Abstract
Background
Wiskott-Aldrich Syndrome protein (WASP)-deficient patients and mice are immunodeficient and can develop inflammatory bowel disease. The intestinal microbiome is critical to the development of colitis in most animal models, in which, Helicobacter spp. have been implicated in disease pathogenesis. We sought to determine the role of Helicobacter spp. in colitis development in WASP-deficient (WKO) mice.
Methods
Feces from WKO mice raised under specific pathogen free conditions were evaluated for the presence of Helicobacter spp., after which, a subset of mice were rederived in Helicobacter spp.-free conditions. Helicobacter spp.-free WKO animals were subsequently infected with Helicobacter bilis.
Results
Helicobacter spp. were detected in feces from WKO mice. After re-derivation in Helicobacter spp.-free conditions, WKO mice did not develop spontaneous colitis but were susceptible to radiation-induced colitis. Moreover, a T-cell transfer model of colitis dependent on WASP-deficient innate immune cells also required Helicobacter spp. colonization. Helicobacter bilis infection of rederived WKO mice led to typhlitis and colitis. Most notably, several H. bilis-infected animals developed dysplasia with 10% demonstrating colon carcinoma, which was not observed in uninfected controls.
Conclusions
Spontaneous and T-cell transfer, but not radiation-induced, colitis in WKO mice is dependent on the presence of Helicobacter spp. Furthermore, H. bilis infection is sufficient to induce typhlocolitis and colon cancer in Helicobacter spp.-free WKO mice. This animal model of a human immunodeficiency with chronic colitis and increased risk of colon cancer parallels what is seen in human colitis and implicates specific microbial constituents in promoting immune dysregulation in the intestinal mucosa.
Keywords: Helicobacter species, colitis, animal models, inflammatory bowel disease, Wiskott-Aldrich Syndrome protein
INTRODUCTION
Wiskott-Aldrich syndrome (WAS), is an X-linked immunodeficiency resulting from a lack of functional Wiskott-Aldrich Syndrome protein (WASP) and is characterized by recurrent infections, eczema, and thrombocytopenia.1 WAS patients are at increased risk of lymphoreticular malignancies and autoimmune diseases, including colitis.2 Like human WAS patients, WASP−/− (WKO) mice housed in specific pathogen free (SPF) conditions develop severe chronic colonic inflammation by six months of age with 50% having histologic abnormalities at 4 months of age.3, 4 The colitis in these mice is unique in its markedly elevated levels of IL-4 and IL-13 in addition to IFN-γ in the colonic lamina propria (LP) and its association with defects in regulatory T cell function.4–8
WASP is a hematopoietic-specific molecule that is the founding member of a family of intracellular signaling molecules including the more ubiquitously expressed N-WASP and WAVE proteins that regulate Arp2/3-dependent actin polymerization.9 Interestingly, genome-wide association studies have identified a SNP in components of the Arp2/3 complex that is associated with increased risk of ulcerative colitis (UC).10 Patients and mice deficient in WASP have broad defects in both the adaptive and innate immune systems including abnormalities in T cell receptor-dependent signaling, podosome formation, phagocytosis, and chemotaxis.9, 11 Regulatory T cells, NK-T cells, and marginal zone B cells are particularly sensitive to WASP deficiency.5, 7, 12
Murine models of colitis (approximately 60 in all) have facilitated our understanding of the genetic, immune, microbial, and environmental contributions to the development of inflammatory bowel disease.13 Most models develop colitis spontaneously as a result of genetic mutation/alteration of key regulators of immune homeostasis, while others require a chemical irritant or an adoptive cell transfer.13, 14 Radiation has been shown to accelerate disease severity in a small subset of these colitis models.15, 16 In most colitis models, disease development requires intestinal microflora, since germ-free mice or antibiotic treatment prevents disease.17 In some models, colitis can be transmitted vertically and horizontally as exemplified by T-bet/RAG double KO mice which can transmit colitis susceptibility to WT mice when certain gut resident microbes are transferred.18, 19 Recently, extensive efforts have been made to identify specific microbial community members that can drive or prevent colitis in genetically susceptible hosts.19–21
Helicobacter spp. are gram-negative, microaerobic, helical-shaped bacteria commonly found in research mouse colonies,22 and are linked to the development of typhlocolitis in a number of mouse models.23 Helicobacter hepaticus was initially identified in A/JCr mice with chronic hepatitis and hepatocellular carcinoma24 followed by Helicobacter bilis being isolated from bile, liver, and intestine of aged inbred mice in 1995.25 Flexispira spp. previously isolated from humans and their pets with diarrhea have now been re-classified as H. bilis.26–28 In the mid to late 1990’s, an association between intestinal inflammation and H. hepaticus or H. bilis was recognized.23, 29, 30 Either as a mono- or a co-infection, H. bilis was described to be associated with diarrhea and moderate to severe proliferative typhlocolitis in various strains of SCID mice.31–33 Interestingly, specific strains of Helicobacter spp. may have unique and divergent effects on colitis susceptibility. In mdr1a−/− mice, H. bilis accelerated the development of colitis while H. hepaticus delayed the development of colitis.34 In addition, infection with H. hepaticus leads to colitis-associated colon cancer in RAG KO (independent of T cell transfer)35 and potentiates tumorigenesis in some colon cancer-prone models of colitis.36, 37 Co-infection of mdr1a−/− mice with H. bilis and H. hepaticus can result in high-grade dysplasia and neoplasia.38 Likewise, H. bilis infection or co-infection with H. hepaticus leads to both colitis development and colorectal cancer in Smad3−/− mice and, with greater severity, in Smad3−/−Rag2−/− mice.39, 40
Herein, we explored the role of Helicobacter spp. infection in three distinct models of colitis including spontaneous, T-cell transferred, and radiation-induced colitis by rederiving WKO mice in Helicobacter spp.-free conditions. The development of colitis was also examined following H. bilis infection. We noted that Helicobacter spp. infection was required for the development of spontaneous and T-cell transfer-induced, but not radiation-induced, colitis in WKO mice. More importantly, H. bilis infection leads to the development of dysplasia and carcinoma in the setting of colonic inflammation in WKO mice.
MATERIALS AND METHODS
Mice
WKO mice were generated as previously described.3 WT (129 SvEv), WKO, and WASP−/−RAG−/− mice were maintained under specific pathogen free (SPF) or Helicobacter spp.-free conditions in animal facilities at Massachusetts General Hospital (MGH, Boston, MA) and at Massachusetts Institute of Technology (MIT, Cambridge, MA). The list of microbes excluded in SPF conditions at MGH is outlined in Table 1. Helicobacter spp.-free conditions at MGH exclude Helicobacter spp. and Pasteurella in addition to those excluded in SPF. Definitions of SPF at MIT were similar to that of Table 1, and Helicobacter spp.-free conditions at MIT also excluded Helicobacter spp. in addition to those in SPF. At the initiation of these studies, Helicobacter spp. were routinely identified by Helicobacter spp. PCR assays in sentinel cages in mice maintained in SPF conditions at MGH. All experiments were conducted upon approval and according to regulations of the Subcommittee on Research Animal Care of MGH and also by the MIT Committee on Animal Care. WKO mice maintained in SPF conditions were rederived by embryo transfer into Helicobacter spp.-free female mice and housed in Helicobacter spp.-free conditions at both MGH and at MIT with feces from sentinel cages and some of the experimental mice confirmed to be Helicobacter spp.-free. Mouse rooms were maintained at constant temperature and humidity on a 12/12-hour light-dark cycle, and mice were provided standard rodent chow (Purina Mills, St. Louis, MO) and water ad libitum.
