Abstract
Selenoproteins are ubiquitously expressed, act on a variety of physiological redox-related processes, and are mostly regulated by selenium levels in animals. To date, the expression of most selenoproteins has not been verified in euryhaline fish models. The Mozambique tilapia, Oreochromis mossambicus, a euryhaline cichlid fish, has a high tolerance for changes in salinity and survives in fresh water (FW) and seawater (SW) environments which differ greatly in selenium availability. In the present study, we searched EST databases for cichlid selenoprotein mRNAs and screened for their differential expression in FW and SW-acclimated tilapia. The expression of mRNAs encoding iodothyronine deiodinases 1, 2 and 3 (Dio1, Dio2, Dio3), Fep15, glutathione peroxidase 2, selenoproteins J, K, L, M, P, S, and W, was measured in the brain, eye, gill, kidney, liver, pituitary, muscle, and intraperitoneal white adipose tissue. Gene expression of selenophosphate synthetase 1, Secp43, and selenocysteine lyase, factors involved in selenoprotein synthesis or in selenium metabolism, were also measured. The highest variation in selenoprotein and synthesis factor mRNA expression between FW- and SW-acclimated fish was found in gill and kidney. While the branchial expression of Dio3 was increased upon transferring tilapia from SW to FW, the inverse effect was observed when fish were transferred from FW to SW. Protein content of Dio3 was higher in fish acclimated to FW than in those acclimated to SW. Together, these results outline tissue distribution of selenoproteins in FW and SW-acclimated tilapia, and indicate that at least Dio3 expression is regulated by environmental salinity.
Additional keywords: fish, selenium, selenoprotein, acclimation salinity, tilapia, euryhaline
1. Introduction
Selenium (Se) is a trace element found in the atmosphere and bodies of water worldwide, originating from both natural and anthropogenic sources [1] and an essential micronutrient to most species, from bacteria to higher vertebrates. Levels of Se are usually reported to be higher in seawater (SW) environments than in freshwater (FW) environments [2]. Selenium is naturally found in inorganic (i.e. selenate and selenite) or organic (i.e. selenomethionine, selenocysteine, dimethylselenide and dimethyldiselenide) forms, and is ultimately incorporated into selenoproteins as the amino acid selenocysteine (Sec). Selenocysteine is encoded by the UGA opal codon and, in eukaryotes, the Sec incorporation mechanism requires several factors to be in place [3], including two selenophosphate synthetases, SPS1 and SPS2 [4], and the protein Secp43 [5]. Decomposition of intracellular Sec is catalyzed by the action of Sec lyase (Scly), which releases selenide to then reenter the selenoprotein synthesis pathway [6].
Because Sec is ionized at physiological pH, most selenoproteins function in strong redox reactions, such as peroxidation and deiodination [7]. Early studies have shown Se-dependent glutathione peroxidase (GPx) activity in fish [8, 9]. The gene for GPx was later inferred in zebrafish (Danio rerio) from in silico analysis [10], and confirmed experimentally in zebrafish, coho salmon (Oncorhynchus kisutch), rainbow trout (Oncorhynchus mykiss) and bluefin tuna (Thunnus maccoyii) [11–14]. Deiodination is a key step in thyroid hormone activation, and expression of iodothyronine deiodinases has been previously described in various fish species [15], including the euryhaline killifish (Fundulus heteroclitus) [16]. In addition to redox reactions, Se is transported through the body via selenoprotein P (Sepp1a), a protein that in zebrafish contains 17 Sec residues [17]. Although other selenoproteins evolved various functions in mammals, including the maintenance of endoplasmic reticulum (ER) homeostasis [18], phospholipid biosynthesis [19], and regulation of gene transcription [20], their function in fish remains unknown.
Interestingly, of the twenty-nine selenoproteins identified in eukaryotes to date, three of them, Fep15, SelJ and SeL are only found in fish [21] or aquatic organisms [18, 22]. In fact, the genomes of aquatic organisms, including fish, encode for more selenoproteins than terrestrial animals [23, 24]. Having a larger selenoproteome suggests that fish have developed greater reliance on environmental Se for redox metabolism. In light of this, it is surprising that little information is available on the abundance and distribution of selenoproteins in fish species that can thrive in environments that vary in salinity and Se content.
The Mozambique tilapia (Oreochromis mossambicus) is a euryhaline cichlid fish native to estuarine areas of the coast of Mozambique, eastern Africa [25]. Because this species evolved under exposure to daily tidal changes, it serves as a good model to study opposing osmotic paradigms for survival: a diluting environment in FW and a dehydrating environment in SW [26]. Studies in other fish species have revealed that an increase in environmental salinity can affect the activity of selenoproteins involved in redox reactions [27–29], suggesting that these proteins may play a role in osmotic adaptation. Nevertheless, these studies did not examine the effects of acclimation salinity in the expression of selenoproteins other than GPx and deiodinases.
To address the possible influence of acclimation salinity on the transcriptional regulation of selenoproteins in a euryhaline fish model, we searched for tilapia selenoproteins in an expressed sequence tag (EST) database. We then screened eight tissues and compared the differences in baseline mRNA expression of twelve selenoproteins and three factors involved in selenoprotein synthesis or Se metabolism in Mozambique tilapia acclimated to SW relative to those acclimated to FW. Based on the availability of antibodies able to detect selenoproteins in fish tissues, we compared the protein levels of Dio3 in FW- and SW-acclimated fish. We also measured the effects of salinity transfers on the branchial gene expression of Dio3.
2. Materials and Methods
2.1. Fish
Mature male Mozambique tilapia (50–100 g), Oreochromis mossambicus, were obtained from a population maintained at the Hawaii Institute of Marine Biology, University of Hawaii. Fish were reared in outdoor tanks (700 L) with a continuous flow of FW or SW under natural photoperiod. SW in the tanks was pumped from Kaneohe Bay, Hawaii, USA. SW-acclimated tilapia employed in this experiment were spawned and reared in SW, having never been previously exposed to FW. FW-acclimated tilapia, on the other hand, were spawned and reared in FW, having never been previously exposed to SW. Water temperature was maintained at 24–26 °C. Animals were fed ~5% of their body weight per day with Trout Chow (Skretting, Tooele, UT), which contains ~1 ppm of total Se content according to the manufacturer (personal communication). Fish were fasted for one day prior to sample collection. At the time of sampling, fish were anesthetized with 2-phenoxyethenol (2-PE; 0.3 mL/L). Blood was collected from the caudal vasculature by a needle and syringe coated with ammonium heparin (200 U/mL, Sigma-Aldrich, St. Louis, MO). Plasma was separated by centrifugation and stored at −80 °C for later analyses of Se content. Fish were then rapidly decapitated and the following tissues were collected: brain, eye, gill, kidney, liver, muscle, pituitary and intraperitoneal white adipose tissue (WAT). All tissues were immediately frozen in liquid nitrogen, and subsequently stored at −80 °C prior to RNA extraction or Western blotting analysis. All experiments were conducted in accordance with the principles and procedures approved by the Institutional Animal Care and Use Committee, University of Hawaii.
