Abstract
Hepatitis E virus (HEV) has been reported to cause acute and chronic hepatitis in those with HIV infection and among solid organ transplant recipients in Europe. Limited data indicate that HEV is endemic in the United States, but the prevalence and significance of HEV infection among those with HIV and awaiting solid organ transplantation is unknown. We evaluated anti-HEV IgM and IgG antibodies and HEV RNA in 166 HIV-infected solid organ transplant candidates enrolled in the NIH HIV-Transplant Cohort. Overall prevalence of anti-HEV IgG approached 20% in both liver and renal transplant candidates. Evidence of recent infection was present in approximately 2% of liver transplant candidates and none of the kidney transplant candidates. HEV RNA was not detected in any patient. We conclude that markers of HEV infection are frequent among candidates for transplantation but active, ongoing viremia is not seen. Evidence of recent infection (acute on chronic) liver disease was present in liver but not kidney recipients.
Keywords: HEV, Transplant, Recipient, Renal, Liver
INTRODUCTION
Hepatitis E has been identified as an emerging infection in the U.S., with high exposure rates to human and zoonotic forms of the virus. While clinical infection usually results in mild illness or subclinical features, more severe disease has been described in those with other forms of underlying liver disease. This may result in acute-on-chronic decompensation which has been poorly characterized due to lack of routine testing to exclude all other causes of hepatitis (e.g. HCV) in clinical practice.
Acute and chronic HEV infections have been reported in European solid organ transplant recipients. Overall prevalence varies from 1.8% to 11.3% (using serologic and virologic markers to define exposure). Longitudinal studies of HEV in solid organ transplant recipients report both acute and chronic hepatitis, with histological progression to cirrhosis described in some. Data in U.S. solid organ transplant candidates and recipients are lacking. To further characterize the significance of HEV infection in the setting of liver transplantation, we evaluated HEV antibody status and assayed for viral RNA in HIV-infected patients awaiting liver and kidney organ transplantation who were enrolled from a national distribution of sites in the NIH Solid Organ Transplant Cohort.
METHODS
Patients
The HIV Solid Organ Transplant Study (HIVTR) was initiated in 2003 to evaluate safety and viability of liver and kidney transplantation in people with HIV infection. A total of 317 kidney, and 273 liver transplant candidates (including 13 combined liver/kidney transplant candidates) who became eligible for transplant and study were enrolled in the study. Within this group, pre-transplant samples collected following listing for transplant and enrollment in the study were available at the time of testing for 166 HIV-infected subjects (53 kidney, and 113 liver (including 10 combined liver/kidney)). All patients provided informed consent at their enrollment sites, and indicated whether they gave permission for serum/plasma banking, testing and analysis. De-identified samples were provided to the laboratory testing site at the University of Cincinnati.
HEV EIA Testing
Serum samples from a subset (30%) of transplant wait-listed patients were tested for HEV IgG and IgM antibodies using ELISA-based and validated assays (Wantai, China and Adaltis, Italy respectively). A signal/cutoff (S/C) ratio >1.2 was considered positive, and a S/C value 1 to 1.2 was considered borderline positive.
HEV RNA Testing
TaqMan technology qPCR of HEV was performed using our adaptation of the method of Jothikumar et al., which is able to detect all HEV genotypes.1 Primers amplify a 70bp product located in the highly conserved ORF3 region along with a TaqMan probe (IDT, Inc., Coralville, IA) to provide higher specificity than non-probe-based assays. A second TaqMan method using a modification of Gyarmati et al. which amplifies a 113 bp region of ORF2 that is highly conserved amongst the four HEV genotypes2, was also used. These methods detect 1–20 genome equivalents of HEV plasmid DNA. Samples are run in triplicate with appropriate controls included in each run.
RESULTS
Cohort Characteristics
166 HIV+ subjects were evaluated for HEV antibodies. Within this group, 113 were eligible for liver transplantation (including 10 combined liver-kidney candidates) and 53 for kidney transplantation. These patients are further characterized in Table 1.
Table 1.
