Abstract
Protein prenylation is an important lipid posttranslational modification of proteins. It includes protein farnesylation and geranylgeranylation, in which the 15-carbon farnesyl pyrophosphate or 20-carbon geranylgeranyl pyrophosphate is attached to the C-terminus of target proteins, catalyzed by farnesyl transferase or geranylgeranyl transferases, respectively. Protein prenylation facilitates the anchoring of proteins into the cell membrane and mediates protein-protein interactions. Among numerous proteins that undergo prenylation, small GTPases represent the largest group of prenylated proteins. Small GTPases are involved in regulating a plethora of cellular functions including synaptic plasticity. The prenylation status of small GTPases determines the subcellular locations and functions of the proteins. Dysregulation or dysfunction of small GTPases leads to the development of different types of disorders. Emerging evidence indicates that prenylated proteins, in particular small GTPases, may play important roles in the pathogenesis of Alzheimer’s disease. This review focuses on the prenylation of Ras and Rho subfamilies of small GTPases and its relation to synaptic plasticity and Alzheimer’s disease.
Keywords: Protein prenylation, farnesylation, geranylgeranylation, small GTPases, synaptic plasticity, Alzheimer’s disease
Introduction
Many proteins undergo posttranslational modifications that allow for proper protein folding, trafficking, and function [1]. These modifications often include the addition of functional groups such as phosphates, lipids and carbohydrates. The functions of proteins are regulated by posttranslational modifications. One type of lipid posttranslational modification is prenylation [2]. Prenylation refers to the addition of short-chain lipid molecules called isoprenoids to the C-terminus of target proteins. These lipid attachments facilitate the anchoring of proteins to the cell membrane and mediate protein-protein interactions. Prenylated proteins are involved in regulating a variety of cellular functions including synaptic plasticity and in the pathogenesis of a number of diseases including Alzheimer’s disease (AD).
AD is the most common cause of dementia, affecting approximately 11% of the population aged 65 years and older [3]. AD is a neurodegenerative disorder characterized clinically by impaired episodic memory. The pathological hallmarks of AD are intracellular neurofibrillary tangles and deposits of aggregated amyloid-β protein (Aβ) in neuritic plaques and cerebral vessels [4]. Importantly, failures of synaptic plasticity are thought to represent early events in AD progression [5]. However, the relationship between the neuropathology and the behavioral changes is not fully understood. Emerging evidence indicates that alterations in the level and function of some small GTPases may contribute to the pathogenesis of AD [6,7]. The focus of this review is on the prenylation of small GTPases, in particular the Ras and Rho subfamilies of proteins, and its relation to synaptic plasticity and AD.
Isoprenoids and Protein Prenylation
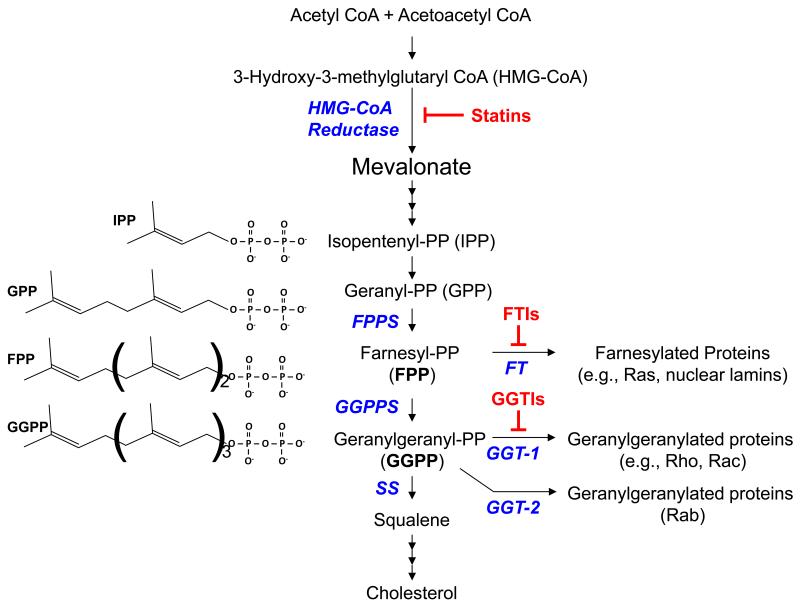
Isoprenoids are short-chain lipid molecules formed in the mevalonate pathway for cholesterol biosynthesis [8] (Fig. 1). The 15-carbon isoprenoid, farnesyl pyrophosphate (FPP), is a major branching point in the mevalonate pathway. FPP serves as a substrate for several enzymes, including squalene synthase (SS), farnesyl transferase (FT), and GGPP synthase (GGPPS) that produces the 20-carbon geranylgeranyl pyrophosphates (GGPP). FPP is also a precursor for the synthesis of long-chain isoprenoids such as dolichol and ubiquinone (coenzyme Q), and heme. FPP and GGPP serve as lipid donors for protein prenylation.
