Summary
The somatic gonad of the nematode Caenorhabditis elegans exhibits highly regulated contractility during ovulation, which is essential for successful reproduction. Non-striated actin filament networks in the myoepithelial sheath at the proximal ovary provide contractile forces to push a mature oocyte for ovulation, but the mechanism of assembly and regulation of the contractile actin networks is poorly understood. Here, we show that actin-interacting protein 1 (AIP1) is essential for the assembly of the contractile actin networks in the myoepithelial sheath. AIP1 promotes disassembly of actin filaments in the presence of actin depolymerizing factor (ADF)/cofilin. C. elegans has two AIP1 genes, unc-78 and aipl-1. Mutation or RNA interference of a single AIP1 isoform causes only minor impacts on reproduction. However, simultaneous depletion of the two AIP1 isoforms causes sterility. AIP1-depleted animals show very weak contractility of the myoepithelial sheath and fail to ovulate a mature oocyte, which results in accumulation of endomitotic oocytes in the ovary. Depletion of AIP1 prevents assembly of actin networks and causes abnormal aggregation of actin as well as ADF/cofilin in the myoepithelial sheath. These results indicate that two AIP1 isoforms have redundant roles in assembly of the contractile apparatuses necessary for C. elegans reproduction.
Keywords: Actin dynamics, actin depolymerizing factor/cofilin, myoepithelial cells, spermatheca, ovulation, sterility
Introduction
Gonads are essential parts of animal reproductive systems. Somatic gonads provide environment for proper maintenance and development of germline and support transport of gametes and subsequent fertilization. In the nematode Caenorhabditis elegans, hermaphroditic somatic gonads exhibit highly coordinated contractile activities during ovulation, a process to expel a mature oocyte out of the ovary (McCarter et al. 1997; McCarter et al. 1999). Parts of the somatic gonad, the myoepithelial sheath and the spermatheca, have highly differentiated contractile actin cytoskeletal structures (Ardizzi and Epstein 1987; Ono et al. 2007; Strome 1986). The myoepithelial sheath cells surround oocytes at the proximal ovary. These cells have lattice-like networks of actin and myosin filaments. When oocytes are premature, the myoepithelial sheath cells show only minimal contraction. Upon maturation of an oocyte, they initiate intense contraction and push the oocyte into the spermatheca (McCarter et al. 1997; McCarter et al. 1999). The spermatheca is a highly elastic tube-like tissue where sperm are stored. During ovulation, it dilates and allows entry of an ovulated oocyte, which then fertilizes with sperm. A fertilized egg is then expelled to the uterus by contraction of the spermatheca (Kovacevic and Cram 2010; Kovacevic et al. 2013). Malfunction of these contractile tissues often results in sterility: defective contraction of the myoepithelial sheath causes retention of oocytes in the ovary (Myers et al. 1996; Obinata et al. 2010; Ono and Ono 2004), and uncoordinated spermathecal dilation and contraction cause retention or rupture of an oocyte (Clandinin et al. 1998; Kovacevic et al. 2013; Wissmann et al. 1999). Therefore, proper assembly and function of contractile actin cytoskeleton in these cells are essential for reproduction of C. elegans.
Actin networks in the myoepithelial sheath are morphologically similar to contractile apparatuses in smooth muscle (Strome 1986). Nonetheless, several contractile proteins are expressed commonly in the myoepithelial sheath and the body wall muscle, which has obliquely striated sarcomeric structures. Two myosin heavy chains, UNC-54 and MYO-3, and paramyosin UNC-15 are common components of thick filaments in the myoepithelial sheath (Ardizzi and Epstein 1987; Ono et al. 2007; Rose et al. 1997) and the body wall muscle (Epstein et al. 1985; Miller et al. 1983). In addition, troponin components (PAT-10 troponin C, UNC-27 troponin I, TNI-1 troponin I, and MUP-2 troponin T) are expressed in the myoepithelial sheath and function with tropomyosin as an actin-linked regulatory system for contraction (Myers et al. 1996; Obinata et al. 2010; Ono and Ono 2004), and these are also known to control contraction of the body wall muscle (Burkeen et al. 2004; Terami et al. 1999). However, how oocyte maturation and sheath contraction are precisely coordinated are not well understood.
By contrast, for assembly of contractile apparatuses in the myoepithelial sheath and the body wall muscle, different isoforms of actin depolymerizing factor (ADF)/cofilin are required. ADF/cofilin enhances actin filament dynamics by severing and depolymerizing actin filaments and involved in actin reorganization in a variety of cells (Ono 2007). C. elegans has two ADF/cofilin isoforms: UNC-60A with weak actin-filament severing and strong actin-monomer sequestering activities and UNC-60B with strong actin-filament severing and negligible actin-monomer sequestering activities (McKim et al. 1994; Ono and Benian 1998; Yamashiro et al. 2005). UNC-60A is required for assembly of actin networks in the myoepithelial sheath (Ono et al. 2008), whereas UNC-60B is required for sarcomeric organization of actin filaments in the body wall muscle (Ono et al. 2003; Ono et al. 1999). Thus, differential regulation of actin dynamics by ADF/cofilin isoforms might be a key to understanding how actin cytoskeleton is differentiated into non-striated and striated contractile apparatuses in these muscles. Although sarcomeres in striated muscle appear stable, dynamic regulation of actin filaments is required for assembly and maintenance of organized sarcomeric structures (Ono 2010). Likewise, actin filaments in non-striated muscle, including smooth muscle, might be regulated dynamically, but the mechanism of assembly of non-striated contractile apparatuses is poorly understood.
In this study, we examined roles of actin-interacting protein 1 (AIP1) in the C. elegans somatic gonad. AIP1 is a conserved actin regulatory protein with WD-repeats, which promotes disassembly of ADF/cofilin-bound actin filaments (Ono 2003). C. elegans has two AIP1 isoforms, UNC-78 and AIPL-1, and these have similar activities to disassemble actin filaments in the presence of UNC-60B (Mohri and Ono 2003; Mohri et al. 2004; Ono et al. 2004; Ono et al. 2011). In the body wall muscle, mutation in unc-78 causes severe disorganization of sarcomeric actin filaments (Mohri et al. 2006; Ono 2001), whereas mutation in aipl-1 does not cause detectable phenotypes (Ono et al. 2011). However, simultaneous depletion of UNC-78 and AIPL-1 causes embryonic lethality with primary defects in sarcomeric actin organization in embryonic muscle and failure of body elongation (Ono et al. 2011), indicating that UNC-78 and AIPL-1 have partially redundant functions. In vitro, both UNC-78 and AIPL-1 preferentially cooperate with UNC-60B for actin disassembly but not with UNC-60A (Mohri and Ono 2003; Ono et al. 2011). Since UNC-60A is the ADF/cofilin isoform essential in the somatic gonad (Ono et al., 2008), whether either UNC-78 or AIPL-1 play any role in the somatic gonad has been unclear. Our study shows that the two AIP1 isoforms have redundant functions in actin organization in the gonad and are essential for reproduction, suggesting that the AIP1 isoforms cooperate with UNC-60A under in vivo conditions.
