Abstract
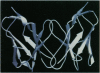
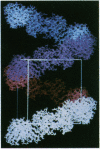
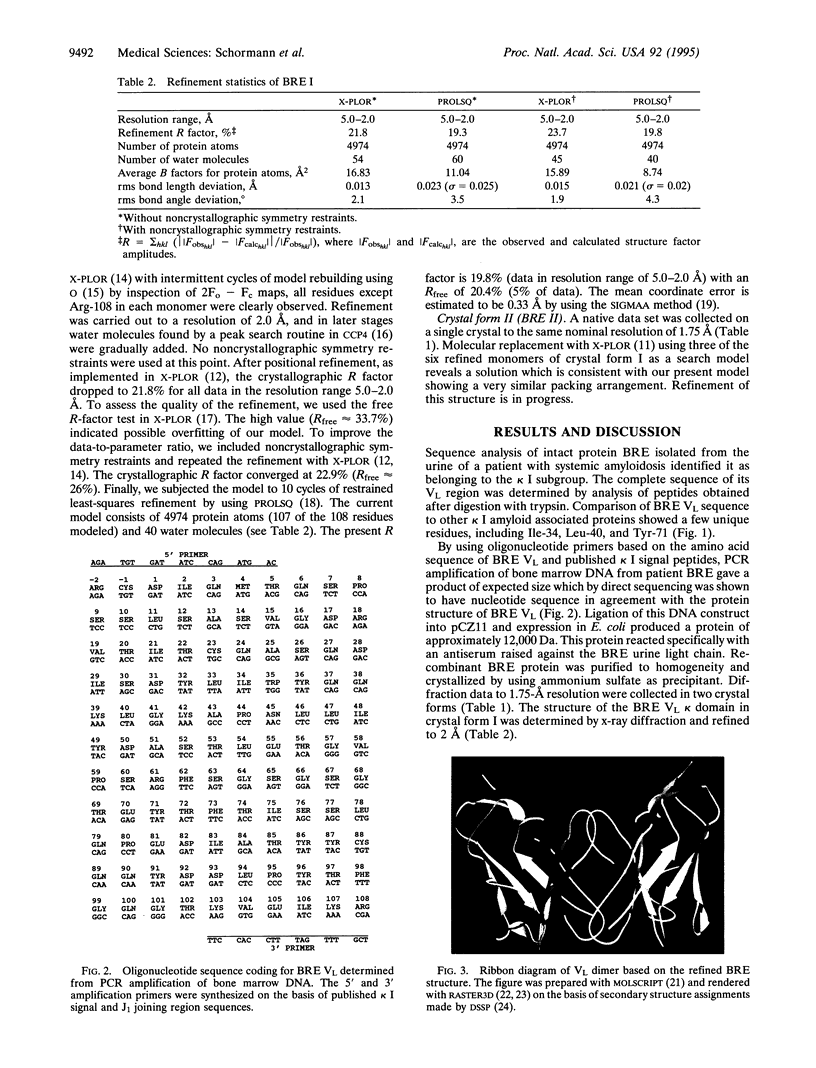
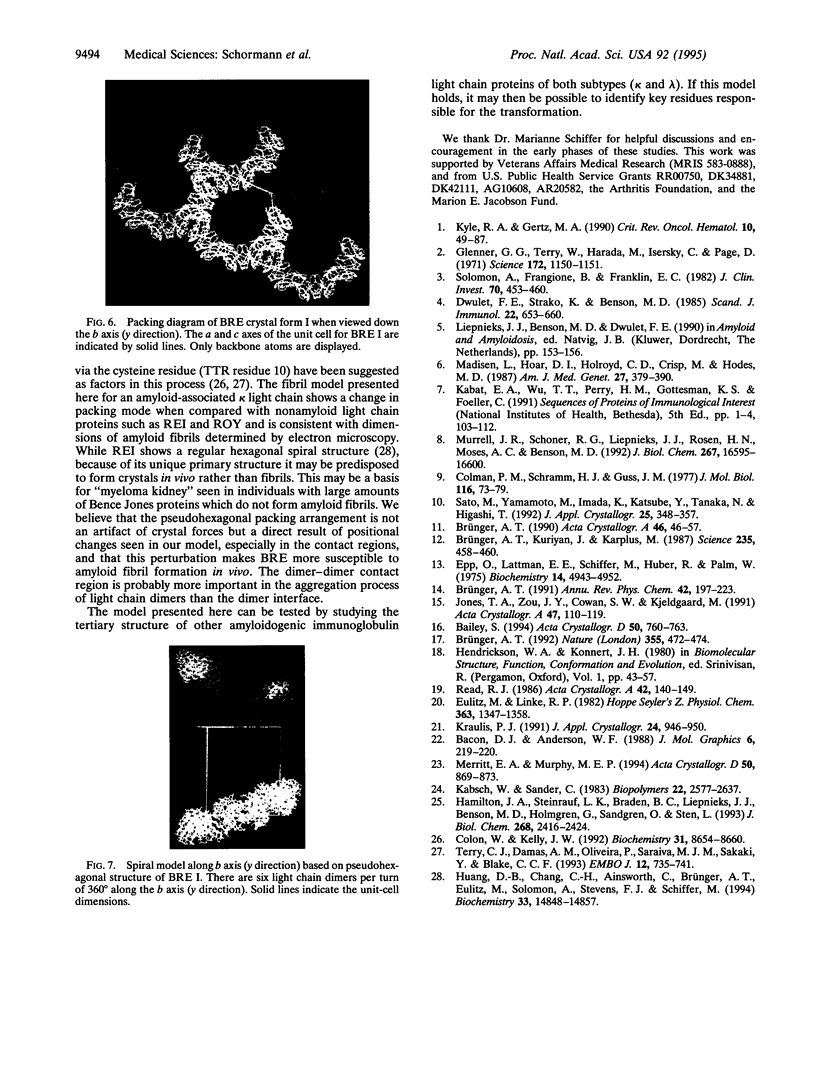
An immunoglobulin light chain protein was isolated from the urine of an individual (BRE) with systemic amyloidosis. Complete amino acid sequence of the variable region of the light chain (VL) protein established it as a kappa I, which when compared with other kappa I amyloid associated proteins had unique residues, including Ile-34, Leu-40, and Tyr-71. To study the tertiary structure, BRE VL was expressed in Escherichia coli by using a PCR product amplified from the patient BRE's bone marrow DNA. The PCR product was ligated into pCZ11, a thermal-inducible replication vector. Recombinant BRE VL was isolated, purified to homogeneity, and crystallized by using ammonium sulfate as the precipitant. Two crystal forms were obtained. In crystal form I the BRE VL kappa domain crystallizes as a dimer with unit cell constants isomorphous to previously published kappa protein structures. Comparison with a nonamyloid VL kappa domain from patient REI, identified significant differences in position of residues in the hypervariable segments plus variations in framework region (FR) segments 40-46 (FR2) and 66-67 (FR3). In addition, positional differences can be seen along the two types of local diads, corresponding to the monomer-monomer and dimer-dimer interfaces. From the packing diagram, a model for the amyloid light chain (AL) fibril is proposed based on a pseudohexagonal spiral structure with a rise of approximately the width of two dimers per 360 degree turn. This spiral structure could be consistent with the dimensions of amyloid fibrils as determined by electron microscopy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994 Sep 1;50(Pt 5):760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Schramm H. J., Guss J. M. Crystal and molecular structure of the dimer of variable domains of the Bence-Jones protein ROY. J Mol Biol. 1977 Oct 15;116(1):73–79. doi: 10.1016/0022-2836(77)90119-x. [DOI] [PubMed] [Google Scholar]
- Colon W., Kelly J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992 Sep 15;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Strako K., Benson M. D. Amino acid sequence of a lambda VI primary (AL) amyloid protein (WLT). Scand J Immunol. 1985 Dec;22(6):653–660. doi: 10.1111/j.1365-3083.1985.tb01927.x. [DOI] [PubMed] [Google Scholar]