Table 1.
Microbes excluded in animal facility at MGH considered to specific pathogen free (SPF).
Viruses:
|
Bacteria:
|
Parasites
|
Helicobacter bilis infection
Weaning Helicobacter spp.-free WKO mice were transferred from MGH to MIT and housed in groups of fewer than five in polycarbonate microisolator cages on hardwood bedding (PharmaServ, Framingham, MA) under specific pathogen free and Helicobacter spp.-free conditions. A total of 49 mice (27 males and 22 females) at 4 weeks of age used in the study were divided into two groups: 18 mice (11 males and 7 females) served as uninfected sham-dosed controls whereas 31 mice (16 males and 15 females) were orally inoculated daily with 0.2 ml of 108 CFU H. bilis Missouri strain in brucella broth for five consecutive days. At 7–9 months post-inoculation (mpi), mouse colons were analyzed for inflammation by histopathologic analysis and feces collected for Helicobacter spp. PCR.
Helicobacter spp. PCR
DNA was extracted from fecal samples of control mice, H. bilis-infected mice, and WKO mice housed under SPF conditions using a High Pure Nucleic Acid Isolation kit from Roche Applied Science (Indianapolis, IN). Helicobacter genus-specific PCR C97 and C05 (species-specific PCR for H. hepaticus, H. bilis, H. typhlonius, and H. rodentium) were used as described previously.22, 41
Irradiation of mice
Two- to four-months-old WKO mice placed in autoclaved irradiation pies received sub-lethal total body irradiation dose of 700 rads in 2 divided doses to induce a more uniform onset of colitis. Mice were then observed for 2 months, necropsied, and assessed histologically. Initial irradiation experiments on WT compared to WKO mice maintained under SPF conditions were done on two-month-old mice but to maximize the probably of finding a difference between non-irradiated and irradiated animals in subsequent experiments, mice at a younger age (2 months, when spontaneous colitis has not manifested) were used for irradiation.
T cell transfer colitis
WASP−/−RAG−/− mice were injected intraperitoneally with 400,000 naïve T cells isolated from spleen, peripheral, and mesenteric lymph nodes of WT mice, which were enriched for CD4 expression by negative selection using Dynabeads (Invitrogen, Carlsbad, CA) and subsequently sorted for CD4+CD25-CD45RBhi by FACS DIVA Vantage SE (BD Biosciences, San Jose, CA). Post-sort purity was typically >98%. Mice were followed for 3 weeks, euthanized and necropsied; colons were evaluated by histology.
Histologic colitis scoring
Histologic analysis was performed using a scale previously described and often used for Helicobacter spp.-associated typhlitis and colitis that rates inflammation, edema, epithelial defects, crypt atrophy, hyperplasia and dysplasia/neoplasia on a scale of 0 to 4; the sum of these subscores makes up the cumulative colitis or typhlitis index.42 Colitis is defined as colitis index of 3 or higher with an inflammation subscore of ≥ 1; typhlitis as typhlitis index of 4 or greater (minor abnormalities are seen at baseline even in a healthy WT mouse) with an inflammation subscore of ≥ 1. Dysplasia is defined as dysplasia score of at least 1 point with moderate-to-high-grade dysplasia defined by a dysplasia score of ≥ 2).
Statistics
Comparisons were made using the Mann-Whitney U test unless otherwise indicated.
ETHICAL CONSIDERATIONS
none
RESULTS
Helicobacter spp. are detectable in WKO mice
To determine if colitis in WKO mice correlates with the presence of a specific bacterial pathogen, initial microbial analyses were performed on fecal samples from several WKO colitic mice greater than 6 months of age by Charles River Laboratories. No specific pathogens other than H. bilis, H. hepaticus, or other unspeciated Helicobacter species were detected by PCR in 6 out of 6 WKO mice. To expand upon these initial analyses, feces of WKO mice were analyzed and 10 out of 11 cages (mouse ages ranging from 3 months to one year) were colonized with Helicobacter spp. In addition, feces collected from six mouse cages were positive for H. bilis, eight for H. rodentium, four for H. hepaticus, and one for H. typhlonius (Table 2). Feces of mice from five cages had co-infections (H. bilis plus another Helicobacter species); the other half was colonized by only one Helicobacter spp. Different Helicobacter spp. were also seen in WT mice (data not shown).
Table 2.
PCR for Helicobacter species in 11 cage of WKO mice in SPF facility demonstrating that most animals were colonized with one or more Helicobacter species.
| Cage # | Helicobacter spp. | H.bilis | H. rodentium | H. typhlonicus | H. hepaticus |
|---|---|---|---|---|---|
| 275 | - | - | - | - | - |
| 289 | + | + | + | - | - |
| 302 | + | + | + | + | + |
| W51 | + | + | + | - | + |
| W52 | + | + | - | - | - |
| W296 | + | + | + | - | + |
| W303 | + | - | + | - | - |
| W303 | + | - | + | - | - |
| W304 | +/- | - | + | - | - |
| W53 | + | - | + | - | - |
| W55 | + | + | - | - | + |
Spontaneous colitis associated with WASP-deficiency is attenuated when mice are rederived in a Helicobacter spp.-free environment
Given the association of Helicobacter spp. colonization and colonic inflammation in several mouse models of colitis,23 and our finding of Helicobacter spp. in the feces of WKO mice housed in SPF conditions, we determined the role of Helicobacter spp. in WASP deficiency-induced colitis by rederivation and maintenance of WKO mice in Helicobacter spp.-free environments in two independent animal facilities (MIT and MGH).