Results and Discussion
The Two AIP1 Isoforms, UNC-78 and AIPL-1, Are Redundantly Required for Ovulation in C. elegans
Simultaneous depletion of the two AIP1 proteins, UNC-78 and AIPL-1, causes ~100 % embryonic lethality (Ono et al. 2011), which precludes us to examine adult phenotypes. To investigate their functions in adult worms, we treated newly hatched L1 larvae with RNAi of the AIP1 isoforms and examined their phenotypes when they grow up to adult worms. We found that depletion of the two AIP1 isoforms caused sterility in adult hermaphrodites. Wild-type worms treated with control RNAi produced 284 ± 26 progeny per worm (brood size) (n=7) (Fig. 1). unc-78(RNAi) in wild-type slightly reduced the brood size (171 ± 62 progeny per worm, n=7). The aipl-1(null) mutant with control RNAi also produced less progeny (211 ± 48 progeny per worm, n=7) than wild-type with control RNAi. When the two AIP1 isoforms are simultaneously depleted in aipl-1(null); unc-78(RNAi), no progeny (0 ± 0 progeny per worm, n=7) were produced from examined animals. Therefore, the two AIP1 isoforms function redundantly and are essential for reproduction of C. elegans.
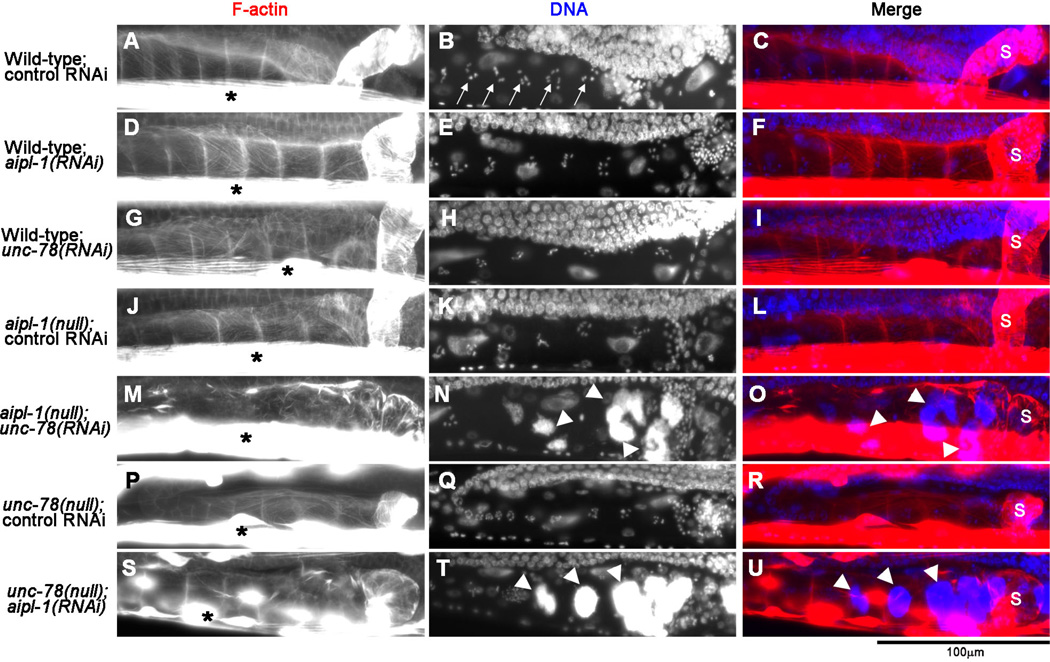
Fig. 1. Depletion of the two AIP1 isoforms causes accumulation of endomitotic oocytes in the ovary.
Adult worms with genotypes and RNAi treatments as indicated on the left were fixed and stained with tetramethylrhodamine-phalloidin for F-actin (left column) and DAPI for DNA (middle column), and regions of the ovary and the spermatheca are shown. The spermatheca is on the right side of each micrograph, and its location indicated by “s” in merged images on the right column (red: F-actin; blue: DNA). Arrows in B indicate condensed chromosomes in normal oocytes. Asterisks in the left column show intense staining of F-actin in the body wall muscle. Arrowheads in N and T indicate abnormal accumulation of DNA in endomitotic oocytes. Bar, 100 µm.
To determine the cause of sterility, we examined the gonads in these worms by staining whole worms by tetramethylrhodamine-phalloidin for filamentous (F-) actin and DAPI for DNA, which allowed us to examine morphology and locations of the somatic gonad, oocytes, and embryos (Fig. 1). In wild-type worms with control RNAi (Fig. 1A–C), each oocyte in the ovary had condensed chromosomes (Fig. 1B, arrows), and normal embryos were present in the uterus (data not shown). The gonads did not show detectable phenotypes by depletion of a single AIP1 isoform by aipl-1(RNAi) (Fig. 1D–F) or unc-78(RNAi) (Fig. 1G–I) in wild-type, or single mutation by aipl-1(null) with control RNAi (Fig. 1J–L) or unc-78(null) with control RNAi (Fig. 1P–R). However, simultaneous depletion of both AIP1 isoforms by unc-78(RNAi) in aipl-1(null) (Fig. 1M–O) or aipl-1(RNAi) in unc-78(null) (Fig. 1S–U) caused accumulation of abnormal oocytes with excessive DNA in the proximal ovary (Fig. 1N and T, arrowheads) and depletion of embryos from the uterus in 100 % of examined animals (n=100 each). These are typical Emo (endomitotic oocytes in gonadal arms) phenotypes when ovulation is defective (Iwasaki et al. 1996). During normal ovulation, oocyte maturation is coupled with enhanced contraction of the myoepithelial sheath, which expels a mature oocyte into the spermatheca for fertilization. However, when ovulation does not occur, a mature oocyte remains in the proximal ovary and undergoes endomitotic replication of chromosomal DNA (McCarter et al. 1997; McCarter et al. 1999). Thus, these results strongly suggest that the AIP1 isoforms have redundant functions that are required for ovulation. Since simultaneous depletion of the two AIP1 isoforms by unc-78(RNAi) in aipl-1(null) or aipl-1(RNAi) in unc-78(null) caused indistinguishable phenotypes, not all combinations of RNAi/mutation are shown in the subsequent figures.