2.2. Transfer experiments
Mature male Mozambique tilapia (7– 55 g) were obtained from a population reared in indoor tanks for 4 months, in either FW or SW. The salinity of one of the FW tanks was switched to SW, and the salinity of one of the SW tanks was switched to FW. It took 1.5 hours for a complete salinity transfer. Fish were fed for the duration of the experiment and water temperature was maintained between 24 and 26 °C. Fish (n=6–10) were sampled at 0 h (immediately prior to opening the FW- or SW-valves), 6 h, 24 h, 48 h and 7 days after transfer. At the time of sampling, fish were netted and anesthetized with 2-PE (0.3 mL/L). Plasma was collected as described above and stored in −20°C until measurement of osmolality. Plasma osmolality was determined using a vapor pressure osmometer (Wescor 5100C, Logan, UT). Filaments from the second gill arch on the left side of the fish were harvested. Gill samples were frozen in liquid nitrogen and stored at −80°C prior to RNA extraction.
2.3. Selenium levels
Selenium content in the tank water, with or without fish, and in the plasma of tilapia was measured by Inductively-Coupled Plasma-Mass spectrometry (ICP-MS), performed by Exova, Inc. (Santa Fe Springs, CA, USA). Briefly, water samples were mixed with 0.5 ml of nitric acid and internal standards, producing 5g of solution for ICP-MS analysis, and assay detection limit was 0.0003 ppm. Plasma sample portions (~0.18 g) were mixed with internal standards and then diluted to a mass of 5 g with a solution of 0.1% ammonium hydroxide, 0.05% EDTA and 0.05% Triton X100. After complete dissolution, samples were analyzed by ICP-MS and assay detection limit was 0.02 ppm.
2.4. Extraction of RNA, cDNA synthesis and real-time quantitative PCR (qPCR)
Total RNA was extracted from all tissues with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol, followed by purification employing the RNeasy clean-up kit (Qiagen, Valencia, CA). Total RNA concentrations were determined by measuring the absorbance of purified RNA samples at 260 nm wavelength with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Complementary DNA (cDNA) was synthesized by reverse transcription with 1 µg total RNA using the High Capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. qPCR reactions were carried out on 384-well plates, with a cDNA amount equivalent to 10ng total RNA, 100 nM forward and reverse primers, and SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA) in a total volume of 5µl. Cycling conditions were: 2 min at 50 °C, 5 min at 95 °C followed by 45 cycles at 95 °C for 10 sec, 55 °C for 10 sec and 72 °C for 10 sec. Relative quantification was carried out by the ΔCt method. Assays were run on a LightCycler 480 system (Roche, Mannheim, Germany).
2.5. Sequences
Sequences for tilapia selenoproteins and selenoprotein synthesis or Se metabolism factors were identified by searching the translated nucleotide online database using a protein query (tBLASTn), at the NCBI Genbank database (http://www.ncbi.nlm.nih.gov). The tilapia mRNA sequences for twelve selenoprotein and selenoprotein synthesis factor genes were identified by using templates from known protein sequences of zebrafish; two genes were identified from the protein sequence and cDNA library of Nile tilapia; one was identified from the protein sequence of rainbow trout. The mRNA sequence of SelW was employed as previously published [30]. Accession numbers for each cDNA sequence found after conducting tBLASTn are provided in Table 1. ClustalW program was used for multiple sequence alignments and phylogenetic analyses to ensure sequence conservation among species [31].
Table 1.
Primer sequences of selenoproteins and selenoprotein synthesis factors found in the EST database and their respective accession numbers.
| Gene | Primers | Amplicon (bp) |
GenBank accession numbers (EST) |
|---|---|---|---|
| Dio1 | F- AACTATGAGGATTGGGGTCT | 117 | GR608944.1, GR661324.1 |
| R- TGAGTCTGGAGCTTCTCCT | |||
| Dio2 | F- CTTCTGTTTGTGCGTTTACA | 132 | GR660528.1, GR663328.1 |
| R- TTCCAAACACTTTTCTCGTT | |||
| Dio3 | F- AGAAACTGGCTGGAACAATA | 160 | Y11111.1 |
| R- ATGGGTGAACATCTGATAGC | |||
| Fep15 | F- GAGGTGTGTGGATGAAAACT | 105 | GR672000.1, GR666049.1, GR660980.1, GR609035.1 |
| R- GCCTCTGACGTACTTGATCT | |||
| GPx2 | F- GCTGTTGTCTGGAGAAACTC | 187 | GR660798.1, GR681215.1, GR639987.1, GR701620.1, GR606241.1, GR695782.1 |
| R- CTCCTGATGTCCAAACTGAT | |||
| SelJ | F- CCGATAGACAGAATCCTCAG | 170 | GR632526.1, GR655559.1, GR658891.1, GR605549.1, GR606300.1 |
| R- GTTGAGTGTCAGGACTCCAT | |||
| SelK | F- TAGAAGCCAAGGAAAGATGA | 156 | GR638190.1, GR646820.1, GR653210.1, GR649728.1, GR646585.1, GR682968.1, GR650246.1 |
| R- ATGAGGGAAGAAGTGATGTG | |||
| Sel | L F- GTTCTCCTCAGACATTTTGG | 161 | GR695431.1, GR668977.1, GR666688.1, GR697243.1 |
| R- AAGGTACATCCAGTCTGCTC | |||
| SelM | F- TAATGATCTTCGCCTCATTT | 163 | GR611511.1, GR667195.1, GR609923.1, GR621658.1, GR610284.1 |
| R- TGCACTTGAGTCTTTTTGTG | |||
| SelS | F- GTTCTACCTGCTCATTCTGC | 138 | XM_003459754.1 |
| R- TCTTCTTGCATCTTCATCCT | |||
| Sepp1a | F- ATGTTTGGCAGACACTGG | 100 | GR619607.1, GR663172.1 |
| R- CCAATGATGGAGTATGGAAG | |||
| SelW | F- GAATATTGTGGTGGATGAGG | 174 | AY737049.1 |
| R- ATAAGGGAATCCACCAGTTT | |||
| SPS1 | F- GACAAGAACTTCAGGCTCAC | 127 | GR694970.1, GR676170.1 |
| R- GGAACTGTTCATCCTCTTGA | |||
| Secp43 | F- GGGTTACTGTTTTGTGGAGA | 153 | GR609029.1, GR620171.1, GR700905.1 |
| R- GAATACATCTGTCCGCTTTC | |||
| Scly | F- ATATAGCAGGTGCTAAAGCTAA | 80 | GR660440.1, GR669885.1, BJ693872.1 |
| R- CTCTGCTTTCCCTCCAAC | |||
2.6. Primers
Primers were designed using the online software, Primer 3 [32] and checked for consensus sequences using Clustal W. Primer sequences and the accession numbers of the cDNAs for primer design are listed in Table 1. All primers were purchased from IDT Technologies (San Diego, CA). Lyophilized primers were resuspended with RNAse-free water to a concentration of 100 µM and further diluted to 10 µM working stocks. Elongation factor 1α (EF1-α) was employed as the housekeeping gene used for normalization of all target genes as previously described [33], after it was confirmed that there was no salinity effect on its expression. Specificity and efficiency of all new primers was inferred by performing melting curve analyses, and standard concentration curves, respectively.