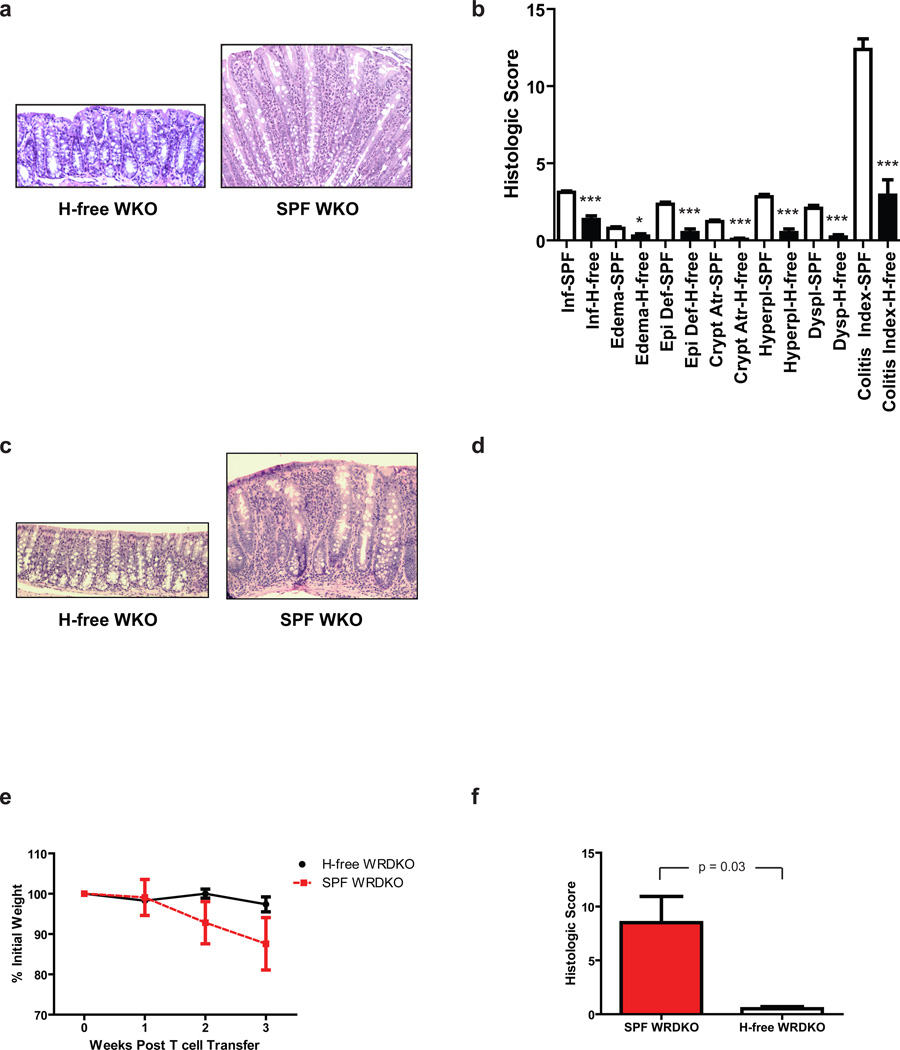
After rederivation at MGH, stool from founders were confirmed negative for Helicobacter spp. on PCR by BioReliance (Rockville, MD) and from multiple animals after subsequent breedings by our laboratories (data not shown). Mice were analyzed at 4 to 15 months of age (n = 23), and none had macroscopic signs of colitis (i.e. gross colonic thickening or soft stools). Microscopic analysis of mice aged 10–15 months (n = 7) revealed a mean histologic colitis index of 2.9 ± 1.0 compared to a mean index of 12.4 ± 0.7 in WKO mice housed in SPF conditions (Figure 1a-b). Similarly, WKO mice rederived in a Helicobacter spp.-free facility at MIT had little to no colitis at 5 months of age (mean colitis index of 2.8, n = 4) compared to WKO mice concurrently maintained in SPF facility that had moderate disease (mean colitis index of 8.5, n = 4) (Figure 1c). Moreover, even at 14 months of age, there was minimal colonic inflammation in Helicobacter spp.-free mice with a mean colitis index of 4.2 (n = 13) compared to 8.25 in WKO mice maintained concurrently under SPF conditions (n = 8) (Figure 1d). Taken together, these data indicate that the prominent colitis associated with WKO mice requires persistent Helicobacter spp. colonization.
Figure 1. Helicobacter spp. are required for colitis induction in WKO mice.
(a) Representative H&E sections and (b) mean histologic scores ± SEM of WKO mice at 10–15 months of age from Helicobacter spp.-free rederived group (n = 7) and of 6 months-old WKO mice in SPF conditions (n = 30) at MGH. (c) Representative H&E sections and (d) mean histologic scores ± SEM of WKO mice rederived in Helicobacter spp.-free conditions at MIT (n = 13) at 5 months and 14 months old, respectively, compared to age-matched WKO mice housed in SPF conditions (n = 8). (e) Naïve CD4+ T cells were transferred into 4–6 month-old WRDKO mice to induce colitis. Percent of initial weight of WRDKO mice from SPF (n = 4) compared to those from Helicobacter spp.-free (n = 4) rooms after WT naïve CD4+ T cell transfer. (f) Mean histologic colitis indices of animals ± SEM in (e), p = 0.017. Data are representative of two independent experiments. H&E stained sections taken at 20x objective. *p < 0.05, **p < 0.01, ***p < 0.005 on Mann-Whitney test comparing mice in SPF and Helicobacter-free rooms. H-free = Helicobacter spp.-free; SPF = specific pathogen free; WKO = WASP−/− mice; WRDKO = WASP−/−RAG-2−/− mice.
Innate immune cell-mediated colitis is also dependent on the presence of Helicobacter spp.
We recently demonstrated that even when WASP deficiency is limited to the innate immune cell compartment, colitis develops after WT CD4+ T-cell transfer.43 In this setting, WASP−/−RAG−/− (WRDKO) recipients of WT native CD4+ T cells develop severe colitis within 3 weeks compared to absence of disease in RAG−/− recipients.43 To assess the role of Helicobacter spp. in this accelerated model of colitis, WT naïve CD4+ T cells (isolated from animals purchased from Taconic negative for Helicobacter spp.) were transferred into WRDKO recipients maintained in either an SPF facility (documented to be Helicobacter spp. positive) or in a Helicobacter spp.-free room (documented Helicobacter spp. negative). Similar to that observed for WKO animals described above, colitis did not develop in this transfer model in Helicobacter spp.-free WRDKO recipients (Figure 1e-f).