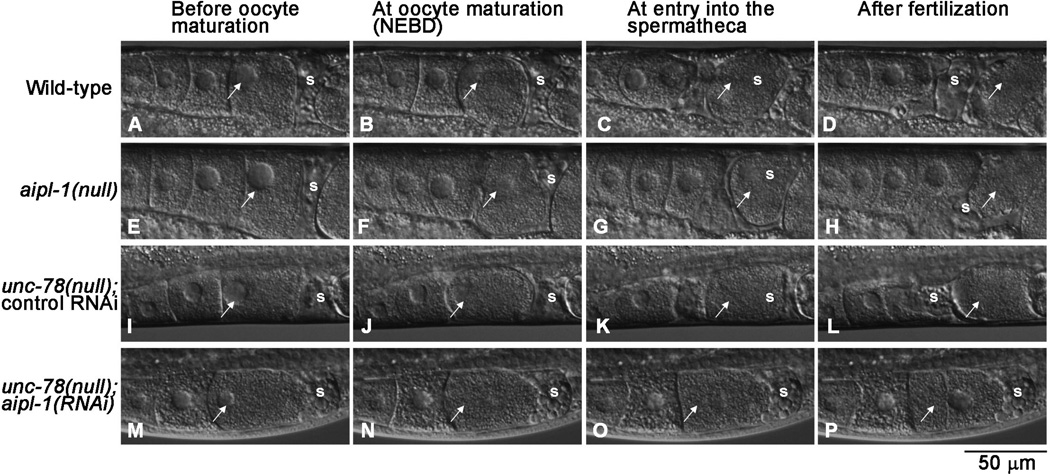
Normal ovulation in C. elegans involves several processes including intense contraction of the myoepithelial sheath, dilation of the spermatheca, and exit of a fertilized egg from the spermatheca (McCarter et al. 1997). To determine which process(es) of ovulation is disturbed by AIP1 depletion, ovulation in live worms was observed by time-lapse imaging (Fig. 2). Wild-type worms showed normal ovulation processes (13/13) (Fig. 2A–D, Supplemental Movie 1). Single mutants, aipl-1(null) (16/16) (Fig. 2E–H, Supplemental Movie 2) and unc-78(null) (with control RNAi) (16/16) (Fig. 2I–L, Supplemental Movie 3) also showed normal ovulation. However, when the two AIP1 isoforms were simultaneously depleted in unc-78(null); aipl-1(RNAi) worms, worms were not successful in ovulation (18/18) (Fig. 2M–P, Supplemental Movie 4): contractile activity of the myoepithelial sheath was significantly weaker and less frequent than wild-type and insufficient to push an oocyte into the spermatheca (Fig. 2M–P, Supplemental Movie 4). Spermatheca did not appear to dilate, but this could be due to lack of oocyte entry. The oocyte, which was retained in the ovary, underwent nuclear envelope breakdown (Fig. 2N, arrow), which is an indication of oocyte maturation as observed during normal ovulation (Fig. 2B, F, and J, arrows). This suggests that oocyte maturation was not affected by the depletion of the AIP1 isoforms. The retained oocyte reformed the nuclear envelope (Fig. 2P, arrow), indicating that endomitotic cycles are repeated. Together, the live imaging demonstrates that lack of strong contractility in the myoepithelial sheath is the major cause of the ovulation defect in AIP1-depleted worms.
Fig. 2. Depletion of the two AIP1 isoforms causes ovulation defects.
Ovulation processes in live worms with genotypes and RNAi treatments as indicated on the left were recorded by time-lapse Nomarski microscopy. Snapshots at key steps are shown: before oocyte maturation (A, E, I and M), at oocyte maturation (nuclear envelope breakdown: NEBD) (B, F, J, and N), at entry of an oocyte into the spermatheca (C, G, K, and O), and after fertilization (D, H, L, and P). Arrows indicate the most mature oocytes being ovulated. Positions of the spermatheca are indicated by “s”. Note that the oocyte in unc-78(null);aipl-1(RNAi) was not ovulated (M-P), and snapshots at equivalent timing to wild-type worms are shown. Bar, 50 µm. See corresponding Supplemental Movies 1– 4 in supplemental materials.
The AIP1 isoforms are Essential for Assembly of Contractile Actin Networks in the Myoepithelial Sheath and Spermatheca
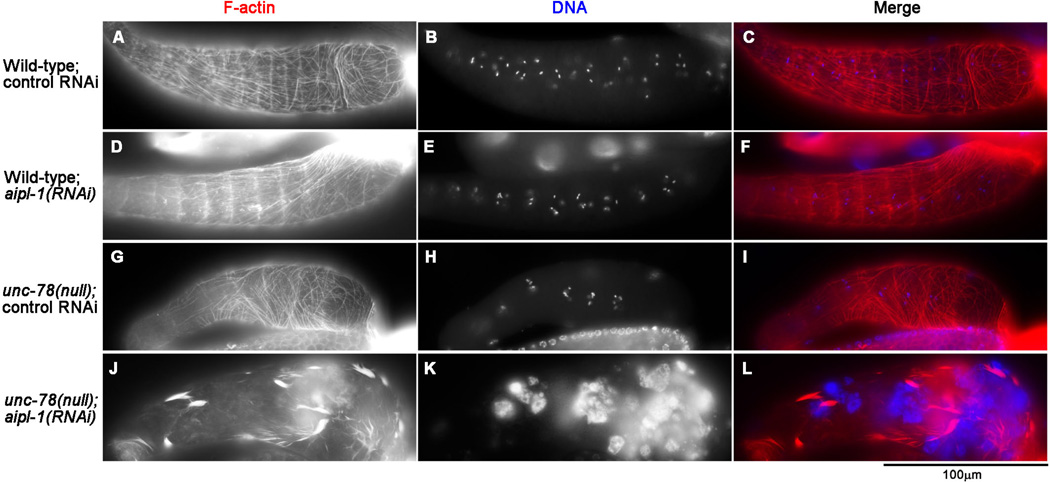
Non-striated actin networks in the myoepithelial sheath are responsible for contractility of the C. elegans ovary (Ono et al. 2007). Therefore, we dissected the gonads out of the body and examined organization of actin filaments in the myoepithelial sheath (Fig. 3). In wild-type worms with control RNAi, actin filaments were organized into normal non-striated networks in the myoepithelial sheath (Fig. 3A–C). Single AIP1 depletion by aipl-1(RNAi) (Fig. 3D–F) or unc-78(null) with control RNAi (Fig. 3G–I) did not cause detectable alterations in the actin organization in the myoepithelial sheath. However, simultaneous depletion of the two AIP1 isoforms by unc-78(null); aipl-1(RNAi) caused severe disorganization of the actin networks in the myoepithelial sheath (Fig. 3J–L). In these AIP1-depleted cells, fine actin networks were absent, and most of actin filaments were found in thick bundles and aggregates (Fig. 3J). This phenotype is consistent with the lack of contractile activity during ovulation as shown by live imaging (Fig. 2M–P). These results indicate that the two AIP1 isoforms have redundant functions and are essential for organization of the contractile actin networks in the myoepithelial sheath.
