Abstract
Importance
Information on age-specific risk for Parkinson disease (PD) in Gaucher disease (GD) patients and glucocerebrosidase (GBA) heterozygotes is important for understanding the pathophysiology of the genetic association and for counseling these populations.
Objective
To estimate the age-specific risk of PD in Ashkenazi Jewish (AJ) patients with Type-1 GD and in GBA heterozygotes.
Setting
Two tertiary Gaucher centers and a Parkinson’s tertiary center.
Participants
GD patients at Shaare Zedek, Jerusalem, Israel (n=332) and Mount Sinai School of Medicine, NY (n=95), and GBA non-carrier non-PD spouse controls at Columbia University, NY (n=77). All were AJ and most GD patients (98.1%) carried at least one N370S mutation.
Main outcome measure
Main outcome measure was a diagnosis of PD. Diagnosis was established in GD patients on examination. We used a validated family history interview that identifies PD with a sensitivity of 95.5% and specificity of 96.2% to identify PD in family members. Kaplan-Meier survival curves were used to estimate age-specific PD risk among GD patients (n=427), among their parents, who are obligate GBA mutation carriers (heterozygotes, n=694) and among non-carriers (parents of non-PD non-GD controls, n=154). The age-specific risk was compared among groups using the log-rank test.
Results
Among those who developed PD, patients with GD had a younger age-at-onset than GBA heterozygotes (54.2, versus 65.2, p=0.003). Estimated age-specific risk for PD at ages 60 and 80 was 4.7% and 9.1% among GD patients, 1.5% and 7.7% among heterozygotes, and 0.7% and 2.1% among non-carriers. PD risk was higher in GD patients than non-carriers (p=0.008, log-rank test) and in heterozygotes than non-carriers (p=0.026, log-rank test) but did not reach significance between GD patients and GBA heterozygotes (p=0.074, log-rank test).
Conclusion
GD patients and GBA heterozygotes have an increased age-specific risk for PD compared to controls, with a similar magnitude of PD risk by age 80; however, the number of mutant alleles may play an important role in PD age-at-onset.
INTRODUCTION
Mutations in the glucocerebrosidase (GBA) gene have emerged as a common risk factor for the development of Parkinson disease (PD). This association was originally reported in case reports and case series involving patients with Gaucher disease (GD),1,2 an autosomal recessive lysosomal storage disease resulting from mutations in both alleles of GBA. GD is the most common genetic disorder in Ashkenazi Jews (AJ). Among AJ, the vast majority of GD patients carry at least one N370S allele, a missense mutation, associated with Type-1 GD, the milder non-neuronopathic form. GBA heterozygosity is also associated with PD, based on case-control studies comparing mutation frequency in PD cases and controls in different ethnic groups.1,3–10 Among PD patients, at least 15% of AJ and 3% of non-AJ carry one of the two most common GBA mutations (N370S and L444P).7 A multicenter analysis estimated the odds ratio for carrying a GBA mutation in PD patients versus controls at 5.43 (95% CI, 3.89 to 7.57).7 However, data on the reverse association—the risk of PD in GBA heterozygotes—has been limited and widely variable, with penetrance estimations between 10.9% and 29.7% by age 80.11–13 Furthermore, it is unclear whether the risk of PD is different among those carrying two mutant GBA alleles (i.e. GD patients) and those with one mutant allele (i.e. heterozygote carriers).
Here, we aimed to estimate the age-specific risk for PD associated with either one or two mutant GBA alleles among AJ by obtaining PD history and family history data from two of the world’s largest AJ-GD centers, Shaare-Zedek Medical Center (SZMC) in Israel and Mount Sinai School of Medicine (MSSM) Comprehensive Gaucher Disease Treatment Center in New York. A major advantage of this approach was the ability to easily access a large number of GBA heterozygotes: GD patients’ parents, who are obligate carriers of a GBA mutation. We compared the estimated risk of PD between Type-1 GD patients who have two GBA mutations, carriers of a single mutation (parents of GD patients), and presumed non-carriers (parents of non-PD non-GBA carrier AJ participants). We hypothesized that age-specific risk for PD would increase with the number of mutated alleles, i.e. lowest in controls, higher in heterozygotes and highest in GD patients.