Radiation-induced colitis does not require the presence of Helicobacter spp. in WKO mice
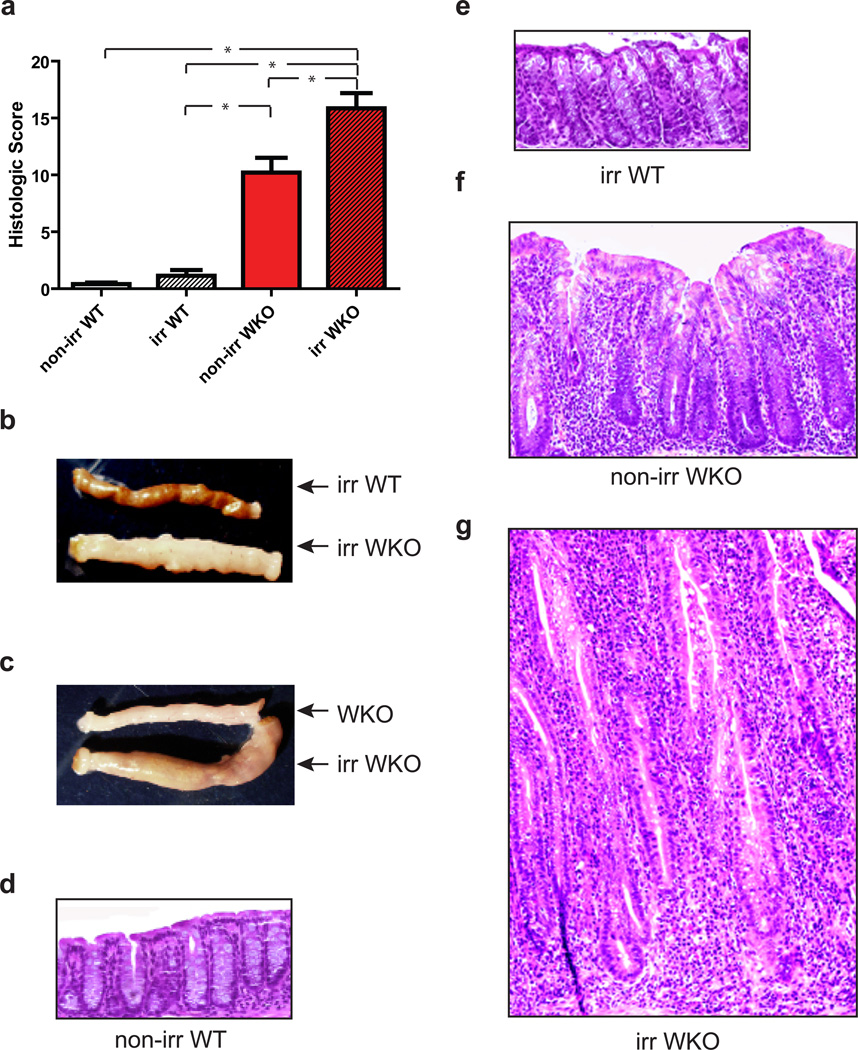
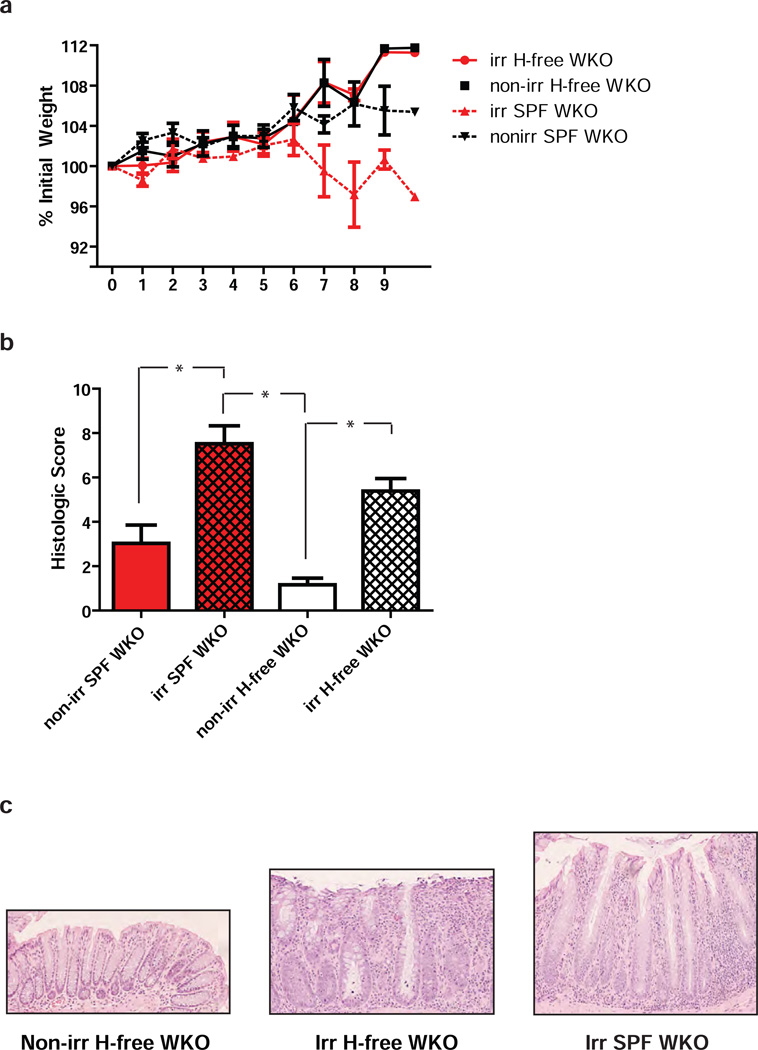
We next sought to determine whether colitis development could be accelerated by epithelial injury (i.e., sub-lethal irradiation) in WKO mice and, if so, whether disease in this condition would also be dependent on Helicobacter spp. Previously, bone marrow transplantation experiments revealed that WKO recipients raised in SPF conditions developed severe colitis with high rates of mortality within 2–4 weeks after lethal irradiation.44 Therefore, we investigated whether sub-lethal irradiation was sufficient to rapidly induce colitis. Indeed, 700 rads of whole body irradiation led to more severe colitis in 4-month-old WKO mice when compared to non-irradiated WKO mice (Figure 2a, c, f, g). All non-irradiated and irradiated WT mice remained colitis-free (Figure 2a, b, d, e). While the pathophysiology underlying this radiation-induced colitis remains unclear, we hypothesized that irradiation, even at sub-lethal doses, leads to a transient breach in the epithelial barrier resulting in microbial translocation and immune activation. To examine whether Helicobacter spp. are required for colitis under this condition, we assessed whether Helicobacter spp.-free WKO mice were susceptible to colitis in this radiation-accelerated model. Sub-lethally irradiated Helicobacter spp.-free WKO mice did not lose weight; however, they developed colitis in contrast to their non-irradiated Helicobacter spp.-free WKO littermates (Figure 3). These data indicate that Helicobacter spp. colonization is not required for disease initiation in a genetically susceptible host when the epithelial barrier is injured by radiation.
Figure 2. Sublethal irradiation led to colitis in WKO mice in SPF conditions.
(a) Mean histologic colitis indices ± SEM and macroscopic thickening of (b) irradiated WKO mice compared to irradiated WT mice and (c) irradiated WKO mice compared to non-irradiated WKO mice at six months of age two months after irradiation. Representative H&E images of colons from (d) non-irradiated WT mice, (e) irradiated WT mice, (f) non-irradiated WKO mice, and (g) irradiated WKO mice taken at 10x magnification. Kruskal-Wallis analysis with post-hoc Dunn’s test showed * p < 0.05. Irr = irradiated.
Figure 3. Radiation-induced colitis in WKO mice is not dependent on the presence of Helicobacter spp.
(a) Mean percent of initial weights ± SEM of sublethally irradiated and non-irradiated WKO mice in SPF and Helicobacter spp.-free conditions over 9 weeks after irradiation starting at 2 months of age. (b) Mean histologic colitis indices ± SEM in sublethally irradiated or non-irradiated Helicobacter spp.-free or SPF WKO mice. Data were pooled from five independent experiments (n = 22 for each irradiated group; n = 15 for non-irr H-free; n = 8 for non-irr SPF). Kruskal-Wallis analysis with post-hoc Dunn’s test showed * p < 0.05. (c) Representative H&E images of non-irradiated (left) and irradiated (middle) Helicobacter spp.-free WKO mice and irradiated SPF WKO (right), 10x objective. H-free = Helicobacter spp.-free; Irr = irradiated.