Fig. 3. Depletion of the two AIP1 isoforms disrupts contractile actin networks in the myoepithelial sheath.
Gonads from worms with genotypes and RNAi treatments as indicated on the left were dissected out and stained with tetramethylrhodamine-phalloidin for F-actin (left column) and DAPI for DNA (middle column), and the regions of the myoepithelial sheath are shown. Merged images are shown in the right column (red: F-actin; blue: DNA). Bar, 100 µm.
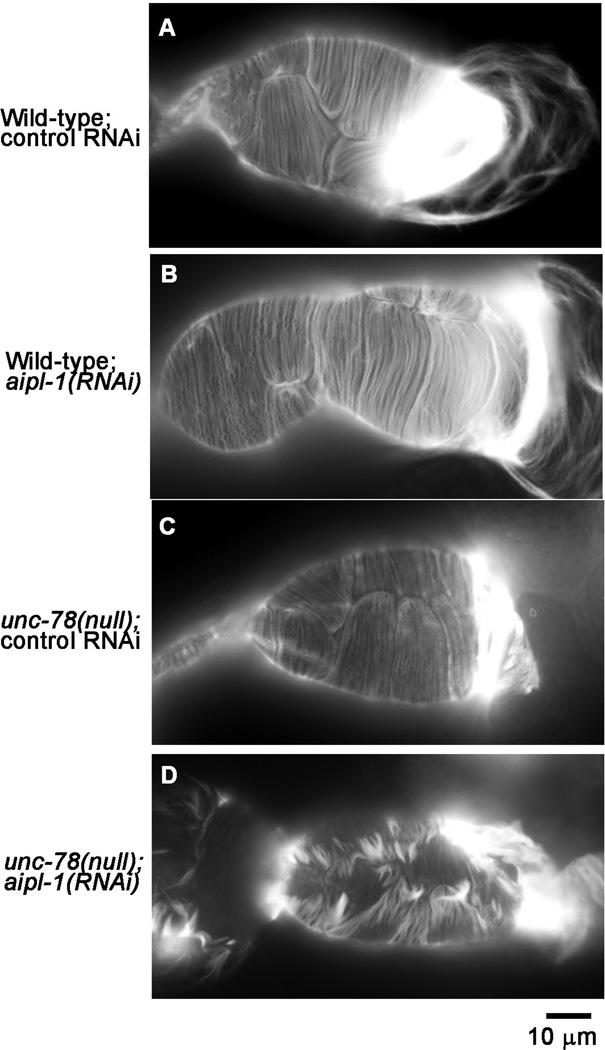
The spermatheca is adjacently located to the myoepithelial sheath in the somatic gonad, and its dilation is required for successful ovulation (Clandinin et al. 1998). We found that simultaneous depletion of the two AIP1 isoforms also disrupted the actin organization in the spermatheca (Fig. 4). In wild-type with control RNAi, thin actin bundles were aligned in a nearly parallel manner (Fig. 4A). This pattern of actin filaments was not altered by single AIP1 depletion by aipl-1(RNAi) (Fig. 4B) or unc-78(null) with control RNAi (Fig. 4C). However, simultaneous depletion of the two AIP1 isoforms by unc-78(null); aipl-1(RNAi) caused formation of gaps among actin bundles, accumulation of actin into thick bundles, and reduction in thin parallel actin fibers (Fig. 4D). Therefore, the two AIP1 isoforms are also redundantly required for proper organization of actin filaments in the spermatheca.
Fig. 4. Depletion of the two AIP1 isoforms disturbs actin organization in the spermatheca.
Gonads from worms with genotypes and RNAi treatments as indicated on the left were dissected out and stained with tetramethylrhodamine-phalloidin for F-actin, and the regions of the spermatheca are shown. Note that the spermatheca is a highly flexible tissue, and the difference in the overall shape of the spermatheca is not a significant phenotype. Rather, parallel actin fibers seen in wild-type with control RNAi (A), wild-type with aipl-1(RNAi) (B), and unc-78(null) with control RNAi (C), were highly disorganized in unc-78(null) with aipl-1(RNAi) (D). Bar, 10 µm.
Live observation of ovulation processes strongly suggested that weak contraction of the myoepithelial sheath in AIP1-depleted worms is the major cause of ovulation defects (Fig. 2). This is consistent with disorganized actin networks in the myoepithelial sheath of AIP1-depleted worms (Fig. 3). In these cells, most of the actin filaments were found in abnormal thick bundles and were very unlikely to produce coordinated contractile activity of the myoepithelial sheath. Likewise, actin filaments in the spermatheca were also disorganized in AIP1-depleted worms (Fig. 4). Although dysfunction of the spermatheca is probably not the major cause of ovulation defects, it may enhance the phenotype by not allowing entry of an oocyte into the spermatheca. The spermatheca is composed of a single layer of highly elastic epithelial cells and undergo repeated dilation and contraction during ovulation (Kovacevic et al. 2013). Although the mechanism of regulation of the contractile activities of the spermatheca is not well understood, contractility or elasticity of the spermatheca should be affected when the actin organization is disrupted as observed in AIP1-depleted worms. Although different sets of cytoskeletal proteins are expressed in the myoepithelial sheath and the spermatheca (Ardizzi and Epstein 1987; Ono et al. 2007; Rose et al. 1997), the two AIP1 isoforms are essential factors for assembly of contractile actin cytoskeleton in both parts of the somatic gonad.
