Abstract
In multiple sclerosis (MS4) B cell depleting therapy using monoclonal anti-CD20 antibodies, including rituximab (RTX) and ocrelizumab (OCR), effectively reduces disease activity. Based on indirect evidence, it is generally believed that elimination of the antigen presenting capabilities and antigen non-specific immune functions of B cells underlie the therapeutic efficacy. However, a small subset of T lymphocytes (T cells) was shown to also express CD20, but controversy prevails surrounding the true existence of this T cell subpopulation. Using single-cell imaging flow cytometry and expression profiling of sorted lymphocyte subsets, we unequivocally demonstrate the existence of CD3+CD20dim T cells. We show that in MS patients increased levels of CD3+CD20dim T cells are effectively depleted by RTX. The pathological relevance of this T cell subset in MS remains to be determined. However, given their potential pro-inflammatory functionality, depletion of CD20-expressing T cells may also contribute to the therapeutic effect of RTX and other monoclonal antibodies targeting CD20.
Introduction
Since the first phase II clinical trials demonstrated rapid and sustained reduction of inflammatory disease activity following a single course of rituximab (RTX) treatment(1, 2), B cell depletion has emerged as a most promising therapeutic approach in multiple sclerosis (MS). Rituximab is a chimeric monoclonal anti-CD20 antibody of the IgG1 isotype that triggers rapid complement and natural killer (NK) cell-mediated depletion of CD20-expressing B cells (3). B cell depletion using RTX does not affect the CD19+CD20− pro-B cell and CD20−CD138+ plasma cell populations, and within 6 to 8 months following RTX treatment the CD20+ B cell compartment begins to replenish (4), mainly composed of naïve B cells (4). B cells of the CD27+ memory phenotype remain at significantly lower levels in peripheral blood, often times beyond 12 months, possibly accounting for a long-lasting beneficial effect of anti-CD20 therapies on MS disease activity that is sustained following repletion of circulating B cells (5).
Low percentages of CD20-expressing T cells in human blood were first described in 1993 (6), but the existence of this rather rare T cell subset has been disputed (7). Others have found that CD20-expressing T cells can exhibit pro-inflammatory capacity (8, 9). In rheumatoid arthritis (RA), CD20+ T cells make up a larger percentage of Th17 cells when compared to healthy individuals (9). However, the overall percentage of CD20+ T cells among all T cells does not differ between RA patients and healthy individuals, and the pathological relevance, if any, of CD20+ T cells in autoimmune diseases remains entirely unknown. Almost expectedly, during clinical trials in RA, it was noted that CD3+ T cells expressing low levels of CD20 are depleted by RTX (4).
Here, we were interested in unequivocally demonstrating the existence of CD20+CD3+ cells and determining if these cells indeed belong to a T cell lineage. Furthermore, we sought to evaluate whether CD3+CD20+ cells were differentially present in the peripheral blood of MS patients compared to healthy donors, and to determine their level of depletion in response to RTX treatment in MS patients. To address these questions, we performed extensive flow cytometric phenotypic characterization of B and T lymphocytes, and gene expression profiling of CD20− T cells, B cells, and CD20+ T cells, from peripheral blood of healthy control subjects, untreated MS patients, and MS patients at different time points following RTX treatment.
Materials and Methods
Patients and samples
Peripheral blood obtained from patients with a confirmed diagnosis of MS who were untreated or had received standard dose RTX therapy (two infusions 1 g IV each, two weeks apart) at different time points prior to sample acquisition, or from healthy donors; see Table I for sample details. Peripheral blood mononuclear cells (PBMC) were prepared using a Ficoll paque density gradient following standard protocols. These studies were approved by the UCSF Committee on Human Research (CHR).
Table I. Samples and experiments.
Shown are patient IDs, diagnosis (Dx), treatment, age, sex, and experiments performed per sample. B cell subsets: multicolor B cell subset panel; T cell subsets, multicolor T cell subset panel; CD3CD20, additional samples studied using limited flow cytometry panel; ImageStream: samples used for single cell flow cytometry imaging; Microarray, samples used for expression profiling using Affymetrix GeneChip2.0.