METHODS AND PARTICIPANTS
GD participants were recruited from two outpatient centers: SZMC and MSSM. GD diagnosis was established at both sites in a similar fashion. Initial work up included glucocerebrosidase enzymatic activity and a panel of the common AJ mutations (of 6–8 mutations). In cases where enzymatic activity was consistent with GD, but the panel revealed only heterozygous or no mutations, full sequencing was offered. Control participants were recruited from the Center for Parkinson’s Disease at Columbia University Medical Center (CUMC), NY, who were spouses of PD cases. In order to compare participants with similar genetic background (given that controls were available only in NY), participants were included only if they reported all four grandparents were Jewish. All study procedures were approved by the local ethics committees, and all participants signed an informed consent.
Shaare Zedek Medical Center, Jerusalem, Israel
Adult patients (age≥18) with confirmed GD evaluated at SZMC from October 2011 to March 2013 were approached irrespective of family history of PD, GD severity or frequency of visits (e.g. patients who were asymptomatic or mildly symptomatic were also included). Information was collected on 332 adult GD patients. One patient was not included in the study due to a personal history of dementia and inability to provide informed consent, and one patient was not included due to an inability to speak either Hebrew or English. Three (0.9%) declined to participate. Seven interviews of GD participants who live remotely and who are not treated with enzyme replacement were conducted over the phone.
Mount Sinai Medical Center, NY
Participants from the Comprehensive Gaucher Disease Treatment Center, NY, have been previously described.11 For the current analysis, only data from adult (age≥18) participants with AJ ancestry (n=95) are included, including three participants not previously reported.
Columbia University Medical Center, NY
Non-GD non-PD control participants were recruited among spouses of PD patients from the Center for Parkinson’s Disease at CUMC between 2010–2013. Controls were screened for the eight most common GBA mutations in the AJ population;14 when combined, these account for over 96% of GBA mutations in AJ.15 Only participants (n=77) who tested negative for GBA mutations and reported AJ ancestry were included.
CLINICAL EVALUATION
Demographics and GBA genotype were obtained from the charts of all GD participants. GD participants stating a previous diagnosis of PD were examined by a movement disorders specialist (RNA) to confirm PD diagnosis. Control participants reported not having PD and were clinically examined (RNA) to rule out the presence of PD. A validated family history interview (FHI) was used to elicit family history of PD from GD patients and controls in each of their first-degree relatives.11,16 Although relatives were not genotyped or examined, the survey identifies PD with a sensitivity of 95.5% and specificity of 96.2%, using an algorithmic “conservative” diagnosis of PD.16 The same FHI was previously used for Parkin17 and LRRK218 penetrance estimations. Participants reported their first-degree relatives’ current age or age-at-death as well as the age-at-onset of PD symptoms/signs, if applicable.
STATISTICAL ANALYSIS
Descriptive statistics (means, standard deviations, Student t test and ANOVA for continuous variables; proportions, frequencies, chi square test and Fisher exact tests for categorical variables) were used to compare the demographics of GD participants, their parents, and non-carrier parents of controls as well as to compare demographics of participants from SZMC and MSSM. When GD participants were related to one another (multiplex families), only one FHI per family was included. Primary analyses were restricted to parents of GD patients and non-GD non-PD controls, whom we estimated to be independent observations (none of the participants reported consanguinity), and in the case of GD parents, they were each obligate carriers of GBA mutations. In all survival analyses, time to event was defined as age-at-onset of PD. Participants were censored either at their current age or age at death, if they did not reach an event (PD). We used Kaplan-Meier survival curves to estimate survival free of PD (penetrance) in patients with GD who by definition carry two GBA mutations (GD probands from MSSM and SZMC), carriers of a single GBA mutation (parents of GD participants from MSSM and SZMC) and non-carrier individuals (parents of controls from CUMC). We used the log-rank test to compare survival free of PD between GD patients, GBA heterozygotes and non-carrier controls. We then analyzed whether the following two characteristics of the GD probands are associated with increased risk for PD. First, we used Cox proportional hazards models, adjusted for recruitment site, parent’s sex and proband’s sex to compare the risk for PD between parents of GD patients with and without a diagnosis of PD. Second, we compared the risk for PD in parents of GD N370S homozygotes (whose parents are obligate N370S carriers) and parents of compound heterozygote GD patients (where at least one of the parents carries a non-N370S mutation) using similarly adjusted Cox models. Analyses were performed using SPSS Statistics version 19.0 software (Chicago, IL).