2.7. Western Blotting
Gills were lysed using CelLytic MT buffer (Sigma-Aldrich, St. Louis, MO) with protease inhibitors (Calbiochem, Darmstadt, Germany), sonicated in ice bath, centrifuged at 14,000 × g for 15 min, 4°C, and supernatant was collected. Supernatant protein concentration was determined by the colorimetric Bradford assay using the Bio-Rad protein assay dye reagent (Bio-Rad, Hercules, CA). Samples consisting of 30 µg of total protein were separated by 4–20% SDS-PAGE (Bio-Rad), transferred to polyvinylidene difluoride membranes (Millipore) and incubated with specific antibody for Dio3 (Novus Biologicals) or β-actin (Cell Signaling) for 1 hour at room temperature, under agitation. Membranes were washed with PBS containing 0.01% Tween-20, incubated with secondary antibodies coupled to infrared fluorophores (Li-Cor Biosciences) for 30 min at room temperature, and blots were visualized using the Odyssey Infrared Imager (Li-Cor Biosciences). The expression of other selenoproteins was not measured due to the lack of specificity of commercially available antibodies.
2.8. Statistical analysis
For each gene and tissue, comparisons between different acclimation salinities were analyzed by Student’s t-test (P<0.05). Salinity transfer experiments were analyzed by one-way analysis of variance (ANOVA), followed by Fisher’s LSD test (P< 0.05). Individual values were log-transformed to meet assumptions of normality and equal variance. Statistical calculations were performed using statistical software programs, Prism 5.0 (GraphPad, La Jolla, CA) and Minitab 13.1 (Minitab Inc., State College, PA).
3. Results
3.1. Selenium content
Fresh water employed in this study was found to have non-detectable levels of Se, both in tanks with and without fish. Se content of Kaneohe Bay SW was 0.0049 µg/g (ppm) and 0.0032 µg/g (ppm), taken from SW tanks with and without fish, respectively. Plasma Se content was found to be 0.2033 ± 0.014 µg/g (ppm) in FW-acclimated fish and 0.2175 ± 0.002 µg/g (ppm) in plasma from SW-acclimated fish. These values were not significantly different from each other (n=4, Student’s t-test).
3.2. Tilapia selenoproteins
Fifteen selenoprotein and selenoprotein synthesis or Se metabolism factor mRNA sequences were identified in the EST database for tilapia. The tilapia mRNA sequences for iodothyronine deiodinases 1, 2 and 3, (Dio1, Dio2 and Dio3), Fep15, glutathione peroxidase 2 (GPx2), selenoprotein J (SelJ), selenoprotein K (SelK), selenoprotein L (Sel L), selenoprotein M (SelM), selenoprotein P (Sepp1a), selenoprotein S (SelS), selenoprotein W (SelW), selenophosphate synthetase 1 (SPS1), Secp43, and Sec lyase (Scly) were found from known protein sequences from zebrafish, Nile tilapia, or rainbow trout. These sequences were highly conserved by comparing consensus regions between different fish species. The sequence for both selenoprotein N (SelN) and thioredoxin reductase 1 (TrxR1) were found in the EST Nile tilapia database (accession numbers GR649079.1 and GR669725.1 for SelN, and GR589931.1 and GR641653.1 for TrxR1) but alignment analysis with the zebrafish sequence (GR694733.1 and AAH54599.1 for SelN and TrxR1, respectively) revealed low similarity; for this reason, SelN and TrxR1 were not included in this study. Other selenoprotein genes, including the expected glutathione peroxidases 1 and 4, were not analyzed due to the lack of available tilapia ESTs at the time of this study.
3.3. Selenoprotein mRNA levels
Differences in selenoprotein mRNA levels between FW and SW-acclimated tilapia were compared in eight tissues (Figure 1, with numerical values in Supplementary Table 1). Sepp1a, SelM and SelS were the only selenoproteins whose mRNA expression was detected in all tissues examined. All three deiodinases (Dio1, Dio2, and Dio3) were most highly expressed in the gill, followed by the brain and the liver (Figure 1A–C). Branchial expression of Dio3 mRNA was higher in FW- than in SW-acclimated tilapia. Expression of Dio1 mRNA was higher in the liver of SW-acclimated fish, while hepatic Dio2 and Dio3 expression did not change according to acclimation salinity. While Dio2 mRNA expression was not detected in the pituitary, Dio1 expression was significantly higher in the pituitary of FW-acclimated fish; pituitary Dio3 expression did not change with salinity.
Figure 1.
Expression of selenoproteins of male Mozambique tilapia acclimated to FW (open bars) or SW (solid bars). Selenoprotein mRNA levels are shown as the ratio of target genes to EF1α. The following genes were measured: Dio 1 (A), Dio2 (B), Dio3 (C), Fep15 (D), GPx2 (E), SelJ (F), SelK (G), Sel L (H), SelM (I), Sepp1a (J), SelS (K), and SelW (L). *, **, *** correspond to significant differences between FW and SW at P<0.05, P<0.01 and P<0.001, respectively, using unpaired Student’s t-test, n=6–36.
Gene expression of Fep15 was highest in the brain and kidney. Salinity-dependent differences were only observed in the kidney and liver where gene expression was higher in SW-acclimated fish (Figure 1D). While GPx2 was mostly expressed in the gill, salinity effect was only observed in the kidney, where expression was higher in SW-acclimated fish (Figure 1E). SelJ mRNA expression was highest in the kidney, brain and gill, but a salinity effect was only observed in the brain, where expression was higher in FW-acclimated fish (Figue 1F). SelK was most highly expressed in the gill. A significant effect of acclimation salinity was observed in the muscle, where mRNA levels were higher in SW-acclimated fish (Figure 1G). Although Sel L mRNA levels were greater in the brain there was no salinity effect (Figure 1H).
Gene expression of SelM was highest in the gill, but salinity effects were only observed in the kidney, where mRNA levels were higher in SW-acclimated fish (Figure 1I). Sepp1a was the most highly expressed selenoprotein mRNA in most tissues, with target to housekeeping gene expression ratios of approximately one order of magnitude higher than those of other selenoprotein. Sepp1a expression was higher in the kidney and gill of SW-acclimated fish when compared with those of FW-acclimated fish (Figure 1J). SelS mRNA expression was highest in the pituitary, and salinity effects were observed in the gill, where expression was higher in FW-acclimated tilapia (Figure 1K). SelW expression was highest in the brain, with higher expression in FW-acclimated fish when compared with SW fish (Figure 1L).
