Abstract
Background
Most research on dietary patterns and health outcomes does not include longitudinal exposure data. We used an innovative technique to capture dietary pattern trajectories and their association with hemoglobin A1c (HbA1c), homeostasis model of insulin resistance (HOMA-IR), and prevalence of newly diagnosed diabetes.
Methods
We included 4,096 adults with three to six waves of diet data (1991–2006) and biomarkers measured in 2009 from the China Health and Nutrition Survey. Diet was assessed with three 24-hour recalls and a household food inventory. We used a dietary pattern previously identified with reduced rank regression that positively predicted diabetes in 2006 (high in wheat products and soy milk and low in rice, legumes, poultry, eggs, and fish). We estimated a score for this dietary pattern for each subject at each wave. Using latent class trajectory analysis, we grouped subjects with similar dietary pattern score trajectories over time into five classes.
Results
Three trajectory classes were stable over time, and in two classes the diet became unhealthier over time (upward trend in dietary pattern score). Among two classes with similar scores in 2006, the one with the lower (healthier) initial score had an HbA1c 1.64% lower (−1.64 [95% confidence interval= −3.17, −0.11]) and nonsignificantly a HOMA-IR 6.47% lower (−6.47 [−17.37, 4.42]) and lower odds of diabetes (0.86 [0.44, 1.67]).
Conclusions
Our findings suggest that dietary pattern trajectories with healthier scores longitudinally had a lower HbA1c compared to those with unhealthier scores, even when the trajectories had similar scores in the end point.
Keywords: dietary pattern, diabetes, latent class trajectory analysis, China
INTRODUCTION
China has experienced a rapid increase in diabetes prevalence among adults, from 3%1 in 1994 to 11.6% in 2010 (about 113.9 million diabetics).2 Addressing lifestyle behaviors such as diet is important for prevention efforts, and dietary patterns may be particularly useful, as estimated effects of single nutrients or foods may be too small to detect and diet patterns may closely reflect actual eating behavior.3–8 In China food patterning indicates that rice and wheat are key distinct characteristics that belong to different dietary patterns, and patterns containing wheat are characterized as more heterogeneous and Westernized (i.e., fruits, nuts, soy milk, instant noodles, cakes, fried wheat products).9–11
Most of the studies associating dietary patterns with health outcomes include diet from only one point in time3 and are not able to capture longitudinal changes in dietary patterns. To address this gap and represent long-term exposure to a dietary pattern, we used latent class trajectory analysis (LCTA) to identify subgroups of subjects sharing similar trajectories over 15 years.12, 13
We used a dietary pattern previously identified to be associated with diabetes in this population (Batis et al., unpublished data, 2013). We derived this pattern with reduced rank regression (RRR) using the 2006 diet and hemoglobin A1c (HbA1c), homeostasis model of insulin resistance (HOMA-IR) and glucose in 2009 as response variables. This pattern was behaviorally meaningful, as it shared many characteristics with patterns from principal component analysis. It was positively associated with wheat noodles, wheat buns and breads, deep-fried wheat products, and soy milk and negatively associated with rice, fresh legumes, poultry and game, eggs, and fish and seafood. Because we knew this pattern was associated with diabetes, our research question was no longer about that association itself but about the association of its long-term trajectories with diabetes. Individuals may have similar dietary pattern scores at one point in time but varying diet histories. It is important to understand the health outcomes of different trajectories.
We estimated a score for the dietary pattern in six repeated diet measures over 15 years of follow-up (1991–2006) and identified groups of individuals with similar score trajectories over time with LCTA. We used these latent classes to examine the association between long-term dietary patterns and HbA1c, HOMA-IR, and the prevalence of newly diagnosed diabetes in 2009.
METHODS
Study design and participants
The China Health and Nutrition Survey (CHNS) is an ongoing longitudinal study that has conducted surveys in nine provinces in 1991, 1993, 1997, 2000, 2004, 2006, and 2009.14 These surveys were conducted according to the guidelines in the Declaration of Helsinki. All waves have identical clinical, dietary, and anthropometric measures. However, blood samples were collected for the first time in 2009. Each wave maintains a desired range of economic and demographic circumstances, even if new participants are recruited to compensate loses to follow-up. Therefore our goal was to make inferences about all the participants in wave 2009. To be included in our analytic sample, respondents had to be 18 to 65 years old during at least three waves between 1991 and 2006. (For example, if a subject was 15 years old in 1997, he or she only entered the analysis in 2000. If a subject was 65 years old in 2006, he or she was not included in the analysis, because he or she qualified during only one wave, not three). There were 7,646 eligible participants aged 27–68 in 2009, not previously diagnosed with diabetes, and not pregnant in 2009. We did not consider subjects with previously diagnosed diabetes, because the diagnosis could affect their dietary habits. We excluded those who did not have dietary data in at least three waves (1991–2006) (n = 2,712), who did not have complete biomarkers in 2009 (n = 604) or did not fast before blood collection (n = 185), or who had missing information for covariates (n = 49). Among the 4,096 subjects in our final sample, 40% had complete dietary data in all six waves, and 17% had complete dietary data in only three waves. We addressed selection bias by using inverse probability weights in a sensitivity analysis (see the Online Supplemental Material).
