orally administered GT3 in the systemic circulation is not sufficient to provide radioprotection.
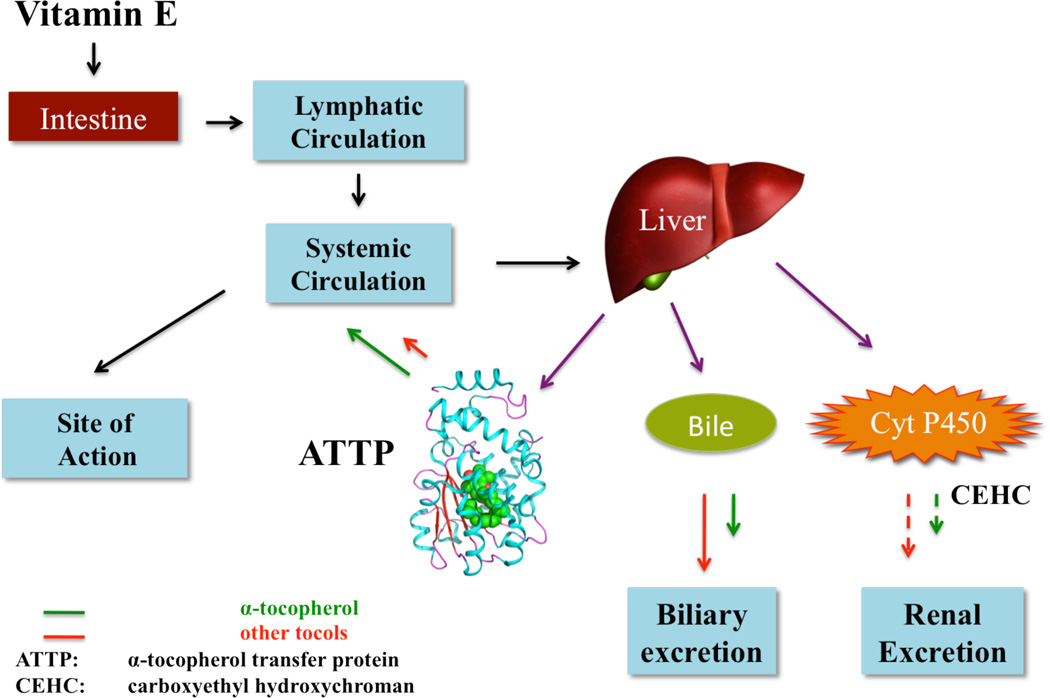
The limited systemic residence of the tocotrienols is due largely to their limited binding to ATTP, which is responsible for transporting tocols from the liver into the systemic circulation (Fig. 3) [Meier et al., 2003; Singh et al., 2013]. This step is considered to be the major determinant of tocol plasma levels in humans. GT3, DT3, and other tocotrienols have a much lower affinity for ATTP than does AT. Thus, they are trapped in the liver, where they are subject to clearance by metabolism and biliary excretion [Meier et al. 2003]. On the other hand, AT is secreted actively back into the circulation by ATTP. The key role of ATTP in regulating the pharmacokinetics of tocols has been demonstrated elegantly by Traber [2007], who showed that a vitamin E component that does not bind efficiently to ATTP has a reduced plasma half-life.
Fig. 3.
Schematic representation of the fate of vitamin E in the body. By selectively recirculating tocols ATTP plays a central role in maintaining the plasma levels of the tocols.
Tocotrienols have short elimination half-lives and thus limited oral bioavailability. The apparent elimination half-lives of exogenously administered tocotrienols in humans have been estimated to be between 2 – 4.4 hours; whereas, the half-life for AT is longer than 70 hours [Yap et al., 2001; Schwedhelm et al., 2003]. The short elimination half-life of tocotrienols requires them to be administered more frequently or in large doses by the subcutaneous route. A radioprotective agent in an oral formulation with longer half-life and larger AUC will be more suitable for use for the general population. In addition, an oral formulation will be easier to stockpile and can be made readily available in case of emergencies [Pellmar et al., 2005]. Thus, it would be highly desirable to develop a new vitamin E analogue with improved pharmacokinetic properties, specifically increased elimination half-life and increased area under the plasma level vs. time curve. In this paper we report the development of one such compound, tocoflexol, which maintains the superior level of bioactivity of the tocotrienols and has the potential to achieve a half-life and AUC comparable to that of the tocopherols.
The process of drug development is a nearly overwhelming task, in which failure is much more likely than success. In this regard, we have optimized the chances of success by designing tocoflexol via a rigorous computer-aided drug design process. The very specific binding requirements of ATTP cause AT to bind more efficiently than tocotrienols. A prominent reason for this selectivity is the flexible tail of the AT molecule which allows it to bend in a conformation that can be enclosed in the binding pocket of the ATTP protein. In contrast, the rotationally restricted tail of GT3 cannot bend in the appropriate conformation for binding. These observations prompted us to design a series of novel compounds with enhanced tail flexibility. Accordingly, we have named them “tocoflexols”. In tocoflexols, the tridienyl chain of the farnesyl tail of the corresponding tocotrienol is substituted with a mono- or dienyl chain. Depending on the position and the number of the double bonds in the tail, there can be 10 groups of tocoflexols. Taking into consideration the number of possible variations on the head, we can have hundreds of different compounds that could bind to ATTP with various degrees of efficiency.
To select the tocoflexols with better possibilities to bind to ATTP, potential candidates were screened using molecular dynamics simulations [Friedman et al., 2013]. This approach allowed us to reject numerous compounds with lesser potential, and to focus on the more promising candidates. From this analysis we were able to select the candidates with the highest potential to bind ATTP. It follows that these tocoflexols should have enhanced transport from the liver and improved distribution via the systemic circulation throughout the body to the various sites of action. By overcoming the rate-limiting step of transport from the liver to the systemic circulation, tocoflexols will have extended elimination half-lives, and thus larger AUCs, in comparison to the existing tocotrienols.