3.4. mRNA expression of selenoprotein synthesis or Se metabolism factors
Genes encoding the factors involved in the selenoprotein synthesis or Se metabolism measured in this study were SPS1, Scly and Secp43 (Figure 2, with numerical values in Supplementary Table 2). Of the three factors, mRNA expression of SPS1 and Scly were the most abundant. SPS1 expression was highest in the gill, followed by the brain. SPS1 levels in the muscle were higher in FW-acclimated fish (Figure 2A). Secp43 mRNA expression was undetected but in the liver, brain, kidney and muscle; no effects of acclimation salinity were observed in any tissue analyzed. Scly mRNA expression was most abundant in the gill, followed by pituitary. Salinity effects were only observed in the kidney, where Scly expression was higher in FW-acclimated fish (Figure 2C).
Figure 2.
Expression of selenoprotein synthesis factors of male Mozambique tilapia acclimated to FW (open bars) or SW (solid bars). Selenoprotein synthesis factor mRNA levels are shown as the ratio of target genes to EF1α. The following genes were measured: SPS1 (A), Secp43 (B) and Scly (C).*, ** correspond to significant difference between FW and SW at P<0.05 and P<0.01, respectively, using unpaired Student’s t-test, n=6.
3.5. Protein levels of Dio3
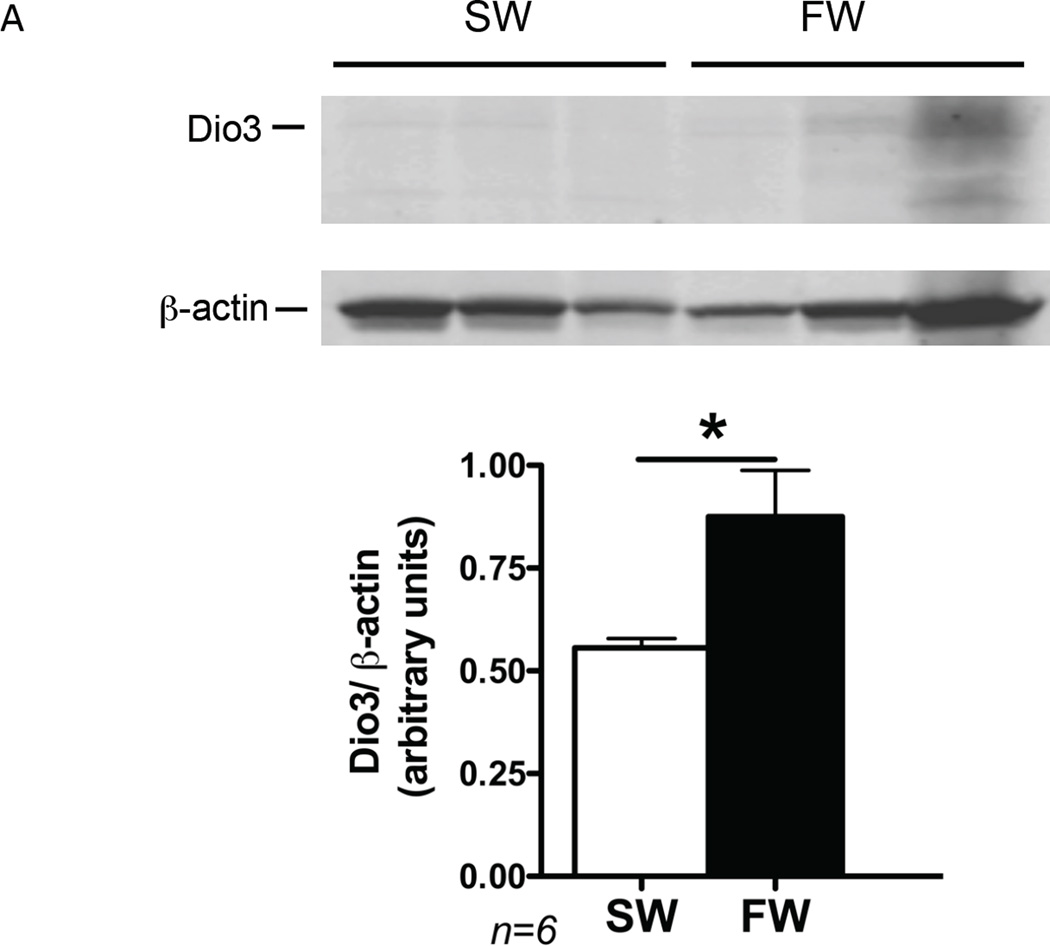
To characterize whether the effect of acclimation salinity on selenoprotein gene expression was also observed on their gene products, we compared the branchial protein abundance of Dio3 between FW- and SW-acclimated fish. Protein levels of Dio3 were significantly (P<0.05) higher in the gills of FW-acclimated tilapia than in those of SW-acclimated tilapia (Figure 3).
Figure 3.
Expression of Dio3 in gill of Mozambique tilapia acclimated to either FW or SW. Dio3 protein intensity levels were normalized by the β-actin levels, and ratios are plotted as average ± SEM (n=6). White bar represents FW-acclimated fish; black bar represents SW-acclimated fish. *P<0.05 by analysis with Student’s t-test.