| ID | Dx | Therapy | Sex | Age | B cell subsets |
T cell subsets |
CD3 CD20 |
Image Stream |
Micro- array |
|---|---|---|---|---|---|---|---|---|---|
| 13311d | HC | N/A | M | 43 | x | x | x | ||
| 13411e | HC | N/A | M | 40 | x | x | |||
| 27112a | HC | N/A | F | 33 | x | ||||
| 28112a | HC | N/A | F | 40 | x | ||||
| 29312a | HC | N/A | M | 27 | x | ||||
| 29412a | HC | N/A | M | 37 | x | ||||
| 29512a | HC | N/A | M | 22 | x | ||||
| 29812a | HC | N/A | F | 34 | x | x | x | x | |
| 30212a | HC | N/A | M | 21 | x | ||||
| 36613a | HC | N/A | M | 37 | x | x | |||
| 36813a | HC | N/A | F | 26 | x | x | |||
| 36913a | HC | N/A | F | 23 | x | x | |||
| 41113a | HC | N/A | F | 51 | x | x | |||
| 42113a | HC | N/A | F | 64 | x | x | |||
| 47913a | HC | N/A | F | 62 | x | ||||
| 48313a | HC | N/A | M | 38 | x | x | x | ||
| 49413a | HC | N/A | F | 21 | x | x | |||
| 50013a | HC | N/A | M | 45 | x | x | |||
| 50613a | HC | N/A | F | 53 | x | x | |||
|
| |||||||||
| 33112a | RRMS | untreated | F | 41 | x | x | x | x | |
| 46413b | RRMS | untreated | M | 44 | x | x | x | x | |
| 47413b | PPMS | untreated | M | 44 | x | x | x | ||
| 47713a | RRMS | untreated | M | 25 | x | x | x | x | |
| 48413a | CIS | untreated | F | 47 | x | x | x | ||
| 48613a | RRMS | untreated | F | 48 | x | x | x | ||
| 48713a | RRMS | untreated | F | 20 | x | ||||
| 48813b | RRMS | untreated | F | 42 | x | x | x | ||
| 49013a | RRMS | untreated | F | 31 | x | x | |||
| 49913a | RRMS | untreated | M | 45 | x | x | |||
| 50513a | RRMS | untreated | F | 61 | x | x | |||
| 50913a | CIS | untreated | F | 51 | x | x | |||
|
| |||||||||
| 35012a | PPMS | RTX 0-12 w | M | 53 | x | x | |||
| 39713a | PPMS | RTX 0-12 w | M | 63 | x | x | |||
| 40813a | SPMS | RTX 0-12 w | M | 53 | x | x | |||
| 45713a | RRMS | RTX 0-12 w | M | 36 | x | x | |||
| 46613a | RRMS | RTX 0-12 w | F | 39 | x | x | |||
| 48113a | SPMS | RTX 0-12 w | F | 71 | x | x | |||
| 48213a | RRMS | RTX 0-12 w | F | 54 | x | x | |||
|
| |||||||||
| 11911a | SPMS | RTX 13-24 w | M | 59 | x | ||||
| 17212b | PPMS | RTX 13-24 w | F | 38 | x | x | |||
| 35112a | SPMS | RTX 13-24 w | F | 39 | x | x | |||
| 35913a | PPMS | RTX 13-24 w | F | 57 | x | x | |||
| 39013a | SPMS | RTX 13-24 w | M | 43 | x | x | |||
| 39813a | PPMS | RTX 13-24 w | M | 49 | x | x | |||
| 40413a | SPMS | RTX 13-24 w | F | 64 | x | x | |||
| 40513a | PRMS | RTX 13-24 w | F | 68 | x | x | |||
| 48513a | RRMS | RTX 13-24 w | F | 34 | x | ||||
|
| |||||||||
| 6211b | SPMS | RTX 25-36 w | F | 47 | x | x | |||
| 11611a | SPMS | RTX 25-36 w | M | 59 | x | ||||
| 35813a | PPMS | RTX 25-36 w | M | 42 | x | x | |||
| 36313a | PPMS | RTX 25-36 w | F | 62 | x | x | |||
| 40613a | RRMS | RTX 25-36 w | M | 49 | x | x | |||
|
| |||||||||
| 34812a | PRMS | RTX 37-52 w | F | 48 | x | x | |||
| 35212a | SPMS | RTX 37-52 w | F | 70 | x | x | |||
| 35713a | RRMS | RTX 37-52 w | F | 53 | x | x | |||
| 36513a | SPMS | RTX 37-52 w | F | 51 | x | x | |||
| 38713a | PPMS | RTX 37-52 w | F | 43 | x | x | |||
Multicolor Flow Cytometry
Phenotypic analysis of B cells and T cells was performed using multicolor FACS; see Table I for experiments performed per sample. PBMC were resuspended in PBS/1% BSA, FcR-blocking was performed using mouse serum (Jackson Laboratories). For B cell analyses cells were stained with pre-titrated volumes of fluorescent labeled antibodies: CD19 (APC-Cy7), IgD (PE Cy7), CD27 (Qdot 605), CD24 (PE Alexa 610), CD38 (PerCP Cy5.5), IgM (PE Cy5), IgG (APC), CD20 (FITC), CD138 (PE) and CD3 (Pacific blue). DAPI was added to discriminate dying/dead cells; samples were analyzed on a 4-laser FACS Aria III (BD Biosciences). CD19+ B cells were gated from singlet lymphocytes after exclusion of CD3+ T cell and dead cells (DAPI+). T cell subsets were stained using the following antibodies: CD3 (APC), CD4 (PerCP Cy5.5), CD8 (APC-Alexa Fluor 750), CD20 (FITC), CD27 (Qdot 605), CCR7 (PE), CD45RA (PE Cy5). CD3+ T cells were identified on singlet lymphocytes and further divided into subsets. The CD20 cut-off for negative versus positive was determined by using fluorescent-minus-one control.
Single Cell Imaging Flow Cytometry (Imagestream)
PBMC were stained with anti-CD20 FITC (Beckman), anti-CD19 PE (Beckman), and anti-CD3 APC (Beckman); dead cells were labeled with DAPI (Invitrogen). Cytometry gating was performed first on lymphocytes, then live cells (DAPI-negative), then on either CD19+ or CD3+, and lastly on CD20+ among CD19+ or CD3+ cells.
