Abstract
Forty cultivable, visually distinct bacterial cultures were isolated from four Baltic microalgal cultures Chlorella pyrenoidosa, Scenedesmus obliquus, Isochrysis sp., and Nitzschia microcephala, which have been maintained for several years in the laboratory. Bacterial isolates were characterized with respect to morphology, antibiotic susceptibility, and 16S ribosomal DNA sequence. A total of 17 unique bacterial strains, almost all belonging to one of three families, Rhodobacteraceae, Rhizobiaceae, and Erythrobacteraceae, were subsequently isolated. The majority of isolated bacteria belong to Rhodobacteraceae. Literature review revealed that close relatives of the bacteria isolated in this study are not only often found in marine environments associated with algae, but also in lakes, sediments, and soil. Some of them had been shown to interact with organisms in their surroundings. A Basic Local Alignment Search Tool study indicated that especially bacteria isolated from the Isochrysis sp. culture were highly similar to microalgae-associated bacteria. Two of those isolates, I1 and I6, belong to the Cytophaga–Flavobacterium–Bacteroides phylum, members of which are known to occur in close communities with microalgae. An UniFrac analysis revealed that the bacterial community of Isochrysis sp. significantly differs from the other three communities.
Keywords: Associated bacteria, characterization, diatom, green algae, marine bacteria, phycosphere
Introduction
Species-specific interactions between bacteria and microalgae have been reported (Yoshinaga et al. 1995, 1997; Hasegawa et al. 2007), but remain underinvestigated due to their complexity. Interactions between bacteria and algae are essential in regulating algae accumulation and degradation of organic matter (Cole et al. 1988; Smith et al. 1995). The ability of algae-associated bacteria to flocculate algae increases sedimentation and removal of organic matter from the water column (Allison and Sutherland 1987). Especially bacterial communities associated with algal blooms play critical roles in carbon and nitrogen cycling through their influence on the formation and fate of dissolved organic matter (Cole et al. 1982; Azam and Ammerman 1984).
Studies on harmful algal blooms have revealed that marine bacteria are able to either stimulate phytoplankton growth (Ferrier et al. 2002) by releasing vitamins or growth factors (Haines and Guillard 1974; Maruyama et al. 1986), inhibit phytoplankton growth, or even destroy phytoplankton (Imai et al. 1995; Yoshinaga et al. 1997; Lovejoy et al. 1998; Amaro et al. 2005). More recent studies demonstrate that compositions of bacterial communities are not necessarily algae species-specific but depend to a high extent on the algae's extracellular products (Sapp et al. 2007). These results are in line with observations that diatom-attached bacteria are mainly algae species-specific while most free-living bacteria in the algae's surrounding are nonspecific (Grossart et al. 2005).
Exudates are among the key factors that influence the algal phycosphere, the zone directly surrounding algal cells. Within this zone, microbial activity is altered compared to that of the surrounding seawater (Bell and Mitchell 1972). Bacterial communities in phycospheres have been analyzed in several studies (Green et al. 2004; Jasti et al. 2005; Garces et al. 2007; Goecke et al. 2013). In particular, the growth-promoting effects for economically interesting algae have been extensively investigated (Gonzalez and Bashan 2000; Rivas et al. 2010). Usually, those studies are performed with algae samples taken directly from the sea. In most of the studies denaturing gradient gel electrophoresis was used (DGGE; Grossart et al. 2005; Rooney-Varga et al. 2005). With this method, large amounts of bacterial DNA can be identified, with the drawback that alive or dead bacteria cannot be distinguished. Furthermore, identified bacteria cannot be cultivated and characterized by classical microbiology methods.
Many microalgae cultures that are used in experiments, for example, for enhancing biomass production or lipid concentration for biofuel production, are cultures that are maintained for years in the respective laboratories. Bacterial communities in microalgae cultures do have an influence on metabolism of algae, and therefore it is necessary to study these bacteria in microalgae cultures regularly used in research or industrial applications.
In the present study, we examined bacteria coexisting in four microalgae cultures: Chlorella pyrenoidosa, Scenedesmus obliquus, Isochrysis sp., and Nitzschia microcephala. All cultures had been originally obtained from the Baltic Sea and were maintained for 5–8 years in standardized medium before this study was performed. Altogether we collected 40 bacterial isolates from these algal cultures and performed microbiological characterization and 16S ribosomal DNA (rDNA) analyses. With these experiments we gained a first insight into the phycosphere of cultivated microalgae.
Experimental Procedures
Microalgae cultures
The Baltic microalgae C. pyrenoidosa (strain code TV216), S. obliquus (strain code TVK-SOB-1), Isochrysis sp. (strain code TV-ISOCHR), and N. microcephala (strain code TV141) were obtained from the culture collection of the Tvärminne Zoological Station of the University of Helsinki, Finland, where they had been maintained for several years as liquid cultures (Hällfors and Hällfors 1992). By default, algal cultures were incubated in 250 mL Erlenmeyer flasks containing 50 mL of modified, sterilized f/2 medium (Guillard and Ryther 1962), denominated as T2 (Spilling et al. 2011). The cultures were incubated at 25°C with 90 rpm shaking and a 16:8 h light–dark cycle with 80 μmol sec−1 m−2 illumination. The algal cultures were subcultured monthly.
Isolation of associated bacteria
To isolate pure bacterial cultures from the algae, the four native algal cultures C. pyrenoidosa, S. obliquus, Isochrysis sp., and N. microcephalia were subcultured in fresh T2 medium and cultivated for 3 days. One milliliter of each culture was suspended in 9 mL of Marine Broth (BD Difco, Sparks, MD), and these suspensions were further diluted in the same way to 10−3 or 10−4. A 0.1 mL aliquot of each dilution was spread onto Marine Agar plates (Marine Agar 2216; BD Difco), and the plates were incubated according to the culturing temperature of the algae cultures at 25°C for 7 days. From each algal culture 10 single, visually distinct bacterial colonies were subcultured on fresh Marine Agar plates and incubated for 7 days. For long-term storage, the bacteria were conserved on beads (Protect Bacterial Preservers; Technical Service Consultants Ltd., Lancashire, UK) at −80°C. Isolated bacteria were labeled according to their algal culture origin, for example, C1–C10 for bacteria isolated from Chlorella culture, S1–S10 from Scenedesmus culture, and so on (Table 1).
Table 1.