MATERIALS AND METHODS
Structure preparation
The initial geometry of AT was taken from the crystal structure of the complex of AT with human ATTP [Min et al., 2003], entry 1R5L from the Protein Data Bank [Bernstein et al., 1977]. The coordinates of AT were extracted from the complex with the transfer protein, and hydrogens were added. α-Tocotrienol (AT3) was built by combining the chroman ring of AT, prepared as described above, with the hydrocarbon chain taken from the SQUALN structure from the Cambridge Structural Database. The final charges, geometry and energy of the molecules were determined using the program Gaussian03 [Frisch et al., 2004]. The B3LYP hybrid function and the 3–21 G basis set were used for approximation of the geometry. Other tocol structures in this study were built based on the structure of AT or AT3 using standard bonds and angles.
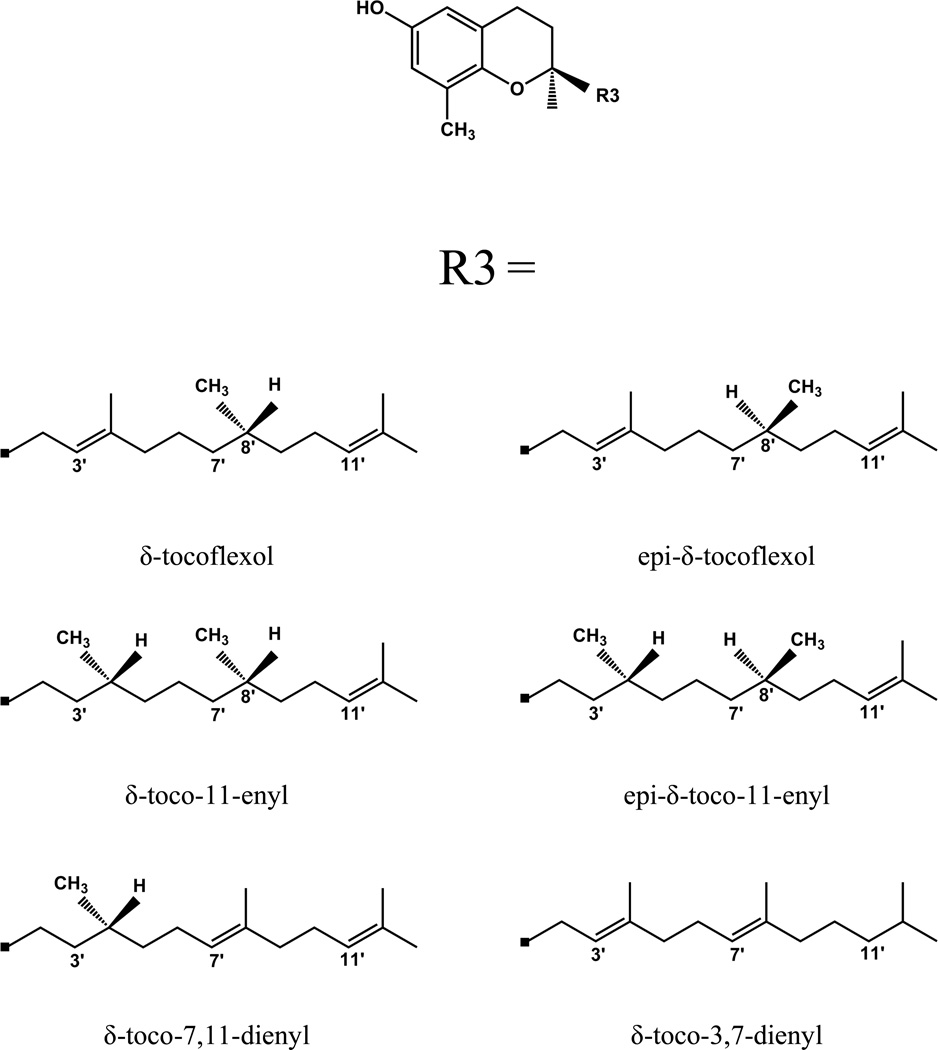
The δ-analogues were built by modifying the chromanol head of AT by deleting the methyl groups at the 5 and 7 positions. The tocoflexol structures were generated by removing the double bond and then adding hydrogens at specific positions as described in Fig. 4. R/S stereoisomers were generated using the invert routine in SYBYL 8.1 and geometry optimization was done for each structure using Gaussian03 as described earlier.
Fig. 4.
Structures of δ-tocoflexol and various representative analogues analyzed by molecular dynamics for potential binding to ATTP
Molecular dynamics simulations
Dynamics simulations were performed with the TRIPOS force field using SYBYL 8.1 (Tripos, St. Louis, MO). The Gaussian-generated geometry files described above were used for the simulations. The simulations were run in vacuum with an explicit dielectric constant of 4 using the NTV ensemble, with the SHAKE algorithm option applied to all the bonds involving hydrogens. The initial system was heated for 100 ps at 300 K with initial atom velocities set to random with a 32759 seed, and no scaling. The system was then equilibrated for 1 ns using the velocities from the previous set with the scale velocities option turned on. Finally, for the production run, the system was allowed to evolve for 20 ns at 300 K with no scaling of the velocities. The structures generated during the production step were saved at 200 fs intervals to produce sets of 100,000 conformations for every molecule. These sets were considered to be representative of the universe of possible conformations that the molecules can adopt at room temperature.
Binding analysis
The AT structure in the binding pocket of ATTP was used as a guideline to fit other tocols. We generated the topology files for AT and tocoflexol using the PRODRG server [Emsley et al., 2010]. Energy minimizations of tocols and the protein were performed using INSIGHT-II. Tocoflexol was incorporated in the binding cavity of ATTP by aligning to AT using Coot [Schüttelkopf and van Aalten, 2004] and the topology files generated by PRODRG.