RESULTS
Demographics
GD participants
Three-hundred and thirty-two AJ-GD probands from SZMC and 95 from MSSM participated in the study (Table 1). Comparisons of the demographics and genotypes of GD participants between the two sites are described in Supplementary Table 1. Participants at MSSM were older (p=0.033), but sex and genotype were similar between SZMC and MSSM participants. The vast majority (98.1%) carried at least one N370S mutation, and 67.3% (65.2% SZMC versus 74.5% MSSM, p=0.060) were N370S homozygotes. The other mutations included 84GG (n=53), L444P (n=21), IVS2+1 (n=15), R496H (n=14), V394L (n=11), del55bp (n=4) and others. Frequency of PD events was similar between MSSM and SZMC for both GD patients (Supp Table 1) and heterozygote parents (5.1% at SZMC versus 4.4% at MSSM, p=0.843). The PD phenotype of the GD/PD participants has been previously reported.11,19
Table 1.
Demographics of GD participants, their parents (obligate carriers), and controls
| GD patients n=427 |
Obligate GBA carriers (parents) n=694 |
Controls’ parents (presumed non- carriers) n=154 |
p-value | |
|---|---|---|---|---|
| Mean age in years, (SD) | 47.1 (16.2) | 69.1 (12.8) | 76.0 (13.7) | <0.001 |
| Percent male, (n) | 47.1% (201) | 50.0% (347) | 50.0% (77) | 0.638 |
| Percent diagnosed with Parkinson disease, (n) | 2.6% (11) | 4.9% (34) | 1.9% (3) | 0.063 |
| Among PD cases, mean PD age-at-onset (SD) | 54.2 (8.6) | 65.2 (10.7) | 72.0 (19.3) | 0.008 |
| Among PD cases, percent male, (n) | 72.7% (8) | 52.9% (18) | 33.3% (1) | 0.367 |
Family members
Of the 427 GD probands recruited at SZMC and MSSM, family history of PD was analyzed in 347 unrelated GD families, including 257 families from SZMC and 90 families from MSSM. Eighty GD participants’ family data were not included because they belonged to multiplex families in which more than one sibling or a parent and child were enrolled. The data included information on 1766 first-degree relatives. None of the 460 children of GD patients (obligate GBA carriers) and 1.3% (8 of the 612) of siblings were diagnosed with PD. Subsequent analyses, as discussed in the next section, were restricted to the 694 parents. As a control group, we studied parents of non-carrier, non-PD AJ controls (n=154); this group was older than the parents of GD participants (Table 1).
PD risk in GD patients, GBA heterozygotes and non-carriers
Eleven of the 427 GD patients (2.6%), 34 of the 694 heterozygote parents (4.9%) and 3 of the 154 non-carriers (1.9%) were diagnosed with PD (Table 1). Among those diagnosed with PD, GD patients had an earlier age of onset of PD compared to both GBA heterozygotes (p=0.003) and non-carrier controls (p=0.030), suggesting that carrying two mutant GBA alleles leads to the onset of PD at a younger age.