Measurement of variables
The CHNS assessed diet with three consecutive 24-hour recalls randomly allocated to start between Monday and Sunday. These were supported and complemented by data from a household food inventory conducted during the same three-day period. Our food groups were based on a system developed specifically for the CHNS15 that separates foods into nutritional and behavioral food groups.
The CNHS collected blood samples by venipuncture after an overnight fast. Glucose, HbA1c, and insulin were measured with standard procedures. We estimated the homeostasis model of insulin resistance (HOMA-IR = [(fasting insulin (µU/ml) * fasting glucose (mmol/l))/22.5].16 We used HbA1c ≥ 6.5% as the diagnostic criteria for type 2 diabetes.17 Compared to a single measure of glucose, HbA1c captures long-term glycemic exposure, and it has been shown to be reliable for diabetes diagnosis among Chinese.18–20
We included several covariates in the analysis. Some, such as gender, geographic region, and age in 2006 (equivalent to using birth year), did not vary by time. For the time-varying covariates, we used a measure that represented the entire exposure period (1991–2006), that is, the mean of all the repeated measures (income, physical activity, urbanicity index,21 and BMI), the proportion of the participating waves in which they were reported (currently smoking and consuming three or more alcoholic beverages per week), and the highest level attained during the follow-up (education).
Statistical analysis
We used the loadings of all food groups on the pattern previously identified with RRR (Batis et al., unpublished data, 2013) to calculate the pattern score of each subject in this sample in all waves (1991–2006). This dietary pattern score represents how much each subject adhered to the dietary pattern: the higher the score, the more closely the participant’s diet conforms to the dietary pattern. (See the Online Supplemental Material and supplemental table 1 for more details on our methods, including the RRR dietary pattern).
To group individuals with similar dietary pattern score trajectories, we used LCTA (Mplus 6.1, Muthén and Muthén, Los Angeles, California). Contrary to conventional growth model approaches, in which the trajectories of all individuals are described using a single estimate of growth parameters and the heterogeneity is captured only by variations in the slopes and the intercepts (random effects), LCTA allows different parameters to represent the trajectories of various classes.12, 13 For the model fitting, the analyst specifies the functional form of the growth trajectories and the number of classes.12 We found that the observed mean trajectories were fairly linear. We compared one to seven classes (supplemental table 2) and decided to retain five classes. The fit of the model was better between five and six classes, but the six-class model included a class with only 1.5% of the sample. In addition, models with two to four classes yielded parallel trajectories, which are less interesting to compare than trajectories that intersect each other, reflecting that the classes share a similar diet at that point.
We ran multiple linear (for HbA1c and HOMA-IR) and logistic (for diabetes) regressions with class trajectory membership as an indicator variable. First we adjusted by all covariates except BMI, and in a second model we additionally adjusted by BMI. The clustering at the household level was accounted for in the estimation of the variance. As a sensitivity analysis, we repeated the analysis including only subjects with four or more waves of dietary data (instead of three) (see the Online Supplemental Material).
RESULTS
We confirmed that in 2006, in the analytic sample of this study, the dietary pattern previously identified had a positive association with HbA1c, HOMA-IR, and the prevalence of newly diagnosed diabetes (supplemental table 3). We also confirmed that most of the pattern’s key food groups had independent associations with HbA1c consistent with the directions of their factor loadings (supplemental table 4). Only soy milk, which is frequently consumed with deep-fried wheat products, was inconsistent. It had a positive loading in the dietary pattern, but its independent association with HbA1c tended to be negative. In sum, the score for this dietary pattern could be interpreted as a weighted sum of healthy (negative weight) and unhealthy (positive weight) food groups. Subjects with total negative scores consumed more of the healthy foods, those with positive scores consumed more of the unhealthy foods, and subjects with scores near zero had similar intakes of both unhealthy and healthy foods.