Characterization of bacterial isolates from Baltic microalgae Nitzschia microcephala, Isochrysis sp., Chlorella pyrenoidosa, and Scenedesmus obliquus
| 16S rDNA analysis | Antibiotic susceptibility (antibiotic μg/disk) | High similarity with | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteriacode | Morphology | Highest BLAST hit | % highest BLAST hit | Gentamycin 50 | Streptomycin 25 | Kanamycin 25 | Rifampicin 5 | Meropenem 5 | Ticarcillin 50 | Cefotaxime 50 | Ampicillin 50 | |
| Nitzschia microcephala | ||||||||||||
| N11 | Slender rod; beige | Loktanella sp. | 100 | 1 | 1.8 | – | 4.1 | 1.8 | 4.1 | 5 | 3.3 | N2, N4, N7 |
| N2 | Slender rod; beige | Loktanella sp. | 100 | 1.5 | 1.8 | – | 4.1 | 1.8 | 4 | 5 | 3 | N1, N4, N7 |
| N31 | Slender rod; beige | Loktanella sp. | 99 | – | 1.6 | – | 42 | 2 | 4.2 | 5 | 3 | N53 |
| N4 | Slender rod; beige | Loktanella sp. | 99 | 1.5 | 1.7 | – | 4.1 | 2.1 | 4.5 | >5 | 2.6 | N1, N2, N7 |
| N51 | Slender rod; beige | Loktanella sp. | 100 | – | 1.5 | – | 4 | 2.2 | 3.7 | 4.7 | 3.9 | N33 |
| N61 | Plump pleomorphic rod; beige | Loktanella sp. | 99 | 2.4 | 1.5 | – | 4.02 | 3.3 | 3.6 | 5 | 2.6 | N9 |
| N7 | Slender rod; beige | Loktanella sp. | 100 | 1.5 | 2 | – | 3.82 | 2 | 3.8 | 5 | 3 | N1, N2, N4 |
| N81 | Short rod; beige | Loktanella sp. | 98 | – | 1.6 | – | 4 | 1.7 | 3.9 | 5 | 2.8 | |
| N9 | Plump pleomorphic rod; beige | Agrobacterium sp. | 99 | 32 | 1.6 | – | 4.2 | 2.1 | 4 | 5 | 3 | N6 |
| N101 | Very short rod, beige | Loktanella sp. | 100 | – | 1.5 | – | 2.6 | 1.5 | 2.8 | 5 | – | |
| Isochrysis sp. | ||||||||||||
| I11 | Pale slender rod; rust/orange | Flexibacter sp. | 95 | – | – | – | – | 1.4 | 2.5 | 1.5 | – | I2 |
| I2 | Pale slender rod; rust/orange | Flexibacter sp. | 100 | – | – | – | – | 1.4 | 2.3 | 1.5 | – | I1 |
| I31 | Short rod; light pink | Rhodobacteraceae bacterium JAM-ALO110 | 99 | 3.8 | 2.2 | – | 3.5 | 2.9 | 4.5 | >5 | 4.4 | |
| I41 | Large pleomorphic rod; cream | Seohaeicola saemankumensis SD-15 | 98 | 3.72 | 2.52 | 1.5 | 4 | 3.82 | 5 | >5 | 4.3 | |
| I51 | Large pleomorphic rod; cream | Roseobacter sp. | 99 | 3.72 | 2 | 1.7 | 4.5 | 4.5 | >5 | >5 | >5 | |
| I61 | Rods with cysts; cream | Flexibacter sp. | 99 | 3 | 2 | 1.4 | 4 | 3.5 | 5 | >5 | 4 | |
| I71 | Short rod, red/orange | Erythromicrobium sp. B04 | 100 | 2.4 | – | – | 4.7 | 4 | 4.5 | >5 | 3 | I8, I9, I10 |
| I8 | Short rod, red/orange | Erythromicrobium sp. B04 | 100 | 2.3 | – | – | 4.5 | 3.7 | 4.5 | >5 | 2.5 | I7, I9, I10 |
| I9 | Short rod, red/orange | Erythromicrobium sp. B04 | 99 | 2.3 | – | – | 4.5 | 4.3 | 4.5 | >5 | 3 | I7, I8, I10 |
| I10 | Short rod, red/orange | Erythromicrobium sp. B04 | 100 | 2.2 | – | – | 4 | 4 | 4.5 | >5 | 2.8 | I7, I8, I9 |
| Chlorella pyrenoidosa | ||||||||||||
| C11 | Short rod; pale | Agrobacterium vitis M63038 | 99 | 3.4 | 2.5 | 2 | >4 | >4 | >5 | >5 | 4.5 | C3, C9, S8 |
| C21 | Short rod; pale | Agrobacterium vitis M63038 | 96 | 3.5 | 2.4 | 1.6 | 3.6 | >4 | >5 | >5 | 4 | |
| C3 | Short rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 3.4 | 2.2 | 1.6 | 3.4 | 4 | >4.5 | 4.5 | 4.2 | C1, C9, S8 |
| C41 | Plump pleomorphic rod; pale | Loktanella vestfoldensis IMCC6033 | 99 | 2.8 | 1.5 | – | 4 | 3.2 | 3.8 | >5 | 2.7 | C6, C10 |
| C51 | Plump pleomorphic rod; pale | Agrobacterium vitis M63038 | 98 | 2.5 | 1.4 | – | 4 | 3.6 | 4 | >5 | 3.2 | C73, C83 |
| C6 | Plump pleomorphic rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 2.5 | 1.4 | – | 4.32 | 3.5 | 4 | >5 | 3.5 | C4, C10 |
| C71 | Plump pleomorphic rod; pale | Agrobacterium vitis M63038 | 98 | 3 | 1.5 | – | 4.3 | 3.5 | 3.6 | >52 | 2.5 | C53, C83 |
| C81 | Plump pleomorphic rod; pale | Agrobacterium vitis M63038 | 98 | 2.7 | 1.6 | – | 4.5 | 3.2 | 4 | >5 | 2.7 | C53, C73 |
| C9 | Short rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 3.4 | 2.1 | 1.7 | 3.5 | 4 | 5 | 4.4 | 3.7 | C1, C3, S8 |
| C10 | Plump pleomorphic rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 2.6 | 1.4 | – | 4.1 | 3.5 | 4 | 5.1 | 3.2 | C4, C6 |
| Scenedesmus obliquus | ||||||||||||
| S11 | Short rod; pale | Agrobacterium vitis M63038 | 98 | 3 | 2.2 | 1.5 | 3.2 | 2.64 | 5 | 4.4 | 4 | S2 |
| S2 | Short rod; pale | Agrobacterium vitis M63038 | 98 | 3.1 | 2.2 | 1.5 | 3 | 4 | 5 | 4.5 | 3.6 | S1 |
| S31 | Slender rod; beige | Rhizobium sp. W7 | 98 | 2.6 | 1.5 | – | 4.3 | 3.5 | 4 | 54 | 34 | S4, S6, S9, S10 |
| S4 | Slender rod; beige | Loktanella vestfoldensis IMCC6033 | 100 | 2.6 | 1.5 | – | 4 | 3.4 | 4.2 | 5.3 | 3.24 | S3, S6, S9, S10 |
| S51 | Short rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 2.8 | 1.5 | 2.42 | 3.2 | 3.64 | >5 | 4.2 | 3.7 | S73 |
| S6 | Slender rod; beige | Loktanella vestfoldensis IMCC6033 | 99 | 2.8 | 1.5 | – | 4.22 | 34 | 4.2 | 54 | 34 | S3, S4, S9, S10 |
| S71 | Short rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 3.6 | 3 | 2 | 3 | 3.54 | >5 | 4.2 | 3.5 | S53 |
| S8 | Short rod; pale | Loktanella vestfoldensis IMCC6033 | 100 | 3.3 | 2.5 | 2.82 | 3.2 | 3.84 | >5 | 4.4 | 4 | C1, C3, C9 |
| S9 | Slender rod; beige | Agrobacterium vitis M63038 | 98 | 3 | 1.6 | – | 4.4 | 44 | 4.7 | 5.5 | 3.5 | S3, S4, S6, S10 |
| S10 | Slender rod; beige | Loktanella vestfoldensis IMCC6033 | 100 | 3 | 1.6 | – | 4.54 | 44 | 5 | 5.5 | 3.8 | S3, S4, S6, S9 |
BLAST, Basic Local Alignment Search Tool.