Characteristics of HIV-infected Kidney and Liver Transplant Candidates Evaluated for HEV Antibodies
| Kidney (N=53) | Liver (N=113) | |
|---|---|---|
| Age – yr (median [IQRa]) | 46 [41, 51] | 49 [44, 54] |
| Male Gender – no. (%) | 38 (72) | 95 (84) |
| Caucasian Race – no. (%) | 12 (23) | 80 (71) |
| African-American Race – no. (%) | 40 (75) | 25 (22) |
| HCV Coinfection – no. (%) | 12 (23) | 74 (65) |
| ALTb (median [IQR]) | 21 [12–31] | 52 [33–81] |
| ASTb (median [IQR]) | 21 [17–32] | 85 [55–139] |
| MELDb (median [IQR]) | N/A | 16 [12–27] |
| CD4+ T-Cell (cells/mm3) b – median [IQR] | 491 [320–609] | 286 [197–435] |
| Detectable HIV RNA b – no. (%) | 0 (0) | 16 (14) |
Interquartile range
At study enrollment
Prevalence of HEV IgG
Positive anti-HEV IgG was present in 19.5% and 18.9% of liver and kidney transplant candidates, respectively. The median [IQR] IgG S/C was 0.040 [0.020–0.100] in kidney and 0.089 [0.032–0.450] in liver cases (Kruskal-Wallis; p=0.01). Median ALT was 21 for both IgG positive and negative kidney candidates (Kruskal-Wallis; p=0.99). In liver, median ALT was 54 in IgG positive and 51 in IgG negative (p=0.34). IgG prevalence did not differ by geographic region (Northeast, Midwest, South, and West) (kidney: p=0.64; liver: p=0.65). Kidney transplant candidates with a positive IgG were significantly older than those without an IgG response (median age 51 vs. 45 years; p=0.049), but this was not seen among liver transplant candidates. Among kidney cases, the proportion of subjects with baseline anti-HEV IgG response was significantly higher in HCV-infected group (5/12 (42%)) compared to HCV non-infected group (5/41 (12%)) (Fisher’s exact; p=0.04). There were no other significant associations between anti-HEV IgG response and demographic factors, liver function tests, MELD score, detectable HIV RNA and CD4+ T-cell count pre-transplant.
Prevalence of HEV-IgM
Anti-HEV IgM prevalence was 0.9% (1.8% including borderline positive) among liver and 0.0% among kidney cases. The median [IQR] IgM S/C was 0.154 [0.136–0.171] in kidney and 0.199 [0.123–0.347] in liver cases (Kruskal-Wallis; p=0.05). The primary diagnosis for the subject with positive IgM was reported as “HCV/HCC”. The primary diagnosis for the subject with borderline positive IgM was HCV cirrhosis.
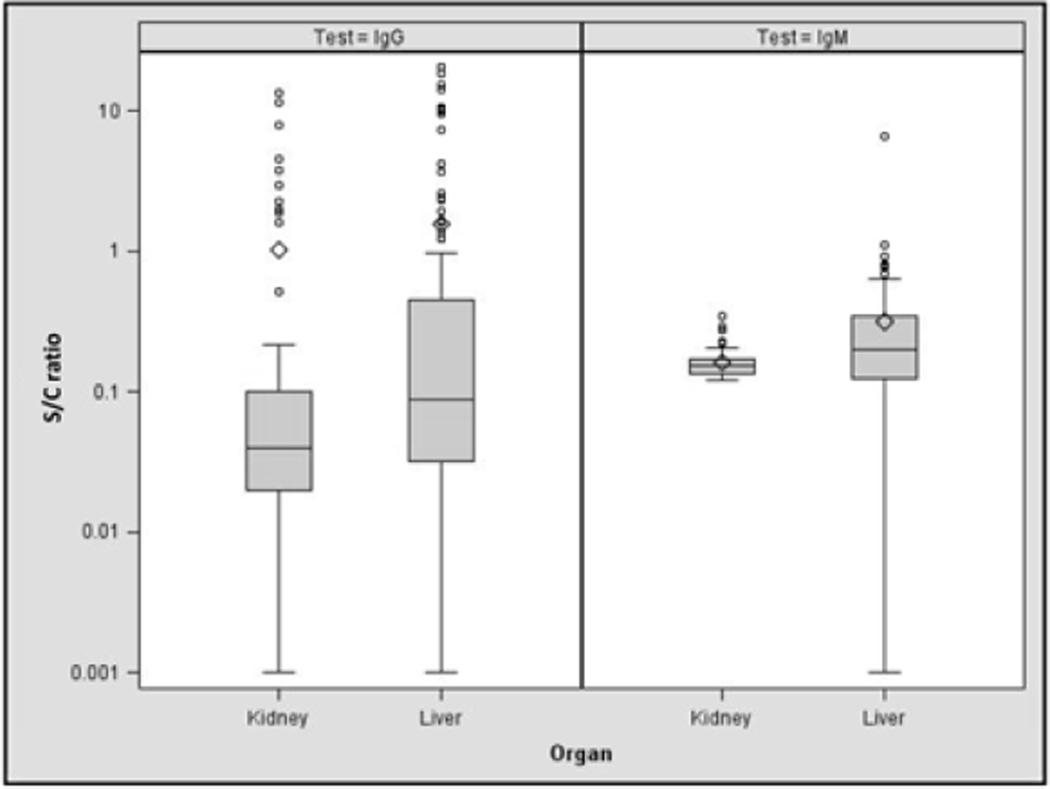
Figure 1 shows the distribution of HEV IgG and IgM levels, as defined by the signal:cutoff ratio. Overall levels of IgG and IgM were higher among liver transplant candidates than kidney transplant candidates, suggesting more recent infection. HEV RNA was not detected in any sample, including those with IgM positivity or borderline positive IgM values.
Figure 1.
Signal-cutoff ratios for HIV-infected liver and kidney transplant candidates. In the box and whisker plots, the line inside the box indicates the median value whereas the bottom and top edges of the box indicate the intraquartile range. Outlier values are shown in circles; means are indicated by diamonds.