Fig. 1. The mevalonate pathway.
HMG-CoA reductase is a rate-limiting enzyme in the mevalonate pathway. Statins inhibit the activity of HMG-CoA reductase and limit the production of isoprenoid intermediates and cholesterol. Farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) serve as lipid donors for protein prenylation. Farnesyl transferase inhibitors (FTIs) and geranylgeranyl transferase-1 inhibitors (GGTIs) block protein farnesylation and geranylgeranylation, respectively (see text for details). PP, pyrophosphate; FPPS, farnesyl PP synthase; GGPPS, geranylgeranyl PP synthase; SS, squalene synthase; FT, farnesyl transferase; GGT-1, geranylgeranyl transferase-1; GGT-2, geranylgeranyl transferase-2 (a.k.a., RabGGT).
During protein farnesylation and geranylgeranylation, collectively called protein prenylation, FPP and GGPP are covalently attached to the C-terminus of target proteins, respectively [2]. Farnesylation is catalyzed by protein farnesyl transferase (FT) and occurs on cysteine residues present in tetrapeptide recognition sequences (CaaX, in which C is cysteine, a is an aliphatic amino acid and X is variable) located at the C-termini of their cognate protein substrates. In contrast, geranylgeranylation is catalyzed by two different protein geranylgeranyl transferases. Geranylgeranyl transferase-1 (GGT-1) acts on substrates that contain C-terminal tetrapeptide sequences similar to but distinct from FT substrates, whereas geranylgeranyl transferase-2 (GGT-2 or RabGGT) recognizes more structurally complex sequences and exclusively prenylates Rab proteins [2,9]. Protein prenylation is an important posttranslational modification that allows proteins to anchor to the cell membrane or other subcellular locations and mediates protein-protein interactions [10].
Statins are drugs commonly used to regulate cholesterol levels. Stains work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, a rate limiting step in the mevalonate pathway for cholesterol biosynthesis that converts acetyl-CoA to mevalonate (Fig. 1). Inhibition of HMG-CoA reductase results in a decreased level of FPP and GGPP, and thus, may lead to decreased farnesylation and geranylgeranylation of proteins [11,12]. As such, it is difficult to dissect the roles of farnesylation and geranylgeranylation using statins. To this end, drugs have been developed to specifically target FT and GGT-1 [13]. Farnesyl transferase inhibitors (FTIs) and geranylgeranyl transferase-1 inhibitors (GGTIs) are powerful tools for studying the function of specific prenylation pathways. These drugs are currently under investigation for the treatment of cancers and other disorders [14].
Over 100 proteins are known to undergo prenylation [10,13]. They include heterotrimeric G protein subunits and nuclear lamins but the largest and most extensively studied group is the Ras superfamily of small GTPases.