Considered unique and used for the tree and UniFrac analysis.
Single colonies in inhibition zone.
Result of the maximum likelihood tree.
Inhibition zones were not totally clear.
Characterization of marine bacteria
Morphological characterization
For morphological characterization, all 40 bacterial isolates were cultivated on Marine Agar 2216 plates as pure cultures. The colors of the bacterial colonies were recorded, and shape and Gram reaction of the bacterial cells were evaluated using a Polyvar microscope (Reichert-Jung, Wien, Austria) with 800× magnification. The Gram stain reaction of the bacteria was determined using a BD Gram Stain Kit (BD Diagnostics, Sparks, MD) according to the manufacturer's instructions. For the oxidase test a piece of filter paper was soaked in aqueous 1% N,N,N′,N′-tetramethyl-p-phenylenediaminedihydrochloride solution. Freshly grown bacteria were scraped from the plate and rubbed onto the filter paper. Development of blue color within 10 s was an indication of oxidase-positive isolates.
Antibiotic susceptibility
In order to test the antibiotic susceptibility of the isolated bacteria their pure cultures were cultivated in liquid medium (Marine Broth 2216; BD Difco) for 26–27 h at 25°C under shaking (120 rpm). Aliquots of 0.1 mL were spread evenly onto Marine Agar plates, allowing the liquid to permeate into the medium. Solutions of gentamycin, streptomycin, kanamycin, rifampicin, meropenem, ticarcillin, cefotaxim, and ampicillin (all antibiotics from Duchefa or Sigma, concentrations in Table 1) were prepared and 0.1 mL of each solution was added to separate disks (Antibiotika-Testblättchen; ø 12.7 mm, Schleicher & Schüll, Dassel, Germany). The disks were placed onto bacterial lawns and the plates were incubated at 25°C for 6 days. Clear inhibition zones were measured.
16S ribosomal DNA (rDNA) analyses
For performing 16S ribosomal DNA (rDNA) analyses beads with preserved bacterial isolates were cultivated in liquid medium (Marine Broth 2216; BD Difco) for 26–27 h at 25°C under shaking (120 rpm). DNA was isolated by a modified cetyl trimethylammonium bromide protocol (Doyle and Doyle 1987). 16S rDNA (1450 bp) was amplified by PCR with Eubac27F and the 1492R primers according to Lane (1991).
Resulting DNA sequences were compared to known DNA sequences in the NCBI database (Basic Local Alignment Search Tool, nucleotide blast). Sequences were aligned with MUSCLE 3.7 and a maximum likelihood tree was calculated using PhyML 3.0 aLRT and drawn by TreeDyn (Dereeper et al. 2010). All sequences used for phylogenetic analyses were deposited in the EMBL Nucleotide Sequence Database (for accession numbers see Fig. 1). To analyze the similarity of 16S rDNA sequences of the bacterial isolates with known sequences of phytoplankton-associated bacteria, 16S rDNA sequences were compared with known sequences in the NCBI database by using the Basic Local Alignment Search Tool (BLAST) function. All sequences with ≥99% similarity to our sequences were examined with respect to their origin. If the origin of the bacterial DNA sequence was a native algae culture, then this sequence was regarded as sequence from bacteria with algae association.
Figure 1.
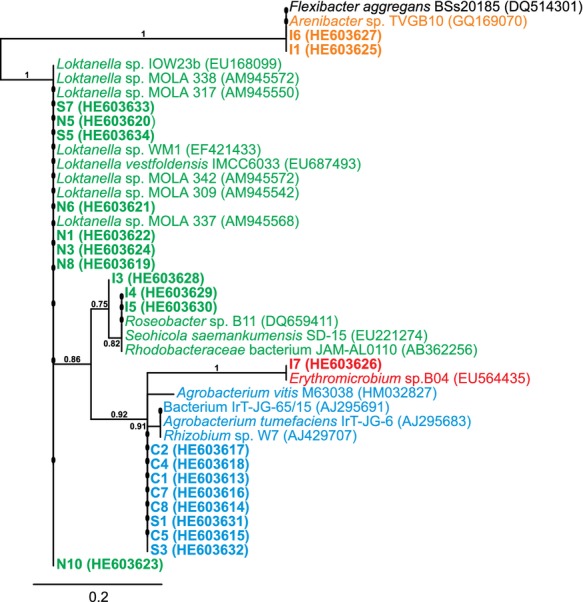
Maximum likelihood tree showing the relationships of the 22 marine bacteria (bold) isolated in this study and their closest relatives based on their 16S rDNA sequences. The DNA sequence accession numbers are shown in brackets. Strains belonging to different families are indicated by colors: Flexibacteraceae black, Flavobacteraceae orange, Rhodobacteraceae green, Erythrobacteraceae red, and Rhizobiaceae blue. Numbers indicate bootstrap values. The scale bar corresponds to 20 base substitutions per 100 nucleotide positions.
Statistical evaluation
Statistical analyses were performed with the program SigmaPlot 11.0 (Systat Software, GmbH, Erkrath, Germany). A t-test (Mann–Whitney rank-sum test) was performed to test the pooled differences between the percentages of algae-associated sequences of bacteria isolated from Isochrysis and the three other algae cultures (Nitzschia, Chlorella, and Scenedesmus).
For comparing the four bacterial communities of Isochrysis, Nitzschia, Chlorella, and Scenedesmus unweighted UniFrac Significance tool (type of test: each environment individually) was used (Lozupone and Knight 2005). In general, UniFrac measures the distance between two environments in terms of the fraction of evolutionary history that separates the organisms in the two environments. Each algae culture was considered as an environment, each 16S rDNA sequence was assigned to one of the four environments. Calculations were performed with all 22 sequences from isolates considered unique (Table 1).