Pharmacokinetic modeling
Curves were constructed using WinNonlin pharmacokinetic model #103 (one-compartment extravascular model) in the simulation mode. AT and GT3 parameters were taken from values reported in the literature (K = 0.0157 h−1 and F = 0.70 for AT and K = 0.1612 h−1 and F = 0.70 for GT3); for δ-tocoflexol the parameters were estimated. The simulated dose was 100 mg and the time course was 100 hours for all three compounds.
Chemicals
Wistar rat liver microsomes were purchased from BD Biosciences, dimethyl sulfoxide (DMSO) from Fisher, tert-butyl hydroperoxide solution (TBHP) from Sigma Aldrich, TBARS kits from Cayman Chemicals; and AT and GT3 from Yasoo Health. δ-Tocoflexol was synthesized through a series of chemical transformations starting from the corresponding δ-tocotrienol (DT3), which was obtained from annatto seeds. The structure of δ-tocoflexol was confirmed by NMR and GC/MS analyses [Zheng and Compadre, unpublished results].
Inhibition of lipid peroxidation
The antioxidant potential of tocoflexol was evaluated and compared with that of AT and GT3 in microsomes by measuring the inhibition of lipid peroxidation. Microsomes were diluted in 0.1 M phosphate buffer (pH 7.4) at a final protein concentration of 1 mg/ml. Microsomes were incubated with 200 µM TBHP in the presence and absence of tocoflexol, AT, or GT3, and were tested at concentrations between 0.5–7.5 µM. Stock solutions of the test compounds were prepared in DMSO, stored at −20° C, and used within a week. Stock solutions of TBHP were prepared immediately before each experiment. After incubation with TBHP, samples and standards were mixed with 200 µl acetic acid and 200 µl TBARS reagent and incubated for 45 min above 90 °C. TBARS reagent contained 0.67% TBA in 10% NAOH (w/v). Samples were then centrifuged for 20 min at 10,000 g at 4° C. Aliquots of 200 µl of each sample were loaded in duplicate in a 96-well plate and the absorbance was recorded at 535 nm using a Spectramax Microplate reader. The concentration of malondialdehyde (MDA), a product of lipid peroxidation, was calculated for each sample from a standard curve.