Gene Expression Profiling
PBMC were stained with antibodies against CD3, CD19, and CD20; three populations of lymphocytes were sorted on a MoFlo Astrios (Beckman) directly into RLT lysis buffer (Qiagen): 1) CD19+CD20+; 2) CD3+CD20−; 3) CD3+ CD19−CD20+. Immediately after sorting, cells were frozen in RLT buffer at -80°C. RNA was extracted using the Qiagen micro RNA kit with DNAse treatment. RNA concentrations were measured using a UV spectrophotometer (Nanodrop) and RNA quality was assessed using Pico-RNA chips (Agilent 2100 Bioanalyzer). Microarray hybridizations were performed at the UCSF-affiliated Gladstone Institutes’ Genomics Core Facility. The NuGEN Pico V2 kit, was used for cDNA amplification, fragmentation, and biotinylation; biotinylated cDNA was hybridized to Human GeneChipR Gene 2.0 ST microarrays (Affymetrix). The signal intensity fluorescent images were read using the Affymetrix Model 3000 Scanner and converted into GeneChip probe results files (CEL). Microarray data was analyzed using the statistical programming language R/Bioconductor. Quality of the analyzed arrays was ascertained using the “arrayQualityMetrics” package (10). Raw data were processed using the rma function in the “oligo” package (11). Log2 transformed data were filtered for variance using the “genefilter” package (keeping only probes showing a difference between the 10% and 90% quantiles greater than 1). Hierarchical clustering was performed using the heatmap.2 function of the “gplots” package.
Statistics
Statistical analyses were performed using GraphPad Prism 6.0. Statistical methods are indicated in the text and figure legends where applicable.
RESULTS
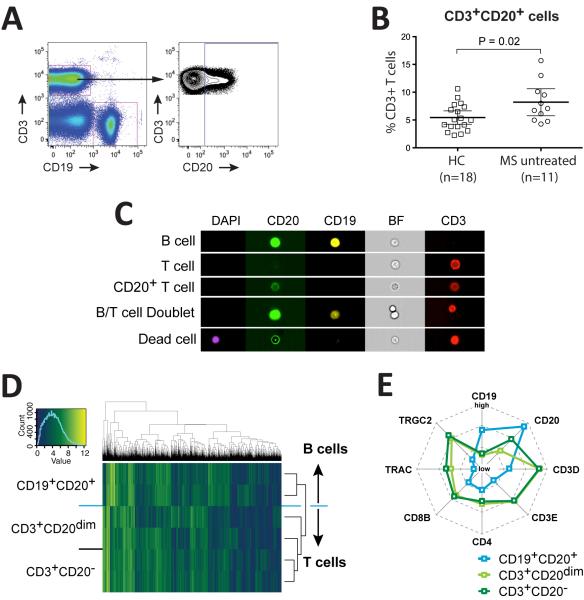
CD3+CD20dim cells are a common subpopulation of CD3+ T cells and are increased in MS patients
We studied T cell and B cell subsets in healthy control subjects (HC) and treatment-naïve MS patients (Table I). In all subjects (HC and MS) we found CD3+ T cells co-expressing low levels of CD20 (Figure 1A), generally representing less than 10% of total CD3+ cells (Figure 1B). Given their potentially pro-inflammatory function (9), we sought to determine if the frequency of CD3+CD20dim T cells in peripheral blood was increased in MS; indeed, the frequency of CD3+CD20dim among all CD3+ T cells was modestly higher in MS (7.2±3.6%, mean ± SD; n=11) compared to 5.4±2.4% (mean ± SD) in HC (n=18) (P=0.02, one-tailed t-test) (Figure 1B). To clearly define the lymphocyte lineage of CD3+CD20dim cells, we performed fluorescent flow cytometry imaging (Amnis ImageStream) on HC (n=3) and treatment-naïve MS patients (n=3), unequivocally revealing CD3 and CD20 expression at the single cell level (Figure 1C); >100 CD3+CD20dim T cells were imaged for this experiment (not shown). Lack of expression of CD19 on these cells confirms that they do not belong to a canonical B cell phenotype (Figure 1C). Transcription profiling and hierarchical clustering of sorted CD3+CD20−, CD19+CD20+, and CD3+CD20dim cells from HC (n=2) further confirmed the T cell lineage of CD3+CD20dim population (Figure 1D); they express both α/β- and γ/δ-TCRs as indicated by increased expression of TRAC (TCR α constant chain) and TRGC2 (TCR γ constant chain) (Figure 1E) compared to B cells. CD20+ T cells expressed lower levels of CD20 compared to CD19+ B cells (Figure 1E), hence their designation as CD3+CD20dim T cells.
Figure 1. CD3+CD20dim cells belong to the T cell lineage and are increased in MS vs. HC.
Flow cytometry gating strategy to identify CD19−CD3+CD20dim cells (A). Percentages of CD3+CD20dim T cells of total CD3+ T cells in HC (n=18) and untreated MS (n=11), P=0.03, one-tailed t-test (B). ImageStream single cell flow cytometry imaging of the cell types indicated on the left. Each image per row shows the same cells as visible in the “BF” column; DAPI, marker for apoptotic (dead) cells; CD20, FITC labeled anti-CD20 antibody; CD19, PE labeled anti-CD19 antibody; BF, brightfield image; CD3, APC labeled anti-CD3 antibody (C). Unsupervised hierarchical clustering of different cell types (B cells: CD19+CD20+, T cells: CD3+CD20−, CD3+CD20dim T cells) from 2 healthy donors according to the expression of variance filtered transcripts. The columns are different genes, the rows reflect different samples. Blue depicts low and yellow high expression. The expression profiles of CD3+CD20dim cells and CD3+ T cells are more similar to each other than to the profile of B cells, hence T cell subtypes cluster together (D). Radar-graph representation of microarray expression levels of indicated transcripts. Note that CD3+CD20dim cells have intermediate levels of CD20 transcripts, and that overall, CD3+CD20dim T cells overlap with CD3+ T cells; both types of T cells have transcripts for CD4 and CD8, and TCR α-chain (TRAC) and γ-chain (TRGC2) (E).