Results
Characterization of cultivable algae-associated bacteria
On the Marine medium plate of N. microcephala, Isochrysis sp., C. pyrenoidosa, and S. obliquus cultures 36, 386, 143, and 106 colonies, respectively, were observed. Ten separate bacterial colonies with different appearances were selected from each of the four microalgal cultures. All bacterial isolates were Gram-negative and oxidase-positive rods with variable susceptibility against selected antibiotics (Table 1). Furthermore, 16S rDNA analysis of all 40 bacterial isolates was performed, and on the basis of morphological characterization, antibiotic susceptibility, and comparison of 16S rDNA sequences to known sequences via BLAST, the collection of 40 bacterial isolates was clustered into 22 distinct groups of isolates. Results of the microbiological and genetic characterization are compiled in Table 1 and Figure 2. These sequence data have been also submitted to the EMBL database. The accession numbers are found in Figure 1.
Figure 2.
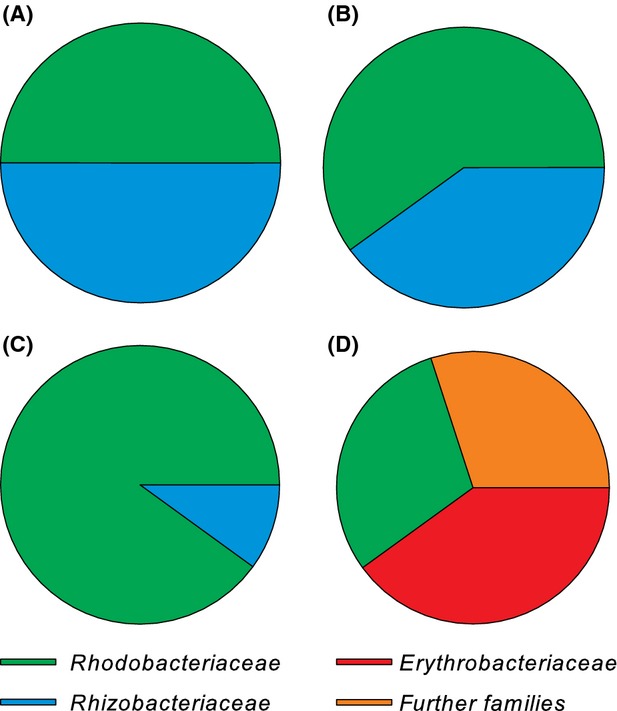
Families of bacteria isolated from microalgae cultures (A) Chlorella pyrenoidosa, (B) Scenedesmus obliquus, (C) Nitzschia microcephala, and (D) Isochrysis sp.
The 16S rDNA sequences of these 22 strains, and those sequences from NCBI database with highest similarity to the sequences, were used for calculating the maximum likelihood tree (Fig. 1). Considering also the results of the tree, 17 of 22 isolated bacteria are unique. According to the combined results, 15 of the 17 bacterial strains were identified as belonging to the families Rhodobacteraceae, Rhizobiaceae, and Erythrobacteraceae, placed in the phylum Proteobacterium, and the remaining two isolates were localized in the Cytophaga–Flavobacterium–Bacteroides phylum (Fig. 1).
Rhodobacteracea
All bacterial strains isolated from the Nitzschia culture, coded from N1 to N10, showed very high 16S rDNA sequence similarity. Combining sequence similarity analysis with results from morphological characterization and the antibiotic susceptibility assays, six bacterial strains (N1, N3, N6, N8, and N10) were considered unique; while N5 is most probably similar to N3. Although all strains isolated from Nitzschia culture show a resistance against kanamycin, differences in antibiotic susceptibility were observed for gentamycin. No susceptibility for gentamycin was observed for strains N1 (similar to N2, N4, and N7) and N6 (similar to N9), while N3, N5, N8, and N10 were resistant against gentamycin. N1 and N6 were considered different strains because of their differences in microscopic appearance. Furthermore, the two strains showed a different antibiotic susceptibility against gentamycin and meropenem. The majority of isolates from Nitzschia showed typically slender rod morphology but N6 and N10 were plump and pleomorphic rods or very short rods, respectively. Additionally, the phylogenetic studies showed that strains S5 and S7 are most probably similar strains. This strain, isolated from Scenedesmus, clustered together with several strains isolated from the Nitzschia culture as well as with several Loktanella species offered by the NCBI database. Furthermore, their cell morphologies (plump pleomorphic/short rods) confirmed that, in addition to the Nitzschia isolates, also S5, S7, and C4 belong to the family of Rhodobacteraceae.
Bacterial strains I3, I4, and I5 isolated from Isochrysis sp. most probably belong to the family Rhodobacteraceae, too, but form a separate cluster. The 16S rDNA sequences of these isolates showed high similarity to the 16S rDNA of unclassified strains of Rhodobacteraceae (strain JAM-AL0110, AB362256), Seohaeicola saemankumensis and two Roseobacter sp. strains (B11 and DG869). In contrast to the other Rhodobacteraceae strains in this study, I4 and I5 were large pleomorphic rods without kanamycin sensitivity. Strain I3 differs from I4 and I5 by a short rod form, color, and sensitivity to kanamycin.
Rhizobiaceae
The second largest group was formed by the bacterial strains C1 (similar to C3, C9, and S8), C2, C5, C7, C8, isolated from the C. pyrenoidosa culture, together with S1 (similar to S2) and S3 (similar to S4, S6, S9, and S10), isolated from the S. obliquus culture. The maximum likelihood tree revealed that most probably C5 is similar to C7 and C8. Because of their high similarity with Agrobacterium vitis (strain M63038), Rhizobium sp. (strain W7), and Agrobacterium tumefaciens (strain IrT-JG-6), those bacteria might belong to the Rhizobiaceae. Cells of these strains varied between short rods (C1, C2, and S1) with sensitivity to kanamycin, slender rods (S3), and plump pleomorphic rods (C5, C7, and C8), both resistant to kanamycin.
Erythrobacteraceae
The bacterial strain I7 (similar to I8, I9, and I10) showed 100% similarity at the nucleotide level with Erythromicrobium sp. (phylum: Proteobacteria, order: Sphingomonadales). The colonies of I7 were colored from red to orange and the cells were short rods with resistance to streptomycin and kanamycin, which was not observed for any other isolated strains.
Further families
On the basis of phylogenetic analysis the strains I1 and I6 showed closest similarity to Arenibacter sp. (Flavobacteraceae) and Flexibacter aggregans (Flexibacteraceae), respectively, both being families of the Cytophaga–Flavobacterium–Bacteroides phylum. The slender rods of I1 (similar to I2) formed rust to orange-colored colonies and showed resistance to gentamycin, streptomycin, kanamycin, rifampicin, and ampicillin. I6 appeared as rods with cysts and was sensitive to all tested antibiotics.
