Abstract
Extracellular vesicles (EVs), including exosomes, microvesicles and apoptotic bodies, are released by almost all cell types, including tumour cells. Through transfer of their molecular contents, EVs are capable of altering the function of recipient cells. Increasing evidence suggests a key role for EV-mediated intercellular communication in a variety of cellular processes involved in tumour development and progression, including immune suppression, angiogenesis and metastasis. Aspects of EV biogenesis or function are therefore increasingly being considered as targets for anti-cancer therapy. Here, we summarize the current knowledge on the contributions of EVs to cancer pathogenesis and discuss novel therapeutic strategies to target EVs to prevent tumour growth and spread.
Keywords: Extracellular vesicles, exosomes, microvesicles, cancer therapy, tumour microenvironment, metastasis
Extracellular vesicles: Novel mediators of cell-to-cell communication
Intercellular communication is fundamental to survival and maintenance of homeostasis in all multicellular systems. In contrast, dysregulated pathways of communication appear to drive cancer development and progression. The development of successful anti-cancer treatments will therefore depend crucially on increasing our understanding of the complexity of interactions between tumour cells and other cells. Communication between cells takes place via direct cell-to-cell contact, for example through adhesion molecules, gap junctions and nanotubes, or via soluble communication signals such as cytokines, growth factors and hormones secreted by both tumour and non-tumour cells. However, an additional novel mechanism that can operate over both short and long distances has recently emerged, based on the release and uptake of membrane-bound vesicles termed extracellular vesicles (EVs) [1]. The recent discovery that EVs are able to convey complex multi-molecular biological messages between cells has the potential to revolutionize our understanding of the communication circuitry in cancer. Further, EV research is anticipated to directly advance various areas of clinical cancer science, including cancer diagnostics (Box 1) and therapy [2].
Box 1: Extracellular vesicles: Novel cancer biomarkers.
The content of EVs found in bodily fluids is closely related to the nature and status of the cells from which the EVs are derived. As tumour- and stromal cell-derived EVs carry signatures and effectors of tumour development, EVs are increasingly considered as novel sources of biomarkers with diagnostic or prognostic value [2]. Additionally, they could be used to predict or monitor patients’ response to treatment. Compared to monitoring via biopsy, which does not allow for frequent and longitudinal sampling, EVs offer non-invasive and almost continuous access to circulating information on the tumour’s status [1]. Depending on the tumour type and location, EVs can be isolated from plasma/serum, urine, cerebral spinal fluid and even saliva.
Numerous reports have already aimed to characterize the components of EVs derived from a variety of cellular sources and bodily fluids, the results of which are often made available through community databases of high-throughput datasets of EV cargo [74, 75]. These studies have shown that EVs derived directly from tumour cells or from the extracellular fluids of cancer patients have a distinct molecular signature on the protein [11, 76–78], DNA [79], mRNA [8] and non-coding RNA [80, 81] level, which could allow their potential use as biomarkers. However, technological challenges related to EV isolation, purification and content analysis remain. For example, for multicentre validation studies, standardized isolation and characterization methods are necessary, yet largely lacking [58]. To overcome some of these challenges, a novel platform has recently been described for rapid protein profiling of EV samples. EVs are introduced onto a microfluidic chip and labelled with target-specific magnetic nanoparticles, which allows for highly sensitive detection of antigens by micro-nuclear magnetic resonance (μ-NMR) [82]. In addition, novel, sensitive approaches based on BEAMing and droplet digital PCR have recently been described to reliably detect and quantify mutant transcripts in EVs, which may contribute to solve challenges related to the detection level of mutant RNA/DNA in tumour-derived EVs in a background of EVs from normal cells [83]. Further technological improvements may allow EV-based diagnostics to become routine clinical practice.