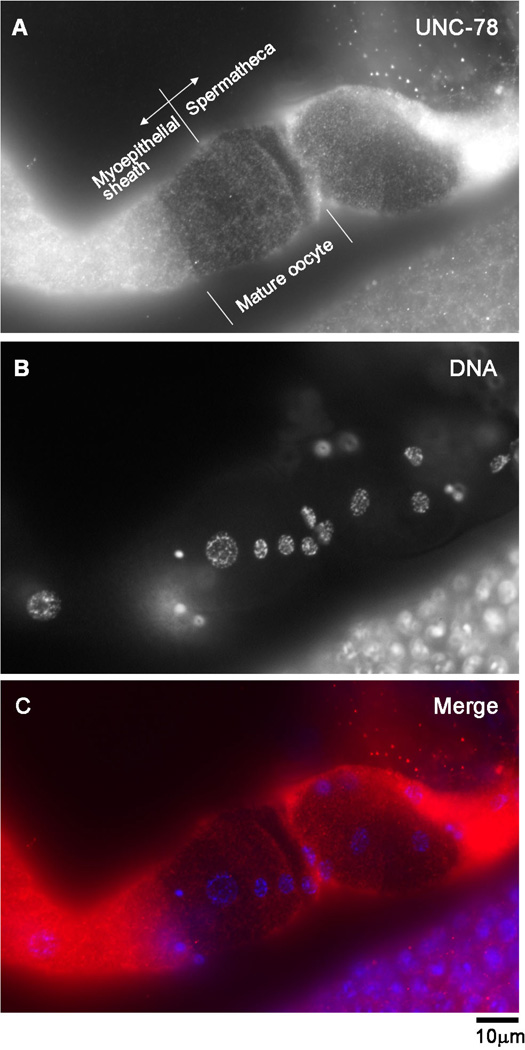
AIP1 Is Expressed in the Myoepithelial Sheath and the Spermatheca and Localized in the Diffuse Cytoplasm
Our previous analysis of the promoters of aipl-1 and unc-78 shows that both AIP1 isoforms are expressed in the somatic gonad (Ono et al. 2011). Here, we determined localization of AIP1 in the myoepithelial sheath and the spermatheca by immunofluorescence microscopy. We have not been successful in generating a specific antibody against AIPL-1. Therefore, we only examined localization of the UNC-78 protein using rabbit polyclonal anti-UNC-78 antibody (Mohri and Ono 2003) in the myoepithelial sheath and the spermatheca. This antibody did not cross-react with AIPL-1 (data not shown). In dissected gonads, the anti-UNC-78 antibody stained diffuse cytoplasm of the myoepithelial sheath, the spermatheca, and oocytes (Fig. 5A, Supplemental Fig. 1). These fluorescent signals were not detected in the same tissues from unc-78(null) mutant worms (Supplemental Fig. 1), indicating that the antibody specifically recognized the UNC-78 protein. In Fig. 5, agonad was fixed when a mature oocyte was being ovulated and located at the junction between the myoepithelial sheath and the spermatheca. Such an oocyte is often impermeable and provides a clear background for better observation of immunofluorescent signals in the surrounding somatic gonad. These micrographs showed that UNC-78 is diffusely distributed in the cytoplasm in both myoepithelial sheath and spermatheca lacking nuclear enrichment (Fig. 5A). This localization pattern suggests that UNC-78 functions in the soluble cytoplasm as a regulator of actin dynamics rather than a structural component of the actin networks. Given the redundant functions of UNC-78 and AIPL-1, AIPL-1 is expected to localize to the diffuse cytoplasm in a similar manner to UNC-78.
Fig. 5. UNC-78 is diffusely localized in the cytoplasm in the somatic gonad.
Gonads from wild-type worms were dissected out and stained with anti-UNC-78 antibody (A) and DAPI for DNA (B). Merged image is shown in C (red: UNC-78; blue: DNA). UNC-78 was detected in oocytes, the myoepithelial sheath, and the spermatheca (also see Supplementary Fig. 1). A mature oocyte is impermeable and was present at the junction between the myoepithelial sheath and the spermatheca (A), providing a clear background to show diffuse localization of UNC-78 in both myoepithelial sheath and spermathecal cells. Bar, 10 µm.
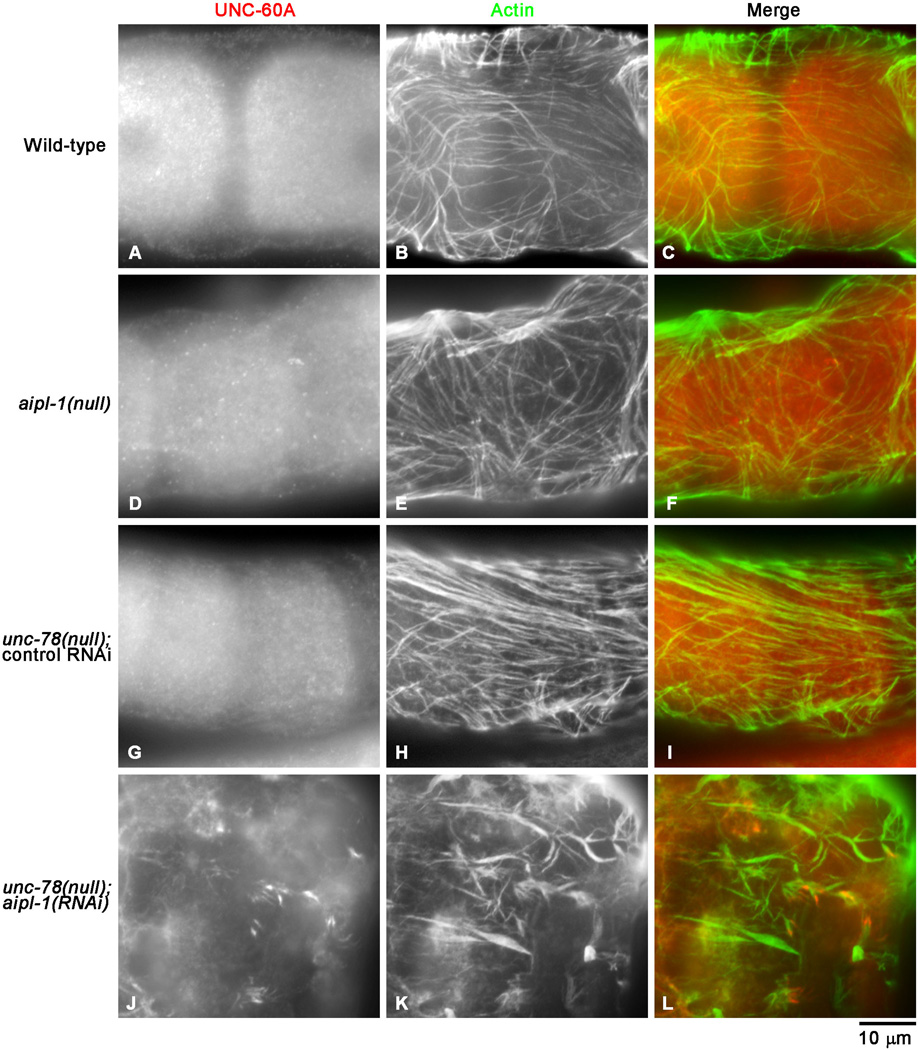
Depletion of AIP1 Causes Abnormal Aggregation of UNC-60A (ADF/Cofilin) and Actin in the Myoepithelial Sheath
AIP1 functions closely with ADF/cofilin to promote disassembly of actin filaments (Ono 2003). C. elegans has two ADF/cofilin isoforms: UNC-60A and UNC-60B with distinct activities (McKim et al. 1994; Ono and Benian 1998), and UNC-60A is an isoform that is expressed in the somatic gonad and essential for organization of the actin networks in the myoepithelial sheath (Ono et al. 2008). Indeed, the AIP1-depletion phenotypes in the actin organization in the somatic gonad were very similar to the UNC-60A-depletion phenotypes (Ono et al. 2008), suggesting that UNC-60A functions together with AIP1. In wild-type worms, UNC-60A was present in the diffuse cytoplasm in the myoepithelial sheath (Fig. 6A). Similar pattern of UNC-60A was observed in aipl-1(null) (Fig. 6D) and unc-78(null) with control RNAi (Fig. 6G). However, when the two AIP1 isoforms were simultaneously depleted by unc-78(null); aipl-1(RNAi), UNC-60A was mislocalized to aggregates (Fig. 6J) where actin was also accumulated (Fig. 6K and L). Interestingly, UNC-60A was not accumulated in all actin aggregates but rather concentrated in a subset of aggregates (Fig. 6J–L), showing heterogeneous nature of actin aggregates in AIP1-depleted cells. Similar abnormal accumulation of UNC-60A in aggregates was also detected in the AIP1-depleted spermatheca (Ono and Ono, unpublished data). Overall levels of UNC-60A did not appear different. These phenotypes suggest that UNC-60A-bound actin filaments are excessively accumulated in the absence of UNC-78 and AIPL-1 because their normal function is to promote disassembly of ADF/cofilin-bound actin filaments. These results provide genetic evidence that UNC-60A, UNC-78, and AIPL-1 cooperate to assemble contractile actin networks in the myoepithelial sheath.