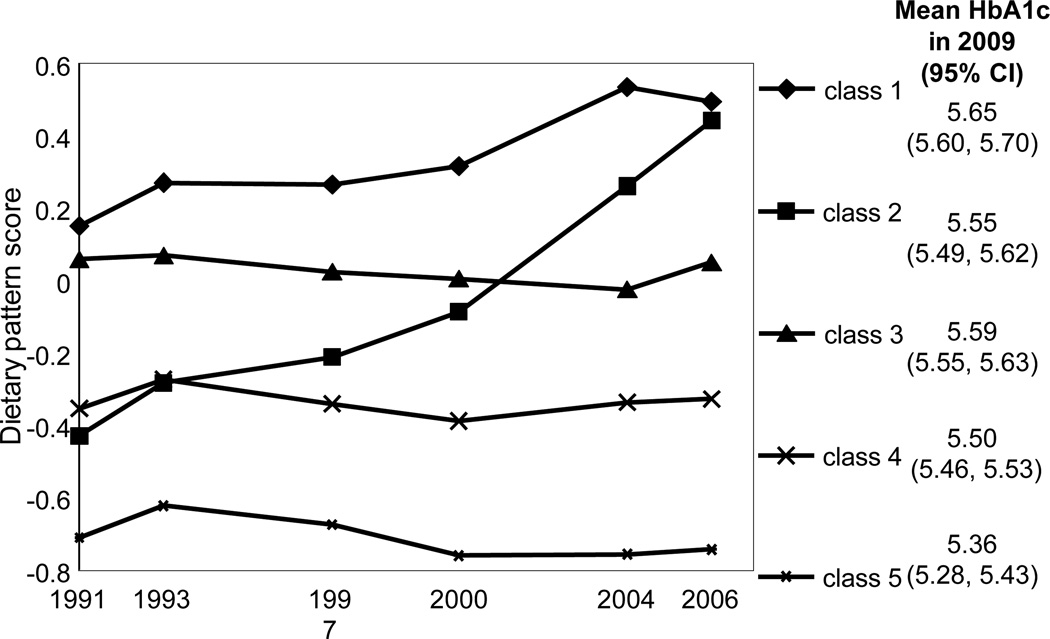
Using LCTA, we identified five classes of subjects with similar dietary pattern score trajectories from 1991 to 2006 (figure 1). To show each class’ changes in food group intake, table 1 presents the proportion of consumers and the mean number of food groups consumed over time. In all trajectory classes the diversity of diet increased, as reflected by the mean number of food groups. Class 1 and 2 changed over time, whereas the other classes were more stable. Classes 1 and 2 showed an increase in unhealthy foods, such as wheat noodles, wheat buns and breads, and deep-fried wheat products. Class 2 also showed a decline in intake of healthy foods, such as fresh legumes, poultry and game, and fish and seafood, which explains why this class had the most dramatic score increase. Classes 3, 4, and 5 had relatively constant scores over time. Their intake of rice decreased, but for all other relevant food groups the proportion of consumers increased. Because the increases in both healthy and unhealthy foods were similar, the balance between the positive and the negative weights and hence the dietary pattern scores for these groups remained stable over time.
Figure 1.
Dietary pattern score trajectory classes from 1991 to 2006. The lines are the mean observed trajectory of each class; the mean HbA1c in 2009, adjusted by age, gender, geographic region, income, urbanicity index, physical activity, smoking, and alcohol intake, is shown next to each class.
Table 1.
Number of food groups and proportion of consumers of food groups associated with dietary pattern (factor loadings > |0.20|) by wave and trajectory class
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1991 | 2000 | 2006 | 1991 | 2000 | 2006 | 1991 | 2000 | 2006 | 1991 | 2000 | 2006 | 1991 | 2000 | 2006 | |
| Number of food groups | 5.9 | 7.7 | 8.7 | 7.2 | 8.1 | 9.4 | 6.8 | 7.9 | 8.8 | 7.4 | 8.3 | 9.1 | 8.3 | 9.7 | 10.5 |
| Proportion of consumers | |||||||||||||||
| Food groups with positive factor loadings (≥ 0.20) | |||||||||||||||
| Wheat noodles | 0.12 | 0.43 | 0.63 | 0.15 | 0.47 | 0.71 | 0.39 | 0.53 | 0.57 | 0.21 | 0.37 | 0.48 | 0.06 | 0.23 | 0.30 |
| Wheat buns and breads | 0.03 | 0.39 | 0.69 | 0.00 | 0.21 | 0.68 | 0.03 | 0.14 | 0.35 | 0.01 | 0.06 | 0.17 | 0.00 | 0.01 | 0.05 |
| Deep-fried wheat products | 0.06 | 0.19 | 0.27 | 0.05 | 0.11 | 0.30 | 0.06 | 0.06 | 0.11 | 0.04 | 0.03 | 0.08 | 0.04 | 0.03 | 0.11 |
| Soy milk | 0.01 | 0.08 | 0.15 | 0.00 | 0.04 | 0.21 | 0.01 | 0.03 | 0.08 | 0.00 | 0.02 | 0.06 | 0.00 | 0.01 | 0.06 |
| Food groups with negative factor loadings (≤ −0.20) | |||||||||||||||
| Rice* | 0.08 | 0.13 | 0.10 | 0.78 | 0.60 | 0.40 | 0.64 | 0.56 | 0.52 | 0.92 | 0.77 | 0.67 | 0.95 | 0.78 | 0.61 |
| Fresh legumes | 0.23 | 0.36 | 0.45 | 0.65 | 0.47 | 0.45 | 0.27 | 0.50 | 0.60 | 0.38 | 0.49 | 0.57 | 0.60 | 0.71 | 0.68 |
| Poultry and game | 0.02 | 0.05 | 0.03 | 0.12 | 0.13 | 0.04 | 0.01 | 0.07 | 0.07 | 0.13 | 0.22 | 0.25 | 0.35 | 0.49 | 0.50 |
| Eggs and egg products | 0.19 | 0.46 | 0.56 | 0.45 | 0.51 | 0.56 | 0.17 | 0.41 | 0.54 | 0.36 | 0.53 | 0.60 | 0.64 | 0.66 | 0.78 |
| Fish and seafood | 0.08 | 0.11 | 0.10 | 0.36 | 0.26 | 0.15 | 0.12 | 0.17 | 0.22 | 0.44 | 0.50 | 0.53 | 0.66 | 0.79 | 0.86 |
Proportion below and above the median.