Biogenesis, composition and function of EVs
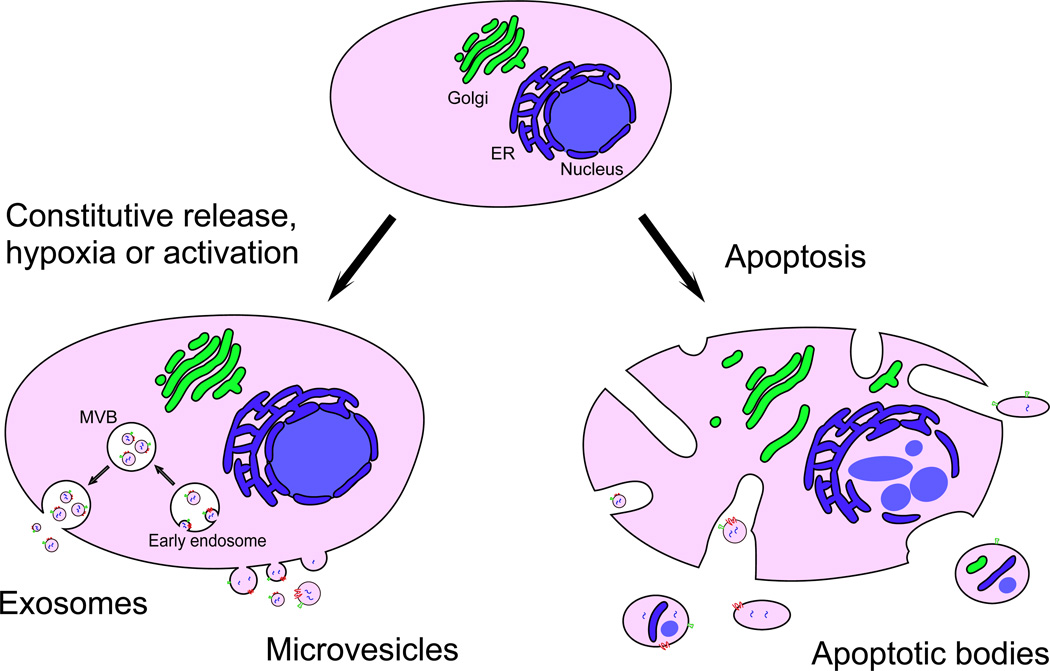
Over the last decade, research efforts into EV biology, function and application have increased dramatically. It has now become clear that virtually all cell types release EVs, constitutively and/or upon activation (for example as a result of hypoxia or shear stress). EVs have been traditionally classified based on their cell or tissue of origin, for example prostasomes are derived from prostate cells, and oncosomes are derived from tumour cells. More recently however, different classifications of EVs are being used, based on the intracellular origin or biogenesis mechanism. Using this approach, although there is currently little consensus in the field regarding nomenclature due to differences in classification criteria, three main classes of EVs can be distinguished: exosomes, microvesicles (also referred to as ectosomes or microparticles) and apoptotic bodies [3–5]. Exosomes have been defined as originating from multivesicular bodies (MVBs) and are secreted upon fusion of MVBs with the plasma membrane. Exosomes are believed to range between 40 and 150 nm in size with a buoyant density of 1.13–1.19 g/cm3, and are often characterized using marker proteins such as ALG-2-interacting protein X (ALIX) and tumour susceptibility gene 101 (TSG101), which indicate an endocytic origin [6]. Microvesicles are shed from the plasma membrane through direct outward budding and are generally more heterogeneous in size (50–2000 nm). Apoptotic bodies are released upon fragmentation of cells undergoing apoptosis. They vary in size between 50 and 5000 nm and can contain DNA and histones. The biogenesis and characteristics of each type of EVs derived from tumour cells are summarized in Figure 1 and Table 1. A strict separation between the EV classes by size, density, markers or morphology has however not yet been established [7]. Moreover, current isolation and detection techniques for EVs do not allow for a clear distinction between different vesicular subpopulations, therefore the term EV will be used throughout this review to include all classes of cell-derived extracellular vesicles.
Figure 1. Types of EVs released by tumour cells.
Exosomes and microvesicles are released constitutively and/or upon activation. Exosomes are formed from endosomes through inward budding to generate multivesicular bodies (MVBs) and are released upon fusion of MVBs with the plasma membrane. Exosomes are relatively homogeneous in size and, because of their endocytic origin, contain proteins involved in endosomal-lysosomal sorting which are used as exosomal markers. Microvesicles on the other hand are formed through direct outward budding of the plasma membrane. Tumour cells undergoing apoptosis release apoptotic bodies, which are formed by random blebbing of the plasma membrane. Apoptotic bodies are heterogeneous in size and may contain nuclear fragments as well as fragments of cytoplasmic organelles. Abbreviations: ER, endoplasmic reticulum; MVB, multivesicular body.
Table 1.
Characteristics of different EV types
| Exosomes | Microvesicles | Apoptotic bodies | |
|---|---|---|---|
| Intracellular origin | Multivesicular bodies | Plasma membrane | Plasma membrane |
| Size | 40–150 nm | 50–2000 nm | 50–5000 nm |
| Suggested markers | ALIX, TSG101, tetraspanins | Unknown | DNA, histones |
| Density | 1.13–1.19 g/cm3 | Unknown | 1.16–1.28 g/cm3 |
| Appearance in transmission electron microscopy | Cup-shaped | Heterogeneous | Heterogeneous |
| Refs | [6, 84, 85] | [84, 86] | [87, 88] |
EVs are lipid bilayer limited vesicles and carry a broad repertoire of cargoes, including proteins (e.g. cytokines, membrane receptors and receptor ligands), nucleic acids (e.g. DNA, mRNA, long and short noncoding RNA) and lipids. Although their content generally reflects the nature and status of the cell of origin, enrichment of specific proteins and nucleic acids suggests at least a degree of specific cellular sorting into EVs, although the mechanisms underlying this remain to be defined [8]. Release of EVs is thought to have various biological roles, including disposal of superfluous or harmful cellular contents [9]. A more recently discovered and likely important role however, is to emit signalling and regulatory molecules that can be recognized by, or transferred to, other cells in a selective manner, thereby influencing the phenotype and function of the recipient cell.