Ras Superfamily of Small GTPases
The Ras GTPase superfamily consists of over 150 known members, divided between five major subfamilies: Ras, Rho, Rab, Arf/Sar and Ran [15]. Specific small GTPases regulate a number of effector proteins and may have different final intracellular locations, and in some cases, differential prenylation can affect the subcellular distribution and function of small GTPases [16,17].
In general, small GTPases act as molecular switches that are activated, or ‘turned on’ by guanine nucleotide exchange factors (GEFs) and inhibited, or ‘turned off’ by GTPase-activating proteins (GAPs) [18,19]. GEFs promote the dissociation of guanosine diphosphate (GDP) from small GTPases [20]. This dissociation step allows the exchange of GDP for guanosine triphosphate (GTP). A small GTPase is considered ‘active’ when GTP is bound. Antagonistically, GAPs enhance the rate of the weak intrinsic GTP hydrolysis activity of small GTPases [21]. Small GTPases depend on prenylation for proper cellular localization and function [10]. Inhibiting small GTPase prenylation affects many cellular functions such as cytoskeletal stability and the efficiency of vesicular transport [22]. More recent studies have also revealed that the interplay between small GTPase GEFs and GAPs regulates spine morphogenesis and synapse development [23]. Interestingly, GGT-1 itself has been shown to have a direct role in neuromuscular junction formation and maintenance by controlling acetyl choline receptor rearrangement during development [24]. In addition to morphological changes, small GTPases are involved in multiple signaling pathways that regulate synaptic plasticity [25].
Synaptic Plasticity
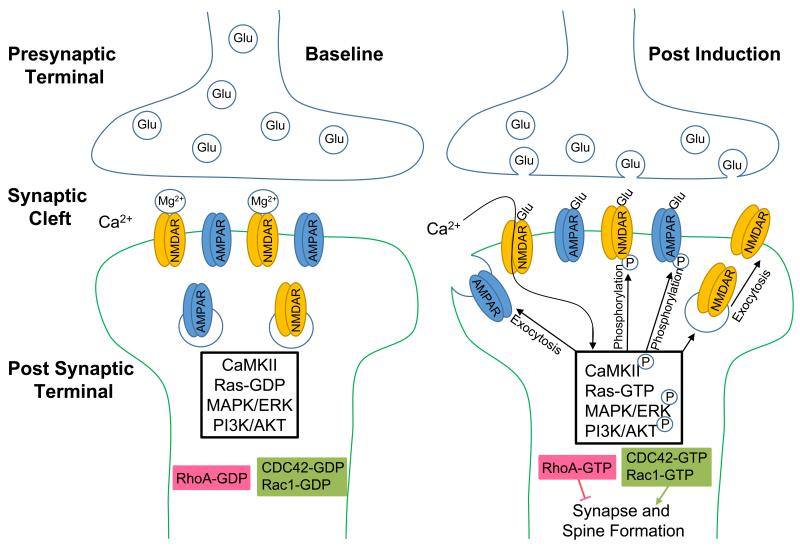
In its most general definition, synaptic plasticity is the strengthening or weakening of the synapse between two neurons over time [26]. Synaptic plasticity has many underlying mechanisms, including pre-synaptic changes regulating the amount of neurotransmitter released, and post-synaptic changes such as the incorporation of new neurotransmitter receptors [27]. In general, both the strengthening and weakening of a synapse depends on calcium uptake. Two major molecular mechanisms of synaptic plasticity in the hippocampus include the regulation and activity of N-methyl D-aspartate (NMDA) and α-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors [28] (Fig. 2). Notably, before NMDA receptors open their ion channel, glutamate must be present at the receptor while the post-synaptic cell is depolarized [29]. This is due to a magnesium block in the NMDA channel pore that must be expelled by a reduction in the voltage across the post-synaptic cell membrane [30]. Once open, NMDA channels allow for calcium to rush into the cell and trigger downstream signaling cascades that alter the synaptic strength between the two cells [30,31]. These NMDA receptors act as a ‘coincidence detector’ because two events need to occur within a narrow temporal window to allow calcium to flow into the post-synaptic cell [32].