Statistical analysis
Data are presented as mean ± SEM. Inhibition concentration (IC50) values for tocoflexol, AT and GT3 were determined using WinNonlin pharmacodynamic model 104 and plotted using R.
RESULTS
Dynamics simulations
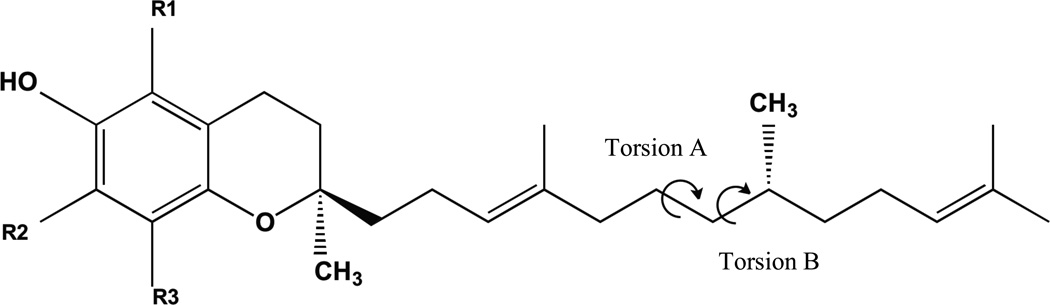
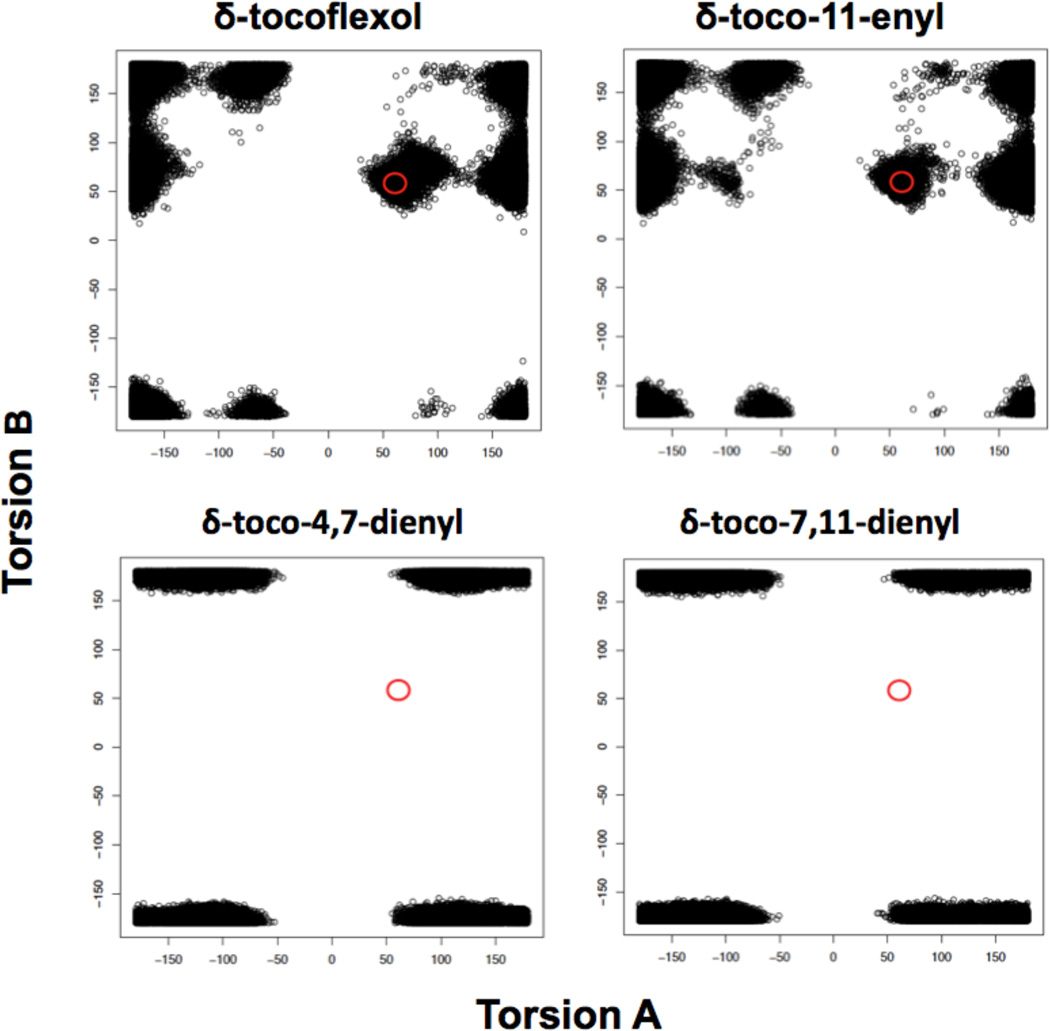
An analysis of the torsion angles of each set of conformations was performed to determine whether the molecules could adopt conformations capable to fit in the binding cavity of ATTP. This analysis is based on the assumption that AT is the best ligand for ATTP, and that molecules which can adopt conformations similar to that of AT in the binding cavity of ATTP would be able to bind effectively. Inspection of the structure of AT in the binding pocket of ATTP suggests that rotations around the bonds between C(6’) and C(7’) (torsion A) and C(7’) and C(8’) (torsion B) of the side chain are key for determining the feasibility of effective binding (Fig. 5). In the crystal structure of AT bound to ATTP the values of torsion angle A and torsion angle B are 61° and 58°, respectively. Thus, to determine whether a molecule has possibility of binding with ATTP, we made plots of torsion angle A against torsion angle B for each one of the molecular dynamics-derived sets (100,000 conformations for each analogue) (Figs 6 – 8). Using the values of AT torsion angles [61°, 58°] as a reference, a threshold was specified with a range ± 10° (represented with red circles). The conformations in the range were considered as allowed conformations. These conformations can bend to fit inside the ligand-binding pocket of ATTP in a manner similar to AT.
Fig. 5.
General structure of the tocoflexols showing the position of key torsion angles A and B. In this general strcuture R1, R2, and R3 can be H or CH3 groups.
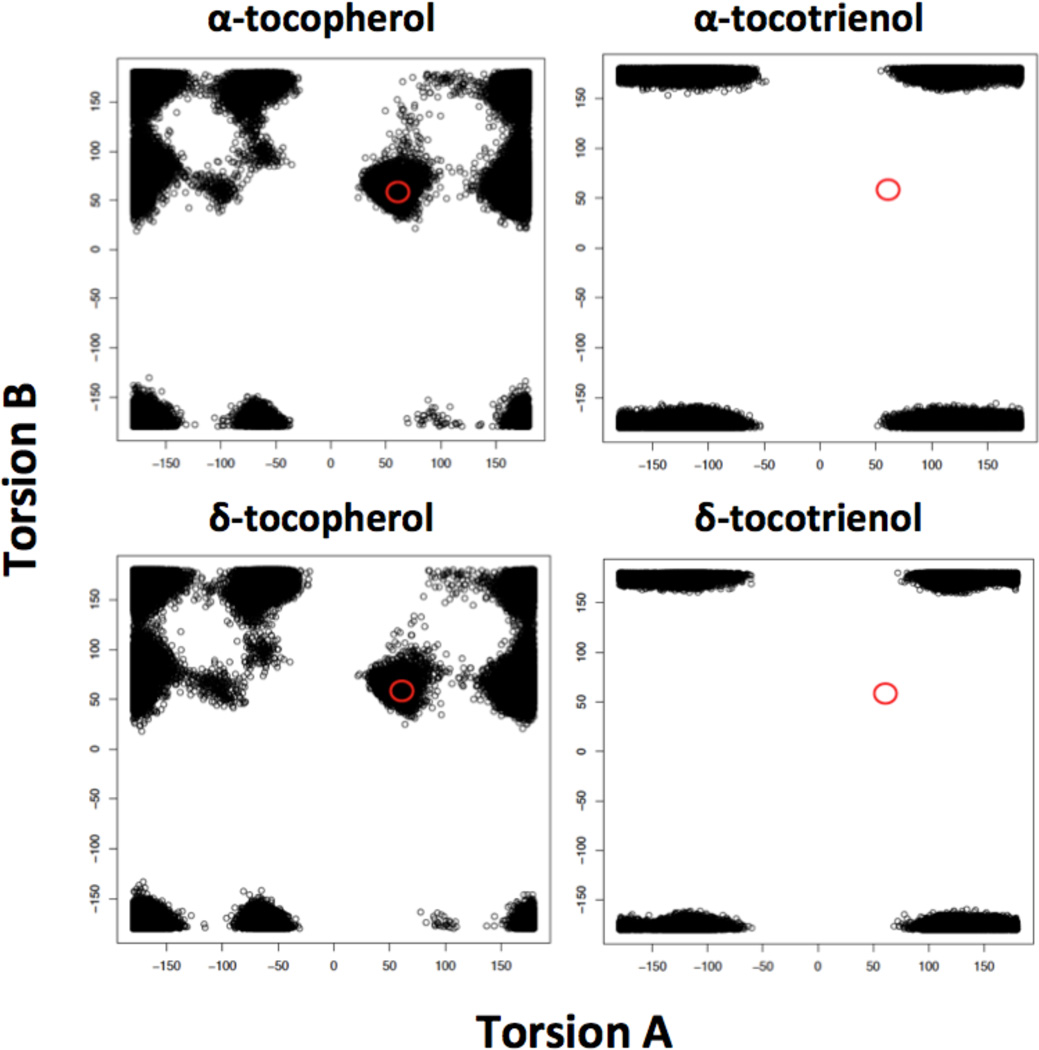
Fig. 6.