- Epp O., Lattman E. E., Schiffer M., Huber R., Palm W. The molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI refined at 2.0-A resolution. Biochemistry. 1975 Nov 4;14(22):4943–4952. doi: 10.1021/bi00693a025. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Linke R. P. Primary structure of the variable part of an amyloidogenic Bence-Jones Protein (Mev.). An unusual insertion in the third hypervariable region of a human kappa-immunoglobulin light chain. Hoppe Seylers Z Physiol Chem. 1982 Nov;363(11):1347–1358. doi: 10.1515/bchm2.1982.363.2.1347. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Steinrauf L. K., Braden B. C., Liepnieks J., Benson M. D., Holmgren G., Sandgren O., Steen L. The x-ray crystal structure refinements of normal human transthyretin and the amyloidogenic Val-30-->Met variant to 1.7-A resolution. J Biol Chem. 1993 Feb 5;268(4):2416–2424. [PubMed] [Google Scholar]
- Huang D. B., Chang C. H., Ainsworth C., Brünger A. T., Eulitz M., Solomon A., Stevens F. J., Schiffer M. Comparison of crystal structures of two homologous proteins: structural origin of altered domain interactions in immunoglobulin light-chain dimers. Biochemistry. 1994 Dec 13;33(49):14848–14857. doi: 10.1021/bi00253a024. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Gertz M. A. Systemic amyloidosis. Crit Rev Oncol Hematol. 1990;10(1):49–87. doi: 10.1016/1040-8428(90)90021-j. [DOI] [PubMed] [Google Scholar]
- Madisen L., Hoar D. I., Holroyd C. D., Crisp M., Hodes M. E. DNA banking: the effects of storage of blood and isolated DNA on the integrity of DNA. Am J Med Genet. 1987 Jun;27(2):379–390. doi: 10.1002/ajmg.1320270216. [DOI] [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Murrell J. R., Schoner R. G., Liepnieks J. J., Rosen H. N., Moses A. C., Benson M. D. Production and functional analysis of normal and variant recombinant human transthyretin proteins. J Biol Chem. 1992 Aug 15;267(23):16595–16600. [PubMed] [Google Scholar]
- Solomon A., Frangione B., Franklin E. C. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982 Aug;70(2):453–460. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry C. J., Damas A. M., Oliveira P., Saraiva M. J., Alves I. L., Costa P. P., Matias P. M., Sakaki Y., Blake C. C. Structure of Met30 variant of transthyretin and its amyloidogenic implications. EMBO J. 1993 Feb;12(2):735–741. doi: 10.1002/j.1460-2075.1993.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]