Fig. 6. UNC-60A (ADF/cofilin) is mislocalized to actin aggregates in AIP1-depleted myoepithelial sheath.
Gonads from worms with genotypes and RNAi treatments as indicated on the left were dissected out and stained with anti-UNC-60A antibody (left column) and anti-actin antibody (middle column), and the regions of the myoepithelial sheath are shown. Merged images are shown in the right column (red: UNC-60A; green: actin). UNC-60A was expressed in both oocytes and the myoepithelial sheath. Although the focuses were adjusted to the levels of the myoepithelial sheath, staining of oocytes were also visible. Bar, 10 µm.
Our previous in vitro analysis showed that both UNC-78 and AIPL-1 promote actin filament disassembly in the presence of UNC-60B but not in the presence of UNC-60A (Mohri and Ono 2003; Ono et al. 2011). However, in the somatic gonad, UNC-60A, but not UNC-60B, is required for assembly of actin networks (Ono et al. 2008). Our current study shows that the two AIP1 isoforms are also required for actin organization in the somatic gonad, as well as for proper localization of UNC-60A, suggesting strongly that UNC-78 and AIPL-1 cooperate with UNC-60A in vivo. Expression of UNC-60B was not altered in the gonad when the two AIP1 isoforms were depleted (Ono and Ono, unpublished observation). The discrepancy between the in vitro and in vivo results suggests the presence of an unknown factor that mediates cooperative action of UNC-60A and UNC-78 or AIPL-1. AIP1 preferentially interacts with ADF/cofilin-bound actin filaments (Ono 2003). Since UNC-60B can stay bound to actin filaments, UNC-60B-bound actin filaments are rapidly disassembled by UNC-78 or AIPL-1 (Mohri and Ono 2003; Ono et al. 2004; Ono et al. 2011). A C-terminal truncation of UNC-60B only abolishes its F-actin binding but not G-actin binding (Ono et al. 2001), and UNC-78 is not able to promote actin disassembly in the presence of UNC-60B with this truncation (Mohri and Ono 2003). Similarly, UNC-60A poorly binds to F-actin in vitro (Ono and Benian 1998; Yamashiro et al. 2005), and this is a probable reason why UNC-60A does not cooperate with AIP1 in vitro. Therefore, a factor that can stabilize association of UNC-60A with F-actin may promote actin disassembly by UNC-60A and an AIP1 isoform. In AIP1-depleted cells, UNC-60A is accumulated to actin aggregates, implying the presence of such a factor. Coronin is known to enhance F-actin binding of cofilin in mammals and yeast (Brieher et al. 2006; Gandhi et al. 2009). C. elegans has a gene encoding coronin (cor-1) (Yonemura and Mabuchi 2001), but genome-wide RNAi studies did not find a detectable phenotype for cor-1(RNAi) (Kamath et al. 2003; Sonnichsen et al. 2005), and its function is currently unknown. Thus, additional studies are required to understand how ADF/cofilin and AIP1 work together in the somatic gonad.
Conclusion
AIP1 genes are not essential in relatively simple organisms such as the budding yeast Saccharomyces cerevisiae (Iida and Yahara 1999; Rodal et al. 1999), the slime mold Dictyostelium discoideum (Aizawa et al. 1999; Konzok et al. 1999), and moss Physcomitrella patens (Augustine et al. 2011). However, AIP1 is essential for development of more complex organisms, and knockout or knockdown of AIP1 has been shown to be lethal in the fruit fly Drosophila melanogaster (Ren et al. 2007), the nematode C. elegans (Ono et al. 2011), the green plant Arabidopsis thaliana (Ketelaar et al. 2004), and mouse (Kile et al. 2007). Here, our study revealed another essential function of AIP1 in the reproductive system by promoting assembly of the contractile actin cytoskeleton in the somatic gonad. Somatic tissues within gonads support development of gametes and are essential for reproduction in multicellular organisms, and some of them require contractile actin cytoskeleton. The Drosophila ovaries contain highly branched striated muscles, which provide contractile forces for ovulation (Hudson et al. 2008; Middleton et al. 2006; Rodriguez-Valentin et al. 2006). Mammalian ovaries also contain smooth muscle cells (Espey 1978), and their endothelin-induced contraction promotes ovulation (Bridges et al. 2011). However, regulation of assembly and contraction of these actin cytoskeletal structures is poorly understood. The studies in C. elegans strongly suggest that ADF/cofilin and AIP1 are important molecules for understanding how cell contractility is generated and utilized in animal reproduction.