Molecular dynamics-based screening accurately discriminates between strong ATTP binding molecules and weak ATTP binding molecules. The figure shows the distribution of conformations around torsion angles A and B for 100,000 conformations of each molecule. In molecules with favorable conformations for binding to ATTP, such as the tocopherols, both torsion angles must be in the region delineated by the circles in the figures. Tocotrienols show no conformations in this region.
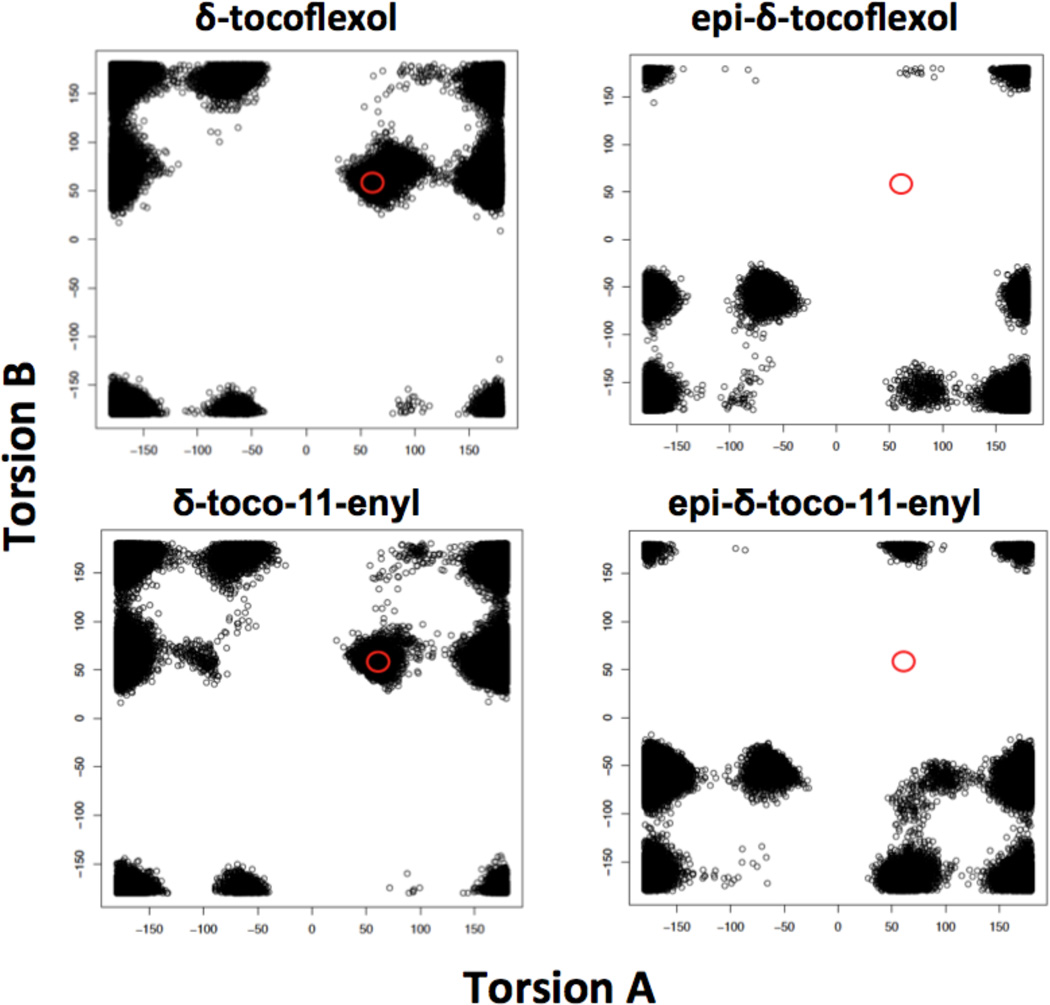
Fig. 8.
Molecular dynamics-based screening predicts that stereochemistry of the tocoflexols is critical for strong binding to ATTP. The figure shows the distribution of conformations around torsion angles A and B for 100,000 conformations of each molecule. The analysis suggests that tocoflexols lacking an unsaturation in position 7 are capable of strong binding to ATTP, only if their stereochemistry is equivalent to that of naturally occurring AT.
Molecular dynamics-based screening was able to discriminate accurately between strong ATTP binding molecules such as AT and δ-tocopherol and weaker binding molecules such as AT3 and DT3. As shown in Fig. 6, the stronger binding molecules have substantial populations of conformations with combination of torsion angles A and B in the allowed conformation area, while the weaker binding molecules have no conformations in that region. These computational results agree very well with the experimentally determined relative affinity of AT and AT3 for ATTP [Hosomi et al., 1997].
Molecular dynamics-based screening predicted that the number and position of double bonds in the side chain of the tocotrienols is critical for strong binding to ATTP. As shown in Fig. 7, of the various possible tocoflexol analogues, only the ones in which there is no double bond between C(7’) and C(8’) have populations of conformations in the allowed conformation area.
Fig. 7.
Molecular dynamics-based screening predicts that the number and position of double bonds in the side chain of the tocotrienols is critical for strong binding to ATTP. The figure shows the distribution of conformations around torsion angles A and B for 100,000 conformations of each molecule. The analysis suggests that tocols lacking an unsaturation in position 7 are capable of strong binding to ATTP.