Class 5 had the lowest (healthiest) score and class 1 the highest (unhealthiest). Comparing the classes with lower (healthier) and higher (unhealthier) scores, those with lower scores tended to have higher education, income, and urbanicity index and lower physical activity and BMI (table 2). There was a strong regional difference among the classes: 80% of class 1 lived in the central region, and only 1.5% of class 5 lived in the North.
Table 2.
Demographic characteristics by dietary pattern trajectory class
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | P valuea | |
|---|---|---|---|---|---|---|
| N (%) | 745 (18.2) | 245 (6.0) | 994 (24.3) | 1,911 (46.7) | 201 (4.9) | |
| Mean dietary pattern score ± SD | 0.36 ± 0.14 | 0.00 ± 0.12 | 0.03 ± 0.09 | −0.34 ± 0.14 | −0.73 ± 0.11 | 0.000 |
| Age in 2006 (years), mean ± SD | 48.9 ± 9.5 | 47.9 ± 8.9 | 48.8 ± 9.5 | 47.9 ± 9.4 | 50.2 ± 9.7 | 0.002 |
| Region, % | 0.000 | |||||
| South | 5.2 | 42.5 | 43.4 | 64.6 | 41.3 | |
| Central | 80.0 | 26.9 | 26.7 | 18.0 | 57.2 | |
| North | 14.8 | 30.6 | 30.0 | 17.4 | 1.5 | |
| Male, % | 45.8 | 46.9 | 46.1 | 47.9 | 49.3 | 0.775 |
| BMI,b mean ± SD | 23.4 ± 2.8 | 23.1 ± 3.0 | 22.8 ± 2.8 | 22.5 ± 2.8 | 22.3 ± 2.4 | 0.000 |
| Overweight (BMI ≥ 25 kg/m2),b % | 25.6 | 19.2 | 21.1 | 16.9 | 13.4 | 0.000 |
| Highest level of education attained,c % | 0.000 | |||||
| None | 21.1 | 13.9 | 17.7 | 12.2 | 11.9 | |
| Primary school | 28.2 | 20.0 | 29.1 | 25.9 | 25.9 | |
| ≥ Lower middle school | 50.7 | 66.1 | 53.2 | 61.9 | 62.2 | |
| Income,d % | 0.000 | |||||
| Low | 46.4 | 24.1 | 35.8 | 27.5 | 10.5 | |
| Medium | 29.1 | 38.4 | 36.2 | 35.0 | 21.4 | |
| High | 24.4 | 37.6 | 28.0 | 37.6 | 68.2 | |
| Urbanicity,d % | 0.000 | |||||
| Low | 47.8 | 33.5 | 37.3 | 27.5 | 6.5 | |
| Medium | 27.9 | 33.5 | 37.0 | 34.3 | 39.3 | |
| High | 24.3 | 33.1 | 25.7 | 38.3 | 54.2 | |
| Not currently smoking in all participating waves, % | ||||||
| Female | 92.8 | 93.9 | 89.9 | 94.9 | 100.0 | 0.000 |
| Male | 16.1 | 22.6 | 17.7 | 18.8 | 18.2 | 0.596 |
| Alcohol intake < 3 times/week in all participating waves, % | ||||||
| Female | 93.6 | 90.8 | 92.0 | 93.2 | 92.2 | 0.745 |
| Male | 42.5 | 35.7 | 37.3 | 43.3 | 32.3 | 0.056 |
| Physical activity,d % | 0.000 | |||||
| Low | 30.1 | 39.2 | 27.9 | 34.5 | 43.8 | |
| Medium | 39.3 | 33.9 | 33.5 | 33.5 | 31.3 | |
| High | 30.6 | 26.9 | 38.6 | 32.0 | 24.9 |
ANOVA for continuous variables and chi-square for categorical variables.
Mean of participating waves, 1991–2006.
Highest level attained in participating waves until 2006.
Tertiles of the mean of participating waves, 1991–2006.
Figure 1 shows the adjusted mean HbA1c for each class, and table 3 shows the unadjusted and adjusted percentage differences between each pair of classes. As expected, the higher the overall 1991–2006 dietary pattern score, the higher the HbA1c in 2009. Interestingly, class 1 and class 2 had similar scores in 2006, but for class 2, which had a lower (healthier) mean score over time, the model adjusted mean HbA1c was −1.64 (95% confidence interval [CI] = −3.17, −0.11) relative to class 1. Similarly, class 2 and class 4 had similar scores in 1991, but for class 4, which had a lower (healthier) score in 2006, HbA1c tended to be slightly lower (−1.02 [95% CI = −2.35, 0.30]). The smallest HbA1c difference (0.62 [95% CI = −0.72, 1.96]) was seen in classes 2 and 3, whose mean scores were the most similar over time, even though one had a positive slope and the other a flat one. Results for HOMA-IR and the odds of being newly diagnosed diabetic were comparable to those for HbA1c, although estimates were less significant. Adjusting by BMI brought all the estimates closer to the null.