CD3+CD20dimcells are a heterogeneous T cell subpopulation
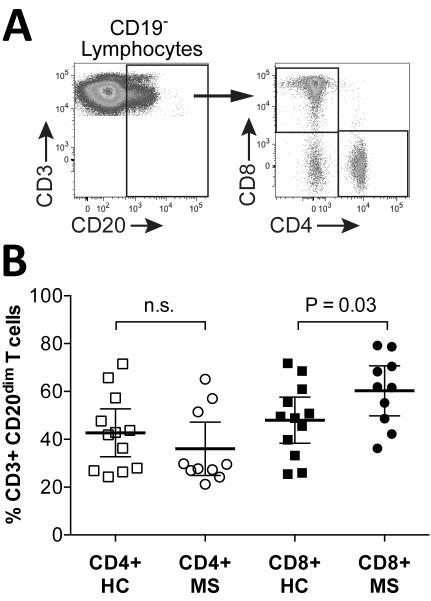
Extensive flow cytometry immunophenotyping of T cells was performed using PBMC from MS patients (n=10) and HC (n=12). The CD3+CD20dim population comprised both CD4+ helper and CD8+ cytotoxic subsets (Figure 2A). Interestingly, among CD3+CD20dim T cells, CD8+ were modestly increased in MS patients (60.3±14.7%) compared to HC (48±15%)(P=0.03, one-tailed t-test) (Figure 2B); no significant difference was found between MS and HC in the percentages of CD4+ among CD3+CD20dim T cells (42.7±15.7% in HC vs. 36±15.6% in MS; P=0.17, one-tailed t-test) (Figure 2B), or of CD4+ or CD8+ total CD3+ T cells (Figure S1).
Figure 2. In MS CD8+ T cells are increased among CD3+CD20dim T cells.
Shown are CD3+CD20dim T cell gating strategy (A) and scatter plots (B; mean and 95% CI) of CD4+ and CD8+ cells among CD3+CD20dim T cells in untreated MS (“MS”) patients and healthy controls (“HC”). Comparisons between MS and HC were made using one-sided t-test; n.s.=not significant.
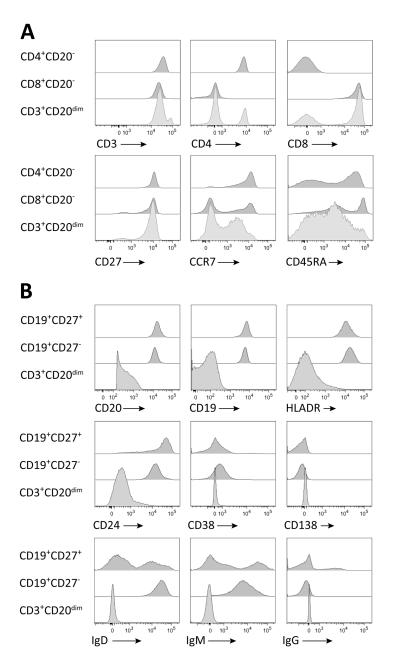
CD3+CD20dim T cells also express CD27, CCR7, and CD45RA (Figure 3A) in different combinations, highlighting the developmental diversity contained within this T cell subset. We evaluated a number of defined subpopulations among CD3+CD20dim T cells; in untreated MS patients the following distribution was found: CD45RA+CCR7+ naïve T cells (28.3±12.3%); CD45RA−CCR7+ central memory T cells (28.8±13.4%); CD45RACCR7− effector memory T cells (16.8±8.6%), CD45RA+CCR7- RA+ memory T cells (27.5±12.1%) (not shown). There was no significant difference between MS and HC for any of these CD3+CD20dim T cell subsets (not shown). Lastly, surface markers commonly found on B cells (CD19, HLADR, CD24, CD38, CD138, IgD, IgM, IgG) were not observed on CD3+CD20dim T cells (Figure 3B).
Figure 3. CD3+CD20dim T cells express typical T cell surface markers but not B cell markers.
Phenotypes of CD3+CD20dim T cells were compared to CD3+CD4+CD20− and CD3+CD8+CD20− T cells (A), and naïve CD19+CD20+CD27− B cells and memory CD19+CD20+CD27− B cells (B) using the indicated surface markers. Shown are FACS histograms of the indicated markers on lymphocytes gated on T and B cell subsets.