16S rDNA sequence similarities of isolates from microalgae with known sequences of phytoplankton-associated bacteria
In order to quantify the association of isolated bacteria to algae, 16S rDNA sequences of the 22 unique bacteria were compared to known sequences in the NCBI database with BLAST function. All sequences with high similarity (≥99%) to 16S rDNA sequences from isolated bacteria were checked as to their origin. Table 2 shows the amount and percentage of those sequences with high similarity and an origin in a phytoplankton culture.
Table 2.
Similarity of 16S rDNA of bacterial isolates from Baltic microalgae to sequences from phytoplankton-associated bacteria in NCBI database
| Bacterial strain | Number of base position compared | Number of sequences with ≥99% similarity1 | Number of sequences isolated from phytoplankton culture | Percentage of phytoplankton-associated sequences (%) |
|---|---|---|---|---|
| N1 | 608 | 100 | 6 | 6 |
| N3 | 632 | 100 | 7 | 7 |
| N5 | 504 | 100 | 7 | 7 |
| N6 | 625 | 100 | 7 | 7 |
| N8 | 298 | 0 | 0 | 02 |
| N10 | 138 | 100 | 3 | 3 |
| I1 | 483 | 0 | 0 | 02 |
| I3 | 620 | 13 | 3 | 25 |
| I4 | 527 | 0 | 0 | 02 |
| I5 | 569 | 14 | 3 | 25 |
| I6 | 527 | 42 | 15 | 37 |
| I7 | 564 | 12 | 3 | 27 |
| C1 | 550 | 49 | 1 | 2 |
| C2 | 522 | 0 | 0 | 02 |
| C4 | 710 | 31 | 8 | 28 |
| C5 | 554 | 31 | 2 | 8 |
| C7 | 572 | 58 | 2 | 4 |
| C8 | 619 | 10 | 0 | 0 |
| S1 | 635 | 9 | 0 | 0 |
| S3 | 520 | 44 | 3 | 8 |
| S5 | 638 | 100 | 7 | 7 |
| S7 | 638 | 100 | 7 | 7 |
Maximal number of sequences shown in NCBI database with BLAST: 100 sequences.
Values are not used for further calculations.
Bacteria isolated from Nitzschia, Chlorella, and Scenedesmus cultures showed high similarity with on average 6.2%, 8.3%, and 5.5% (SE ± 0.8%, 5.0%, and 1.8%) of sequences with algae association. In contrast, 16S rDNA sequences of bacteria isolated from Isochrysis culture revealed a significant (t-test, P = 0.009) higher similarity than sequences isolated from all other microalgae cultures. Sequences of bacteria isolated from Isochrysis culture showed on average 28.5% (SE ± 2.8%) similarity to published sequences of algae-associated bacteria. Highest similarity with sequences of bacteria known to be associated with algae was identified for strain I6. Thirty-seven percent of the published sequences with similarity ≥99% revealed algae associations. Moreover, 16S rDNA sequence of strain C4 revealed a considerably high similarity (28%) to sequences of algae-associated bacteria as compared to other bacteria isolated from Chlorella culture.
Comparison of the four bacterial communities using UniFrac
Based on an alignment of 22 16S rDNA sequences, the online tool UniFrac Significance indicated that the bacterial community of Isochrysis sp. differs significantly from the other communities (P < 0.01). This result is in line with the results of the comparison of the 16S rDNA sequences to sequences from phytoplankton-associated bacteria in NCBI database (Table 2). All other communities are not significantly different from each other (P = 0.72, P = 0.38, and P = 0.85 for Nitzschia, Chlorella, and Scenedesmus, respectively).
Discussion
At the time of initiating a nonaxenic monoalgal culture it is conceivable that two groups of bacteria are present in the medium: Those that were coincidentally near the algae at the time of sampling or those that have some sort of relationship with the algae, ranging from symbiotic to pathogenic (Jasti et al. 2005). Regardless their connection, it is anticipated that bacteria are either favored or suppressed in the artificial situation and that a relatively balanced situation is reached within the years of continued subcultivation.
On the basis of microbiological characterization and sequence similarity, 40 visually distinct bacterial isolates, representing only a selection cultivable at these specific conditions, were assigned to four families, Rhodobacteraceae, Rhizobiaceae, Erythrobacteraceae, and Flavobacteraceae. Members of these families had been previously found worldwide in marine environments associated with algae as well as in lakes, sediments, and soil (Allgaier et al. 2003; Rooney-Varga et al. 2005; Penn et al. 2006; Jordan et al. 2007; Cho et al. 2008; Salka et al. 2008; Shigematsu et al. 2009; Xu et al. 2009).
Rhodobacteraceae
The majority (55%) of the bacterial strains isolated in this study belong to the Rhodobacteraceae and show highest similarities regarding 16S rDNA with Loktanella sp. Although its presence in the Baltic Sea had been reported previously (Salka et al. 2008) Loktanella's microalgae association has not yet been assessed. However, it has been recently shown that Loktanella sp. are more abundant on the surface of the green algae Ulva australis than in the surrounding sea water, which might owe to interaction of both species (Burke et al. 2011). It has been found that a Loktanella sp., isolated from the North Sea, releases a chemical compound showing high similarity to insect pheromones (Dickschat et al. 2005). In the present study, 16S rDNA sequences of three bacterial strains (I3, I4, and I5) clustered with the Roseobacter sp. B11 and two further Rhodobacteraceae sequences. Roseobacter species dominate among marine algae-associated bacteria and are engaged in intermittent symbiotic relations (reviewed by Buchan et al. 2005). Phaeobacter gallaeciensis belongs to the Roseobacter clade and produces antibacterial compounds in the biofilm of U. australis, thus preventing the growth of other bacteria on the algae (Ruiz-Ponte et al. 1998; Brinkhoff et al. 2004; Bruhn et al. 2005). It has been shown that breakdown compounds of the senescent algae stimulate P. gallaeciensis to produce potent and selective algicides, which ultimately kill the algae (Seyedsayamdost et al. 2011). Therefore, it is likely that the Isochrysis isolates I3, I4, and I5 also interact with Isochrysis sp. and probably with other microalgae and bacterial species as well.
Erythrobacteriaceae
The bacterial strain I7 showed 100% 16S rDNA sequence similarity with Erythromicrobium sp. B04, which was isolated in the eastern Gotland Basin, Baltic Sea (Salka et al. 2008). Another study demonstrated the growth-inhibiting effects of Erythromicrobium ramosum, isolated from a bloom, on the cyanobacterium Microcystis aeruginosa (Shi et al. 2009). On the basis of 16S rDNA sequence analysis, many of the bacterial isolates described in this study are very close, or even identical with known bacterial species, such as strain I7 representing the cluster of isolates I7, I8, I9, and I10 from Isochrysis sp. These new isolates might even have potential in environmental biotechnology, because strains of Erythromicrobium are known to be highly tolerant toward toxic heavy-metal oxide tellurite by accumulating metallic crystals inside the cells (Yorkov et al. 1996). Also, several environmental bacterial isolates that are potentially useful for biodegradation and cleaning polluted sites are described as close relatives of Erythromicrobium, such as Sphingomonas (Nohynek et al. 1996; Tao et al. 2007). A detailed taxonomic study is still needed to identify these isolates, which would both increase information on associated cultures in marine environments and supplement the taxonomic scenery of marine bacteria.