3.6. Effects of salinity transfer on Dio3 gene expression
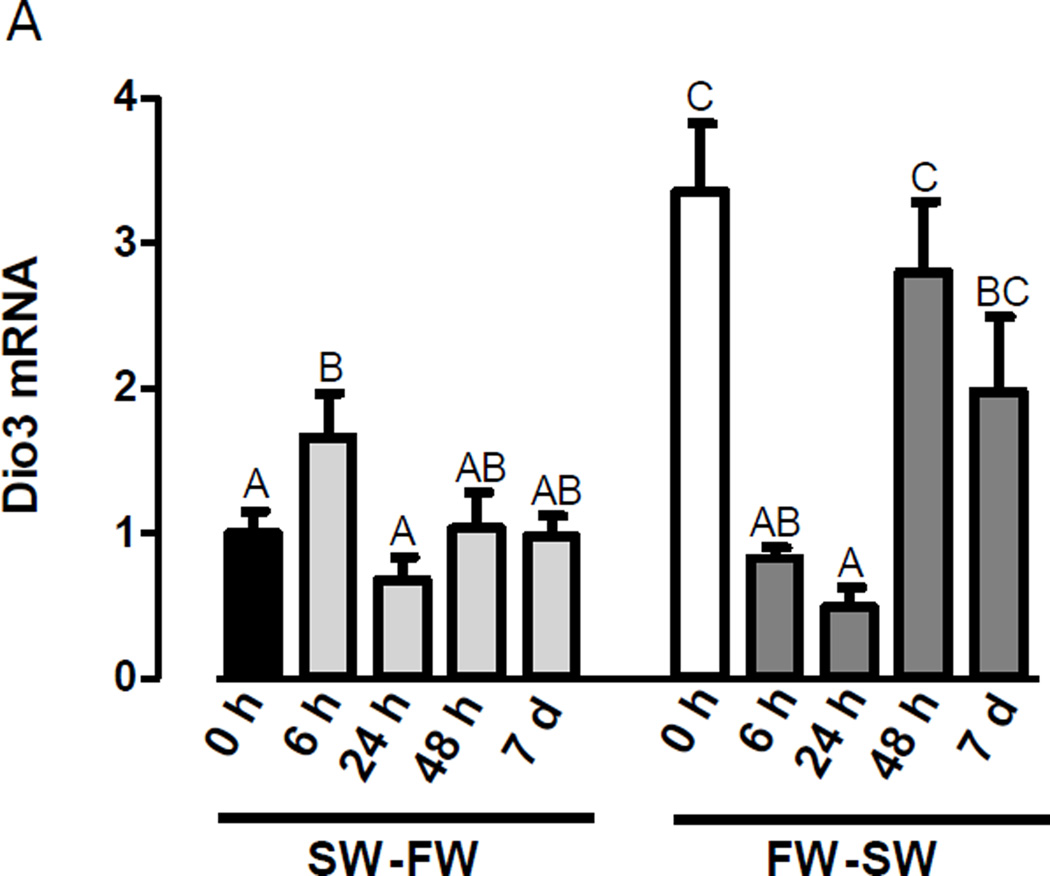
The effects of salinity transfer on the branchial expression of Dio3 was characterized by collecting gill samples from tilapia at 0 h, 6 h, 24 h, 48 h, and 7 d of transfer from SW to FW and vice-versa. Plasma osmolalities (mean ± S.E.M.) were 335.3 mOsm/kg ± 1.87, 315.3 mOsm/kg ± 1.26, 310.8 mOsm/kg ± 8.69, 303.6 mOsm/kg ± 3.73, and 313 mOsm/kg ± 2.79, at 0 h, 6 h, 24 h, 48 h, and 7 d, respectively, following transfer from SW to FW, and 316.6 mOsm/kg ± 1.64, 490.9 mOsm/kg ± 19.05, 434.3 mOsm/kg ± 14.46, 353.3 mOsm/kg ± 5.02, and 337.8 mOsm/kg ± 1.61 at 0 h, 6 h, 24 h, 48 h, and 7 d, respectively, following transfer from FW to SW. Branchial expression of Dio3 increased by 6 h of transfer from SW to FW, but returned to baseline levels throughout the remainder of the transfer period (Figure 4). Consistent with the first experiment, branchial expression of Dio3 was higher in FW- than in SW-acclimated tilapia, when comparing time 0 h of both transfers. Expression of Dio3 declined upon transfer from FW to SW by 6 h and 24 h. By 48 h and 7 d, Dio3 expression was similar to that of the 0 h FW control (Figure 4).
Figure 4.
Changes in Dio3 mRNA expression after transfer of Mozambique tilapia from SW to FW and FW to SW. Bars represent branchial mRNA expression ± SEM (n=6–29). Gene expression is presented relative to the time 0 SW group (black bar). Differences among groups were evaluated by one-way ANOVA, followed by Fisher’s LSD test. Means not sharing the same letter are significantly different (P<0.05).
4. Discussion
Although Se levels vary widely among aquatic habitats, little is known on the effects of environmental salinity on selenoprotein mRNA expression in fishes. Using a public EST library database, we were able to identify the genes for twelve expressed selenoproteins and three factors involved in Se metabolism or selenoprotein synthesis, and characterize their expression pattern in eight tissues of Mozambique tilapia reared in either FW or SW since hatching. We followed up by measuring Dio3 protein content in the gills of fish acclimated to either salinity, and found that the acclimation-salinity effects observed on protein levels follow those observed on branchial gene expression. The effects of salinity transfer on Dio3 gene expression were also measured, revealing both transient and long-term patterns of transcriptional modulation by environmental salinity. To our knowledge, this is the first study to comparatively screen the differential expression between selenoproteins and selenoprotein synthesis factors found in tissues of a euryhaline fish acclimated to either FW or SW, and to characterize the effects of salinity on Dio3 protein levels in the gill.
Previous studies have demonstrated that aquatic organisms have larger selenoproteomes when compared with terrestrial ones. It has been conjectured that a greater diversity of selenoproteins may stem from a life history in aquatic environments [23]. In silico analysis using all the available vertebrate genome sequences revealed that the ancestral vertebrate selenoproteome possibly contained twenty-eight selenoproteins, including all the deiodinases, four glutathione peroxidases and two thioredoxin reductases [34]. Only eighteen selenoproteins were previously found in the EST database for zebrafish [10]. Based on the available EST library database, however, we identified twelve of these genes in tilapia and characterized their mRNA expression in response to environmental salinity.
In mammals, the possible molecular mechanisms underlying the effects of Se in the regulation of selenoprotein expression have been discussed [35–38]. An optimal range of Se intake (40–100 µg per day) appears to be required for the maintenance of physiological homeostasis, where deviations from this narrow range increase the probability of health risks, such as Se toxicity or Se deficiency [39, 40]. In fingerling channel catfish (Ictalurus punctatus), a daily intake between 0.1 and 0.5 ppm of Se was demonstrated to be ideal for maintenance of liver and plasma GPx activity [41], while in rainbow trout, Se diets containing 0.07 ppm to 4 ppm did not incur signs of Se deficiency nor toxicity [8]. Nonetheless, the daily requirement of Se to prevent Se deficiency or toxicity, and whether this requirement changes with acclimation salinity, is unknown in tilapia. In the present study, although Se levels in SW were higher than those in FW, Se plasma levels in FW- and SW-acclimated tilapia were similar, at around 0.02 ppm. In rainbow trout, liver and kidney Se content was found to be proportional to dietary Se intake [8], and selenomethionine treatment resulted in Se accumulation in the liver, kidney, gills, muscle and brain [42]. The similarity in plasma Se levels between tilapia acclimated to both salinities may be driven by the availability of dietary Se in the commercial chow (~1 ppm), which was three orders of magnitude greater than Se levels found in the water. Thus, the effects of acclimation salinity on selenoprotein expression appear to be independent of circulating Se levels. Albeit in the ppb range, the discrepancy between FW and SW Se levels raises the possibility that selenoprotein expression in response to salinity may be driven by the external concentration of Se. It has been suggested that environmental Se uptake by rainbow trout may take place in FW, where levels are as low as 0.4 ppb [8]. Alternatively, the difference in acclimation salinity per se, independent of water Se levels, appears to underlie our observed differences in selenoprotein expression.
Expression patterns and functions of selenoproteins have been most extensively described in mammalian models, with little available information in fish [43]. Our study unveiled the expression patterns of twelve selenoproteins, including those that are only found in fish and aquatic organisms, such as Fep15, SelJ and Sel L. Fep15, SelK, SelM, and SelS are ER-resident proteins, and thought to share a role in mitigation of ER stress [21, 44]. In mammals, SelK is important for Ca2+ flux in macrophages [45], while SelM acts on Ca2+ regulation in neurons [46]. We observed SelM and SelK mRNA expression mostly in the gills. Whether these selenoproteins are associated with Ca2+ metabolism in the gill remain to be investigated. In the case of SelS, glucose levels were previously shown to inversely regulate its expression in human hepatoma cells [47]. In the present study, SelS increased 5-fold in the gill of FW-acclimated fish compared with SW fish. It is worth noting that while in mammals the brain has high metabolic demand, in fish metabolic demand is high in the gills [48]. Inasmuch as SelS is tied to energy metabolism in fish as it is in mammals, higher levels in the FW gill suggests a difference in branchial metabolic demand between FW and SW-acclimated tilapia.