Rituximab effectively depletes CD3+CD20dim T lymphocytes in MS patients
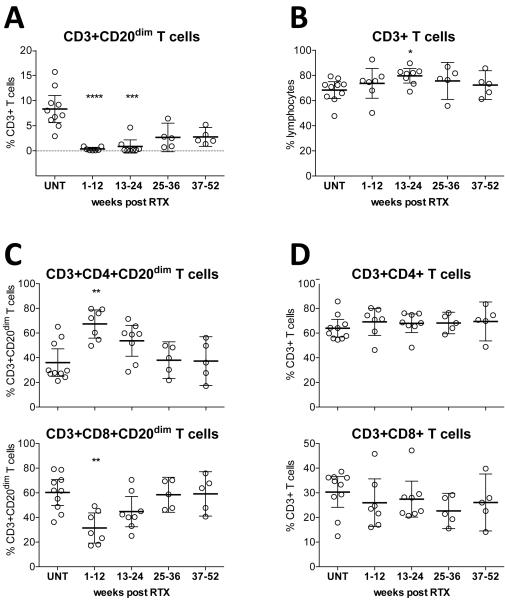
To study the effect of RTX on CD3+CD20dim T cells and other lymphocyte subsets, we collected PBMC from cross-sectional cohorts of untreated (UNT) MS patients and compared lymphocyte subsets to their distribution in RTX-treated patients at different time points: 1-12 weeks post RTX treatment (n=7); 13-24 weeks post RTX (n=8); 25-36 weeks post RTX (n=5); and 37-52 weeks post RTX (n=5). We found near-complete depletion of CD3+CD20dim T cells during weeks 1-12, with mean frequencies of 0.36% (±0.36) compared to 7.8% (±3.7) in UNT patients; during weeks 25-36 and weeks 57-52, partial repletion of CD3+CD20dim T cells was observed, with values of 2.7% (±2.3), and 2.8% (±1.5) respectively (Figure 4A). Apart from a slightly increased proportion of overall CD3+ T cells during weeks 13-24, no significant effect of RTX on the overall CD3+ T cell compartment was present (Figure 4B), suggesting that following depletion of CD3+CD20dim T cells by RTX there was rapid homeostatic repopulation with CD20− T cells. A typical representation of the depletion and gradual repopulation of CD3+CD20dim T cells is depicted in Figure S2. As also observed for B cells, very small numbers of CD3+CD20dim T cells remained detectable in PBMC even during RTX treatment (Figure 4A). Interestingly, the non-depleted CD3+CD20dim T cells that remained in the circulation 1-12 weeks following RTX infusion were predominantly comprised of CD4+ T cells (Figure 4C). In contrast, the overall distribution of CD3+CD4+ and CD3+CD8+ T cells remained unchanged at all time points following RTX therapy (Figure 4D).
Figure 4. CD3+CD20dim T cells are effectively depleted from peripheral blood of MS patients.
Shown are scatter plots (mean and 95% CI) for CD3+CD20dim T cells as percentages of CD3+ T cells (A), and CD3+ T cells as percentages of lymphocytes (B), and of CD4+ and CD8+ cells among CD3+CD20dim (C) and all CD3+ (D) T cells at different time points after RTX treatment (UNT, untreated; and indicated weeks after treatment). RTX almost completely depletes CD3+ CD20dim T cells (A) but has only a small effect on the overall CD3+ T cell compartment (B); RTX leads to significantly higher depletion of CD8+ than CD4+ cells among CD3+CD20dim T cells (C) but not among overall CD3+ T cells. Comparisons between UNT samples and cohorts at different time points after RTX treatment were made using Kruskal-Wallis test; significance levels are: *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.
Rituximab effectively depletes B cells and skews the B cell compartment
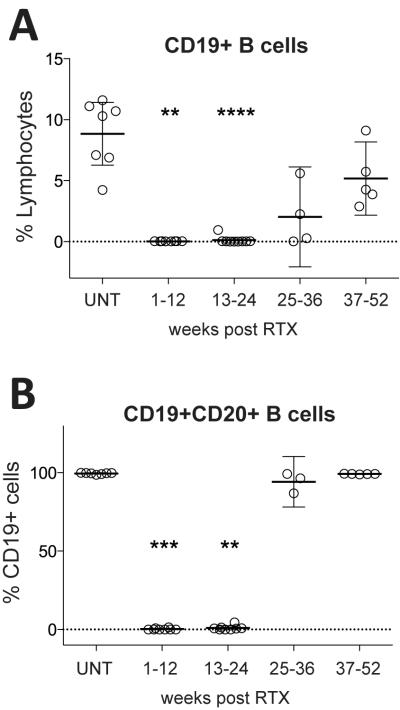
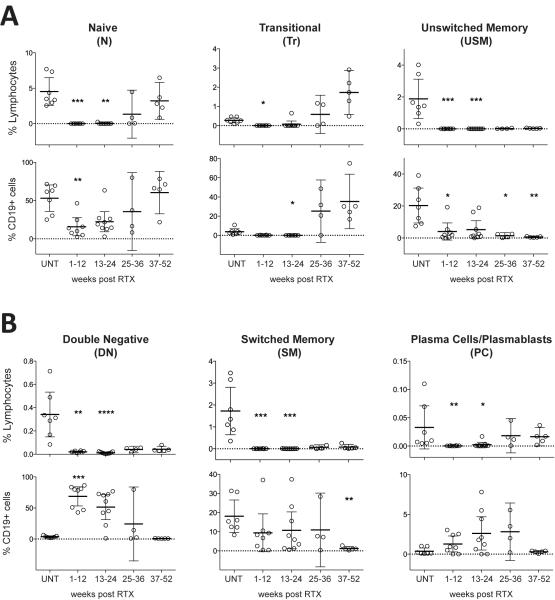
We were also interested in determining the response of CD19+ B cells (Figure 5A) and CD19+CD20+ B cells (Figure 5B), and of other B cell subpopulations (Figure 6) to RTX treatment in our patient cohort. Representative flow cytometry plots and gating strategies for B cell subpopulation analysis are shown in Figure S3. In the untreated MS group, the overall CD19+ B cell fraction ranged between 4.2 to 11.6% of lymphocytes (8.9±2.8%) (Figure 5A). As expected (4), RTX induced near-complete depletion of CD19+ and CD19+CD20+ B cells with replenishment during weeks 25-36 (Figure 5A and B); the majority of B cells in replenishing repertoires were composed of antigen-inexperienced IgD+CD27− naïve and transitional B cell subsets, suggesting influx from CD20- proB cells (Figure 6A). Memory B-cell populations and IgD−CD27− B cells remained depleted for significantly longer and were low even during weeks 37-52 (Figure 6A and B). We found small percentages of residual B cells at every time point after RTX treatment; interestingly, in the 1-12 weeks cohort, the residual B cell compartment was almost entirely composed of IgD−CD27− (i.e. double negative, DN) B cells (Figure 6B). We also found that the percentage of CD19+CD27hiCD38hi plasma cells/plasmablasts (PC) was significantly reduced by RTX during weeks 1-12 and 13-24 (Figure 6B). Among CD19+ cells, PC did not show significant changes in either cohort, but trended towards making up higher numbers among residual CD19+ cells (Figure 6B).