Infection of Helicobacter spp.-free WKO mice with H. bilis is associated with typhlo-colitis and ceco-colonic carcinoma
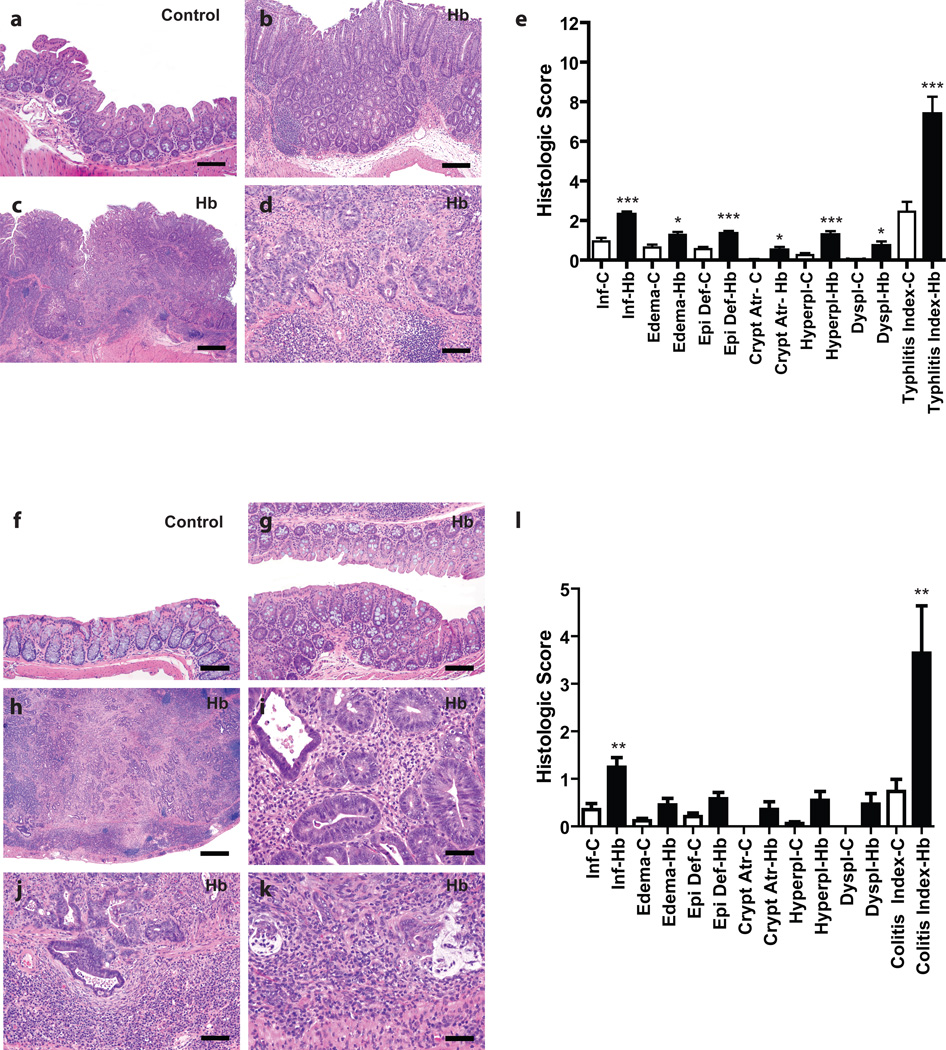
Since H. bilis is found frequently in the feces of WKO mice housed in SPF conditions, we investigated whether infection of Helicobacter spp.-free WKO mice with H. bilis would result in colitis development. PCR of fecal DNA confirmed lack of Helicobacter spp. in all mice before initiation of an experiment (data not shown) and in uninfected control WKO mice at necropsy (Supplemental Figure 1). A subset of WKO mice were infected with H bilis at 4 weeks of age with subsequent confirmation of infection by PCR at various time points post-infection: 2 weeks, 4 weeks, 3 months, and at necropsy at 7–9 months (Supplemental Figure 1 and data not shown). At 7–9 months post infection (mpi), the cecum of H. bilis-infected WKO mice (n = 31: 16 males, 15 females) had significantly higher mean inflammatory, edema, epithelial defects, crypt atrophy, hyperplasia, and dysplasia subscores and cumulative typhlitis index compared to uninfected controls (n = 18: 11 males, 7 females) (Figure 4a-e). In the colon, the H. bilis-infected mice had significantly higher inflammation subscore and cumulative colitis index than uninfected WKO controls, with an increasing trend for all other parameters, namely epithelial defects, edema, crypt atrophy, hyperplasia and dysplasia (Figure 4f-i,l). The inflammatory process in both the cecum and colon was composed of prominent aggregates of a mixture of mononuclear cells (lymphocytes, plasma cells and macrophages) and varying proportions of neutrophils and few eosinophils with associated epithelial degeneration/necrosis, crypt abscesses, and crypt atrophy/loss (Figure 4b-d,g-h,k). The location of cellular infiltrates varied, with aggregates localized either in a multifocal or patchy distribution, and found in the mucosa or submucosa (with occasionally transmural involvement).
Figure 4. Rederived Helicobacter spp.-free WKO mice infected with H. bilis develop typhlitis and colitis at 7–9 months post infection.
(a) Representative cecum from an uninfected control mouse at 7 mpi with sparse inflammatory cells in the lamina propria and lack of other significant epithelial changes (bar = 80 µM). (b) Representative non-neoplastic cecum of a H. bilis-infected mouse at 7 mpi showing moderate mucosal and submucosal inflammation, edema, surface epithelial tethering, epithelial hyperplasia and minimal dysplasia (bar = 160 µM). (c) Low magnification image of the cecum of a H. bilis-infected male at 7 mpi that developed a broad-based mucosal proliferative lesion (diagnosed as intramucosal carcinoma) showing prominent inflammation, edema, epithelial defects, epithelial hyperplasia, and disorganized glandular architecture (bar = 400 µM). (d) Higher magnification view of (c) showing high-grade dysplastic glands with invasion into the muscularis mucosa and extension/herniation into the submucosa (bar = 80 µM). (e) Mean subscores ± SEM of inflammation (inf), edema, epithelial defect (epi def), crypt atrophy (crypt atr), hyperplasia (hyperpl), dysplasia (dyspl), contribute to the cumulative typhlitis index of all mice. (f) Representative H&E image of the colon from an uninfected control mouse at 7 mpi with no significant mucosal inflammation or other epithelial alterations (bar = 80 µM). (g) Representative image of a non-neoplastic colon from an H. bilis-infected at 7 mpi showing mild mucosal inflammation and minimal hyperplasia (bar = 80µM). (h) Low magnification H&E image of a grossly thickened colon from a H. bilis-infected male at 7 mpi showing severe transmural mixed inflammation, lympho-follicular formation, edema, epithelial defects, fibrosis, crypt loss, marked epithelial hyperplasia, and severely disorganized glandular architecture with high grade dysplasia and multifocal submucosal invasion/herniation (bar = 400 µM). (i) Higher magnification view of (h) showing dysplastic glands with frequent mitotic activity and luminal cellular debris in a background of granulocytic inflammation (bar = 40µM). (j) Higher magnification of (h) showing invasive high grade dysplastic/neoplastic glands in the muscularis mucosa and submucosa surrounded by proliferating fibroblasts and dense lympho-plasmacytic inflammation (bar = 80µM). (k) Higher magnification of (h) showing a focus of invasive glands deep in the submucosa with associated partial loss of epithelial lining, disorganized pools of mucin-like material, and associated dense inflammatory cellular aggregates (bar = 40µM). (l) Subscores contributing to the colitis total index of all mice. *p < 0.05, **p < 0.01, ***p < 0.005 on Mann-Whitney test comparing un-infected and infected mice in that subscore. C = uninfected control mice; Hb = H. bilis-infected mice; mpi = months post infection.