Table 3.
Association between dietary pattern trajectory classes 1991–2006 and HbA1c, HOMA-IR, and prevalence of newly diagnosed diabetes in 2009
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |
|---|---|---|---|---|---|
| HbA1c, % change (95% CI)* | |||||
| Unadjusted | 0 (Ref.) | −5.34 (−6.81, −3.86) | −4.51 (−5.62, −3.41) | −7.41 (−8.47, −6.36) | −6.85 (−8.58, −5.11) |
| Adjusted model 1 (changing reference class) | |||||
| Reference class: 1 | 0 (Ref.) | −1.64 (−3.17, −0.11) | −1.02 (−2.18, 0.14) | −2.66 (−3.86, −1.46) | −5.24 (−6.93, −3.55) |
| Reference class: 2 | — | 0 (Ref.) | 0.62 (−0.72, 1.96) | −1.02 (−2.35, 0.30) | −3.60 (−5.44, −1.76) |
| Reference class: 3 | — | — | 0 (Ref.) | −1.64 (−2.54, −0.74) | −4.22 (−5.80, −2.65) |
| Reference class: 4 | — | — | — | 0 (Ref.) | −2.58 (−4.09, −1.07) |
| Adjusted model 2 | 0 (Ref.) | −1.64 (−3.13, −0.15) | −0.83 (−1.95, 0.30) | −2.37 (−3.53, −1.21) | −4.32 (−6.00, −2.65) |
| HOMA-IR, % change (95% CI)* | |||||
| Unadjusted | 0 (Ref.) | −11.77 (−22.45, −1.10) | −13.85 (−20.94, −6.76) | −16.47 (−22.72, −10.21) | −5.22 (−15.66, 5.23) |
| Adjusted model 1 | 0 (Ref.) | −6.47 (−17.37, 4.42) | −6.07 (−13.73, 1.58) | −9.74 (−17.31, −2.18) | −9.96 (−20.72, 0.79) |
| Adjusted model 2 | 0 (Ref.) | −6.46 (−16.55, 3.62) | −4.45 (−11.64, 2.74) | −7.29 (−14.50, −0.09) | −2.23 (−12.64, 8.17) |
| Diabetes, odds ratio (95% CI) | |||||
| Unadjusted | 1 (Ref.) | 0.51 (0.28, 0.95) | 0.61 (0.42, 0.88) | 0.46 (0.33, 0.65) | 0.36 (0.16, 0.79) |
| Adjusted model 1 | 1 (Ref.) | 0.86 (0.44, 1.67) | 0.99 (0.67, 1.47) | 0.96 (0.65, 1.42) | 0.46 (0.20, 1.06) |
| Adjusted model 2 | 1 (Ref.) | 0.80 (0.40, 1.61) | 1.03 (0.69, 1.54) | 1.01 (0.68, 1.50) | 0.60 (0.26, 1.40) |
Because HbA1c and HOMA-IR were natural log transformed, the regression coefficients were multiplied by 100 and interpreted as the percentage of change in the outcome for being in a given class compared to the reference class.
Adjusted model 1: age in 2006, gender, geographic region, mean income, mean urbanicity index, mean physical activity, highest education attained, proportion of waves with smoking, proportion of waves with alcohol intake ≥ 3 times/week.
Adjusted model 2: model 1 plus mean BMI.
DISCUSSION
Comparing two classes that had similar dietary pattern score trajectories in 2006, we found that the one that previously had lower scores and hence a healthier diet had lower HbA1c in 2009. This suggests that even if the recent diet scores were similar, the different dietary trajectories that subjects followed to get to that point might also affect HbA1c levels. Additionally, comparing two classes that cumulatively had similar mean dietary pattern scores over time, we found no difference in HbA1c, even if one class had a stable score and the other had an increase over time. This suggests that the cumulative dietary pattern score is more important than the shape of the dietary pattern score trajectory (i.e., changing or maintaining a constant dietary pattern score).
Only a few studies have looked at the association between dietary patterns and health outcomes with repeated measures of dietary intake. As there are many approaches to studying repeated measures, it is important to understand which is the most meaningful way to represent the long-term exposure to a dietary pattern. Several studies have used longitudinal mixed models with the goal of obtaining an average dietary pattern–health outcome association adjusted by the interindividual correlation of repeated measures.22, 23 One study assessed the independent effect on the outcome of the dietary pattern measured at each point. The aim was to evaluate the duration of the dietary pattern effect.24 None of these analyses evaluated the interindividual changes or cumulative exposure to dietary patterns. Among other approaches, one study evaluated the sequence of transition between different clusters of dietary patterns (i.e., staying in the same dietary pattern, change to a healthier or unhealthier one, etc.).25 Others have looked at the effect of the change in dietary patterns’ scores between two points in time26, 27 or the effect of the intercept and the slope of three measures of dietary pattern scores.28 The limitation of the last two approaches is that they can only evaluate either the effect of the dietary score at baseline or that of the change (slope) at one time.