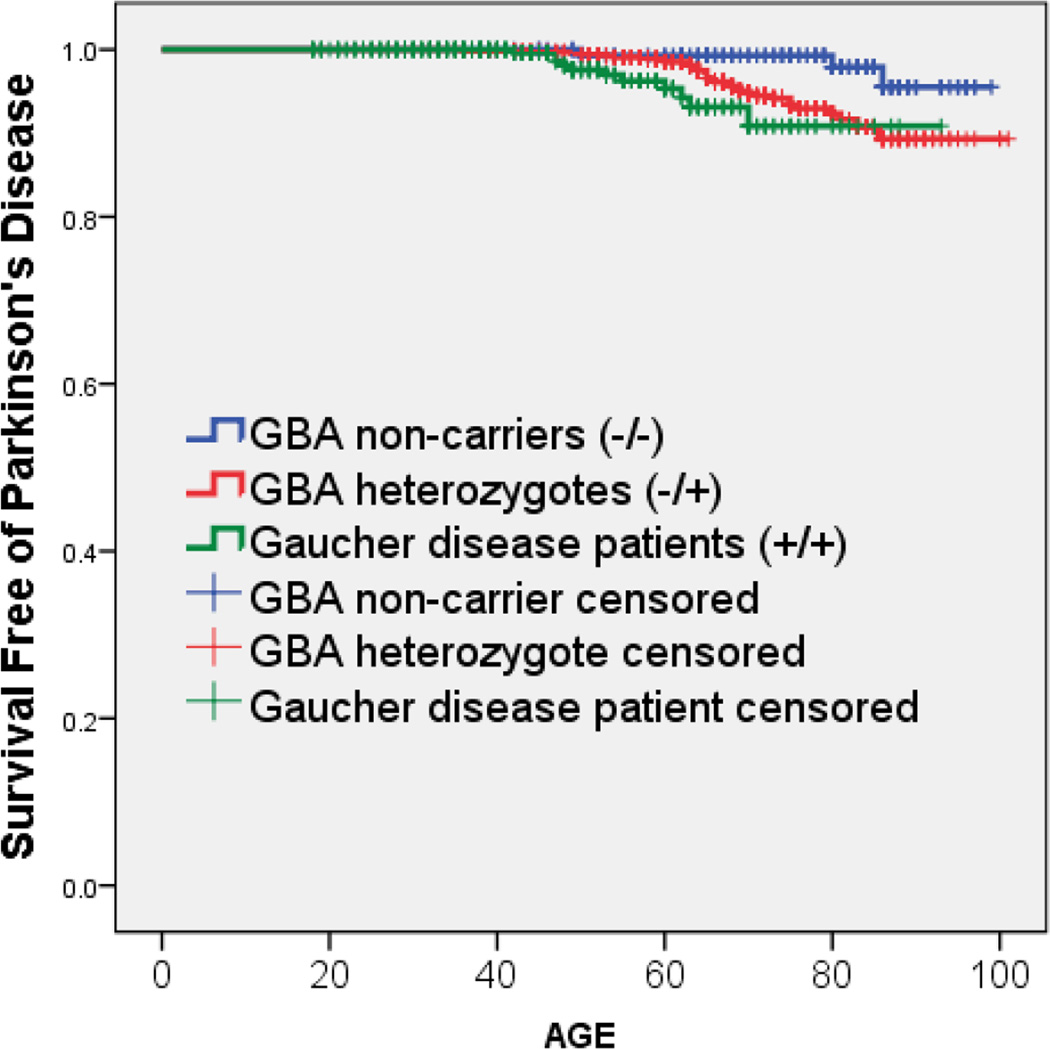
The probability of having PD by a specific age (using Kaplan Meier plots) among GD patients, GBA heterozygotes and non-carriers is presented in Table 2 and Figure 1. Log-rank tests confirm higher age-specific risk for PD in GD patients compared to controls (p=0.008) and in heterozygotes compared to controls (p=0.028), but differences between GD patients and GBA heterozygotes did not reach statistical significance (p=0.074). Taken together, these data suggest that both GD patients and GBA heterozygotes are at increased risk of PD. However, the overall risk of developing PD is not substantially greater for those with two mutant alleles (i.e. GD patients) compared to heterozygotes with one mutant allele.
Table 2.
Age-specific risk (penetrance) for PD among Ashkenazi Jews with and without GBA mutations using Kaplan Meier plots
| Age 60 | Age 70 | Age 80 | |
|---|---|---|---|
| Non-carriers (n=154) | 0.7% ±1.37% | 0.7% ±1.37% | 2.1% ±2.94% |
| Obligate GBA carriers (n=694) | 1.5% ±0.98% | 5.2% ±1.96% | 7.7% ±2.74% |
| Obligate N370S carriers (n=464) | 1.2% ±0.98% | 3.5% ±1.96% | 5.9% ±3.14% |
| Gaucher patients (N370S homozygote or compound heterozygote carriers), (n=427) | 4.7% ±3.33% | 9.1% ±6.07% | 9.1% ±6.07% |
± represents confidence intervals
Figure 1.