Rhizobiaceae
Members of the Rhizobiaceae were first isolated from soil (Conn 1938), later on also from marine sediments (Rüger and Höfle 1992; Süss et al. 2006; Jordan et al. 2007) and snow (Gonzalez-Toril et al. 2009). The genus Agrobacterium was affiliated to the genus Rhizobium, followed by renaming Agrobacterium tumefaciens to Agrobacterium radiobacter and Agrobacterium rhizogenes as Rhizobium rhizogenes (Young et al. 2001). However, in the present study the bacteria of this genus are named in accordance with the respective literature. In our study we isolated several bacteria with high similarity to Agrobacterium sp. at the 16S rDNA level. Agrobacterium rhizogenes (Rhizobiaceae) is known for its natural ability to insert bacterial DNA into the genome of higher plants (Tepfer 1984), a mechanism facilitating genetic engineering of plants. For a number of years Agrobacterium has also been used in the transformation of microalgae such as Chlamydomonas reinhardii (Kumar and Rajam 2007), Haematococcus pluvialis (Kathiresan et al. 2009), and Nannochloropsis sp. (Cha et al. 2011). Furthermore, a recent study revealed probiotic and growth-promoting properties of a Rhizobium sp. on the microalgae Botryococcus braunii (Rivas et al. 2010).
Flavobacteriaceae
The 16S rDNA sequences of I1 and I6 showed highest similarity to Arenibacter sp. TVGB10 and Flexibacter aggregans BSs20185, both of which are members of the Flavobacteriaceae, belonging to the diverse Cytophaga–Flavobacterium–Bacteroides (CFB) phylum. Flexibacter aggregans is known as a fish pathogen belonging to a family of marine species with unclear taxonomic positions (Nedashkovskaya et al. 2005). Arenibacter was discovered in sandy sediment in the South China Sea and classified as a new genus of Flavobacteriaceae (Ivanova et al. 2001). Interestingly, further species of that genus, Arenibacter palladensis and Arenibacter certesii, were isolated from green algae and sea urchins (Nedashkovskaya et al. 2004, 2006; Urvantseva et al. 2006). Recently, Arenibacter algicola sp. nov., a polycyclic aromatic hydrocarbon degrading bacterium, was isolated from the marine diatom Skeletonema costatum which was obtained from a culture collection (Gutierrez et al. 2014). Despite their isolation from microalgae, interaction between algae and Arenibacter has not been described so far. Furthermore, Grossart and coworkers showed impressively that most bacteria which were associated with two diatoms belong to the CFB phylum (Grossart et al. 2005).
From all the bacterial isolates obtained in this study, those isolated from Isochrysis sp. culture showed highest similarity to known bacteria with algae association. This may indicate that bacteria that were found in the Isochrysis sp. culture have higher tendency of association or interference with the microalgae than other isolated bacteria. Results were confirmed by the UniFrac calculations and by the BLAST analysis (Table 2). Independently, both methods indicate that the bacterial community of Isochrysis sp. significantly differs from the three other communities.
In summary, the present study revealed that many of the bacterial strains isolated from Baltic microalgae cultures show high similarity to bacteria with confirmed algae association or even interaction. The isolated bacteria had survived several years of continuous algae maintenance in artificial medium, indicating that those bacteria benefit from their coexistence with microalgae. Especially the isolates I1 and I6 are likely to live associated with microalgae and might interfere with them. Possible interferences have not been well elucidated yet, and should be subject of future studies. For controlled and repeatable conditions in experiments with high-precision measurements of metabolic compounds such as lipids or glycosides the usage of axenic algae cultures should be considered working with microalgae. If axenic microalgae cultures are not available, it may be recommended to characterize the phycosphere of algae before performing other experimental work.
Acknowledgments
We thank the Finnish Environmental Institute for providing the native microalgal cultures. We are grateful to Jaana Rikkinen and Airi Hyrkäs for their excellent technical assistance, Outi Priha for performing the morphological characterization and microbiological tests, and Erna Storgårds for helpful discussions about the isolated bacteria. This work was supported by the European Commission (grant no. 245137, project acronym: MAREX, large-scale integrated project within KBBE-2009-3-2-01); the Academy of Finland Research Programme “Sustainable Energy – SusEn” (grant no. 124409, project acronym: Algiesel); and the VTT Technical Research Centre of Finland.
Conflict of Interest
None declared.