Selenoprotein J has only been reported in fish and sea urchins. In zebrafish, it was found to form the structural crystallin in the eye lens during development, with broader expression throughout the body in adult stages [22]. Although the expression of SelJ in the eye of tilapia was lower than in most tissues, higher expression in the brain of FW-acclimated tilapia suggests other possible roles for this protein. The biological function of Sel L, which is structurally unique amongst selenoproteins and also restricted to aquatic organisms, is unknown [18]. In the present study, Sel L was most highly expressed in the tilapia brain, and was not affected by acclimation salinity. Sepp1 plays a major role in Se transport and delivery to various organs in the body [49], and in mammals plays an essential role in neurological function [50]. In tilapia, Sepp1 did not change with salinity in the brain, but its role in neurological function remains to be investigated. Expression of SelW, on the other hand, was highest in the brain of FW-acclimated tilapia. Although SelW, which is highly expressed in the developing mammalian brain [51] and is sensitive to changes in Se levels, was one of the first selenoproteins to be identified, little is known about its function. Further studies are required to determine the roles of SelJ, Sel L, Sepp1, and SelW in fish physiology. Together, the differential expression of these selenoproteins between FW and SW-acclimated tilapia suggests that their function may be regulated by acclimation salinity.
We also characterized the tissue distribution of three selenoprotein synthesis and Se metabolism factors in fish acclimated to either FW or SW. Although several mechanistic studies of selenoprotein translation were discovered using zebrafish as a model organism [17, 37, 52], tissue distribution of SPS1, Scly and Secp43 had not been previously reported in teleosts. In the present study, expression of SPS1 was upregulated in the gill of SW-acclimated fish, and muscle of FW-acclimated fish, suggesting that these tissues may respond to salinity by regulating Se metabolism. In mammals, Scly, the enzyme responsible for Se recycling, is mainly expressed and active in the liver and kidney, indicating that reutilization of Se may be associated to hydromineral balance [53]. In the current study, Scly was only upregulated in the kidney of FW-acclimated fish, suggesting that plasma Se levels may be differentially regulated by acclimation salinity with participation of renal mechanisms to maintain plasma Se homeostasis. It must be noted that although total plasma Se concentration was the same between FW and SW-acclimated tilapia, levels of blood-borne Se carrier proteins and molecules, including Sepp1, may vary and affect the availability of Se for selenoprotein synthesis of various organs according to acclimation salinity.
The tilapia pituitary plays a central role in orchestrating osmoregulatory responses [54–56]. In the present study, Dio1 expression was higher in the pituitary of FW-acclimated fish relative to SW fish. Local conversion of T4 to T3 is performed by deiodinases, which play a key role in thyroid metabolism by locally regulating thyroid hormone availability and activity in peripheral tissues [57]. While Dio1 and Dio2 convert T4 into the active thyroid hormone T3, Dio3 converts T4 to reverse T3 (rT3), which is physiologically inactive. Kinetic deiodination studies in the blue tilapia (Oreochromis aureus), have shown that Dio1 is active in the fish brain, where it plays a role in outer ring deiodination [58]. Circulating T3 levels increase after rainbow trout are transferred from FW to SW [29]. Hence, high Dio1 mRNA expression in the pituitary of FW tilapia may be a response to low plasma T3 levels and serve in the regulation of the thyroid hormone negative feedback. In Mozambique tilapia, like in other euryhaline teleosts, gills play an essential role in osmoregulation, by actively uptaking ions in FW and extruding ions in SW [59, 60]. Interestingly, increased branchial Na+/K+-ATPase (NKA) activity and mitochondria-rich cells size, hallmarks of branchial salinity adaptation, were observed following triiodothyronine (T3) and thyroxine (T4) injections in Mozambique tilapia [61]. It is possible that osmotically-induced local conversion of T4 to T3 followed by the rapid action of T3 on mobilizing ion pumps such as NKA, are major drivers of cellular morphological and functional changes during exposure to increased salinity. Killifish Dio2 has been shown to possess two functional osmotic response elements in its gene promoter [62], which suggests that Dio2 may participate in the osmotic responses in fish. Dio3 mRNA is expressed mainly in the brain and in the gills of Nile tilapia [63]. Although Nile tilapia are less tolerant of high salinity environments than Mozambique tilapia, evidence of Dio3 activity in the tilapia gill [58] suggests that T3 inactivation could play a role in the local regulation of thyroid hormone action during salinity acclimation. In our studies, expression of all deiodinases was higher in the gill of FW-acclimated tilapia than in that of SW fish. This suggests that, through the actions of deiodinases, the local control of T3 availability may be involved in salinity acclimation.
Based on the high expression of selenoproteins in gill and on the availability of antibodies that cross-reacted with selenoproteins in fish, we examined the branchial protein content of Dio3. Consistent with gene expression patterns, protein abundance of Dio3 was higher in the gills of FW-acclimated fish relative to SW fish. These results suggest that, in addition to transcriptional regulation, acclimation salinity may exert a similar effect on protein synthesis of this selenoprotein. Results of transfer experiments also confirmed steady-state acclimation salinity effects on Dio3 mRNA expression. Interestingly, transfer from SW to FW only transiently increased Dio3 expression by 6 h. On the other hand, transfer from FW to SW elicited a transient decrease in the expression of Dio3. Salinity transfers elicit large excursions in plasma osmolality, which in turn triggers mechanisms to restore salt and water balance; such mechanisms are largely mediated by the neuroendocrine system [54–56]. Within days of salinity transfer, deviations in plasma osmolality typically return to baseline levels [64], which range from 305–330 mOsm/kg in FW and 335–360 mOsm/kg in SW (cf. [56]). In the present experiment, changes in Dio3 gene expression in response to salinity transfers were inversely related to changes in plasma osmolality. Nonetheless, whether these changes in expression are under direct osmotic control or involve the mediation of the endocrine system remain to be investigated.
In conclusion, we found that selenoprotein transcripts in tilapia are ubiquitously expressed, and differentially regulated according to acclimation salinity. This indicates that selenoproteins and their synthesis factors may be involved in osmoregulation and in cellular mechanisms that are sensitive to changes in extracellular osmolality. While these results established a baseline of selenoprotein expression patterns in response to acclimation salinity in vivo, further studies are required to elucidate t he mechanisms (i.e. hormonal modulation and direct osmotic response) underlying the regulation of selenoproteins and synthesis factors in fish.