EVs can interact with target cells via different mechanisms. For example, membrane proteins on the surface of EVs can interact directly with receptors on the target cell, thereby activating intracellular pathways. Alternatively, EVs can be internalised by target cells either via membrane fusion or via endocytosis/phagocytosis, with subsequent transfer and release of their cargo [10]. In this manner, EVs can shuttle functional membrane receptors from one cell to another, after which intracellular signalling via these receptors can take place in the recipient cell [11]. mRNAs present in EVs are also transferrable to recipient cells where they can be translated into functional protein [12, 13]. Strikingly, even microRNAs (miRNAs) have been shown to be shuttled between cells by EVs leading to the repression of mRNA translation in recipient cells [14, 15], although recent evidence suggests that miRNAs can also be transported and delivered via other mechanisms [16]. Through this exchange of molecular information, EVs are thought to exhibit pleiotropic biological functions and increasing evidence supports their importance in a variety of fundamental physiological as well as pathological processes [3, 5]. For instance, B lymphocyte-derived EVs present antigens and induce antigen-specific responses in T cells, suggesting a role in adaptive immune responses [6]. During pregnancy, placenta-derived EVs function to circumvent maternal immune-surveillance by suppressing T-cell activation [17]. EVs have also been implicated in neurodegenerative diseases such as Alzheimer’s disease via the intercellular transfer of aberrant protein structures such as beta-amyloid peptides [18]. Not surprisingly, numerous studies to date have also implicated EVs as critical contributors to tumour growth and spread.
Emerging roles of EVs in tumour growth and spread
A variety of EVs can be readily isolated from bodily fluids of cancer patients, including from blood, lymph, urine, saliva, cerebrospinal fluid and ascites. In fact, the number of circulating EVs in cancer patients seems to be higher than in healthy subjects and has been found to correlate with poor prognosis [19]. The vast majority of these circulating EVs seem not to be derived from tumour cells, but arise from activated platelets (or megakaryocytes [20]), lymphocytes, macrophages and erythrocytes [1]. Initially, EVs were thought to be mainly associated with venous thromboembolic events (VTE) in cancer patients because of their ability to carry tissue factor (Tf) and to interact with components of the coagulation system. Several studies have linked increased incidence of VTE to elevated Tf-bearing EV levels [21–23]. Currently, clinical trials are underway to test the possibility of either reducing the number of pro-coagulant EVs in cancer patients, or to use them diagnostically as tools to predict patients’ risk of developing VTEs [24].
Recent large-scale proteomic and transcriptomic studies have revealed differences between the protein and nucleic acid content of EVs derived from cancer cells compared to those derived from normal cells (although some caution is required when interpreting these results as EV purification methods that are typically used are unable to completely purify EVs from non-EV contaminants and differ between laboratories). Many of the proteins and RNAs found in tumour-derived EVs are known for their roles in cancer development and progression. These include oncoproteins, oncogenes, chemokine receptors as well as soluble factors and transcripts of proteins involved in angiogenesis or inflammation (reviewed in [9, 25, 26]). As EVs are capable of transferring these molecules to other cells in the tumour microenvironment or at specific distant sites, they have increasingly become recognized as key players in a variety of cellular processes related to cancer pathogenesis (Figure 2). Important examples of the influence of EV-mediated signalling on tumour growth and spread are described below. It should be emphasized that much of our current understanding is based on data from in vitro experiments. Caution must be taken when correlating these results with the physiological situation, as EV concentrations essential for in vitro observations sometimes exceed those found in vivo [27]. Thus, the relevance of EVs in cancer pathology in vivo largely remains to be evaluated.
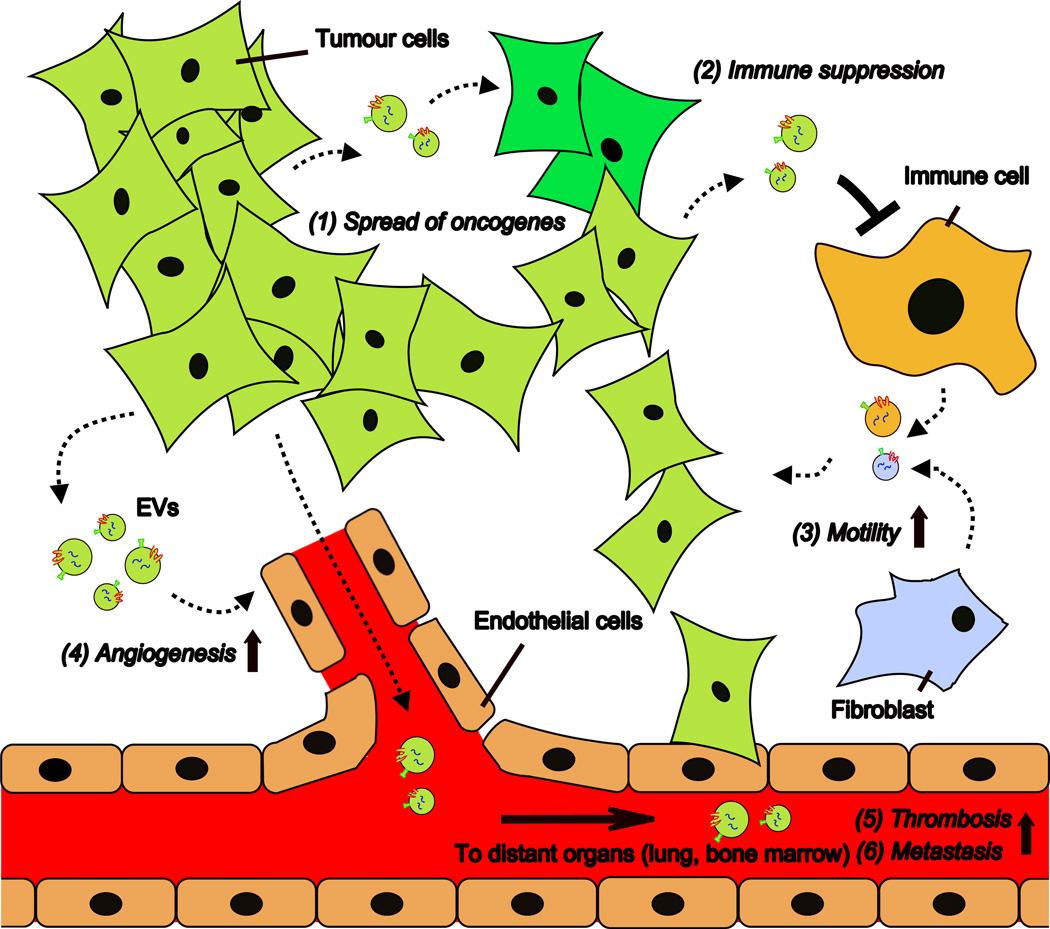
Figure 2. Schematic representation of processes affected by EV-mediated signalling in cancer.