Figure 5. Rituximab efficiently depletes the B cell compartment.
Shown are CD19+ B cells relative to all lymphocytes (% lymphocytes) (A), and CD19+CD20+ B cells as percentages of CD19+ B cells (B) at the indicated time points. In untreated patients (UNT), 8.9±2.8% (mean±SD) of lymphocyte are B cells (A), virtually all of which are CD20+ (B). B cells are efficiently depleted by RTX, with replenishment beginning 25-36 weeks after treatment. Comparisons between UNT samples and cohorts at different time points after RTX treatment were made using the Kruskal-Wallis test; significance levels are: *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.
Figure 6. Composition of the B cell compartment at different time points after RTX treatment.
Shown are B cell subsets relative to all lymphocytes (% lymphocytes) or all B cells (% CD19+ cells) at the indicated time points. Naïve B cells (N), transitional B cells (Tr), and unswitched memory B cells (USM) (A); double negative B cells (DN,), switched memory B cells (SM), and plasma cells/plasmablasts (PC) (B). Indicated are percentages (mean and 95% CI). Please refer to Figure S3 for the flow cytometry gating strategy. Comparisons between samples from untreated patients (UNT) and cohorts at different time points after RTX treatment were made using the Kruskal-Wallis test; significance levels are: *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.
Discussion
In this report, we unequivocally establish the existence of CD3+CD20dim cells belonging to the T cell lineage in healthy donors and MS patients, thus lending strong support to earlier data suggesting the presence of a CD20-expressing T cell population (6). We confirm that CD20-expression is generally dim on these cells when studied by flow cytometry, further supported by lower levels of CD20 transcripts in these cells. While we did not perform functional assays, we show that CD3+CD20dim T cells can be either CD4+ T-helper (Th) cells or CD8+ cytotoxic T cells. Together with previous reports that CD3+CD20dim T cells can assume a pro-inflammatory Th17 phenotype (9), our data suggest that CD3+CD20dim T cells represent a functionally heterogeneous population that may also potentially be involved in the MS disease process.
A vast body of evidence assigns important roles to T cells in the immune pathology of MS. Partially driven by the strong genetic association of MS susceptibility with HLADRB1*1501 and other genes known to be important to Th functions (reviewed in (12)), and by the knowledge that in disease models adoptive transfer of myelin-reactive CD4+ T cells elicits MS-like pathology (reviewed in (13)), Th cells are considered necessary contributors to autoimmune demyelination in MS (reviewed in (14)). Accumulating evidence also suggests an important role of CD8+ T cells in MS immune pathology. Oligoclonal CD8+ T cells are overrepresented in MS lesions (15, 16), and myelin-reactive CD8+ T cells are present in peripheral blood of MS patients (17), outnumbering myelin-reactive CD4+ T cells (18). In animal models, CD8+ T cells reactive against myelin basic protein (MBP) and MOG are encephalitogenic (19), and CD8+ T cells reactive against the glial fibrillary acidic protein (GFAP) were recently shown to mediate relapsing-remitting experimental disease (20). In this context it is interesting that we find increased numbers of CD8+ cells among CD3+CD20dim T cells in untreated MS patients compared to healthy control subjects. In addition, RTX appears to have, overall, a greater impact on the CD8+ than the CD4+ subset of CD3+CD20dim T cells. It is not known if CD3+CD8+CD20dim T cells contribute to autoimmunity in MS and whether depletion of the CD3+CD20dim T cell population contributes to the therapeutic effect of CD20-targeting therapies in MS (21, 22). Based on the preliminary, but highly encouraging clinical results of anti-CD20 therapies in MS, plus the hypothesis that B cell depletion is responsible for this effect, a number of additional B cell-targeting therapeutic approaches are being actively pursued. In particular, a monoclonal antibody targeting the B cell lineage restricted marker CD19 (23) is currently in phase I clinical evaluation for the treatment of MS (MEDI-551) (24). Compared with anti-CD20 approaches, anti-CD19 therapy targets a broader spectrum of B cells at different developmental stages but is not expected to deplete CD3+CD20dim T cells; accordingly, a difference in therapeutic efficacy between anti-CD19 and anti-CD20 approaches may indirectly identify a pathogenic role, should it exist, of CD3+CD20dim T cells in MS.