Dysplasia and frank carcinoma were associated with the inflammation in some H. bilis-infected WKO mice. Three H. bilis-infected males during the course of the study were dehydrated and in poor body condition and had to be euthanized either 2 weeks short of 7 mpi (1 animal) or at 7 mpi (2 animals). Grossly, the ceca and colons of all these three mice were markedly and diffusely thickened (Supplemental Figure 2). Histologically, these thickened ceca/colons were characterized by severe, diffuse, mucosal to occasionally transmural inflammation with associated epithelial defects, multifocal areas of glandular hyperplasia, high grade glandular dysplasia (score > 3), and invasion into muscularis mucosa and/or submucosa consistent with carcinoma (Figure 4c,d,h-k). Frequently, the invasive dysplastic glands split and extended into the muscularis mucosa with occasional herniation into the submucosa. Invasive glands were characterized by lateral spreading of high grade dysplastic glands at various levels of the submucosa with frequent loss of epithelial lining from the mucin-filled glands in a background of inflammation and variable fibroblastic stromal proliferation (Figure 4c,d,h,j-k). Invasive carcinoma (reflected by a dysplasia subscore of 4 out of 4) with submucosal involvement was observed in the colon of 3 mice whereas for the cecum, only one animal had submucosal invasion while the other two had intramucosal carcinoma with dysplastic glands extending into the muscularis mucosa. Besides frank carcinoma, there was also dysplasia (defined as dysplasia subscore of ≥ 1 point) seen in some H. bilis-infected WKO mice both in the cecum and, to a lesser extent, in the colon (Figure 5a-b). In contrast, uninfected controls had none to mild or rarely (1 animal) moderate background inflammation (Figure 4a,e-f,l,) with minimal epithelial defects, edema, and hyperplasia without any evidence of any significant epithelial dysplasia in either the cecum or the colon (Figure 5a-b).
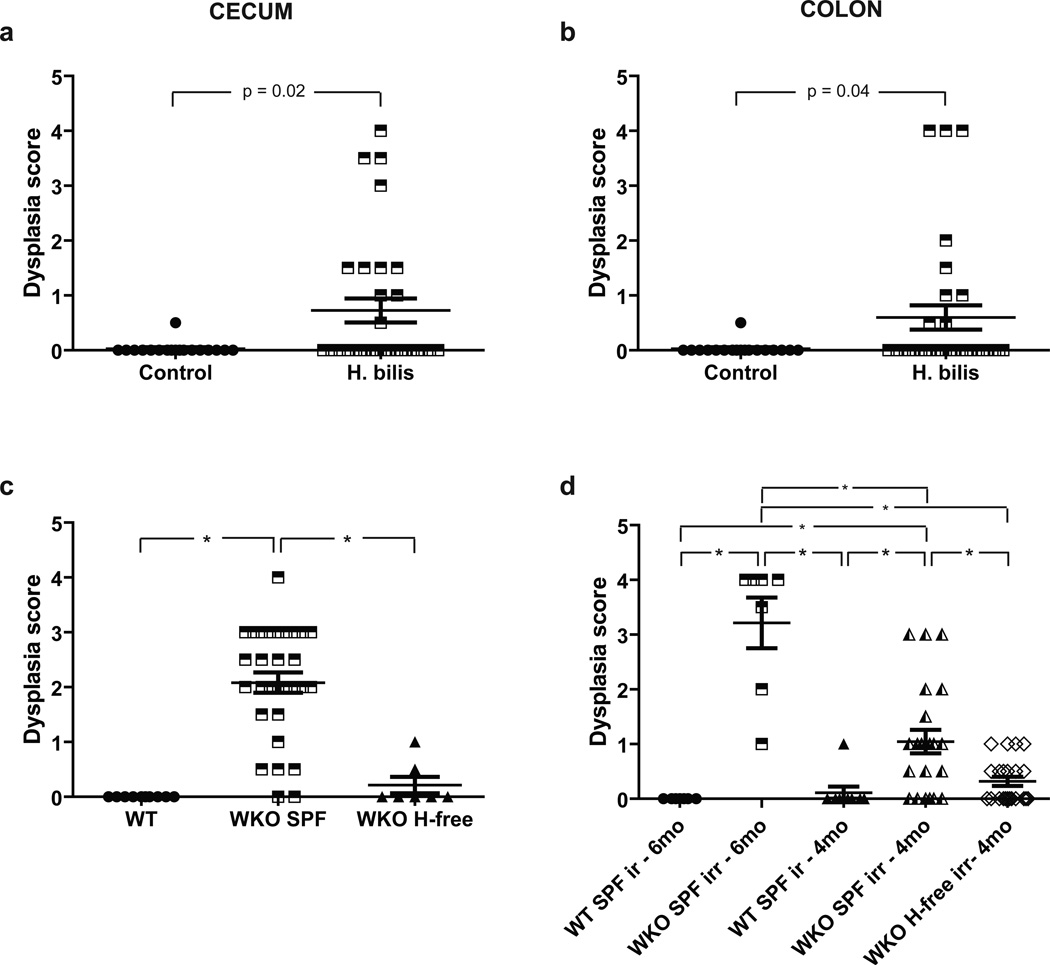
Figure 5. Dysplasia and colon carcinoma found in WKO mice raised under SPF conditions and under Helicobacter spp.-free conditions with H. bilis infection.
Dysplasia subscores ± SEM of individual mice in (a) cecum and (b) colon of rederived WKO animals 7–9 months after infection H. bilis with the horizontal bars representing the mean ± SEM shown in Figure 4e&l. (c) Dysplasia subscores ± SEM of colons from 6-month-old WT, 6-month-old WKO in SPF, and 10–15-month-old WKO mice raised in Helicobacter spp.-free conditions. (d) Dysplasia subscores ± SEM of colons from irradiated 4-month-old and 6-month-old WT or WKO mice (two months after irradiation). H-free = Helicobacter spp.-free; irr = irradiated.
With the observation of dysplasia and colon carcinoma in H. bilis-infected mice, we reexamined WKO mouse colons raised in SPF conditions and identified colonic masses (approximately <5% of > 8-month-old WKO mice), some of which were investigated histologically and found to be carcinomatous lesions in older, severely colitic WASP−/− mice housed in SPF conditions (Supplemental Figure 3). Upon re-review of the histologic slides of the 30 unmanipulated WKO mice maintained under SPF conditions at six months of age, 25 (83%) had dysplasia with 10 (33%) animals having moderate-to-high-grade dysplastic changes (dysplasia subscore ≥ 2). This is in comparison to only 1 out of 7 (17%) Helicobacter spp.-free WKO mice necropsied at a much later age (10–14 months) with low-grade dysplasia (Figure 5c). Likewise, irradiation led to dysplasia in 7 out of 7 (100%) of WKO animals irradiated at 4 months of age (and necropsied at 6 months of age) and 13 out of 22 (59%) WKO mice irradiated at 2 months of age (and necropsied at 4 months of age) when raised under SPF conditions (Figure 5d). In contrast, only 4 out of 22 (18%) irradiated WKO mice raised under Helicobacter spp.-free conditions demonstrated dysplasia (Figure 5d) despite the mean colitis indices not being significantly different between the irradiated SPF compared to irradiated Helicobacter spp.-free WKO mice (5.4 vs 7.5). No dysplasia or carcinoma has been observed in WT mice raised under SPF or Helicobacter-free conditions.