Fig. 2. Schematic diagram of a synapse.
A) At resting membrane potentials, NMDA receptors are blocked by Mg2+. Low intracellular concentrations of Ca2+ prevent the autophosphorylation of Ca2+/calmodulin-dependent protein kinase (CaMKII). B) Post-synaptic depolarization pushes the Mg2+ out of the NMDA channel pore, allowing Ca2+ to enter the cell. Glutamate (Glu) from the pre-synaptic cell binds to AMPA and NMDA receptors to depolarize the post-synaptic cell. The rise in intracellular Ca2+ promotes CaMKII autophosphorylation. Once phosphorylated, CaMKII phosphorylates AMPA subunits to enhance their conductance. CaMKII also promotes the exocytosis of receptor containing vesicles and thereby increases the presence of receptors at the synapse. Furthermore, CaMKII activates RasGEFs (guanine exchange factors) and promotes the turnover of inactive Ras-GDP to active Ras-GTP. Downstream signaling cascades include mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT). When activated (phosphorylated), ERK and AKT facilitate exocytosis of glutamate receptors during synaptic plasticity. In addition, neuronal activity activates Rho GTPases. Active (GTP-bound) Rac1/Cdc42 enhance while RhoA inhibits synapse/spine formation.
Calcium influx from NMDA receptors is necessary for the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) [33]. The activation of NMDA receptors is a mechanism to transfer the information of mutual depolarization across the synapse. If the two cells continue to fire strongly together, their synapses will strengthen over time by the addition of AMPA and NMDA receptors to the post-synaptic cell membrane, or phosphorylation of the subunits of existing AMPA and NMDA receptors in the membrane [34,35]. The changes in synaptic potentiation exist in equilibrium, and therefore, AMPA and NMDA receptors can be removed/dephosphorylated from synapses.
Synaptic plasticity can be divided into two major categories, short-term plasticity, and long-term plasticity. Long-term plasticity can be further separated into long-term depression (LTD), early long-term potentiation (E-LTP), and late long-term potentiation (L-LTP) [36-38]. LTD is the weakening of synaptic strength over time, often by removal or dephosphorylating of post-synaptic receptors. E-LTP is the enhancement of synaptic strength that is not dependent on protein synthesis. L-LTP has the longest lasting effects, and is dependent on synthesis of new proteins in response to increased synaptic activity. Small GTPases are involved in regulating multiple aspects of synaptic plasticity and the following sections will briefly discuss the roles of Ras and Rho subfamilies of proteins in synaptic function.
Ras and Synaptic Plasticity
Ras subfamily GTPases were originally studied for their role in oncogenesis. Constitutively active mutations in Ras small GTPases occur in 8% to 93% of cancers depending on the tumor type [39]. This underscores the importance of proper regulation of small GTPases. Normally, Ras small GTPases play crucial roles in regulating cell proliferation, differentiation, cell survival, and memory formation [40,25]. The well-known members of Ras subfamily of small GTPases include H-Ras, K-Ras, and N-Ras. Ras small GTPases primarily undergo farnesylation but some of them can also undergo geranylgeranylation. For example, while H-Ras is exclusively farnesylated, K-Ras and N-Ras can be geranylgeranylated when farnesyltransferase is inhibited [13]. Major downstream signaling cascades of Ras include mitogen-activated protein kinases (MAPK), such as extracellular signal-regulated kinase (ERK), and phosphoinositide 3-kinase (PI3K), which regulate glutamate receptor trafficking during synaptic plasticity (Fig. 2) [25,41].