A number of studies have also demonstrated effects of anti-CD20 therapy on the T cell compartment. In humans, RTX treatment reduced T cell numbers in the CSF (25, 26), and in the circulation diminished pro-inflammatory Th1 and Th17 responses of CD4+ and CD8+ T cells (27). Similarly, in a B cell-dependent model of experimental autoimmune encephalomyelitis (EAE) induced by immunization of mice with the recombinant extracellular domain of myelin-oligodendrocyte glycoprotein (MOG), anti-CD20 treatment reduced pro-inflammatory T cell responses (28, 29). These effects have generally been thought to result from indirect effects of B-cell depletion on pathogenic T-cells, presumably via blocking B-cell mediated antigen presentation or secretion of proinflammatory B-cell cytokines. The current data raise another possibility, i.e. that some of the known effects of RTX on the T cell compartment (25-27), and perhaps also the beneficial effect of RTX in RA and MS, may result from depletion of CD3+CD20dim T cells. Irrespective of the mechanism, here we expand the spectrum of immunological changes induced by CD20-targeting therapy in MS to include near-complete direct depletion of CD3+CD20dim T cells for at least 1 year following RTX infusion. In this regard, it is of great interest that, in preliminary clinical studies of both RTX and OCR in MS, long-term protection against focal inflammatory disease activity was found even after repletion of circulating B-cells occurred(5), consistent with the hypothesis that CD3+CD20dim T cells could have some pathogenic role in MS.
The long-term depletion of CD3+CD20dim T cells also suggests that this population may represent an early developmental stage of T-cells that is not rapidly replenished, similar to the prolonged depletion of memory B cells observed after RTX treatment (4). In fact, the diversity of T-cell associated markers expressed by CD20dim T cells – including helper, cytotoxic, naïve and memory populations detected by flow cytometry, and α/β- and γ/δ-TCRs by transcriptomics – all suggest that CD20 expression may be an early thymic event in the development of a diverse CD3+CD20dim T cell population. To our knowledge, no murine equivalent to human CD3+CD20dim T cells has been identified. However, a murine CD20 homolog, MS4aB1, that is expressed on T cells but not B cells was recently described (30). In murine EAE, treatment with an anti-MS4aB1 antibody was found to ameliorate disease severity and reduce pro-inflammatory T cell responses (31), theoretically mimicking the therapeutic effect of anti-CD20 mediated CD3+CD20dim T cell depletion, in the absence of B-cell depletion, in humans.
CD20 is traditionally considered a B cell lineage-specific marker. CD20 is a trans-membrane protein (32) expressed on the majority of peripheral B cell subsets, but not on early bone marrow pro B cells or on a subset of terminally differentiated plasma cells primarily residing in secondary lymphoid tissues. There is no known ligand for CD20; functions of CD20 have been proposed to include calcium transport (33) and boosting of T cell-independent B cell responses (34). CD20-targeting therapies are commonly referred to as “B cell-depleting therapy”; accordingly, we also find the previously described significant impact of RTX on the B cell compartment, with long-term-depletion of memory B cells, early replenishment with naïve B cell phenotypes, and also reduction of plasmablasts, possibly owing to the higher turnover rates of short-lived plasmablasts (35, 36). We also show for the first time that normally rather infrequent CD19+CD27-IgD− (double negative, DN) B cells become significantly depleted by RTX but at the same time comprise the majority of the small B cell population present in peripheral blood of RTX-treated patients; this may suggest relative resistance of DN B cells to CD20-targeted lymphocyte depleting therapy. Presently, very little is known regarding immunological function of DN B cells and it has been speculated that this B cell subset may also contribute to autoimmunity, at least in systemic lupus erythematosus (37). The relevance of DN B cells in MS immune pathology is a matter of ongoing investigation.
In summary, we show that in MS increased numbers of a previously neglected T cell subset, CD3+CD20dim T cells, are effectively depleted by RTX. Further studies will be required to understand the developmental origin and evolutionary relevance of CD3+ CD20dim T cells, and whether they differ functionally from CD20− T cells. Understanding the pathological relevance of this T cell subset in MS and other autoimmune disorders will likely broaden our understanding of the pathology of human autoimmunity and may reveal novel therapeutic avenues. Current studies in our laboratory are aimed at delineating the functional properties of CD3+CD20dim T cells, defining their potential contribution to MS immune pathology, and elucidating the dynamics of the CD3+CD20dim T cell subpopulation through longitudinal follow-up studies of individuals treated with CD-20 depleting therapy.
Supplementary Material
Acknowledgements
The authors are deeply thankful to their patients who agreed to participate in this research. We thank Erica Eggers for excellent technical assistance, and the Gladstone Institutes Genomics Core Facility for performing microarray experiments.
Footnotes
Conflict of Interest Statement David Leppert is an employee of F. Hoffmann-La Roche Ltd. H.-Christian von Büdingen has received research funding from F. Hoffmann-La Roche Ltd.
Funding: These studies were supported by grants from the NMSS (RG-4868 to HCvB) and the NIH/NINDS (K02NS072288 to HCvB, R01NS026799 to SLH). HCvB was also supported by an endowment from the Rachleff Family Foundation. SEB is a Harry Weaver Neuroscience Fellow of the National Multiple Sclerosis Society.