Kaplan Meier survival curves demonstrating survival free of PD among Gaucher patients, obligate GBA carriers (parents of Gaucher patients), and non-carriers (parents of non-carrier controls)
We analyzed whether characteristics of the GD proband affected PD risk in their parents. The hazard ratio of PD among parents of GD-PD participants was not significantly different from parents of GD probands without PD (HR=1.2, 95%CI: 0.6–2.3, p=0.822), adjusted for recruitment site, parent’s sex and proband’s sex. When risk for PD was estimated based on the genotype of the proband, being a parent of a GD compound heterozygote (N370S and another mutation) versus a parent of GD N370S homozygote, adjusted for recruitment site, parent’s sex and proband’s sex, non-N370S homozygote state was not associated with a significantly higher age-specific risk for PD (HR: 1.8, 95%CI: 0.9–3.6, p=0.084).
DISCUSSION
This study estimates the age-specific penetrance of PD among GD patients and GBA heterozygotes based on the largest number of obligate carriers published to date. Our age-specific estimation of PD risk among GD patients (9.1% by age 80) is similar to estimations derived from the International Collaborative Gaucher Group (ICGG) Registry data (9–12% by age 80).20 The fact that only 11 GD patients (of 427) were diagnosed with PD is reassuring when compared with prior odds ratio estimations of an almost 20-fold increased life-time risk of developing PD in this population.21 Our estimations of GBA heterozygotes’ risk for PD (7.7% by age 80) are, however, lower than those obtained in the U.K. (15% by age 80) and France (29.7% by age 80).12,13 The large difference between our penetrance estimations and those reported by Anheim et al. from France may be explained by differences in methodology – how GBA heterozygotes were ascertained—and differences in the type of GBA mutations observed. In this study, we collected data on heterozygotes regardless of family history of PD, while heterozygotes in the Anheim et al report were all ascertained through a familial PD cohort, i.e., by family history of PD. Therefore, it is possible that these GBA heterozygotes, by virtue of belonging to families with familial PD, have other risk factors for PD, in addition to the GBA mutations.22 Further, it is possible that the higher prevalence of the non-N370S mutations in the French and British populations, who are mostly non-AJ, conveys a higher risk for PD than those observed in AJ. Two studies reported that “milder” GBA mutations, including the N370S mutation, are associated with lower risk for PD than more “severe” mutations, e.g., L444P or 84GG.23,24 In this study, we examined this issue by comparing the risk of PD in parents of N370S homozygotes to parents of N370S compound heterozygotes. While we did not find a statically significant difference between parents of N370S homozygotes and parents of compound heterozygotes (HR=1.8, p=0.084), our study may have been underpowered to detect such a difference.
The availability of data on both GD patients and GBA heterozygotes provides a unique opportunity to assess the effect of one versus two GBA mutations on PD risk. GD patients, who carry two GBA mutations, developed PD at an earlier age (54.2 versus 65.2, p=0.003) compared to heterozygote carriers, i.e. those with a single mutant GBA allele. However, by age 80, the difference in the age-specific risk of PD between the two groups had diminished (9.1% in GD patients and 7.7% in heterozygotes). Together, these suggest that the incremental risk of carrying a second GBA mutant allele lies in developing PD at an earlier age, rather than having a substantially greater overall risk of developing PD. The mechanisms underlying the link between GBA mutations and PD are unknown. The most common hypotheses linking GBA mutations to PD suggest either a loss of function of glucocerebrosidase (GCase) enzymatic activity or a toxic gain of function.25 It is possible that GCase activity modifies age-at-PD onset, since GD patients had an earlier PD onset than heterozygotes, and heterozygotes’ age-at-onset is earlier than non-carriers’.7 Reduced GCase activity has been implicated in the pathogenesis of neuronal loss and alpha synuclein deposits in a mouse model of GBA homozygotes,26,27 but to our knowledge an animal model of heterozygote animals with neuronal damage resembling PD does not exist. In addition, the similar magnitude of PD risk among GD patients and heterozygotes by age 80 suggests additional, more complex biological mechanisms are involved, perhaps including a toxic gain of function, e.g., disrupted clearance of alpha synuclein by mutated GCase enzyme.25,28
Our findings may help clinicians and genetic counselors provide penetrance estimations for GBA heterozygotes in the AJ population. Many AJ undergo pre-conception and prenatal GBA testing in both Israel and the United States, as recommended by the American College of Medical Genetics (ACMG) and are aware of their genotype.29 Accurate penetrance estimates are important in enabling clinicians to inform heterozygotes of their risk for PD and address concerns about the association.