Tumour cells and stromal cells exchange EVs carrying proteins and nucleic acids that can affect the function of recipient cells. Tumour cell-derived EVs can contribute to spread of the transformed phenotype from transformed (light green) cells to surrounding non-transformed (dark green) cells (1) and contribute to tumours’ ability to escape from immune surveillance (2). EVs derived from stromal cells, such as fibroblast and immune cells, may influence tumour cell motility (3). Moreover, tumour-derived EVs stimulate endothelial angiogenic responses (4) and may enter the circulatory system and reach distant organs, where they promote thrombosis (5) and formation of pre-metastatic niches (6).
Tumour formation involves accumulation of genetic alterations, including inactivating mutations in tumour suppressor genes and activating mutations in proto-oncogenes, as well as epigenetic changes in gene expression. Although the exact underlying mechanisms remain to be elucidated, malignant transformation seems to be associated with increased release of EVs [28, 29]. Interestingly, EVs can contribute to spread of the transformed phenotype by intercellular transfer of oncogenes. It has been shown that apoptotic bodies can transfer tumour DNA from H-RASV12- and human C-MYC-transfected rat fibroblasts to wild-type mouse fibroblasts, leading to development of full tumourigenic potential of the wild-type cells in vivo [30]. Via transfer of mutant K-RAS, EVs from colon cancer cells can transform cells expressing only the wild-type K-RAS allele [31]. Similarly, glioma cells expressing a truncated form of the epidermal growth factor receptor (EGFR), known as EGFRvIII, release EGFRvIII-positive EVs that can be taken up by indolent glioma cells lacking this oncogenic receptor. Upon acquiring EGFRvIII, growth-promoting mitogen-activated protein kinase (MAPK) and AKT signalling pathways are activated and cellular transformation is induced [11].
In order to grow beyond microscopic size, tumours depend on angiogenesis, defined as the formation of new blood vessels out of pre-existing ones [32], and many reports suggest that tumour-derived EVs can promote endothelial angiogenic responses. EVs derived from A431 squamous carcinoma cells can transfer oncogenic EGFR to endothelial cells. EGFR signalling in the recipient cells leads to activation of MAPK and AKT pathways, as well as to increased expression of endogenous vascular endothelial growth factor (VEGF) and subsequent autocrine activation of VEGF receptor 2, which is involved in induction of angiogenesis [33]. Glioblastoma EVs are enriched in angiogenic proteins such as fibroblast growth factor (FGF), interleukin (IL)-6 and VEGF and stimulate angiogenesis in vitro in a brain microvascular endothelial tubule formation assay [8]. Similarly, B16–F10 melanoma-derived EVs induce production of pro-angiogenic cytokines including IL-1α, FGF and tumour necrosis factor alpha (TNFα) by 2F-2B endothelial cells, which results in increased formation of endothelial spheroids and sprouts [34]. In this study, however, the stimulatory components of the EVs were not identified. EVs may also regulate angiogenesis via transfer of genetic information. Hong et al. showed that 241 mRNAs, including 27 mRNAs involved in cell-cycle regulation, are enriched in SW480 colorectal cancer cell-derived EVs compared to the cells of origin. Indeed, treatment of endothelial cells with these EVs significantly stimulated their proliferation [35]. EVs derived from CD105-positive human renal cancer stem cells contain miRNAs implicated in tumour progression, and stimulate blood vessel formation of endothelial cells upon implantation in severe combined immunodeficient (SCID) mice [36]. A recent study suggests that under hypoxic conditions, which have been associated with tumour aggressiveness, effects of EVs on tumour angiogenesis and growth are even more pronounced. In a mouse glioblastoma multiforme (GBM) xenograft model, EVs derived from tumour cells grown in hypoxic conditions significantly enhance tumour growth compared to EVs derived from cells grown in normoxic conditions. This enhanced growth is accompanied by increases in tumour vascularization, pericyte coverage of the vessels, and GBM cell proliferation [37]. Hypoxia also results in acidification of the tumour microenvironment, which may have a profound influence on EV trafficking, as both EV release and uptake have been shown to be increased at lower pH [38].