Non-standard abbreviations: RTX, rituximab; MS, multiple sclerosis; HC, healthy control subject.
REFERENCES
- 1.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Annals of neurology. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, Group HT. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 3.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 4.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL, Li D, Calabresi P, O’Connor P, Bar-Or A, Barkhof F, Sauter A, Leppert D, Masterman D, Tinbergen J, Kappos L. American Academy of Neurology. San Diego, CA: 2013. Week 144 Results of a Phase II, Randomized, Multicenter Trial Assessing the Safety and Efficacy of Ocrelizumab in Patients with Relapsing–Remitting Multiple Sclerosis (RRMS) S31.004. [Google Scholar]
- 6.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 7.Henry C, Ramadan A, Montcuquet N, Pallandre JR, Mercier-Letondal P, Deschamps M, Tiberghien P, Ferrand C, Robinet E. CD3+CD20+ cells may be an artifact of flow cytometry: comment on the article by Wilk et al. Arthritis Rheum. 2010;62:2561–2563. doi: 10.1002/art.27527. [DOI] [PubMed] [Google Scholar]
- 8.Wilk E, Witte T, Marquardt N, Horvath T, Kalippke K, Scholz K, Wilke N, Schmidt RE, Jacobs R. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–3571. doi: 10.1002/art.24998. [DOI] [PubMed] [Google Scholar]
- 9.Eggleton P, Bremer E, Tarr JM, de Bruyn M, Helfrich W, Kendall A, Haigh RC, Viner NJ, Winyard PG. Frequency of Th17 CD20+ cells in the peripheral blood of rheumatoid arthritis patients is higher compared to healthy subjects. Arthritis research & therapy. 2011;13:R208. doi: 10.1186/ar3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25:415–416. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet. 2008;9:516–526. doi: 10.1038/nrg2395. [DOI] [PubMed] [Google Scholar]
- 13.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013;34:410–422. doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 15.Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Annals of neurology. 1986;19:578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida T, Parker KC, Turner RV, McFarland HF, Coligan JE, Biddison WE. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci U S A. 1994;91:10859–10863. doi: 10.1073/pnas.91.23.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 19.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, Peters B, Shen ZT, Vanguri V, Liggitt D, Huseby ES. Relapsing-Remitting Central Nervous System Autoimmunity Mediated by GFAP-Specific CD8 T Cells. J Immunol. 2014 doi: 10.4049/jimmunol.1302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, Yin M, Leppert D, Glanzman R, Tinbergen J, Hauser SL. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 22.Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert opinion on investigational drugs. 2009;18:491–500. doi: 10.1517/13543780902832679. [DOI] [PubMed] [Google Scholar]
- 23.Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, Woods R, Rowe DC, Cheng L, Cook K, Evans K, Sims GP, Pfarr DS, Bowen MA, Dall’Acqua W, Shlomchik M, Tedder TF, Kiener P, Jallal B, Wu H, Coyle AJ. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. The Journal of pharmacology and experimental therapeutics. 2010;335:213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 24.Safety and Tolerability Study of MEDI-551, a B-cell Depleting Agent, to Treat Relapsing Forms of Multiple Sclerosis. ClinicalTrials.gov. NCT01585766.
- 25.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccio L, Naismith RT, Trinkaus K, Klein RS, Parks BJ, Lyons JA, Cross AH. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Archives of neurology. 2010;67:707–714. doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Annals of neurology. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 28.Monson NL, Cravens P, Hussain R, Harp CT, Cummings M, de Pilar Martin M, Ben LH, Do J, Lyons JA, Lovette-Racke A, Cross AH, Racke MK, Stuve O, Shlomchik M, Eagar TN. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e17103. doi: 10.1371/journal.pone.0017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber MS, Prod’homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CC, Martin F, Zamvil SS. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Annals of neurology. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Williams MS, Spain LM. Patterns of expression, membrane localization, and effects of ectopic expression suggest a function for MS4a4B, a CD20 homolog in Th1 T cells. Blood. 2006;107:2400–2408. doi: 10.1182/blood-2005-08-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Y, Li Z, Zhang GX, Williams MS, Carey GB, Zhang J, Rostami A, Xu H. Anti-MS4a4B treatment abrogates MS4a4B-mediated protection in T cells and ameliorates experimental autoimmune encephalomyelitis. Apoptosis : an international journal on programmed cell death. 2013;18:1106–1119. doi: 10.1007/s10495-013-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Current directions in autoimmunity. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 33.Walshe CA, Beers SA, French RR, Chan CH, Johnson PW, Packham GK, Glennie MJ, Cragg MS. Induction of cytosolic calcium flux by CD20 is dependent upon B Cell antigen receptor signaling. J Biol Chem. 2008;283:16971–16984. doi: 10.1074/jbc.M708459200. [DOI] [PubMed] [Google Scholar]
- 34.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. CD20 deficiency in humans results in impaired T cell-independent antibody responses. The Journal of clinical investigation. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hachiya Y, Uruha A, Kasai-Yoshida E, Shimoda K, Satoh-Shirai I, Kumada S, Kurihara E, Suzuki K, Ohba A, Hamano S, Sakuma H. Rituximab ameliorates anti-N-methyl-d-aspartate receptor encephalitis by removal of short-lived plasmablasts. J Neuroimmunol. 2013;265:128–130. doi: 10.1016/j.jneuroim.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.