DISCUSSION
Hepatitis E infection, originally thought to be a disease of developing countries with poor sanitation is being reported with increasing frequency in Western countries. In Europe, rates of infection, as determined by IgG antibody positivity in HIV-infected patients range from 2.6% to 9%.3,4 HEV chronicity has also been described in both HIV-infected and post-transplant patients, where it may cause ALT abnormalities, fibrotic progress and development of cirrhosis.5,6 Beyond case reports, there are limited data available regarding HEV prevalence and incidence among transplant candidates or recipients in the U.S. Drobeniuc et al. evaluated HEV prevalence among 154 persons with acute hepatitis whose clinical specimens were referred to the CDC for HEV testing. Within this group, 15 cases represented solid organ transplant recipients in non-travelers to endemic areas, and 7/15 (47%) were anti-HEV IgM positive.7 There are also limited data among those with HIV infection where both chronicity and progressive liver disease have also been reported. A large repository of HIV-infected patients among U.S. military beneficiaries was evaluated to determine the relationship of HEV to acute serum transaminases abnormalities. 194 patients were sampled yielding a 6.7% prevalence of anti-HEV IgG and/or IgM.8
The study reported herein reports HEV prevalence among a unique cohort of patients with HIV infection, namely those with end-stage liver or kidney disease who are listed for organ transplantation. Anti-HEV prevalence rates in both groups approached 20%, which is similar to that reported (5–21%) in the retrospective NHANES III analysis of HEV antibody prevalence9. However, unlike NHANES III data which suggested that the mid-west U.S. represents a hotspot for HEV exposure, there was no evidence of a geographic prediction in our national distribution of transplant candidates.
Higher levels of anti-HEV IgM were observed among liver candidates; however it remains unclear whether recent acute-on-chronic infection with HEV could contribute to worsened hepatic decompensation. In the two probable or definite HEV IgM positive cases, one had HCV and the other HCV/HCC as their underlying primary liver disease. There are insufficient data to confirm this hypothesis however. Liver transplant listing is highly dependent upon the Model of End Stage Liver Disease (MELD) score, with higher scores boosting rapidity of listing. In contrast, there were no patients with chronic end-stage kidney disease who had evidence of recent acute HEV infection. This is not surprising and indirectly supports our hypothesis, since the methodology of organ allocation for kidneys is not tied to parameters that might be affected by HEV infection.
Despite the relatively low CD4 count, HEV viremia was not detected in any of the tested samples. In the setting of HIV, HEV chronicity has been reported to occur in up to 1% of patients. It is not known if this is directly influenced by CD4 count, though it is clearly affected by exposure to either ribavirin or exogenously administered interferons that lead to viral clearance in a high proportion of patients. In the setting of recent HEV infection, viremic clearance occurs rapidly. Therefore, it is not surprising that we did not detect HEV RNA in patients with positive or borderline anti-HEV IgM.
In conclusion, nearly 20% of HIV-infected patients with end-stage liver and/or kidney disease have evidence of prior HEV infection. Evidence suggesting recent infection are present in approximately 2% of liver transplant candidates and none of the renal transplant candidates, raising the question whether incident HEV infection in those with HIV and chronic liver disease could be associated with hepatic decompensation. Further evaluation of longitudinal samples from this and other transplant cohorts in the United States is indicated.
Acknowledgement
On behalf of the investigators of Solid Organ Transplantation in HIV: Multi-Site Study www.hivtransplant.com; Funding: National Institute of Allergy and Infectious Diseases (AI052748) ClinicalTrials.gov NCT00074386; sponsored by the University of California, San Francisco.
BIBLIOGRAPHY
- 1.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Gyarmati P, Mohammed N, Norder H, Blomberg J, Belák S, Widén F. Universal detection of hepatitis E virus by two real-time PCR assays: TaqMan and Primer-Probe Energy Transfer. Journal of Virological Methods. 2007;146:226–235. doi: 10.1016/j.jviromet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Kenfak-Foguena A, Schoni-Affolter F, Burgisser P, et al. Hepatitis E Virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17:1074–1078. doi: 10.3201/eid1706.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renou C, Lafeuillade A, Cadranel JF, et al. Hepatitis E virus in HIV-infected patients. AIDS. 2010;24:1493–1499. doi: 10.1097/QAD.0b013e32833a29ab. [DOI] [PubMed] [Google Scholar]
- 5.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 6.Schlosser B, Stein A, Neuhaus R, et al. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56:500–502. doi: 10.1016/j.jhep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Drobeniuc J, Greene-Montfort T, Le NT, et al. Laboratory-based surveillance for hepatitis E virus infection, United States, 2005–2012. Emerg Infect Dis. 2013;19:218–222. doi: 10.3201/eid1902.120961. quiz 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum-Cianflone NF, Curry J, Drobeniuc J, et al. Hepatitis E virus infection in HIV-infected persons. Emerg Infect Dis. 2012;18:502–506. doi: 10.3201/eid1803.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]