Molecular dynamic simulation analysis also predicted that the stereochemistry of the tocoflexols is critical for strong binding to ATTP. Fig. 8 suggests that tocoflexols lacking an unsaturation in position 7’ are capable of strongly binding to ATTP only if their stereochemistry is equivalent to that of naturally occurring AT. Thus, while δ-tocoflexol and δ-toco-11-enyl show substantial populations on the allowed region, their epimeric counterparts show no conformations in the same region.
Binding analysis
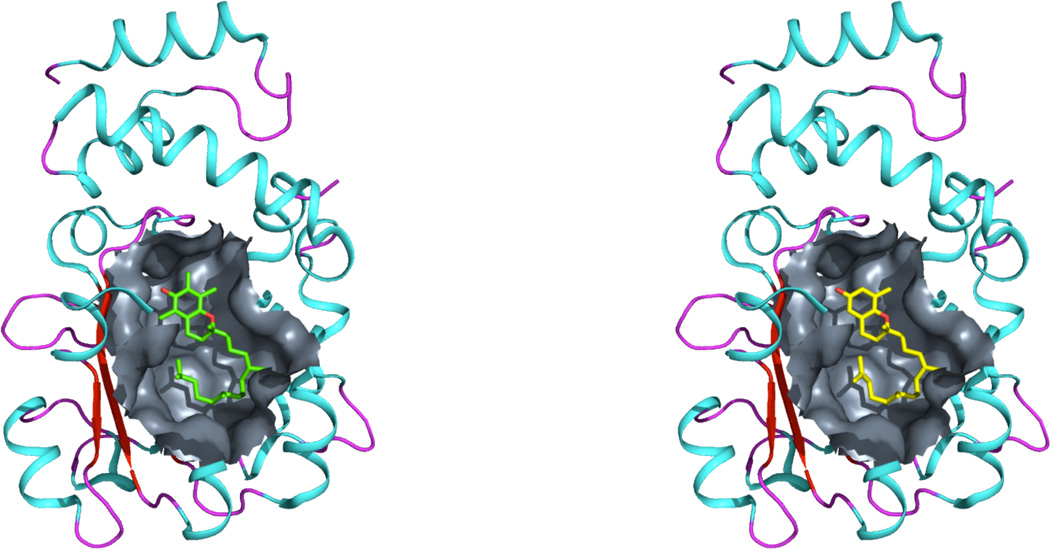
The crystal structure from PDB entry 1R5L shows that AT fits completely inside the binding pocket of ATTP in a bent conformation that was largely unforeseen before the elucidation of the crystal structure. The flexibility of the tail region of AT allows the tail to bend and fit entirely inside the binding pocket of ATTP. To visualize and further analyze the predictions of the dynamics simulations, structural and computational binding analyses were conducted to predict the fit of tocoflexol in the binding pocket of ATTP. The crystal structure of AT in complex with ATTP was used as the scaffold to fit tocoflexol in the binding pocket. Fig. 9 shows the analysis results.
Fig. 9.
Comparison of the binding of AT (green, left) and tocoflexol (right, yellow) in the binding cavity of ATTP. Coordinates of ATTP were obtained from PDB entry 1R5L. Topology files for tocoflexol were generated using the program PRODRG. Tocoflexol was fitted in the cavity of ATTP using the program COOT.
Pharmacokinetic modeling
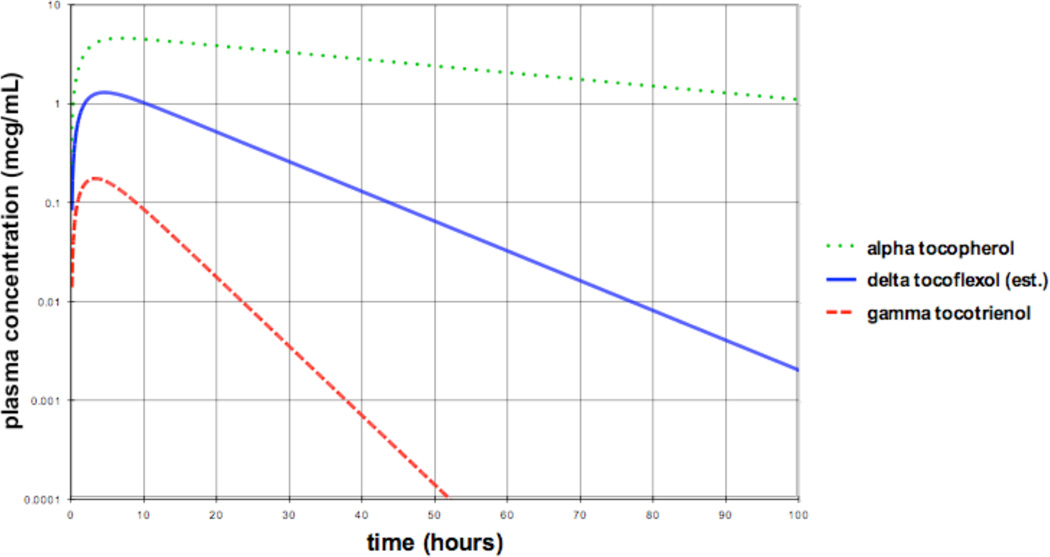
Fig. 10 shows the estimated time course of δ-tocoflexol plasma concentration, as compared to the AT and GT3 plasma concentrations that would occur for equal oral doses of each compound. The δ-tocoflexol curve is based on a half-life estimated to be 38% of that of AT and an oral bioavailability F estimated to be midway between AT and GT3. The former estimate is based on δ-tocoflexol showing 38% of the allowed conformations of AT, as shown in Fig. 7; while the latter estimate is based on the expectation that δ-tocoflexol will have an oil/water partition coefficient intermediate to that of AT and GT3.