Strengths of this study include the large number of GD patients and heterozygotes that were included in the analysis. Six-hundred and ninety-four obligate carriers were analyzed in this study, a three-fold increase from our previous report.11 Furthermore, we used the same validated FHI questionnaire16 in all three genetic groups from an exclusively AJ cohort.
The major limitation of our study is that the information on the parents (obligate heterozygotes and non-carriers) was obtained by proband report, and we neither examined nor directly genotyped the parents. It is possible that probands may not recall the accurate age-at-PD onset of their parents. We could not assess the parents for previously reported risk modifiers,30 including LRRK2 mutations,31 for non-paternity, or for carrying homozygous mutations themselves. However, given the practice of offering genetic counseling and testing to first-degree relatives of GD patients (we had to exclude family data from 80 GD probands who came from multiplex families), it is likely most GD parents had been identified. In addition, we have not genotyped the parents of the non-carrier controls, and it is possible that members of this cohort carry a GBA mutation on the allele not inherited by their genotyped offspring. This limitation, however, would bias our results towards the null hypothesis.
Despite the fact that GBA mutations are among the most common genetic risk factors for PD, our results (as well as others) indicate that most GD patients and GBA heterozygotes will never develop PD.25 In order to refine the estimation of the age-specific risk of PD in these populations and to identify genetic and environmental risk modifiers, long term follow up of GD patients and GBA heterozygotes is required. Longitudinal detailed evaluation of GD/PD and GBA heterozygote/PD patients will allow us to compare disease severity, disease progression, cognition, and other PD characteristics in addition to age-at-onset between these groups.
Supplementary Material
Acknowledgments
Funding for this study provided by the Parkinson’s Disease Foundation, the National Institutes of Health (K02 NS080915, R56NS036630 and UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156, and by grant UL1RR 029887 from the National Center for Research Resources), and by the Department of Genetics and Genomic Sciences at Mount Sinai School of Medicine.
Dr. Alcalay receives research support from the NIH (K02NS080915), the Parkinson’s Disease Foundation, the Smart Foundation and the Michael J Fox foundation.
Dr. Levy is supported by the NIH (K08-NS070608), as well as the Parkinson’s Disease Foundation and the Smart Foundation.
Dr. Waters received speaking honorarium from Teva and UCB. She receives research support from the Parkinson’s Disease Foundation.
Dr. Fahn reports receiving consulting and advisory board membership with honoraria from: Merz Pharma (Jan 2013), Genervon Biotechnology - expects to receive compensation for serving as Principal Investigator of a pilot clinical trial. Grants/Research Support from the Parkinson’s Disease Foundation (no salary support). Grant from the Smart Family Foundation (Dec. 2012). Lecture Honoraria: American Academy of Neurology (April 2012), Columbia University (July 2012), Sun Pharmaceuticals India (Nov 2012). Editor and Author Honoraria: Springer Publishers for serving as co-editor of Current Neurology and Neurosurgery Report (annual); Elsevier Publishers for co-authorship of book Principles and Practice of Movement Disorders.
Dr. Nichols reports receiving support from the NIH/NHLBI (5R01HL102107-04, 5R24HL105333-02).
Dr. Balwani receives NIH support (K23DK095946). Dr. Balwani is a member of the ICGG Gaucher registry advisory board and has received honoraria from Genzyme corp. The Department of Genetics and Genomic Sciences receives support for participation in the ICGG registry.
Dr. Elstein received honoraria and travel expenses to present data from clinical trials by Shire, Lexington MA, USA
Dr. Zimran receives consultancy fees and has options in Protalix Biotherapeutics and is a member of the Scientific Advisory Board; he receives support from Genzyme Corp. for participation in the ICGG registry & from SHGT for GOS; honoraria: Pfizer and SHGT. Travel by Medison.
Footnotes
Conflict of Interest: No conflicts of interests to report with relevance to this publication.
Disclosures:
Dr. Dinur, Mr. Quinn, Ms. Sakanaka and Mr. Dorovski, Mr. Paucilo, Dr. Chung, Dr. Rana and Ms. Bier have nothing to disclose.