References
- Allgaier M, Uphoff H, Felske A, Wagner-Döbler I. Aerobic anoxygenic photosynthesis in Roseobacter clade bacteria from diverse marine habitats. Appl. Environ. Microbiol. 2003;69:5051–5059. doi: 10.1128/AEM.69.9.5051-5059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DG, Sutherland IW. The role of exopolysaccherides in adhesion of freshwater bacteria. J. Gen. Microbiol. 1987;133:1319–1327. [Google Scholar]
- Amaro AM, Fuentes MS, Ogalde SR, Venegas JA, Suárez-Isla BA. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 2005;52:191–200. doi: 10.1111/j.1550-7408.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- Azam F. Cycling of organic matter by bacterioplankton in pelagic marine ecosystems: microenvironmental considerations. In: Fasham MJR, Ammerman JW, editors. Flows of energy and materials in marine ecosystems. New York, NY: Plenum Press; 1984. pp. 345–360. [Google Scholar]
- Bell W, Mitchell R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972;143:265–277. [Google Scholar]
- Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, Simon M. Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl. Environ. Microbiol. 2004;70:2560–2565. doi: 10.1128/AEM.70.4.2560-2565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, Schulz S, et al. Ecology, inhibitory activity, and morphogenesis of a marine antagonistic bacterium belonging to the Roseobacter clade. Appl. Environ. Microbiol. 2005;71:7263–7270. doi: 10.1128/AEM.71.11.7263-7270.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan A, Gonzalez JM, Moran MA. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 2005;71:5665–5677. doi: 10.1128/AEM.71.10.5665-5677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011;5:590–600. doi: 10.1038/ismej.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T-S, Chen C-F, Yee W, Aziz A, Loh S-H. Cinnamic acid, coumarin and vanillin: alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J. Microbiol. Methods. 2011;84:430–434. doi: 10.1016/j.mimet.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Cho Y, Hiramatsu K, Ogawa M, Omura T, Ishimaru T, Oshima Y. Non-toxic and toxic subclones obtained from a toxic clonal culture of Alexandrium tamarense (Dinophyceae): toxicity and molecular biological feature. Harmful Algae. 2008;7:740–751. [Google Scholar]
- Cole JJ, Likens GE, Strayer DL. Photosynthetically produced dissolved organic carbon: an important carbon source for planktonic bacteria. Limnol. Oceanogr. 1982;27:1080–1090. [Google Scholar]
- Cole JJ, Findlay S, Pace ML. Bacterial production in fresh and saltwater: a cross-system overview. Mar. Ecol. Prog. Ser. 1988;43:1–10. [Google Scholar]
- Conn H. Taxonomic relationships of certain non-sporeforming rods in soil. J. Bacteriol. 1938;36:320–321. [Google Scholar]
- Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010;10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickschat JS, Wagner-Döbler I, Schulz S. The chafer pheromone buibuilactone and ant pyrazines are also produced by marine bacteria. J. Chem. Ecol. 2005;31:925–947. doi: 10.1007/s10886-005-3553-9. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- Ferrier M, Martin JL, Rooney-Varga JN. Stimulation of Alexandrium fundyense growth by bacterial assemblages from the Bay of Fundy. J. Appl. Microbiol. 2002;92:706–716. doi: 10.1046/j.1365-2672.2002.01576.x. [DOI] [PubMed] [Google Scholar]
- Garces E, Vila M, Rene A, Alonso-Saez L, Angles S, Luglie A, et al. Natural bacterioplankton assemblage composition during blooms of Alexandrium spp. (Dinophyceae) in NW Mediterranean coastal waters. Aquat. Microb. Ecol. 2007;46:55–70. [Google Scholar]
- Goecke F, Thiel V, Wiese J, Labes A, Imhoff JF. Algae as an important environment for bacteria – phylogenetic relationships among new bacterial species isolated from algae. Phycologia. 2013;52:14–24. [Google Scholar]
- Gonzalez LE, Bashan Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000;66:1527–1531. doi: 10.1128/aem.66.4.1527-1531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Toril E, Amils R, Delmas RJ, Petit JR, Komarek J, Elster J. Bacterial diversity of autotrophic enriched cultures from remote, glacial Antarctic, Alpine and Andean aerosol, snow and soil samples. Biogeosciences. 2009;6:33–44. [Google Scholar]
- Green DH, Llewellyn LE, Negri AP, Blackburn SI, Bolch CJS. Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiol. Ecol. 2004;47:345–357. doi: 10.1016/S0168-6496(03)00298-8. [DOI] [PubMed] [Google Scholar]
- Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T. Marine diatom species harbour distinct bacterial communities. Environ. Microbiol. 2005;76:667–684. doi: 10.1111/j.1462-2920.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- Guillard R, Ryther J. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Rhodes G, Mishamandani S, Berry D, Whitman WB, Nichols PD, et al. Polycyclic aromatic hydrocarbon degradation of phytoplankton-associated Arenibacter spp. and description of Arenibacter algicola sp. nov., an aromatic hydrocarbon-degrading bacterium. Appl. Environ. Microbiol. 2014;80:618–628. doi: 10.1128/AEM.03104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines KC, Guillard RRL. Growth of vitamin B12-requiring marine diatoms in mixed laboratory cultures with vitamin B12-producing marine bacteria. J. Phycol. 1974;10:245–252. [Google Scholar]
- Hällfors G, Hällfors S. The Tvärminne collection of algal cultures. In Tvärminne Studies. University of Helsinki; 1992. pp. 15–17. [Google Scholar]
- Hasegawa Y, Martin JL, Giewat MW, Rooney-Varga JN. Microbial community diversity in the phycosphere of natural populations of the toxic alga, Alexandrium fundyense. Environ. Microbiol. 2007;9:3108–3121. doi: 10.1111/j.1462-2920.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- Imai I, Ishida Y, Sakaguchi K, Hata Y. Algicidal marine bacteria isolated from northern Hiroshima Bay, Japan. Fish. Sci. 1995;61:628–636. [Google Scholar]
- Ivanova EP, Nedashkovskaya OI, Chun J, Lysenko AM, Frolova GM, Svetashev VI, et al. Arenibacter gen. nov., new genus of the family Flavobacteriaceae and description of a new species, Arenibacter latericius sp. nov. Int. J. Syst. Evol. Microbiol. 2001;51:1987–1995. doi: 10.1099/00207713-51-6-1987. [DOI] [PubMed] [Google Scholar]
- Jasti S, Sieracki ME, Poulton NJ, Giewat MW, Rooney-Varga JN. Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton. Appl. Environ. Microbiol. 2005;71:3483–3494. doi: 10.1128/AEM.71.7.3483-3494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan EM, Thompson FL, Zhang X-H, Li Y, Vancanneyt M, Kroppenstedt RM, et al. Sneathiella chinensis gen. nov., sp. nov., a novel marine alphaproteobacterium isolated from coastal sediment in Qingdao, China. Int. J. Syst. Evol. Microbiol. 2007;57:114–121. doi: 10.1099/ijs.0.64478-0. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Chandrashekar A, Ravishankar GA, Sarada R. Agrobacterium-mediated transformation in the green alga Haematococcus pluvialis (Chlorophyceae, Volvocales) J. Phycol. 2009;45:642–649. doi: 10.1111/j.1529-8817.2009.00688.x. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Rajam MV. Induction of Agrobacterium tumefaciens vir genes by the green alga, Chlamydomonas reinhardtii. Curr. Sci. 2007;92:1727–1729. [Google Scholar]