Fig. 10.
Estimated time course of δ-tocoflexol plasma concentration, as compared to the α-tocopherol and γ-tocotrienol plasma concentrations that would occur for equal oral doses of each compound. The δ-tocoflexol curve is based on a half-life estimated to be 38% of that of α-tocopherol and an oral bioavailability F estimated to be midway between α-tocopherol and γ-tocotrienol.
Comparative antioxidant activity of tocoflexol, GT3 and AT in microsomes
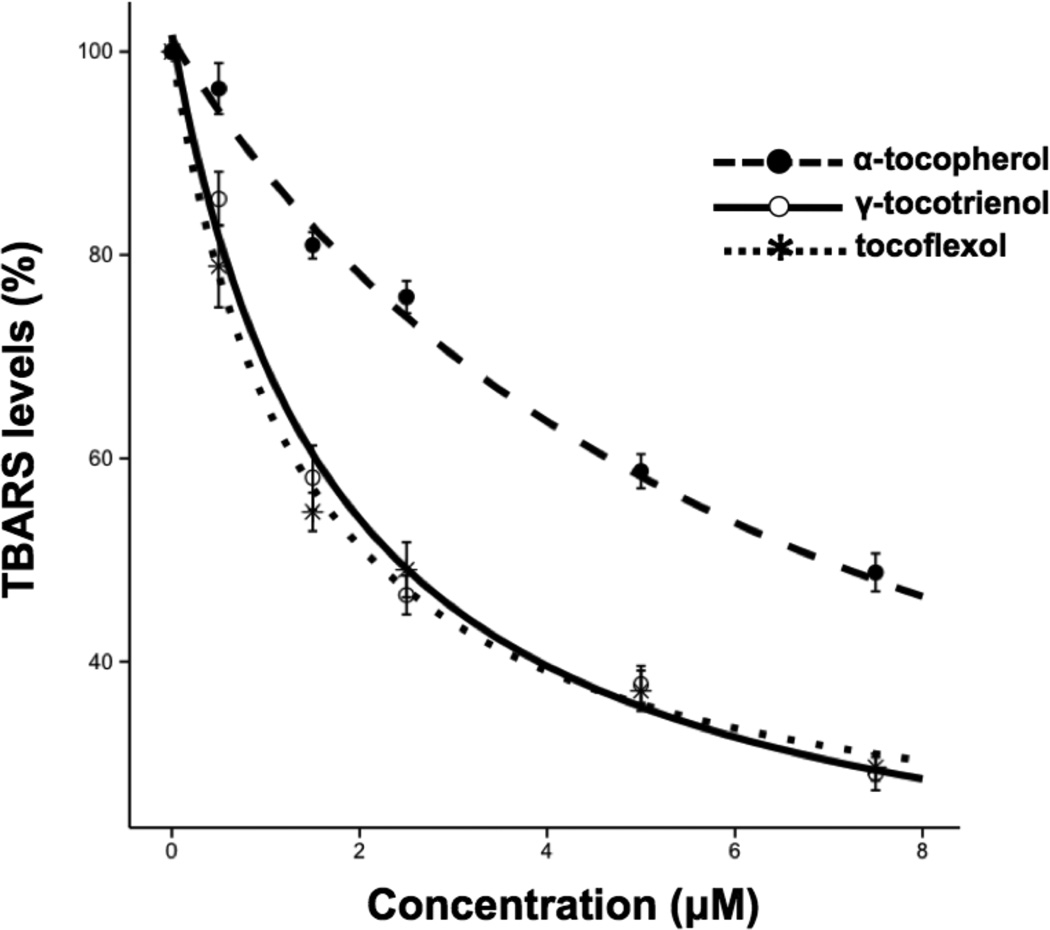
The antioxidant efficacy of tocoflexol was measured for its potential to inhibit lipid peroxidation in microsomal membranes and also compared with AT and GT3 using the thiobarbituric assay with MDA as standard. Tocoflexol, AT, and GT3 were dissolved in DMSO and added to the microsomes at micromolar concentrations, and their effects on TBHP-induced MDA formation were then measured at different concentrations for 30 min. The extent of lipid peroxidation was calculated by the level of TBARS produced during incubation with TBHP, as measured at 535 nm. The tocols used in the study reduced lipid peroxidation in a dose-dependent manner (Fig. 11).
Fig. 11.
Comparison of the antioxidant capacity of AT, GT3 and tocoflexol. Inhibition of TBHP induced lipid peroxidation in Wistar rat liver microsomes. Data are means ± SEM of triplicate experiments
TBARS production increased several fold following the addition of TBHP in the absence of tocols. The presence of tocoflexol, AT, and GT3 significantly reduced lipid peroxidation in a dose-dependent manner. In micro-molar concentrations, all tested compounds were able to inhibit lipid peroxidation in microsomes. Tocoflexol (IC50 = 1.4 µM) was more effective at quenching oxidative damage in microsomes than AT (IC50 = 6.7 µM) and equivalent in activity to GT3 (IC50 = 1.7 µM). These findings clearly demonstrate that tocoflexol is a powerful antioxidant with an IC50 4.9-fold lower than AT.
DISCUSSION
The increasing threat of radiological terrorism and nuclear accidents creates new demands for discovering and developing agents that can provide effective protection against ionizing radiation with minimal side effects. In recent findings tocotrienols have shown various biological activities transcending traditional vitamin E antioxidant activity. In the current study, we have addressed the problem of limited systemic residence of tocotrienols resulting from their lower affinity for the ATTP.