Supplementary Material
Acknowledgements
This work was funded in part by grants from the National Oceanic and Atmospheric Administration, Project R/SS-12 to A.P.S., which is sponsored by the University of Hawaii Sea Grant College Program, SOEST, under Institutional Grant No. NA14OAR4170071 from NOAA Office of Sea Grant, Department of Commerce; the National Science Foundation (IOS-1119693), the Edwin W. Pauley Foundation (2012), and the Binational Agricultural Research Development (BARD) fund (IS-4296-10) to E.G.G.; and the National Institutes of Health (NIH; G12RR003061-27) to M.J.B. The views expressed herein are those of the authors and do not necessarily reflect the views of the aforementioned granting agencies. University of Hawai‘i Sea Grant publication number UNIHI-SEAGRANT-JC-14-04.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wen H, Carignan J. Ocean to continent transfer of atmospheric Se as revealed by epiphytic lichens. Environ Pollut. 2009;157(10):2790–2797. doi: 10.1016/j.envpol.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Nriagu J. A global assessment of natural sources of atmospheric trace metals. Nature. 1989;338:47–49. [Google Scholar]
- 3.Small-Howard A, et al. Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol. 2006;26(6):2337–2346. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squires JE, Berry MJ. Eukaryotic selenoprotein synthesis: mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life. 2008;60(4):232–235. doi: 10.1002/iub.38. [DOI] [PubMed] [Google Scholar]
- 5.Low SC, Harney JW, Berry MJ. Cloning and functional characterization of human selenophosphate synthetase, an essential component of selenoprotein synthesis. J Biol Chem. 1995;270(37):21659–21664. doi: 10.1074/jbc.270.37.21659. [DOI] [PubMed] [Google Scholar]
- 6.Mihara H, et al. cDNA cloning, purification, and characterization of mouse liver selenocysteine lyase. Candidate for selenium delivery protein in selenoprotein synthesis. J Biol Chem. 2000;275(9):6195–6200. doi: 10.1074/jbc.275.9.6195. [DOI] [PubMed] [Google Scholar]
- 7.Seale LA, Ralston NV, Berry MJ. Biochemistry Research Trends. New York: NOVA Science Publishers, Inc.; 2011. The role of selenium in mitigating mercury toxicity; p. 52. [Google Scholar]
- 8.Hilton JW, Hodson PV, Slinger SJ. The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri) J Nutr. 1980;110(12):2527–2535. doi: 10.1093/jn/110.12.2527. [DOI] [PubMed] [Google Scholar]
- 9.Smith J, Shrift A. Phylogenetic distribution of glutathione peroxidase. Comp Biochem Physiol. 1979;63(1):39–44. doi: 10.1016/0305-0491(79)90231-1. [DOI] [PubMed] [Google Scholar]
- 10.Kryukov GV, Gladyshev VN. Selenium metabolism in zebrafish: multiplicity of selenoprotein genes and expression of a protein containing 17 selenocysteine residues. Genes Cells. 2000;5(12):1049–1060. doi: 10.1046/j.1365-2443.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 11.Bain PA, Schuller KA. A glutathione peroxidase 4 (GPx4) homologue from southern bluefin tuna is a secreted protein: first report of a secreted GPx4 isoform in vertebrates. Comp Biochem Physiol. 2012;161(4):392–397. doi: 10.1016/j.cbpb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Benner MJ, Drew RE, Hardy RW, Robison BD. Zebrafish (Danio rerio) vary by strain and sex in their behavioral and transcriptional responses to selenium supplementation. Comp Biochem Physiol. 2010;157(4):310–318. doi: 10.1016/j.cbpa.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, et al. Characterization of phospholipid hydroperoxide glutathione metabolizing peroxidase (gpx4) isoforms in Coho salmon olfactory and liver tissues and their modulation by cadmium. Aquat Toxicol. 2012;114:115, 134–141. doi: 10.1016/j.aquatox.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacitti D, et al. Characterization of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure. Aquat Toxicol. 2013;130–131C:97–111. doi: 10.1016/j.aquatox.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Orozco A, Valverde RC. Thyroid hormone deiodination in fish. Thyroid. 2005;15(8):799–813. doi: 10.1089/thy.2005.15.799. [DOI] [PubMed] [Google Scholar]
- 16.Valverde C, et al. Cloning and expression of a 5'-iodothyronine deiodinase from the liver of Fundulus heteroclitus. Endocrinology. 1997;138(2):642–648. doi: 10.1210/endo.138.2.4904. [DOI] [PubMed] [Google Scholar]
- 17.Tujebajeva RM, Ransom DG, Harney JW, Berry MJ. Expression and characterization of nonmammalian selenoprotein P in the zebrafish, Danio rerio. Genes Cells. 2000;5(11):897–903. doi: 10.1046/j.1365-2443.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 18.Shchedrina VA, Novoselov SV, Malinouski MY, Gladyshev VN. Identification and characterization of a selenoprotein family containing a diselenide bond in a redox motif. Proc Natl Acad Sci U S A. 2007;104(35):13919–13924. doi: 10.1073/pnas.0703448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horibata Y, Hirabayashi Y. Identification and characterization of human ethanolaminephosphotransferase1. J Lipid Res. 2007;48(3):503–508. doi: 10.1194/jlr.C600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Panee J, Stoytcheva ZR, Liu W, Berry MJ. Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification. J Biol Chem. 2007;282(33):23759–23765. doi: 10.1074/jbc.M702267200. [DOI] [PubMed] [Google Scholar]
- 21.Novoselov SV, Hua D, Lobanov AV, Gladyshev VN. Identification and characterization of Fep15, a new selenocysteine-containing member of the Sep15 protein family. Biochem J. 2006;394(Pt 3):575–579. doi: 10.1042/BJ20051569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellano S, et al. Diversity and functional plasticity of eukaryotic selenoproteins: identification and characterization of the SelJ family. Proc Natl Acad Sci U S A. 2005;102(45):16188–16193. doi: 10.1073/pnas.0505146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobanov AV, et al. Evolutionary dynamics of eukaryotic selenoproteomes: large selenoproteomes may associate with aquatic life and small with terrestrial life. Genome Biol. 2007;8(9):R198. doi: 10.1186/gb-2007-8-9-r198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobanov AV, Hatfield DL, Gladyshev VN. Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol. 2008;9(3):R62. doi: 10.1186/gb-2008-9-3-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trewavas E. Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. Dorchester, UK: The Dorset Press; 1983. [Google Scholar]
- 26.Seale AP, Hirano T, Grau EG. In: Osmoreception: a fish model for a fundamental sensory modality, in Fish Endocrinology. Zaccone G, Reinecke M, Kapoor BK, editors. Oxford & IBH Publishing Company; 2006. pp. 419–440. [Google Scholar]
- 27.Martinez-Alvarez RM, et al. Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol. 2002;205:3699–3706. doi: 10.1242/jeb.205.23.3699. [DOI] [PubMed] [Google Scholar]
- 28.Orozco A, Linser PJ, Valverde MA. Salinity modifies hepatic outer ring deiodinating (ORD) activity in Fundulus heteroclitus. Ann N Y Acad Sc. 1998;839:409–411. [Google Scholar]