Materials and Methods
Nematode Strains
Wild-type strain N2 and VC701 aipl-1(ok1019) were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN). aipl-1(ok1019) was described previously (Ono et al. 2011). unc-78(gk27) was described previously (Ono 2001). These alleles were used as homozygotes in this study and referred as aipl-1(null) and unc-78(null), respectively. Nematodes were grown under standard conditions at 20 °C as described previously (Brenner 1974).
RNA Interference Experiments
RNAi experiments were performed by feeding with E. coli expressing double-stranded RNA as described previously (Ono and Ono 2002) with modifications as described (Ono et al. 2007). RNAi clones for unc-78 (clone X-2A18) and aipl-1 (clone V-9L16) were obtained from Source Bioscience (Kamath et al. 2003). To bypass embryonic lethality, embryos were collected from gravid adults by treatment with hypochlorite/NaOH and cultured over night, and newly hatched L1 larvae were transferred to plates that had been seeded with E. coli strains expressing dsRNA. Phenotypes were characterized in the same generation when they became adults.
Fluorescence Microscopy
Immunofluorescent staining of dissected gonads was performed as described previously (Ono et al. 2007). Briefly, gonads were dissected by cutting adult hermaphrodites on polylysine-coated slides. For staining for UNC-78 (Fig. 5), they were fixed with 4 % paraformaldehyde in cytoskeleton buffer (138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 10 mM MES-KOH, pH 6.1) containing 0.32 M sucrose for 15 min at room temperature followed by permeabilization by phosphate-buffered saline (PBS) containing 0.5 % Triton X-100 and 30 mM glycine for 10 min at room temperature. For staining for UNC-60A and actin (Fig. 6), they were fixed with methanol at −20 °C for 5 min. They were washed by PBS for 10 min and stained with mouse anti-actin monoclonal (C4; MP Biomedicals), rabbit anti-UNC-60A (Ono et al. 1999), or rabbit anti-UNC-78 (Mohri and Ono 2003) antibodies diluted in 1 % bovine serum albumin in PBS. The primary antibodies were visualized by staining with Alexa 488-conjugated goat anti-mouse IgG (Invitrogen) and Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch).
Staining of whole worms with tetramethylrhodamine-phalloidin (Sigma-Aldrich) (Fig. 1) was performed as described previously (Ono 2001). Staining of dissected gonads with tetramethylrhodamine-phalloidin (Figs. 3 and 4) was performed as described previously (Ono et al. 2008). 4’6-diamidino-2-phenylindole (DAPI) was mixed at 0.2 µg/ml with primary antibody or tetramethylrhodamine-phalloidin solutions.
Samples were mounted with ProLong Gold (Invitrogen) and observed by epifluorescence using a Nikon Eclipse TE2000 inverted microscope with a CFI Plan Fluor ELWD 40× (Dry; NA 0.60) or Plan Apo 60× (oil; NA 1.40) objective. Images were captured by a SPOT RT monochrome CCD camera (Diagnostic Instruments) and processed by IPLab imaging software (BD Biosciences) and Adobe Photoshop CS3.
Time-lapse Nomarski Microscopy
Worms were anesthetized in 0.1 % tricaine, 0.01 % tetramisole in M9 buffer for 30 min and mounted on 2 % agarose pads (McCarter et al. 1997). Tricaine/tetramisole paralyzes body wall movement but does not block several rounds of oocyte maturation and ovulation. They were set on a Nikon Eclipse TE2000 inverted microscope and observed with a 40 × CFI Plan Fluor objective (N.A. 1.4). Images were captured at room temperature by a SPOT Idea CMOS camera (Diagnostic Instruments) and recorded at 30 frames per minute by the SPOT Imaging Software (Diagnostic Instruments). Movie files were saved in a compressed AVI format at 20 frames per second (40 times as fast as real time), and phenotypes analyzed later. Movie files were edited and converted to Quicktime files using Adobe Premire 6.5.
Supplementary Material
Acknowledgments
Some worm strains were provided by the Caenorhabditis Genetics Center at University of Minnesota, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). This work was supported by a grant from the National Institutes of Health (R01 AR048615) to S. O.
References
- Aizawa H, Katadae M, Maruya M, Sameshima M, Murakami-Murofushi K, Yahara I. Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells. 1999;4:311–324. doi: 10.1046/j.1365-2443.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- Ardizzi JP, Epstein HF. Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J Cell Biol. 1987;105:2763–2770. doi: 10.1083/jcb.105.6.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RC, Pattavina KA, Tuzel E, Vidali L, Bezanilla M. Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell. 2011;23:3696–3710. doi: 10.1105/tpc.111.090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges PJ, Cho J, Ko C. Endothelins in regulating ovarian and oviductal function. Front Biosci. 2011;3:145–155. doi: 10.2741/s140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Kueh HY, Ballif BA, Mitchison TJ. Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J Cell Biol. 2006;175:315–324. doi: 10.1083/jcb.200603149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen AK, Maday SL, Rybicka KK, Sulcove JA, Ward J, Huang MM, Barstead R, Franzini-Armstrong C, Allen TS. Disruption of Caenorhabditis elegans muscle structure and function caused by mutation of troponin I. Biophys J. 2004;86:991–1001. doi: 10.1016/S0006-3495(04)74174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Miller DM, Ortiz I, Berliner GC. Myosin and paramyosin are organized about a newly identified core structure. J Cell Biol. 1985;100:904–915. doi: 10.1083/jcb.100.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey LL. Ovarian contractility and its relationship to ovulation: a review. Biol Reprod. 1978;19:540–551. doi: 10.1095/biolreprod19.3.540. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Achard V, Blanchoin L, Goode BL. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol Cell. 2009;34:364–374. doi: 10.1016/j.molcel.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Petrella LN, Tanaka AJ, Cooley L. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev Biol. 2008;314:329–340. doi: 10.1016/j.ydbio.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Yahara I. Cooperation of two actin-binding proteins, cofilin and Aip1, in Saccharomyces cerevisiae. Genes Cells. 1999;4:21–32. doi: 10.1046/j.1365-2443.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, McCarter J, Francis R, Schedl T. emo-1 a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J Cell Biol. 1996;134:699–714. doi: 10.1083/jcb.134.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Ketelaar T, Allwood EG, Anthony R, Voigt B, Menzel D, Hussey PJ. The actin-interacting protein AIP1 is essential for actin organization and plant development. Curr Biol. 2004;14:145–149. doi: 10.1016/j.cub.