Several lines of evidence indicate that H-Ras plays a negative role in regulating synaptic plasticity and memory function. In a mouse model of neurofibromatosis type 1 mental retardation, which is characterized by the hyperactivity of Ras, learning/memory and synaptic function are severely impaired [42,43]. Treatment with an FTI or a statin rescues hyperactive Ras-induced synaptic and memory impairment [42,43]. Similarly, H-Ras overexpression negatively affects the NMDA receptor transmission by decreasing the level of tyrosine phosphorylation of the NMDA receptor NR2A subunit [44]. In contrast, mice deficient in H-Ras expression display enhanced tyrosine phosphorylation of NMDA receptors and NMDA receptor-mediated hippocampal LTP [45]. These data support the role of Ras small GTPases in regulating NMDA receptor dependent synaptic plasticity.
Rho and Synaptic Plasticity
The Rho subfamily of GTPases primarily undergoes geranylgeranylation, although some are exclusively farnesylated (e.g., RhoE). Others, such as RhoB, can be either farnesylated or geranylgeranylated [46,47]. Interestingly, geranylgeranylated and farnesylated RhoB exhibit distinct and opposite functions. Geranylgeranylated RhoB inhibits cell growth, whereas farnesylated RhoB promotes cell growth and transformation [16,17]. However, for some Rho GTPases, although their functions depend on being prenylated, either geranylgeranylated or farnesylated form works equally. For example, RhoA is exclusively geranylgeranylated under physiological conditions; when RhoA is mutated to become susceptible to farnesylation, the farnesylated RhoA shows similar subcellular location and functions as the geranylgeranylated RhoA [48].
The Rho subfamily of GTPases is one of the major regulators in synaptic plasticity, both in dendrite morphogenesis and stability as well as in growth cone motility [49,50,19]. Rho proteins are well documented for their role in the regulation of actin rearrangement in neuronal cytoskeletons. Specifically, three major Rho proteins, RhoA, Rac1, and Cdc42, regulate neuronal structures and synaptic connectivity [49,50,19]. When activated by GEFs, Rho small GTPases interact with effector proteins, initiating signaling cascades that control actin cytoskeletal rearrangement, microtubule rearrangement, transcription, membrane trafficking, and act as key regulators of dendritic growth and spine morphogenesis [49,50,19] (Fig. 2).
The interaction between the Rho GTPases determines the complexity of the dendritic tree and the formation of spines. RhoA, Rac1, and Cdc42 play differential roles in regulating dendritic growth and spine formation. Activation of Rac1 and Cdc42 promotes dendritic branching/remodeling and spine formation, whereas activation of RhoA exhibits opposite function, reducing dendritic growth/complexity and spine density/length [50]. Rac1 is highly expressed in the hippocampus of adult mice [51]. The hippocampus is well known for its synaptic plasticity and its importance in developing associative memories [37,52]. Rac1 plays an important role in the formation of neuronal synapses at their correct locations. Specifically, in vitro studies show that NMDA receptor activation induces membrane translocation and activation of Rac1 in the CA1 region of the hippocampus [51]. Activation of tyrosine kinase receptor B (TrkB) by brain-derived neurotropic factor (BDNF) leads to the activation of Rac1 and induces changes in cellular morphology [53]. Notably, BDNF-dependent dendritic morphogenesis requires the activation of GGT-1, the enzyme that catalyzes the geranylgeranylation of Rac1 and other Rho proteins [54]. In addition, TrkB is physically associated with GGT-1 and neuronal activity enhances this association and GGT-1 activity, further promoting dendritic spine morphogenesis [54]. Conversely, activation of RhoA inhibits dendritic growth and spine formation in multiple model systems [50]. The negative role of RhoA on dendritic growth and spine morphogenesis is partly mediated by the RhoA effector Rho-kinase (ROCK) [55]. Specific inhibitors of ROCK can block active RhoA-induced dendritic simplification [55]. The balance between the positive and negative effects of Rac1/Cdc42 and RhoA guarantees the proper development of dendrites and dendritic spines that are important postsynaptic structures regulating synaptic plasticity.