- Lane DJ. Nucleic acid techniques in bacterial systematics. New York: Wiley; 1991. [Google Scholar]
- Lovejoy C, Bowman JP, Hallegraeff GM. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella Gynodinium, and Heterosigma. Appl. Environ. Microbiol. 1998;64:2806–2813. doi: 10.1128/aem.64.8.2806-2813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:12. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama A, Maeda M, Simidu U. Occurrence of plant hormone (cytokinin)-producing bacteria in the sea. J. Appl. Microbiol. 1986;61:569–574. [Google Scholar]
- Nedashkovskaya OI, Kim SB, Han SK, Lysenko AM, Mikhailov VV, Bae KS. Arenibacter certesii sp. nov., a novel marine bacterium isolated from the green alga Ulva fenestrata. Int. J. Syst. Evol. Microbiol. 2004;54:1173–1176. doi: 10.1099/ijs.0.02872-0. [DOI] [PubMed] [Google Scholar]
- Nedashkovskaya OI, Kim SB, Lee DH, Lysenko AM, Shevchenko LS, Frolova GM, et al. Roseivirga ehrenbergii gen. nov., sp. nov., a novel marine bacterium of the phylum ‘Bacteroidetes’, isolated from the green alga Ulva fenestrata. Int. J. Syst. Evol. Microbiol. 2005;55:231–234. doi: 10.1099/ijs.0.63341-0. [DOI] [PubMed] [Google Scholar]
- Nedashkovskaya OI, Vancanneyt M, Cleenwerck I, Snauwaert C, Kim SB, Lysenko AM, et al. Arenibacter palladensis sp. nov., a novel marine bacterium isolated from the green alga Ulva fenestrata, and emended description of the genus Arenibacter. Int. J. Syst. Evol. Microbiol. 2006;56:155–160. doi: 10.1099/ijs.0.63893-0. [DOI] [PubMed] [Google Scholar]
- Nohynek L, Nurmiaho-Lassila E-L, Suhonen E, Busse HJ, Mohammadi M, Hantula J, et al. Description of chlorophenol-degrading Pseudomonas sp. strains KF1T, KF3, and NKF1 as a new species in the genus Sphingomonas Sphingomonas subarctica sp. nov. Int. J. Syst. Bacteriol. 1996;46:1042–1055. doi: 10.1099/00207713-46-4-1042. [DOI] [PubMed] [Google Scholar]
- Penn K, Wu DY, Eisen JA, Ward N. Characterization of bacterial communities associated with deep-sea corals on Gulf of Alaska seamounts. Appl. Environ. Microbiol. 2006;72:1680–1683. doi: 10.1128/AEM.72.2.1680-1683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MO, Vargas P, Riquelme CE. Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 2010;60:628–635. doi: 10.1007/s00248-010-9686-6. [DOI] [PubMed] [Google Scholar]
- Rooney-Varga JN, Giewat MW, Savin MC, Sood S, LeGresley M, Martin JL. Links between phytoplankton and bacterial community dynamics in a coastal marine environment. Microb. Ecol. 2005;49:163–175. doi: 10.1007/s00248-003-1057-0. [DOI] [PubMed] [Google Scholar]
- Rüger H-J, Höfle MG. Marine star-shaped-aggregate-forming bacteria: Agrobacterium atlanticum sp. nov.; Agrobacterium meteori sp. nov.; Agrobacterium ferrugineum sp. nov., nom. rev.; Agrobacterium gelatinovorum sp. nov., nom. rev.; and Agrobacterium stellulatum sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1992;42:133–143. doi: 10.1099/00207713-42-1-133. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ponte C, Cilia V, Lambert C, Nicolas JL. Roseobacter gallaeciensis sp. nov., a new marine bacterium isolated from rearings and collectors of the scallop Pecten maximus. Int. J. Syst. Bacteriol. 1998;48:537–542. doi: 10.1099/00207713-48-2-537. [DOI] [PubMed] [Google Scholar]
- Salka I, Moulisova V, Koblizek M, Jost G, Jurgens K, Labrenz M. Abundance, depth distribution, and composition of aerobic bacteriochlorophyll a-producing bacteria in four basins of the central Baltic Sea. Appl. Environ. Microbiol. 2008;74:4398–4404. doi: 10.1128/AEM.02447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp M, Schwaderer AS, Wiltshire KH, Hoppe HG, Gerdts G, Wichels A. Species-specific bacterial communities in the phycosphere of microalgae? Microb. Ecol. 2007;53:683–699. doi: 10.1007/s00248-006-9162-5. [DOI] [PubMed] [Google Scholar]
- Seyedsayamdost MR, Case RJ, Kolter R, Clardy J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011;3:331–335. doi: 10.1038/nchem.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Cai Y, Li P, Yang H, Liu Z, Kong L, et al. Molecular identification of the colony-associated cultivable bacteria of the cyanobacterium Microcystis aeruginosa and their effects on algal growth. J. Freshwater Ecol. 2009;24:211–218. [Google Scholar]
- Shigematsu T, Hayashi M, Kikuchi I, Ueno S, Masaki H, Fujii T. A culture-dependent bacterial community structure analysis based on liquid cultivation and its application to a marine environment. FEMS Microbiol. Lett. 2009;293:240–247. doi: 10.1111/j.1574-6968.2009.01536.x. [DOI] [PubMed] [Google Scholar]
- Smith DC, Steward GF, Long RA, Azam F. Bacterial mediation of carbon fluxes during a diatom bloom in a mesocosm. Deep-Sea Res. Pt. II. 1995;42:75–97. [Google Scholar]
- Spilling K, Seppälä J, Tamminen T. Inducing autoflocculation in the diatom Phaeodactylum tricornutum through CO2 regulation. J. Appl. Phycol. 2011;23:959–966. [Google Scholar]
- Süss J, Schubert K, Sass H, Cypionka H, Overmann J, Engelen B. Widespread distribution and high abundance of Rhizobium radiobacter within Mediterranean subsurface sediments. Environ. Microbiol. 2006;8:1753–1763. doi: 10.1111/j.1462-2920.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- Tao X-Q, Lu G-N, Dang Z, Yang C, Yi X-Y. A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem. 2007;42:401–408. [Google Scholar]
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell. 1984;37:959–967. doi: 10.1016/0092-8674(84)90430-6. [DOI] [PubMed] [Google Scholar]
- Urvantseva AM, Bakunina IY, Nedashkovskaya OI, Kim SB, Zvyagintseva TN. Distribution of intracellular fucoidan hydrolases among marine bacteria of the family Flavobacteriaceae. Appl. Biochem. Microbiol. 2006;42:484–491. [PubMed] [Google Scholar]
- Xu X-W, Wu Y-H, Wang C-S, Wang X-G, Oren A, Wu M. Croceicoccus marinus gen. nov., sp. nov., a yellow-pigmented bacterium from deep-sea sediment, and emended description of the family Erythrobacteraceae. Int. J. Syst. Evol. Microbiol. 2009;59:2247–2253. doi: 10.1099/ijs.0.004267-0. [DOI] [PubMed] [Google Scholar]
- Yorkov V, Jappe J, Vermeglio A. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl. Environ. Microbiol. 1996;62:4195–4198. doi: 10.1128/aem.62.11.4195-4198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga I, Kawai T, Takeuchi T, Ishida Y. Distribution and fluctuation of bacteria inhibiting the growth of a marine red tide phytoplankton Gymnodinium mikimotoi in Tanabe Bay, Wakayama Pref., Japan. Fish. Sci. 1995;61:780–786. [Google Scholar]
- Yoshinaga I, Kawai T, Ishida Y. Analysis of algicidal ranges of the bacteria killing the marine dinoflagellate Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fish. Sci. 1997;63:94–98. [Google Scholar]
- Young JM, Kuykendall LD, Martinez-Romero E, Kerr A, Sawada H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie, 1998 as new combinations: Rhizobium radiobacter R. rhizogenes R. rubi R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 2001;51:89–103. doi: 10.1099/00207713-51-1-89. [DOI] [PubMed] [Google Scholar]