- 29.Orozco A, Villalobos P, Valverde RC. Environmental salinity selectively modifies the outer-ring deiodinating activity of liver, kidney and gill in the rainbow trout. Comp Biochem Physiol. 2002;131(2):387–395. doi: 10.1016/s1095-6433(01)00490-1. [DOI] [PubMed] [Google Scholar]
- 30.Chu SL, et al. Profile analysis of expressed sequence tags derived from the ovary of tilapia, Oreochromis mossambicus. Aquaculture. 2006;251:537–548. [Google Scholar]
- 31.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 32.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 33.Breves JP, Hirano T, Grau EG. Ionoregulatory and endocrine responses to disturbed salt and water balance in Mozambique tilapia exposed to confinement and handling stress. Comp Biochem Physiol. 2010;155(3):294–300. doi: 10.1016/j.cbpa.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Mariotti M, et al. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One. 2012;7(3):e33066. doi: 10.1371/journal.pone.0033066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann PR, et al. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007;35(12):3963–3973. doi: 10.1093/nar/gkm355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, et al. Inhibition of selenocysteine tRNA[Ser]Sec aminoacylation provides evidence that aminoacylation is required for regulatory methylation of this tRNA. Biochem Biophys Res Commun. 2011;409(4):814–819. doi: 10.1016/j.bbrc.2011.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoytcheva Z, Tujebajeva RM, Harney JW, Berry MJ. Efficient incorporation of multiple selenocysteines involves an inefficient decoding step serving as a potential translational checkpoint and ribosome bottleneck. Mol Cell Biol. 2006;26(24):9177–9184. doi: 10.1128/MCB.00856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29(5):329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 40.Schweizer U, Dehina N, Schomburg L. Disorders of selenium metabolism and selenoprotein function. Curr Op Pediat. 2011;23(4):429–435. doi: 10.1097/MOP.0b013e32834877da. [DOI] [PubMed] [Google Scholar]
- 41.Gatlin DM, 3rd, Wilson RP. Dietary selenium requirement of fingerling channel catfish. J Nutr. 1984;114(3):627–633. doi: 10.1093/jn/114.3.627. [DOI] [PubMed] [Google Scholar]
- 42.Misra S, et al. Tissue-specific accumulation and speciation of selenium in rainbow trout (Oncorhynchus mykiss) exposed to elevated dietary selenomethionine. Comp Biochem Physiol. 2012;155(4):560–565. doi: 10.1016/j.cbpc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66(15):2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shchedrina VA, et al. Structure-function relations, physiological roles, and evolution of mammalian ER464 resident selenoproteins. Antiox Redox Signal. 2010;12(7):839–849. doi: 10.1089/ars.2009.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meiler S, et al. Selenoprotein K is required for palmitoylation of CD36 in macrophages: implications in foam cell formation and atherogenesis. J Leukoc Biol. 2013;93:771–780. doi: 10.1189/jlb.1212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reeves MA, Bellinger FP, Berry MJ. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antiox Redox Signal. 2010;12(7):809–818. doi: 10.1089/ars.2009.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao Y, et al. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress - SelS is a novel glucose-regulated protein. FEBS letters. 2004;563(1–3):185–190. doi: 10.1016/S0014-5793(04)00296-0. [DOI] [PubMed] [Google Scholar]
- 48.Perry SF, Walsh PJ. Metabolism of isolated fish gill cells: contribution of epithelial chloride cells. J Exp Biol. 1989;144:507–520. doi: 10.1242/jeb.144.1.507. [DOI] [PubMed] [Google Scholar]
- 49.Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790(11):1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill KE, et al. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278(16):13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 51.Loflin J, Lopez N, Whanger PD, Kioussi C. Selenoprotein W during development and oxidative stress. J Inorg Biochem. 2006;100(10):1679–1684. doi: 10.1016/j.jinorgbio.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Xu XM, et al. The zebrafish genome contains two distinct selenocysteine tRNA[Ser]sec genes. FEBS letters. 1999;454(1–2):16–20. doi: 10.1016/s0014-5793(99)00767-x. [DOI] [PubMed] [Google Scholar]
- 53.Esaki N, Nakamura T, Tanaka H, Soda K. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J Biol Chem. 1982;257(8):4386–4391. [PubMed] [Google Scholar]
- 54.Grau EG, Helms LMH. The tilapia prolactin cell: A model for stimulus-secretion coupling. Fish Physiol Biochem. 1989;7:11–19. doi: 10.1007/BF00004685. [DOI] [PubMed] [Google Scholar]
- 55.Seale AP, Watanabe S, Grau EG. Osmoreception: Perspectives on signal transduction and environmental modulation. Gen Comp Endocrinol. 2012;176:354–360. doi: 10.1016/j.ygcen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Seale AP, et al. Endocrine regulation of prolactin cell function and modulation of osmoreception in the Mozambique tilapia. Gen Comp Endocrinol. 2013;192:191–203. doi: 10.1016/j.ygcen.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Marsili A, Zavacki AM, Harney JW, Larsen PR. Physiological role and regulation of iodothyronine deiodinases: a 2011 update. J Endocrinol Invest. 2011;34(5):395–407. doi: 10.3275/7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mol KA, et al. Characterization of iodothyronine outer ring and inner ring deiodinase activities in the blue tilapia, Oreochromis aureus. Endocrinology. 1997;138(5):1787–1793. doi: 10.1210/endo.138.5.5130. [DOI] [PubMed] [Google Scholar]
- 59.Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85(1):97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- 60.Hwang PP, Lee TH. New insights into fish ion regulation and mitochondrion-rich cells. Comp biochem Physiol. 2007;148(3):479–497. doi: 10.1016/j.cbpa.2007.06.416. [DOI] [PubMed] [Google Scholar]
- 61.Subash Peter MC, Lock RA, Wendelaar Bonga SE. Evidence for an osmoregulatory role of thyroid hormones in the freshwater mozambique tilapia Oreochromis mossambicus. Gen Comp Endocrinol. 2000;120(2):157–167. doi: 10.1006/gcen.2000.7542. [DOI] [PubMed] [Google Scholar]
- 62.Garcia GC, et al. 3,5-Diiodothyronine in vivo maintains euthyroidal expression of type 2 iodothyronine deiodinase, growth hormone, and thyroid hormone receptor beta1 in the killifish. Am J Physiol. 2007;293(2):R877–R883. doi: 10.1152/ajpregu.00101.2007. [DOI] [PubMed] [Google Scholar]
- 63.Sanders JP, et al. Cloning and characterization of type III iodothyronine deiodinase from the fish Oreochromis niloticus. Endocrinology. 1999;140(8):3666–3673. doi: 10.1210/endo.140.8.6902. [DOI] [PubMed] [Google Scholar]
- 64.Seale AP, et al. Prolactin 177, prolactin 188 and prolactin receptor 2 in the pituitary of the euryhaline tilapia, Oreochromis mossambicus are differentially osmosensitive. J Endocrinol. 2012;213:89–98. doi: 10.1530/JOE-11-0384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.