The co-development of tumours with phenotypic changes in the local tumour microenvironment involves bidirectional communication between tumour cells and the tumour-associated stroma. It has been shown that stromal cells also release EVs, which are thought to play important roles in regulation of tumour cell behaviour. For example, activated platelets release EVs that stimulate proliferation and trans-matrigel invasion of lung cancer cells [39]. Macrophages promote invasiveness of breast cancer cells via EV-mediated transfer of miR-223, which targets the myocyte enhancer factor (Mef)2c-β-catenin pathway. Interestingly, this effect seems to be specific for macrophages activated by IL-4, which is the major cytokine that induces macrophage differentiation into tumour-promoting, M2-like macrophages [40]. Moreover, fibroblast-derived EVs were shown to increase breast cancer cell motility and protrusion by activating autocrine Wnt-planar cell polarity signalling [41]. Together, these data suggest the importance of EV-mediated crosstalk between tumour and stromal cells in cancer progression.
One of the most striking features of tumour-derived EVs is their potential to facilitate formation of the pre-metastatic niche, the specialized microenvironment that forms at a distant organ site in preparation for future tumour metastasis. Common sites of metastasis include lymph nodes or distant organs such as lung, bone marrow and liver. EVs from tumour cells with high metastatic potential have been shown to carry significantly different cargoes than EVs from poorly metastatic cells, including differences in profiles of proteins (e.g. MET, CD44, heat shock protein (Hsp) 70 and annexin A6 [42]) and miRNAs [43] known to have key roles in tumourigenesis and metastasis. It has been shown that melanoma EVs, in contrast to control liposomes, can travel long distances through the lymphatic system and home to sentinel lymph nodes. EV accumulation in lymph nodes resulted in increased recruitment of melanoma cells, which might be driven by EV-induced expression of metastatic factors such as integrin αv, MAPK and TNF-α in cells in the lymph node microenvironment [44]. Using the same melanoma model, it was shown that intravenously injected EVs extravasate into common sites of melanoma metastasis and enhance endothelial permeability in the lungs. Again, EV accumulation at the site of metastasis resulted in differential expression of genes involved in extracellular matrix remodelling, inflammation and vascular permeability and increased metastasis formation after subcutaneous implantation of tumour cells. In addition, EVs could horizontally transfer MET to bone marrow progenitor cells, thereby enhancing bone marrow cell mobilization to pre-metastatic sites to promote angiogenesis and metastatic progression [42].
In addition to uncontrolled cell proliferation, sustained angiogenesis and metastasis, another hallmark of cancer is the advanced defensive strategies of tumour cells that allow escape from the immune surveillance as well as resistance to treatment. In both scenarios, EV signalling has been suggested to play an important role. Tumour-derived EVs seem to display a multitude of actions that function collectively to suppress immune surveillance [3]. For instance, EVs from a variety of cancer cell lines or isolated from mesothelioma patients impair the cytotoxic capacity of natural killer (NK) cells or CD8+ T cells via downregulation of the stimulatory receptor natural-killer group 2, member D (NKG2D) [45]. Alternatively, EV-mediated signalling can promote generation of cells with immunosuppressive properties, such as myeloid-derived suppressor cells, or stimulate their suppressive functions via signal transducer and activator of transcription 3 (Stat3) activation by EV-associated Hsp72 [46, 47].
Vesicle shedding by cells may also contribute to drug resistance during chemotherapy. Anti-cancer drugs and even excipients contained in drug formulations have been shown to directly affect EV release [48]. In aggressive lymphoma, CD-20-positive EVs protect tumour cells from therapeutic anti-CD20 antibody attack both by binding antibodies in the circulation and via consumption of complement [49]. In addition, drug-resistant cells display enhanced release of chemotherapeutic drugs such as doxorubicin [50] and cisplatin [51] via EV pathways as compared to chemosensitive cells, although the importance of the contribution of drug release via EVs to the observed differences in drug accumulation in cells may vary among drug and cell types. Strikingly, tumour-derived EVs even seem capable of spreading drug resistance. EVs isolated from drug-resistant cancer cells were shown to transfer P-glycoprotein (P-gp), a membrane multidrug efflux transporter, to drug-sensitive cells during co-culture. Using drug accumulation assays, transferred P-gp was shown to be functional in the recipient cells, stimulating efflux of drugs, indicating that cross-resistance had been acquired [52].