The predicted binding of vitamin E components to ATTP using commonly docking tools such as AutoDock, does not correlate well with published results [Upadhyay and Mishra, 2009]. Therefore, in this study we used molecular dynamic simulations to explore the binding of different vitamin E components and novel analogs to ATTP. Based on these simulations we designed the tocoflexols as novel vitamin E analogues with potential for enhanced residence in the systemic circulation. Our data, based on torsion-angle analysis, predicted that tocoflexol will have 38% more conformations in the allowed region and is expected to have better binding to ATTP than its parent tocotrienol. These results also support the notion that the novel compound tocoflexol has greater flexibility to acquire conformations similar to AT, as compared to tocotrienols. Further analysis of the structure of tocotrienol demonstrates that its tail region is not as flexible as that of AT. The tocoflexol tail, however, does demonstrate flexibility similar to AT. The structurally novel analogues are likely to be more bioactive and have longer half-lives and larger AUCs than the tocotrienols.
Radiation exposure leads to severe damage to multiple cellular components such as DNA, proteins, and lipids and also is believed to be a major factor governing the pathophysiology of radiation-induced damages. One of the mechanisms by which radioprotectants exert their beneficial effect includes their antioxidant potential. Current knowledge indicates that tocotrienols play a significant role in the prevention of diseases where free radicals are involved. Tocotrienols are powerful natural antioxidants, do not affect normal tissue and organs, and have favorable toxicity profiles [Nowak et al. 2012; Satyamitra et al., 2011].
Our results support the hypothesis that tocoflexol, a novel synthetic analogue of tocotrienol, maintains its antioxidant potential even after side-chain modification. If, as expected, tocoflexol residence time in the body is extended, this compound will be a practical countermeasure for the prevention and mitigation of radiation-induced damage.
The structural modification of drugs to take advantage of endogenous transport systems is a novel and intriguing concept whose potential is just starting to emerge. Successful demonstration of its usefulness in this application is likely to encourage development of similar strategies for future drug design and development in other areas of biomedical sciences.
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Center for Research Resources award 1ULl RR029884, the IDeA Networks of Biomedical Research Excellence (INBRE), UAMS start-up funds, and NIH/NIAID grant AI67798. The University of Arkansas has applied for patent protection on the tocoflexols. A potential royalty stream may occur consistent with the University of Arkansas policy
REFERENCES
- Berbée M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total body irradiation by an HMG-CoA Reductase- Dependent Mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: What is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54–59. doi: 10.1097/SPC.0b013e32834e3bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein FC, Koetzle TF, Williams GJ, Meyer EE, Brice MD, Rodgers J, Kennard O, Shimanouchi T, Tasumi M. The Protein Data Bank: A computer-based archival file for macromolecular structures. J Mol Biol. 1977;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and Development of Coot. Acta Crystallogr. 2010;D66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R, Boye K, Flatmark K. Molecular modelling and simulations in cancer research. Biochim Biophys Acta - Rev Cancer. 2013;1836:1–14. doi: 10.1016/j.bbcan.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai HM, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Wallingford CT: Gaussian, Inc; 2004. [Google Scholar]
- Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao T, Hauer-Jensen M, Kumar KS. Gammatocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of tocols. FEBS Lett. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, Gambles K, Toles R, Kao T-C, Hauer-Jensen M, Kumar SK. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- Kumar B, Jha MN, Cole WC, Bedford JS, Prasad KN. D-alpha tocopheryl succinate (vitamin E) enhances radiation-induced chromosomal damage levels in human cancer cells, but reduces it in normal cells. J Am Coll Nutr. 2002;21:339–343. doi: 10.1080/07315724.2002.10719232. [DOI] [PubMed] [Google Scholar]
- Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin e retention: structure of human a-tocopherol transfer protein. J Mol Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- Min KC, Kovall RA, Hendrickson WA. Crystal structure of human alpha-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci USA. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Cela J, Asensi-Fabado MA, Munné-Bosch S. Tocotrienols in plants: Occurrence, biosynthesis, and function. In: Tan B, Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E Beyond Tocopherols. 2nd ed. CRC Press; 2013. pp. 1–15. [Google Scholar]
- Nowak G, Bakajsova D, Hayes C, Hauer-Jensen M, Compadre CM. Gamma-tocotrienol protects against mitochondrial dysfunction and renal cell death. J Pharmacol Exp Ther. 2012;340:330–338. doi: 10.1124/jpet.111.186882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- Prince EW, Little JB. The effect of dietary fatty acids and tocopherol on the radiosensitivity of mammalian erythrocytes. Radiat Res. 1973;53:49–64. [PubMed] [Google Scholar]
- Satyamitra MM, Kulkarni S, Ghosh SP, Mullaney CP, Condliffe D, Srinivasan V. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin e isoform δ-tocotrienol. Radiat Res. 2011;175:736–745. doi: 10.1667/RR2460.1. [DOI] [PubMed] [Google Scholar]
- Schüttelkopf AW, van Aalten DMF. PRODRG - a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. 2004;D60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Maas R, Troost R, Boger RH. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet. 2003;42:437–459. doi: 10.2165/00003088-200342050-00003. [DOI] [PubMed] [Google Scholar]
- Singh A, Breen PJ, Ghosh S, Kumar KS, Varughese KI, Crooks PA, Hauer-Jensen M, Compadre CM. Structural modification of tocotrienols to improve bioavailability. In: Tan B, Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E Beyond Tocopherols. 2nd ed. CRC Press; 2013. pp. 359–370. [Google Scholar]
- Traber MG. Vitamin E bioavailability. In: Preedy VR, Watson RR, editors. The Encyclopedia of Vitamin. ECABI; 2007. pp. 221–230. [Google Scholar]
- Upadhyay J, Misra K. Towards the interaction mechanism of tocopherols and tocotrienols (vitamin E) with selected metabolizing enzymes. Bioinformation. 2009;3:326–331. doi: 10.6026/97320630003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol. 2001;53:67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]