Implications for Alzheimer’s Disease
AD is a progressive neurodegenerative disease with a behavioral characterization of impaired episodic memory. Pathologically, AD is defined by amyloid plaques and tau tangles that have been seen in post-mortem brain tissues. However, the relationship between the neuropathology and the behavioral changes is not completely understood.
In the brain of AD patients, Aβ accumulates as the disease progresses. The structural integrity of synapses degrades rapidly during β-amyloidosis [56], with the longer amyloidogenic Aβ42 being more potent than Aβ40 in disrupting synaptic plasticity [57]. One of the mechanisms by which Aβ impairs synaptic function is by promoting endocytosis of NMDA receptors and thereby reducing the presence of NMDA receptors at the cell surface [58]. Importantly, the impairment of synaptic function in the hippocampus occurs prior to the appearance of insoluble amyloid plaques and neuronal cell death [5]. However, inhibition of Aβ-producing enzymes under normal conditions results in abnormalities in synaptic function [59]. These findings suggest that Aβ itself may have normal physiological functions which are disrupted by abnormal accumulation of Aβ during AD pathology.
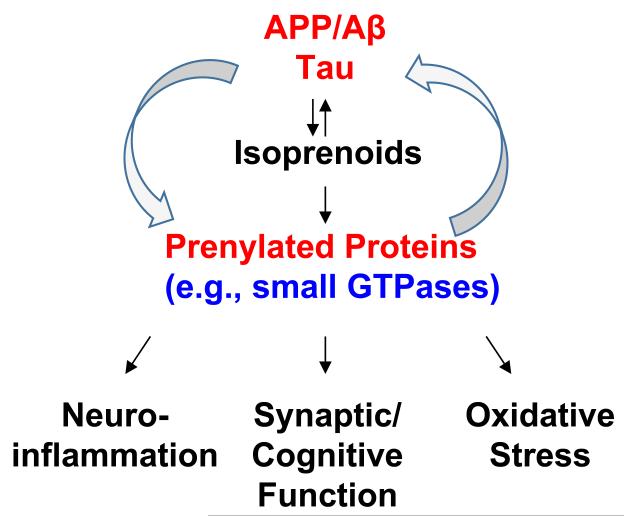
Emerging evidence indicates that isoprenoids/protein prenylation and small GTPases affect multiple aspects of AD (Fig. 3) [6,7]. For example, statin-induced depletion of isoprenoids leads to reduced levels of protein prenylation and promotes non-amyloidogenic processing of APP and reduces the production of Aβ [60-63]. Interestingly, while geranylgeranylated RhoA-mediated activation of ROCK increases Aβ secretion via modulation of γ-secretase [64], specific inhibition of farnesylated RhoB/ROCK pathway promotes α-secretase activity [60]. Of note, although inhibitors of ROCK reduce total Aβ secretion, targeting ROCK by expression of dominant-negative or constitutively active ROCK mutants failed to modulate Aβ secretion [65]. Additional in vitro experiments show that statin-induced low isoprenoid conditions cause the accumulation of intracellular APP, the C-terminal fragment of APP produced by β-secretase cleavage (β-CTF), and Aβ, which can be rescued by GGPP supplementation, suggesting the involvement of geranylgeranylated target proteins [61]. The study also shows that low isoprenoid levels inhibit the trafficking of APP through the secretory pathway [61]. A more recent study further demonstrates that low isoprenoid conditions induced by physiologically relevant doses of statins preferentially inhibit the geranylgeranylation of Rab family proteins involved in vesicle trafficking and thereby affects the trafficking and intracellular localization of APP [62]. Inhibition of Rac also regulates APP expression and processing [66,67]. In contrast, supplementation of FPP and/or GGPP stimulates the production of Aβ [63,64,68].
Fig. 3. Schematic diagram of interplay between prenylated proteins and AD pathology.