Extracellular vesicles: Emerging targets for cancer therapy
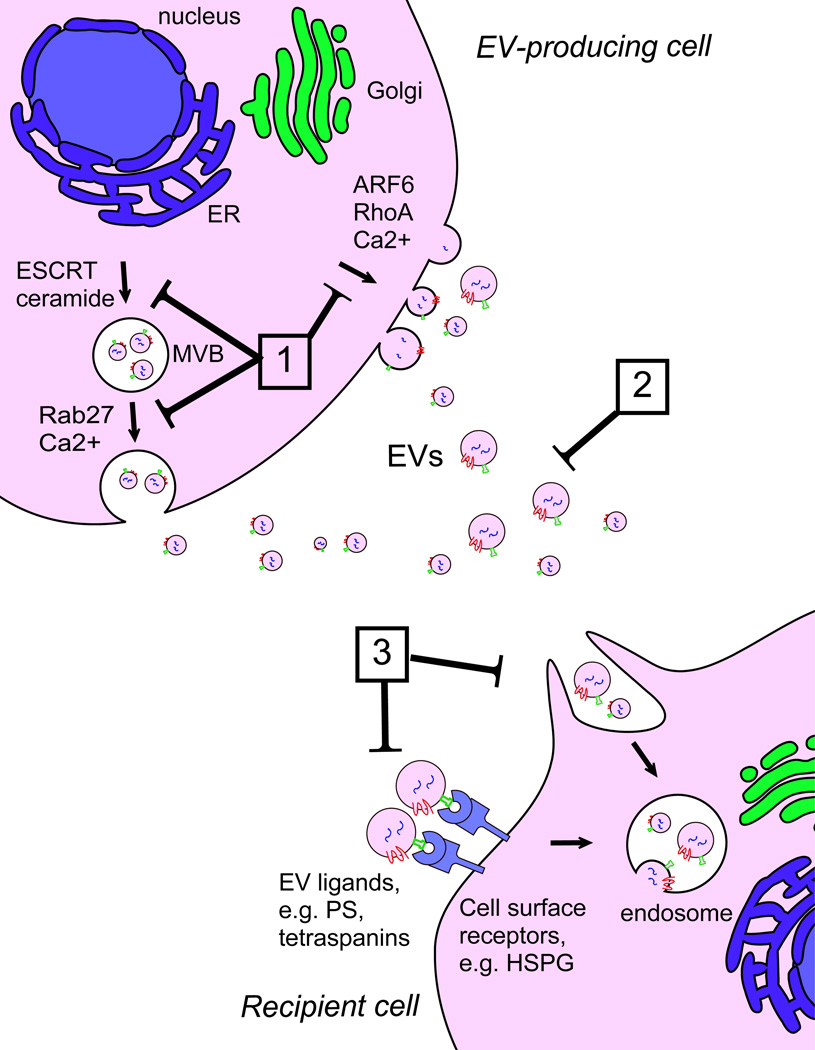
The importance of EV-mediated signalling in cancer progression renders EVs a potential novel class of therapeutic targets, focused on inhibition of a key component of the tumour cell communication network. Different possibilities, although still largely theoretical, to interrupt EV-mediated crosstalk can be envisioned, including interfering with EV biogenesis and/or release, EV removal from the circulation and inhibition of EV uptake by the target recipient cell [1, 5] (Figure 3).
Figure 3. Therapeutic targeting of EV signalling in cancer.
Different potential strategies to interfere with EV-mediated intercellular communication can be envisioned. (1) Inhibition of EV biogenesis or release through interference with components of pathways involved in EV formation (e.g. ESCRT, ceramide) or release (e.g. Rab27, ARF6, RhoA). (2) EV removal from the circulation by extracorporeal hemofiltration. (3) Inhibition of EV uptake in recipient cells by blocking EV ligands (e.g. PS, tetraspanins) or cell surface receptors involved in EV binding or internalization (e.g. HSPGs). Abbreviations: ARF6, ADP-ribosylation factor 6; Ca, calcium; ER, endoplasmic reticulum; ESCRT, Endosomal Sorting Complex Required for Transport; HSPG, heparan sulfate proteoglycans; MVB, multivesicular body; PS, phosphatidylserine; RhoA, Ras homolog family member A.
Recent studies have begun to identify key proteins that are involved in EV biogenesis. For exosomes, components of the Endosomal Sorting Complex Required for Transport (ESCRT) are known to be involved in formation of MVBs and intralumenal vesicles [53]. In HeLa cells, knockdown of the ESCRT-0/1 proteins hepatocyte-growth-factor-regulated tyrosine kinase substrate (HRS), signal-transducing adaptor molecule 1 (STAM1) and TSG101 reduces EV secretion. Interestingly, inhibition of these components also modulates the nature and content of the vesicles, suggesting that various subtypes of EVs may have different release mechanisms with differential roles in cargo sequestration [54]. ESCRT-independent mechanisms of exosome formation have also been described. In some cells, exosome release requires the sphingolipid ceramide and is reduced after inhibition of neutral sphingomyelinase (n-SMase), an enzyme central to ceramide production [15, 55]. In fact, mice treated with the n-SMase inhibitor GW4869 produce a lower number of lung multiplicities after injection of Lewis lung carcinoma (LLC) cells compared to controls [56]. Multiplicity formation could be rescued by intravenous injection of LLC-derived exosomes, indicating that the observed effect of GW4869 was at least partly mediated by inhibition of exosome production.