All in all, these data demonstrate an association between Helicobacter spp. infection in WKO mice with colitis and dysplasia and frank carcinoma in some animals.
Discussion
Several mouse models of colitis have demonstrated that colonization with Helicobacter spp. can lead to colitis development in predisposed hosts. Rederivation of genetically-susceptible animals (RAG-2−/−; NF-κB p65+/−p50−/−; TCR-α−/−; Smad-3−/−; mdr1a−/−; IL-10−/−, scid ICR-defined flora) in a Helicobacter spp.-free environment can prevent colitis development with re-introduction of one particular Helicobacter species permitting disease development.29, 30, 33–35, 45, 46 Here, we report that the colitis associated with WASP deficiency is abolished after rederivation in Helicobacter spp.-free settings and reintroduction of one specific species, H. bilis, can lead to re-emergence of typhlocolitis. It is possible that H. bilis itself does not directly lead to colitis and cancer development but rather indirectly modulates the intestinal microbial community; nevertheless, our observations suggest that the presence or absence of Helicobacter spp. alters the overall inflammatory milieu in genetically susceptible animals.
Unlike most colitis models where mono- or co-infection with H. hepatitis was associated with disease induction, this is the only colitis model other than mdr1a−/− and scid mice where mono-infection with H. bilis can trigger colitis.31, 33, 34 The colitis in the infected WKO mice is milder than the disease observed in WKO mice raised in SPF conditions suggesting that other Helicobacter species may potentiate colitogenicity as has been previously reported.38, 47 However, unlike mdr1a−/− mice and scid mice, we investigated three different types of colitis associated with WASP deficiency and demonstrated radiation-induced colitis in WKO animals occurs independent of Helicobacter colonization suggesting that not all types of intestinal inflammation in WKO mice require Helicobacter spp.
The most notable finding in our study is the development of dysplasia in our WKO mice raised under SPF conditions and high-grade dysplasia and carcinoma in a few WKO mice upon H. bilis infection after rederivation in Helicobacter spp.-free conditions. Only a small (<20%) subset of Helicobacter spp.-free WKO mice developed low-grade dysplasia with irradiation at 6 months of age or spontaneously at old age (10–14 months of age). In contrast, most (>80%) 6-month-old WKO (infected with Helicobacter spp.) housed mice in SPF rooms developed dysplasia with a third having moderate-to-high-grade dysplasia. In addition, H. bilis infection of Helicobacter spp.-free WKO mice led to high-grade dysplasia and frank carcinoma in 3 animals, suggestive of not only a colitogenic but also tumorigenc potential of Helicobacter bilis. Similarly, H. bilis infection in mdr1a−/− mice also leads to dysplasia and rarely carcinoma.38 Likewise, IL-10−/− mice develop colon cancer upon infection with other Helicobacter species (specifically a combination of H. typhlonius and H. rodentium).47, 48 Helicobacter spp. induction of colonic adenocarcinoma has been described in Smad3−/− mice where infection with several Helicobacter species led to colon cancer in 50–66% of animals.39 However, the Smad3−/− model differs from WKO mice in that multiple Helicobacter species had to be used to trigger tumorigenesis and colitis and colon cancer development were independent of the adaptive immune system as colitis and colon cancer are still present in Smad3−/−RAG-2−/− mice, consistent with what has been observed for RAG-2−/− mice and RAG-2-KO/APC(Min/+) mice.35, 37 In contrast, WKO mice devoid of adaptive immune cells (WRDKO) that are raised in SPF conditions do not develop colitis4 or tumors (data not shown).
A critical analysis of the pathology data illustrates several interesting observations. There was variation in the pathology observed within the H. bilis-infected WKO animals and the severity of pathology did not always correlate with the post-infection interval. The mice that developed the most severe H. bilis-induced pathology had to be euthanized at 7 mpi due to their poor clinical condition resulting from the lower bowel carcinoma. While not statistically significant, it is noteworthy that all three mice with either high-grade dysplasia or carcinoma were males. Irradiation uniformly induced colitis with high penetrance in WKO mice at only 4 months of age, which is a higher penetrance than that observed for non-irradiated WKO mice under SPF conditions.4 Several studies also noted an increase in the incidence of colitis in irradiated mice.15, 16 Given that it has been demonstrated that radiation causes increased intestinal permeability,49 we hypothesize this radiation-induced colitis is related to mucosal injury leading to increased access of the intestinal mucosa to luminal antigens, including those of a microbial source, resulting in inflammation. Our studies demonstrate that this pathophysiologic process is not dependent on the presence of intestinal Helicobacter spp. We hypothesize that the non-Helicobacter spp. microbial exposure is adequate to induce inflammation in the setting of dysfunctional adaptive and/or innate immune cells in WKO mice upon disruption of the epithelial barrier.
In summary, our observations re-emphasize the critical importance of the microbial environment in development of intestinal disease in genetically-predisposed mice and likely in humans. In fact, in this murine model of WASP deficiency, the presence or absence of Helicobacter spp. dictates whether disease develops. Whether this occurs through alterations in the intestinal microbial community is actively being investigated. Furthermore, H. bilis infection triggers not only typhlocolitis but also dysplasia and carcinoma. Our current study demonstrates that, in addition to H. hepaticus, H. rodentium, and H. typhlonius that have been previously shown, 47, 48 H. bilis has the ability to lead to inflammation-induced tumorigenesis either due to a direct effect or indirectly through alterations in the intestinal microbial community.
Supplementary Material
Supplemental Figure 1. Helicobacter spp.-free rederived WKO control mice are negative while H. bilis-infected mice are positive for Helicobacter spp. by PCR. Examples of PCR products with primers that recognize Helicobacter spp. in fecal DNA from a Helicobacter spp.-free uninfected WKO control mouse (09-6591) and one that was infected with H. bilis (09-6418) at necropsy.
Supplemental Figure 2. Infection of Helicobacter spp.-free WKO mice with H. bilis led to severe typhlo-colitis with marked splenomegaly. Grossly thickened colon associated with marked enlargement of the spleen (triangle) and mesenteric lymph nodes (asterisk) in an H. bilis-infected WKO mouse previously rederived in Helicobacter spp.-free conditions mouse at 7 mpi.
Supplemental Figure 3. Carcinoma in old and severely colitic WKO mouse raised in SPF conditions. (a) Colonic mass consistent with invasive mucinous cystic adenocarcinoma involving the submucosa and muscularis as shown at low magnification (bar = 400 µM). (b) Higher magnification of (a) showing invasive glands with mucin pools and associated inflammation (bar = 80 µM). (c) Higher magnification of the mucosa from (a) showing well differentiated glandular proliferation in the mucosa and inflammatory aggregates in the lamina propria. (bar = 80 µM).
ACKNOWLEDGEMENTS
Funding for this work included: 1) National Institutes of Health: K08DK083430 to DDN; R01AI50950, P01 HL059561, and P30 DK34854 to SBS; and R01OD011141, R01CA067529, P01CA026731, P30ES02109 to JGF, 2) Crohn’s and Colitis Foundation of America Career Development Award 2193 to DDN.
Abbreviations
- WRDKO
WASP−/−RAG−/−
- Treg
regulatory T cells
- WKO
WASP-deficient
- WASP
Wiskott-Aldrich Syndrome protein
- SPF
specific pathogen free
Footnotes
DISCLOSURE
The authors have no financial conflict of interest to declare.