Our analysis applied LCTA for dietary exposures. A similar method, a growth mixture model, has been previously used to identify trajectories of sodium adherence over a six-month period in 279 adults with heart failure.29 The advantage of this approach is that a single variable (trajectory class membership) encompasses both the starting point (intercept) and the change (slope) in dietary pattern score, and hence it implicitly summarizes also the score at any given point (i.e., midpoint, last point) and the mean score of the follow-up. Therefore the trajectory class gives a comprehensive representation of the long-term exposure to a dietary pattern score. Hence examining the relation of the trajectories and the predictors and outcomes is straightforward, as there is no need to separately examine the association of the intercept and the slope. However, each of these aspects (slope, mean, intercept or score at any given time) might have a different and independent effect that is not possible to separate, as they are all interrelated. We can only exclude the effect of one of these aspects at a time (i.e., comparing trajectory classes with similar end point or mean scores); however, the comparison options available depend on the identified trajectories. It is of particular interest to understand if the slope has an effect by itself, because otherwise just evaluating the score at each time independently or the mean score would be equally informative. Our results suggest that with a similar mean score, the slope (and the last score) do not have an effect.
In terms of demographic factors, we found that region was an important associated factor, as 80% of class 1 (the class with the highest score over time) lived in the central region. This is probably related to the lower consumption of rice and the higher consumption of wheat buns and breads in this region. It is also interesting that the class with the largest change in dietary pattern score (class 2) had a smaller change in urbanicity level and income. Our numbers for income and urbanicity level represent the mean from 1991 to 2006, yet both variables increased over this period in all classes. In 1991 these variables were similar in classes 2 and 4, however, the increase over time was less pronounced in class 2 (data not shown).
The differences we found in HbA1c between the trajectory classes are meaningful and comparable to what other dietary studies have found. Frequent versus never or seldom intake of fruits and vegetables, moderate alcohol versus abstainers, and lowest versus highest quintile of total fat intake had differences in HbA1c of 0.09, 0.2 and 0.2 respectively.30–32 However, our results were not meaningful for HOMA-IR and diabetes prevalence. Dietary intake might be less associated with HOMA-IR, because it includes glucose information, which is a shorter-term measure. For prevalence of newly diagnosed diabetes, perhaps more power was needed, as only 5.6% of the sample was newly diagnosed as diabetic, and the analysis required information for many strata of the sample.
Our main interest was in the total effect of dietary pattern on diabetes, including the effect of diet mediated through BMI. Still, we additionally adjusted for BMI to get an approximation of the direct effect of diet. We found that adjusting by BMI brought our estimates only slightly closer to the null, which suggests that the trajectories of our dietary pattern scores were directly associated with HbA1c, independently of the increased energy intake and increased BMI. Proposed mechanisms by which diet relates directly to diabetes include modulation of oxidative stress and inflammation, which in turn affects insulin sensitivity and beta cell function.33, 34 Our dietary pattern score represents high intake of wheat noodles, breads, and buns and low intake of fish and legumes. Foods with a high glycemic index, such as wheat noodles, breads, and buns, promote hyperglycemia and therefore are proinflammatory and increase oxidative stress,34 whereas omega-3 fatty acids, like the ones in fish, are anti-inflammatory and could improve the physical properties of cellular membranes and the binding affinity of insulin receptors.35 Legumes in turn are a source of antioxidants like phenolic acids and fiber, which also have anti-inflammatory properties.34, 36 Although our results are biologically plausible, it is important to acknowledge that our quantification of the direct effect was not thorough, as this was not our main interest. Direct effects obtained by adjusting by the intermediate variable are unbiased only if there is no unmeasured confounding between the exposure and the outcome and between the intermediate and the outcome.37 In addition, the way we adjusted by BMI might have been insufficient, as we used a variable with the mean BMI instead of trajectories of BMI.
We found that even when recent dietary patterns were similar, cumulative dietary exposure had a differential and important effect. This was expected for a disease such as diabetes, which is known to progress gradually. The insulin resistance and subsequent loss in beta cell function related to diet directly and/or increased BMI could precede diabetes onset by ten or more years.38 These findings might be similar for other chronic diseases that progress gradually (e.g., cardiovascular disease and many cancers). In this analysis we only assessed diet trajectories during adulthood and did not address critical life points, such as pregnancy, early infancy, or adolescence. Studies with life course epidemiology approaches could be useful for understanding the role of timing, accumulation, and temporal associations of diet and chronic diseases from conception to adulthood.39
Even when a dietary pattern, compared to single nutrients or food groups, can approximate the complexity of dietary intake, it is still a very limited and nonexhaustive representation of diet. For instance, our dietary pattern score did not capture diet diversity, as the score remained constant even in the context of increased diet diversity. Additionally, diet was measured over a three-day period only, which limits its representation of usual intake. However, the dietary assessment methodology has remained unchanged in all the surveys; this is a key strength that enabled us to assess the longitudinal effects of dietary intake. We were able to use an innovative method, LCTA, to evaluate trajectories of dietary patterns over a 15-year follow-up period for the first time.