The Rab27 family of small GTPases, as well as their effector proteins synaptotagmin-like protein 4 (Slp4) and synaptotagmin-like protein homologue lacking C2 domains b (Slac2b), have been found to be important regulators of exosome release [57, 58]. Alternatively, formation of microvesicles via direct shedding from the cancer cell surface may be controlled by specific signalling pathways triggered by Ras homolog family member A (RhoA) [59] and/or ADP-ribosylation factor 6 (Arf6) [60]. Preliminary results suggest that targeting these pathways may have direct therapeutic significance. Rab27a knockdown via RNA interference (RNAi) results in reduced tumour growth and decreased lung dissemination of breast carcinoma cells, at least partly by preventing exosome-mediated neutrophil mobilization [61]. Similarly, in a melanoma model, Rab27a RNAi reduces exosome release, primary tumour growth and lung metastasis, associated with a decreased number of bone marrow progenitor cells [42].
In most cell types, EV secretion can also be regulated by stimuli that induce a rise in intracellular Ca2+ concentration. It has been shown that in the human erythroleukemia cell line K562, increasing intracellular Ca2+ stimulates EV secretion, and dimethyl amiloride (DMA), an inhibitor of Na+/H+ and Na+/Ca2+ exchangers, decreases constitutive, as well as stimulated EV release [62]. DMA also reduces EV secretion in mice bearing EL4 lymphoma tumours, which abolishes their T-cell-suppressive functions via Stat3 activation. Moreover, in three different murine tumour models, combination therapy of DMA and the alkylating agent cyclophosphamide reduces tumour growth when compared with cyclophosphamide alone [47]. Finally, EV secretion could potentially be modulated by interfering with trafficking and function of endolysosomal compartments in cells through proton pump inhibitors [63].
Together, these preliminary data support the hypothesis that inhibition of EV biogenesis or release may have beneficial effects in the treatment of cancer. One major challenge is to find therapeutic approaches that interfere with these pathways with sufficient specificity in tumour cells without affecting normal cell function, although temporary disruption of normal functions might be tolerated. Encouragingly, recent evidence suggests that malignant cells secrete specialized sets of EVs that are not released by normal cells [64]. In some cases, these are large (1- to 10-µm diameter) vesicles termed oncosomes [65]. Further research may therefore lead to the discovery of pathways specifically involved in biogenesis of unique tumour cell-derived EVs.
Recently, a different strategy to target EV activities has been proposed which consists of removal of EVs from the entire circulatory system, similar to the removal of circulating antibodies in autoimmune disease. Using an affinity plasmapheresis platform such as the ADAPT™ device, developed by Aethlon Medical Inc., it might be possible to specifically capture tumour cell-derived EVs on an antibody-coated matrix during extracorporeal dialysis. For example, in human epidermal growth factor receptor-2 (HER-2) overexpressing breast cancer, where HER-2-expressing EVs have been shown to interfere with therapy and are associated with tumour aggressiveness, anti-HER-2 antibodies could be used to remove HER-2-expressing EVs from circulation with the aim of improving therapeutic outcome [66]. In principal, this approach could be tailored for other tumour types, as long as the tumour cell-derived EVs are enriched for tumour-specific proteins. However, whether the level and duration of EV depletion after ADAPT™ therapy would be sufficient to achieve a clinically relevant outcome remains to be determined.
With regard to inhibition of EV internalization by recipient cells, examples to date are few due to insufficient understanding of the mechanisms involved, highlighting the importance of establishing fundamental knowledge in this area. Nevertheless, in some cases, the uptake of tumour-derived EVs seems to be mediated by phosphatidylserine exposed on the EV surface which can be blocked with Annexin V or its homodimer Diannexin [11, 33]. Daily intraperitoneal injections of Diannexin impair growth and angiogenesis of A431 squamous carcinoma xenografts in SCID mice, which may result partly from interference with EV communication [33]. EVs from human glioblastoma cells are taken up via heparan sulfate proteoglycans (HSPGs) present on recipient cells. This uptake pathway seems to be important for EV function, as treatment with heparin, to compete with HSPGs for EV binding, significantly inhibited EV-induced target cell migration [67]. Heparin also inhibited oncogenic EGFRvIII mRNA transfer by interfering with EV binding to recipient cells [68]. In an attempt to clarify whether EV targeting and uptake is EV source- and recipient cell-dependent, the contribution of EV tetraspanin webs to target cell selection has also been studied [69]. Comparing four EV types derived from rat pancreatic adenocarcinoma cell lines only differing in tetraspanin-8 and CD104 expression, remarkable differences in in vitro as well as in vivo target selectivity were found. Colocalization and pull down experiments subsequently showed specific roles for target cell markers such as CD54 in EV binding. These data suggest that EV binding and uptake is a selective process, examples of which have also been shown in a non-tumour context [70, 71]. Further advances in our understanding of the basis for EV targeting and uptake will most likely provide additional novel targets for anti-cancer therapy. In addition, future studies should indicate if therapies targeting uptake of tumour-derived EVs by recipient cells are sufficiently specific in order to prevent deleterious side effects.
Concluding Remarks
The interest of the scientific community in the role of EVs in cancer progression has expanded rapidly over the last few years. This has led to recognition of the fundamental importance of EVs as key mediators of information transfer, underpinning important processes involved in tumour growth and spread. However, we are only beginning to understand some of the molecular mechanisms underlying EV release and their physiological relevance in vivo. In fact, recent observations expanding the repertoire of types of vesicles and particles secreted by tumour cells have added yet new layers of complexity [64, 72]. A major challenge facing research in the field is to demonstrate the physiological functions of EVs in vivo, due to a lack of tools to specifically induce or interfere with EV release, without affecting release of other EV subtypes or other signalling molecules [73]. Increasing knowledge of EV biology and release will help address this issue and, together with further insights into EV uptake mechanisms and cell targeting behaviour, should also elucidate novel therapeutic strategies, based on inhibition of EV-mediated intercellular communication in cancer.