References
- 1.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis-Girod S, Medioni J, Haddad E, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–e627. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 3.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–90. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen DD, Maillard MH, Cotta-de-Almeida V, et al. Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein. Gastroenterology. 2007;133:1188–1197. doi: 10.1053/j.gastro.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maillard MH, Cotta-de-Almeida V, Takeshima F, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marangoni F, Trifari S, Scaramuzza S, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humblet-Baron S, Sather B, Anover S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adriani M, Aoki J, Horai R, et al. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clin Immunol. 2007;124:41–48. doi: 10.1016/j.clim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma G, Burns SO, Thrasher AJ. Wiskott-Aldrich Syndrome: Immunodeficiency resulting from defective cell migration and impaired immunostimulatory activation. Immunobiology. 2009;214:778–790. doi: 10.1016/j.imbio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 11.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 12.Westerberg LS, de la Fuente MA, Wermeling F, et al. WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood. 2008;112:4139–4147. doi: 10.1182/blood-2008-02-140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaser A, Blumberg RS. Adaptive immunity in inflammatory bowel disease: state of the art. Curr Opin Gastroenterol. 2008;24:455–461. doi: 10.1097/MOG.0b013e328304d60d. [DOI] [PubMed] [Google Scholar]
- 15.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- 16.Marguerat S, MacDonald HR, Kraehenbuhl JP, et al. Protection from radiation-induced colitis requires MHC class II antigen expression by cells of hemopoietic origin. J Immunol. 1999;163:4033–4040. [PubMed] [Google Scholar]
- 17.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 18.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga P, Gallini CA, Beal C, et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A. 2010;107:18132–18137. doi: 10.1073/pnas.1011737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor NS, Xu S, Nambiar P, et al. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol. 2007;45:2166–2172. doi: 10.1128/JCM.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox JG, Ge Z, Whary MT, et al. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox JG, Dewhirst FE, Tully JG, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox JG, Yan LL, Dewhirst FE, et al. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero S, Archer JR, Hamacher ME, et al. Case report of an unclassified microaerophilic bacterium associated with gastroenteritis. J Clin Microbiol. 1988;26:142–143. doi: 10.1128/jcm.26.1.142-143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer JR, Romero S, Ritchie AE, et al. Characterization of an unclassified microaerophilic bacterium associated with gastroenteritis. J Clin Microbiol. 1988;26:101–105. doi: 10.1128/jcm.26.1.101-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanninen ML, Karenlampi RI, Koort JM, et al. Extension of the species Helicobacter bilis to include the reference strains of Helicobacter sp. flexispira taxa 2, 3 and 8 and Finnish canine and feline flexispira strains. Int J Syst Evol Microbiol. 2005;55:891–898. doi: 10.1099/ijs.0.63245-0. [DOI] [PubMed] [Google Scholar]
- 29.Kullberg MC, Ward JM, Gorelick PL, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox JG, Gorelick PL, Kullberg MC, et al. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;67:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin CL, Riley LK, Livingston RS, et al. Enterohepatic lesions in SCID mice infected with Helicobacter bilis. Lab Anim Sci. 1998;48:334–339. [PubMed] [Google Scholar]
- 32.Shomer NH, Dangler CA, Marini RP, et al. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab Anim Sci. 1998;48:455–459. [PubMed] [Google Scholar]
- 33.Shomer NH, Dangler CA, Schrenzel MD, et al. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infection & Immunity. 1997;65:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggio-Price L, Shows D, Waggie K, et al. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;160:739–751. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erdman SE, Poutahidis T, Tomczak M, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagamine CM, Rogers AB, Fox JG, et al. Helicobacter hepaticus promotes azoxymethane-initiated colon tumorigenesis in BALB/c-IL10-deficient mice. Int J Cancer. 2008;122:832–838. doi: 10.1002/ijc.23175. [DOI] [PubMed] [Google Scholar]
- 37.Nagamine CM, Sohn JJ, Rickman BH, et al. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2(−/−) Apc(Min/+) mouse. Infect Immun. 2008;76:2758–2766. doi: 10.1128/IAI.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, et al. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793–1806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggio-Price L, Treuting P, Zeng W, et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maggio-Price L, Treuting P, Bielefeldt-Ohmann H, et al. Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor-beta dysregulation as a model for studying inflammation-associated colon cancer. Am J Pathol. 2009;174:317–329. doi: 10.2353/ajpath.2009.080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox JG, Dewhirst FE, Shen Z, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 42.Rogers AB, Houghton J. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol Biol. 2009;511:267–295. doi: 10.1007/978-1-59745-447-6_11. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen DD, Wurbel MA, Goettel JA, et al. Wiskott-Aldrich Syndrome Protein Deficiency in Innate Immune Cells Leads to Mucosal Immune Dysregulation and Colitis in Mice. Gastroenterology. 2012;143:719–729. doi: 10.1053/j.gastro.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein C, Nguyen D, Liu CH, et al. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–2166. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
- 45.Erdman S, Fox JG, Dangler CA, et al. Typhlocolitis in NF-kappa B-deficient mice. J Immunol. 2001;166:1443–1447. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- 46.Chin EY, Dangler CA, Fox JG, et al. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alphabeta mutant mice. Comp Med. 2000;50:586–594. [PubMed] [Google Scholar]
- 47.Chichlowski M, Sharp JM, Vanderford DA, et al. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58:534–541. [PMC free article] [PubMed] [Google Scholar]
- 48.Hale LP, Perera D, Gottfried MR, et al. Neonatal co-infection with helicobacter species markedly accelerates the development of inflammation-associated colonic neoplasia in IL-10(−/−) mice. Helicobacter. 2007;12:598–604. doi: 10.1111/j.1523-5378.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 49.Mihaescu A, Santen S, Jeppsson B, et al. Rho kinase signalling mediates radiation-induced inflammation and intestinal barrier dysfunction. Br J Surg. 98:124–131. doi: 10.1002/bjs.7279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Helicobacter spp.-free rederived WKO control mice are negative while H. bilis-infected mice are positive for Helicobacter spp. by PCR. Examples of PCR products with primers that recognize Helicobacter spp. in fecal DNA from a Helicobacter spp.-free uninfected WKO control mouse (09-6591) and one that was infected with H. bilis (09-6418) at necropsy.
Supplemental Figure 2. Infection of Helicobacter spp.-free WKO mice with H. bilis led to severe typhlo-colitis with marked splenomegaly. Grossly thickened colon associated with marked enlargement of the spleen (triangle) and mesenteric lymph nodes (asterisk) in an H. bilis-infected WKO mouse previously rederived in Helicobacter spp.-free conditions mouse at 7 mpi.
Supplemental Figure 3. Carcinoma in old and severely colitic WKO mouse raised in SPF conditions. (a) Colonic mass consistent with invasive mucinous cystic adenocarcinoma involving the submucosa and muscularis as shown at low magnification (bar = 400 µM). (b) Higher magnification of (a) showing invasive glands with mucin pools and associated inflammation (bar = 80 µM). (c) Higher magnification of the mucosa from (a) showing well differentiated glandular proliferation in the mucosa and inflammatory aggregates in the lamina propria. (bar = 80 µM).