Another limitation is that not all subjects had complete follow-up. Fortunately, LCTA can use all of the information available without the need to impute values or delete cases with incomplete data. Even individuals with only one wave of data can be classified in one trajectory class, although one point in time does not seem informative enough. To balance both internal and external validity, we included individuals with at least three waves of complete data. Additionally, our sensitivity analysis indicates that the potential for selection bias was minimal (see the Online Supplemental Material). Finally, compared to the thorough and complex longitudinal modeling of our main exposure with LCTA, the modeling of time-varying confounders was simplified, and residual confounding might be present.
In sum, the long-term exposure to a dietary pattern seems to be relevant for HbA1c at a given point in time. More research using longitudinal methods is needed to confirm and add evidence or insights about the nature of these long-term relations. Findings like this can have important intervention and clinical implications. A subject can be counseled to make intensive diet and lifestyle changes given that his or her previous diet has already increased the risk of disease. Or better-informed interventions to promote an early start and maintenance of healthy eating patterns throughout adulthood could be implemented.
Supplementary Material
What is already known on this subject?
Dietary patterns are useful in the study of diet-disease associations, and many dietary patterns have been associated with diabetes. However, we do not know how longitudinal exposure and intra-individual changes in dietary patterns relate to diabetes.
What this study adds?
Our results suggest that the longitudinal exposure and intra-individual changes in dietary pattern matter. Among individuals with similar recent dietary patterns, those who had a healthier prior exposure (trajectories with better dietary pattern scores throughout) had lower HbA1c.
Moreover, it seems that, from the longitudinal trajectory, what matters most is the cumulative exposure and not the shape of the trajectory (i.e., changing or maintaining a constant dietary pattern).
Acknowledgments
We thank Ms. Frances L. Dancy for administrative assistance and Mr. Daniel Blanchette for exceptional programming assistance. We also thank the institutional review committees of the University of North Carolina at Chapel Hill and the Chinese Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention.
Funding
This work was supported by the National Institutes of Health (NIH) (R01-HD30880, DK056350, R24 HD050924 and R01-HD38700, R01-HL108427) and the Fogarty International Center. The NIH gave financial support for the CHNS data collection and analysis files from 1989 to 2011. The Mexican council Consejo Nacional para la Ciencia y Tecnologia funded C.B.
Footnotes
License for publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in JECH editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms/).
Competing interests
None declared.
Contributorship Statement
C.B. designed and conducted the analysis and wrote the manuscript, M.A.M, P.G.L, D.S.A., and B.P. contributed to the interpretation of the data analysis and reviewed the manuscript. C.B. and B.P. had primary responsibility for final content.
References
- 1.Pan X-R, Yang W-Y, Li G-W, Liu J. Prevalence of diabetes and its risk factors in China, 1994. Diabetes care. 1997;20(11):1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013 Sep 4;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Moeller SM, Reedy J, Millen AE, et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. Journal of the American Dietetic Association. 2007 Jul;107(7):1233–1239. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann K, Schulze M, Boeing H, Altenburg H. Dietary patterns: report of an international workshop. Public health nutrition. 2002;5(1):89–90. doi: 10.1079/phn2001252. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002 Feb;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004 Nov 8;164(20):2235–2240. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001 Aug 13–27;161(15):1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 8.Newby PK, Muller D, J H, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. The American journal of clinical nutrition. 2003;77:1417–1425. doi: 10.1093/ajcn/77.6.1417. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, He Y, Lai J, et al. Dietary patterns are associated with stroke in Chinese adults. The Journal of nutrition. 2011;141(10):1834–1839. doi: 10.3945/jn.111.143883. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Dagevos H, He Y, Van der Lans I, Zhai F. Consumption and corpulence in China: a consumer segmentation study based on the food perspective. Food Policy. 2008;33(1):37–47. [Google Scholar]
- 11.Batis C, Sotres-Alvarez D, Gordon-Larsen P, Mendez MA, Adair L, Popkin B. Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. The British journal of nutrition. 2013 Dec 13; doi: 10.1017/S0007114513003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung T, Wickrama KAS. An Introduction to Latent Class Growth Analysis and Growth Mixture Modeling. Social and Personality Psychology Compass. 2008;2(1):302–317. [Google Scholar]