Intriguingly, the interplay between isoprenoids/prenylated proteins and APP/Aβ metabolism appears to be reciprocal. It has been shown that Aβ and other APP cleavage products such as APP intracellular domain (AICD) may directly regulate the activities of the enzymes in the mevalonate pathway thereby changing the levels of isoprenoids and other lipids [69,70]. Consistent with these findings, the levels of FPP and GGPP are elevated in the brains of patients with AD [71], suggesting that the abundance of prenylated proteins could be increased in AD brains. Indeed, the level of Ras (both cytosolic and membrane/prenylated fractions) in the brain is increased in the early stage of AD [72,73], suggest that upregulation of Ras may play an important role in the pathogenic cascade leading to AD. Aβ causes cellular dislocation and dysfunction of Rac and its effector protein PAK [74,75]. Also, the level of prenylated RhoA is increased in Aβ-treated neuroblastoma cells and in the neurons surrounding Aβ plaques in AD mice [76]. Conversely, a recent study shows that a toxic level of oligomeric Aβ42 inhibits protein prenylation [77].
In addition to APP/Aβ metabolism, prenylation/GTPases have been shown to be involved in other aspects of AD pathology. For instance, inhibition of prenylation of Rho GTPases leads to attenuation of Aβ-induced neuroinflammation [78,79]. Limiting the availability of isoprenoids for prenylation has been shown to protect neurons from Aβ-induced apoptosis via activating pro-survival signaling pathways [80-82]. Activation of the prenylated protein Rac1 has been shown to contribute to increased oxidative stress in AD [83,84]. Inhibition of Rho prenylation decreases total and phosphorylated tau levels [85]. We and others have also shown that manipulation of isoprenoid and protein prenylation levels modulates synaptic plasticity and cognitive function in animal models [86-88,25,42]. Intriguingly, inhibiting the level of FPP/farnesylation, but not GGPP/geranylgeranylation, enhances hippocampal synaptic plasticity in brain slices of mature C57BL/6 mice [87]. Consistent with these results, our most recent study indicates that haplodeficiency in farnesyl transferase, but not geranylgeranyl transferase-1, rescues cognitive function as well as attenuates Aβ-associated neuropathology and neuroinflammation in a mouse model of AD [89]. Taken together, these findings strongly suggest that alteration of small GTPases is implicated in the pathogenesis of AD and that modulation of protein prenylation, in particular protein farnesylation, may present a potential therapeutic strategy for AD.
Concluding Remarks/Perspectives
Protein prenylation is a critical lipid posttranslational modification of many important proteins. Particularly, it plays a key role in determining the cellular localization and functions of small GTPases. Small GTPases control signaling pathways that regulate a plethora of cellular functions including synaptic plasticity, and dysregulation or dysfunction of small GTPases leads to different types of disorders. Emerging evidence indicates that protein prenylation plays an important role in the development of AD. However, clinical trials using statins in patients with AD have not shown consistent benefits [90,91]. While differences in the blood-brain barrier permeability and the dose of statins, the population of subjects, and the stage of the disease at which statins are administered could all contribute to the discrepancies in clinical outcomes, one critical missing point is the fact that statins inhibit the production of FPP and GGPP simultaneously [71] and thus may affect both farnesylation and geranylgeranylation pathways. Importantly, farnesylated and geranylgeranylated proteins are involved in regulating distinct cellular functions [18]. Results from recent studies suggest that specific inhibition of protein farnesylation but not geranylgeranylation enhances synaptic and cognitive function as well as reduces AD pathology, suggesting the potential of FTIs as therapeutic agents for AD.
Several FTIs are in clinical trials mainly for cancer treatment [14]. FTIs have also been proposed to treat neurodegenerative diseases in a patent application [92] and one FTI (LNK-754) has been tested in Phase I clinical trials for safety, tolerability and pharmacokinetics in healthy elderly volunteers and in subjects with mild AD (http://clinicaltrials.gov).