Highlights.
EVs play important roles in regulating all facets of cancer development and spread
Targeting aspects of EV biogenesis or function could prevent tumour progression
Box 2: Outstanding questions.
What is the relevance of EVs for tumour growth and spread in humans? Is this tumour type-dependent?
Do (sub)populations of EVs serve different physiological functions?
What are the molecular mechanisms mediating tumour cell-derived EV release and uptake in recipient cells?
Does interfering with EV-mediated intercellular communication provide therapeutic benefit in cancer patients?
Acknowledgements
This work was supported by funding from the John Fell Fund (MJW) and NIH NCI CA179563 (Extracellular RNA Consortium) (XOB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
MJW has filed patent applications in relation to extracellular vesicles: WO2010/119256, priority date April 2009; UK1121070.5 and UK1121069.7, filed December 2011. XOB is on the Scientific Advisory Board of Exosome Diagnostics, Inc.
References
- 1.Rak J. Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Front Pharmacol. 2013;4:21. doi: 10.3389/fphar.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corrado C, et al. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 2013;14:5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thery C, et al. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 4.van der Pol E, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 5.El Andaloussi S, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witwer KW, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TH, et al. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular 'debris'. Semin Immunopathol. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 10.Mathivanan S, et al. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers KC, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor DD, et al. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 18.Rajendran L, et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HK, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–191. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 20.Flaumenhaft R, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwicker JI, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JG, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesselaar ME, et al. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 24.Zwicker JI, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study) Br J Haematol. 2013;160:530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2012;2:38. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simona F, et al. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: State of the art and new perspectives. Proteomics. 2013;13:1581–1594. doi: 10.1002/pmic.201200398. [DOI] [PubMed] [Google Scholar]
- 27.Sverdlov ED. Amedeo Avogadro's cry: what is 1 microg of exosomes? Bioessays. 2012;34:873–875. doi: 10.1002/bies.201200045. [DOI] [PubMed] [Google Scholar]
- 28.Yu JL, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 29.Lima LG, et al. Malignant transformation in melanocytes is associated with increased production of procoagulant microvesicles. Thromb Haemost. 2011;106:712–723. doi: 10.1160/TH11-03-0143. [DOI] [PubMed] [Google Scholar]
- 30.Bergsmedh A, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A. 2001;98:6407–6411. doi: 10.1073/pnas.101129998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demory Beckler M, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 33.Al-Nedawi K, et al. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hood JL, et al. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong BS, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 37.Kucharzewska P, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janowska-Wieczorek A, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 40.Yang M, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luga V, et al. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rana S, et al. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hood JL, et al. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 45.Clayton A, et al. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 46.Valenti R, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 47.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vader P, et al. Taxol((R))-induced phosphatidylserine exposure and microvesicle formation in red blood cells is mediated by its vehicle Cremophor((R)) EL. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.12.163. [DOI] [PubMed] [Google Scholar]
- 49.Aung T, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. P Natl Acad Sci USA. 2011;108:15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shedden K, et al. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 51.Safaei R, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 52.Bebawy M, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- 53.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colombo M, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013 doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 55.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 56.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 58.Hendrix A, De Wever O. Rab27 GTPases distribute extracellular nanomaps for invasive growth and metastasis: implications for prognosis and treatment. Int J Mol Sci. 2013;14:9883–9892. doi: 10.3390/ijms14059883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, et al. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740–4749. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muralidharan-Chari V, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bobrie A, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 62.Savina A, et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 63.Iero M, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 64.Palma J, et al. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40:9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Vizio D, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marleau AM, et al. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christianson HC, et al. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atai NA, et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013;115:343–351. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rana S, et al. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 70.Nolte-'t Hoen EN, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 71.Vallhov H, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 72.Bobrie A, Thery C. Exosomes and communication between tumours and the immune system: are all exosomes equal? Biochem Soc Trans. 2013;41:263–267. doi: 10.1042/BST20120245. [DOI] [PubMed] [Google Scholar]
- 73.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathivanan S, et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalra H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smalley DM, et al. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Res. 2008;7:2088–2096. doi: 10.1021/pr700775x. [DOI] [PubMed] [Google Scholar]
- 78.Koga K, et al. Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res. 2005;25:3703–3707. [PubMed] [Google Scholar]
- 79.Balaj L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature Communications. 2011;2 doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 81.Rabinowits G, et al. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 82.Shao HL, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature Medicine. 2012;18:1835. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen WW, et al. BEAMing and Droplet Digital PCR Analysis of Mutant IDH1 mRNA in Glioma Patient Serum and Cerebrospinal Fluid Extracellular Vesicles. Mol Ther Nucleic Acids. 2013;2:e109. doi: 10.1038/mtna.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heijnen HF, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 85.Wolfers J, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 86.Antonyak MA, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hristov M, et al. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 88.Thery C, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]