- 13.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 14.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. International journal of epidemiology. 2010 Dec;39(6):1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popkin BM, Lu B, Zhai F. Understanding the nutrition transition: measuring rapid dietary changes in transitional countries. Public Health Nutr. 2002 Dec;5(6A):947–953. doi: 10.1079/PHN2002370. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and Î2-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.International Expert C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care. 2009 Jul;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin Z, Yuan M-X, Li H-X, et al. Evaluation for Fasting and 2-hour Glucose and HbA1c for Diagnosing Diabetes Based on Prevalence of Retinopathy in a Chinese Population. PloS one. 2012;7(7):e40610. doi: 10.1371/journal.pone.0040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Liu Y, Li X, Liang H, Jiang X. Utility of hemoglobin A1c for the identification of individuals with diabetes and prediabetes in a Chinese high risk population. Scandinavian Journal of Clinical & Laboratory Investigation. 2012;72(5):403–409. doi: 10.3109/00365513.2012.689324. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Ouyang X-J, Lou Q-L, et al. Validity of Glycated Hemoglobin in Screening and Diagnosing Type 2 Diabetes Mellitus in Chinese Subjects. The Korean journal of internal medicine. 2012;27(1):41–46. doi: 10.3904/kjim.2012.27.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med. 2011 Oct;71(8):1436–1446. doi: 10.1016/j.socscimed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNaughton SA, Mishra GD, Stephen AM, Wadsworth ME. Dietary patterns throughout adult life are associated with body mass index, waist circumference, blood pressure, and red cell folate. The Journal of nutrition. 2007 Jan;137(1):99–105. doi: 10.1093/jn/137.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Mikkilä V, Räsänen L, Raitakari OT, et al. Major dietary patterns and cardiovascular risk factors from childhood to adulthood. The Cardiovascular Risk in Young Finns Study. British Journal of Nutrition. 2007;98(01):218–225. doi: 10.1017/S0007114507691831. [DOI] [PubMed] [Google Scholar]
- 24.Northstone K, Joinson C, Emmett P, Ness A, Paus T. Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. Journal of epidemiology and community health. 2012;66(7):624–628. doi: 10.1136/jech.2010.111955. [DOI] [PubMed] [Google Scholar]
- 25.Pachucki MA. Food pattern analysis over time: unhealthful eating trajectories predict obesity. International journal of obesity. 2011;36(5):686–694. doi: 10.1038/ijo.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newby PK, Weismayer C, Akesson A, Tucker KL, Wolk A. Longitudinal changes in food patterns predict changes in weight and body mass index and the effects are greatest in obese women. The Journal of nutrition. 2006 Oct;136(10):2580–2587. doi: 10.1093/jn/136.10.2580. [DOI] [PubMed] [Google Scholar]
- 27.Togo P, Osler M, Sorensen TI, Heitmann BL. A longitudinal study of food intake patterns and obesity in adult Danish men and women. Int J Obes Relat Metab Disord. 2004 Apr;28(4):583–593. doi: 10.1038/sj.ijo.0802598. [DOI] [PubMed] [Google Scholar]
- 28.Smithers LG, Golley RK, Mittinty MN, et al. Do Dietary Trajectories between Infancy and Toddlerhood Influence IQ in Childhood and Adolescence? Results from a Prospective Birth Cohort Study. PloS one. 2013;8(3):e58904. doi: 10.1371/journal.pone.0058904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creber RM, Lee CS, Lennie TA, Topaz M, Riegel B. Using Growth Mixture Modeling to Identify Classes of Sodium Adherence in Adults With Heart Failure. The Journal of cardiovascular nursing. 2013 doi: 10.1097/JCN.0b013e3182834191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sargeant L, Khaw K, Bingham S, et al. Fruit and vegetable intake and population glycosylated haemoglobin levels: the EPIC-Norfolk Study. European journal of clinical nutrition. 2001;55(5):342. doi: 10.1038/sj.ejcn.1601162. [DOI] [PubMed] [Google Scholar]
- 31.Meyer KA, Conigrave KM, Chu N-F, et al. Alcohol consumption patterns and HbA1c, C-peptide and insulin concentrations in men. Journal of the American College of Nutrition. 2003;22(3):185–194. doi: 10.1080/07315724.2003.10719292. [DOI] [PubMed] [Google Scholar]
- 32.Harding A-H, Sargeant LA, Welch A, et al. Fat Consumption and HbA1c Levels The EPIC-Norfolk Study. Diabetes care. 2001;24(11):1911–1916. doi: 10.2337/diacare.24.11.1911. [DOI] [PubMed] [Google Scholar]
- 33.Salas-Salvadó J, Martinez-Gonzalez MA, Bulló M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(S2):B32–B48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Avignon A, Hokayem M, Bisbal C, Lambert K. Dietary antioxidants: Do they have a role to play in the ongoing fight against abnormal glucose metabolism? Nutrition. 2012;28(7–8):715–721. doi: 10.1016/j.nut.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Thomas T, Pfeiffer AFH. Foods for the prevention of diabetes: how do they work? Diabetes/metabolism research and reviews. 2012;28(1):25–49. doi: 10.1002/dmrr.1229. [DOI] [PubMed] [Google Scholar]
- 36.Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. The Journal of nutrition. 2008;138(3):439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 37.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002 Feb;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 38.Champe PC, Harvey RA, Ferrier DR. Biochemistry. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 39.Parekh N, Zizza C. Life course epidemiology in nutrition and chronic disease research: a timely discussion. Adv Nutr. 2013 Sep;4(5):551–553. doi: 10.3945/an.113.004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.