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kile BT, Panopoulos AD, Stirzaker RA, Hacking DF, Tahtamouni LH, Willson TA, Mielke LA, Henley KJ, Zhang JG, Wicks IP, et al. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood. 2007;110:2371–2380. doi: 10.1182/blood-2006-10-055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzok A, Weber I, Simmeth E, Hacker U, Maniak M, Muller-Taubenberger A. DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J Cell Biol. 1999;146:453–464. doi: 10.1083/jcb.146.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic I, Cram EJ. FLN-1/filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev Biol. 2010;347:247–257. doi: 10.1016/j.ydbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic I, Orozco JM, Cram EJ. Filamin and phospholipase C-epsilon are required for calcium signaling in the Caenorhabditis elegans spermatheca. PLoS Genet. 2013;9:e1003510. doi: 10.1371/journal.pgen.1003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- McKim KS, Matheson C, Marra MA, Wakarchuk MF, Baillie DL. The Caenorhabditis elegans unc-60 gene encodes proteins homologous to a family of actin-binding proteins. Mol Gen Genet. 1994;242:346–357. doi: 10.1007/BF00280425. [DOI] [PubMed] [Google Scholar]
- Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Ortiz I, Berliner GC, Epstein HF. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–490. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Mohri K, Ono K, Yu R, Yamashiro S, Ono S. Enhancement of actin-depolymerizing factor/cofilin-dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the Caenorhabditis elegans body wall muscle. Mol Biol Cell. 2006;17:2190–2199. doi: 10.1091/mbc.E05-11-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Ono S. Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J Cell Sci. 2003;116:4107–4118. doi: 10.1242/jcs.00717. [DOI] [PubMed] [Google Scholar]
- Mohri K, Vorobiev S, Fedorov AA, Almo SC, Ono S. Identification of functional residues on Caenorhabditis elegans actin-interacting protein 1 (UNC-78) for disassembly of actin depolymerizing factor/cofilin-bound actin filaments. J Biol Chem. 2004;279:31697–31707. doi: 10.1074/jbc.M403351200. [DOI] [PubMed] [Google Scholar]
- Myers CD, Goh PY, Allen TS, Bucher EA, Bogaert T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J Cell Biol. 1996;132:1061–1077. doi: 10.1083/jcb.132.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata T, Ono K, Ono S. Troponin I controls ovulatory contraction of non-striated actomyosin networks in the C. elegans somatic gonad. J Cell Sci. 2010;123:1557–1566. doi: 10.1242/jcs.065060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ono S. Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol Biol Cell. 2004;15:2782–2793. doi: 10.1091/mbc.E04-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Parast M, Alberico C, Benian GM, Ono S. Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J Cell Sci. 2003;116:2073–2085. doi: 10.1242/jcs.00421. [DOI] [PubMed] [Google Scholar]
- Ono K, Yamashiro S, Ono S. Essential role of ADF/cofilin for assembly of contractile actin networks in the C. elegans somatic gonad. J Cell Sci. 2008;121:2662–2670. doi: 10.1242/jcs.034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yu R, Ono S. Structural components of the nonstriated contractile apparatuses in the Caenorhabditis elegans gonadal myoepithelial sheath and their essential roles for ovulation. Dev Dyn. 2007;236:1093–1105. doi: 10.1002/dvdy.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J Cell Biol. 2001;152:1313–1319. doi: 10.1083/jcb.152.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry. 2003;42:13363–13370. doi: 10.1021/bi034600x. [DOI] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton (Hoboken) 2010;67:677–692. doi: 10.1002/cm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Baillie DL, Benian GM. UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J Cell Biol. 1999;145:491–502. doi: 10.1083/jcb.145.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Benian GM. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J Biol Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Ono S, McGough A, Pope BJ, Tolbert VT, Bui A, Pohl J, Benian GM, Gernert KM, Weeds AG. The C-terminal tail of UNC-60B (ADF/cofilin) is critical for maintaining its stable association with F-actin and is implicated in the second actin-binding site. J Biol Chem. 2001;276:5952–5958. doi: 10.1074/jbc.M007563200. [DOI] [PubMed] [Google Scholar]
- Ono S, Mohri K, Ono K. Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/cofilin-bound actin filaments. J Biol Chem. 2004;279:14207–14212. doi: 10.1074/jbc.M313418200. [DOI] [PubMed] [Google Scholar]
- Ono S, Nomura K, Hitosugi S, Tu DK, Lee JA, Baillie DL, Ono K. The two actin-interacting protein 1 genes have overlapping and essential function for embryonic development in Caenorhabditis elegans. Mol Biol Cell. 2011;22:2258–2269. doi: 10.1091/mbc.E10-12-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Ono K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J Cell Biol. 2002;156:1065–1076. doi: 10.1083/jcb.200110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N, Charlton J, Adler PN. The flare gene, which encodes the AIP1 protein of Drosophila functions to regulate F-actin disassembly in pupal epidermal cells. Genetics. 2007;176:2223–2234. doi: 10.1534/genetics.107.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC. Aip1p interacts with cofilin to disassemble actin filaments. J Cell Biol. 1999;145:1251–1264. doi: 10.1083/jcb.145.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valentin R, Lopez-Gonzalez I, Jorquera R, Labarca P, Zurita M, Reynaud E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 2006;209:183–198. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- Rose KL, Winfrey VP, Hoffman LH, Hall DH, Furuta T, Greenstein D. The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1997;192:59–77. doi: 10.1006/dbio.1997.8728. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terami H, Williams BD, Kitamura S, Sakube Y, Matsumoto S, Doi S, Obinata T, Kagawa H. Genomic organization, expression, and analysis of the troponin C gene pat-10 of Caenorhabditis elegans. J Cell Biol. 1999;146:193–202. doi: 10.1083/jcb.146.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, Mains PE. The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Mohri K, Ono S. The two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins differently enhance actin filament severing and depolymerization. Biochemistry. 2005;44:14238–14247. doi: 10.1021/bi050933d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura I, Mabuchi I. Heterogeneity of mRNA coding for Caenorhabditis elegans coronin-like protein. Gene. 2001;271:255–259. doi: 10.1016/s0378-1119(01)00509-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.