Reference
- 1.Mitsui J, Mizuta I, Toyoda A, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009 May;66(5):571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]
- 2.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996 Sep;89(9):691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 3.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004 Jan;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004 Nov 4;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 5.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008 Oct;65(10):1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood cells, molecules & diseases. 2006 May-Jun;36(3):426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009 Oct 22;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giraldo P, Capablo JL, Alfonso P, et al. Neurological manifestations in patients with Gaucher disease and their relatives, it is just a coincidence? Journal of inherited metabolic disease. 2011 Jun;34(3):781–787. doi: 10.1007/s10545-011-9298-4. [DOI] [PubMed] [Google Scholar]
- 9.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neuroscience letters. 2009 Mar 13;452(2):87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Bras J, Paisan-Ruiz C, Guerreiro R, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2007 Dec 19; doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genetics in medicine : official journal of the American College of Medical Genetics. 2013 Feb;15(2):146–149. doi: 10.1038/gim.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeill A, Duran R, Hughes DA, Mehta A, Schapira AH. A clinical and family history study of Parkinson's disease in heterozygous glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatry. 2012 Aug;83(8):853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anheim M, Elbaz A, Lesage S, et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012 Feb 7;78(6):417–420. doi: 10.1212/WNL.0b013e318245f476. [DOI] [PubMed] [Google Scholar]
- 14.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012 May 1;78(18):1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler E, Gelbart T, Kuhl W, Zimran A, West C. Mutations in Jewish patients with Gaucher disease. Blood. 1992 Apr 1;79(7):1662–1666. [PubMed] [Google Scholar]
- 16.Marder K, Levy G, Louis ED, et al. Accuracy of family history data on Parkinson's disease. Neurology. 2003 Jul 8;61(1):18–23. doi: 10.1212/01.wnl.0000074784.35961.c0. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Clark LN, Louis ED, et al. Risk of Parkinson disease in carriers of parkin mutations: estimation using the kin-cohort method. Arch Neurol. 2008 Apr;65(4):467–474. doi: 10.1001/archneur.65.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology. 2006 Nov 28;67(10):1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. [DOI] [PubMed] [Google Scholar]
- 19.Chetrit EB, Alcalay RN, Steiner-Birmanns B, et al. Phenotype in patients with Gaucher disease and Parkinson disease. Blood cells, molecules & diseases. 2013 Mar;50(3):218–221. doi: 10.1016/j.bcmd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbloom B, Balwani M, Bronstein JM, et al. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood cells, molecules & diseases. 2011 Jan 15;46(1):95–102. doi: 10.1016/j.bcmd.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bultron G, Kacena K, Pearson D, et al. The risk of Parkinson's disease in type 1 Gaucher disease. Journal of inherited metabolic disease. 2010 Apr;33(2):167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidransky E, Hart PS. Penetrance of PD in Glucocerebrosidase Gene Mutation Carriers. Neurology. 2012 Jul 3;79(1):106–107. doi: 10.1212/01.wnl.0000416261.29035.4c. [DOI] [PubMed] [Google Scholar]
- 23.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008 Jun 10;70(24):2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 24.Gan-Or Z, Giladi N, Orr-Urtreger A. Differential phenotype in Parkinson's disease patients with severe versus mild GBA mutations. Brain. 2009 Oct;132(Pt 10):e125. doi: 10.1093/brain/awp161. [DOI] [PubMed] [Google Scholar]
- 25.Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurology. 2012 Nov;11(11):986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sardi SP, Clarke J, Kinnecom C, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sardi SP, Clarke J, Viel C, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullen V, Sardi SP, Ng J, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011 Jun;69(6):940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 29.Gross SJ, Pletcher BA, Monaghan KG. Carrier screening in individuals of Ashkenazi Jewish descent. Genetics in medicine : official journal of the American College of Medical Genetics. 2008 Jan;10(1):54–56. doi: 10.1097/GIM.0b013e31815f247c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribner A, Altarescu G, Zimran A, Elstein D. Osteopontin polymorphic susceptibility factor for Parkinson's disease among patients with Gaucher disease. Mov Disord. 2011 Jun;26(7):1341–1343. doi: 10.1002/mds.23595. [DOI] [PubMed] [Google Scholar]
- 31.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006 Jan 26;354(4):424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.