Further studies are needed to elucidate the role of protein prenylation, in particular farnesylation, on the onset and progression of AD. Until then, FTIs developed originally for the treatment of cancers may translate well to the treatment of AD.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (AG031846), the Alzheimer’s Association (IIRG-09-131791), the BrightFocus Foundation (formerly American Health Assistance Foundation) (A2010328), the Alzheimer’s Drug Discovery Foundation, and the Academic Health Center of the University of Minnesota.
References
- 1.Krishna RG, Wold F. Post-translational modification of proteins. Advances in enzymology and related areas of molecular biology. 1993;67:265–298. doi: 10.1002/9780470123133.ch3. [DOI] [PubMed] [Google Scholar]
- 2.Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47(4):681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. doi:10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. doi:10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 6.Cole SL, Vassar R. Isoprenoids and Alzheimer’s disease: a complex relationship. Neurobiol Dis. 2006;22(2):209–222. doi: 10.1016/j.nbd.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hooff GP, Wood WG, Muller WE, Eckert GP. Isoprenoids, small GTPases and Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):896–905. doi: 10.1016/j.bbalip.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 9.Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. geranylgeranylation of Rab GTPases. J Lipid Res. 2006;47(3):467–475. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63(3):255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110(3):285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond lipid lowering. Am J Cardiol. 2003;91(4A):23B–29B. doi: 10.1016/s0002-9149(02)03270-8. [DOI] [PubMed] [Google Scholar]
- 13.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11(11):775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Zhang W, Cheng S, Cao D, Parent M. Isoprenoids and related pharmacological interventions: potential application in Alzheimer’s disease. Mol Neurobiol. 2012;46(1):64–77. doi: 10.1007/s12035-012-8253-1. doi:10.1007/s12035-012-8253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Viciana P, Sabatier C, McCormick F. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol Cell Biol. 2004;24(11):4943–4954. doi: 10.1128/MCB.24.11.4943-4954.2004. doi:10.1128/MCB.24.11.4943-4954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du W, Lebowitz PF, Prendergast GC. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol Cell Biol. 1999;19(3):1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu A, Du W, Liu JP, Jessell TM, Prendergast GC. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol Cell Biol. 2000;20(16):6105–6113. doi: 10.1128/mcb.20.16.6105-6113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Klooster JP, Hordijk PL. Targeting and localized signalling by small GTPases. Biology of the cell / under the auspices of the European Cell Biology Organization. 2007;99(1):1–12. doi: 10.1042/BC20060071. doi:10.1042/BC20060071. [DOI] [PubMed] [Google Scholar]
- 19.Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 2011;94(2):133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16(13):1587–1609. doi: 10.1101/gad.1003302. doi:10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 21.Bernards A, Settleman J. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 2004;14(7):377–385. doi: 10.1016/j.tcb.2004.05.003. doi:10.1016/j.tcb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2(5):303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. Synaptic plasticity, a symphony in GEF. ACS chemical neuroscience. 2010;1(5):348–365. doi: 10.1021/cn100012x. doi:10.1021/cn100012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo ZG, Je HS, Wang Q, Yang F, Dobbins GC, Yang ZH, Xiong WC, Lu B, Mei L. Implication of geranylgeranyltransferase I in synapse formation. Neuron. 2003;40(4):703–717. doi: 10.1016/s0896-6273(03)00695-0. [DOI] [PubMed] [Google Scholar]
- 25.Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 2010;68(3):340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes JR. Post-tetanic potentiation. Physiol Rev. 1958;38(1):91–113. doi: 10.1152/physrev.1958.38.1.91. [DOI] [PubMed] [Google Scholar]
- 27.Gerrow K, Triller A. Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol. 2010;20(5):631–639. doi: 10.1016/j.conb.2010.06.010. doi:10.1016/j.conb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 29.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological reviews. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 30.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 31.Premkumar LS, Auerbach A. Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron. 1996;16(4):869–880. doi: 10.1016/s0896-6273(00)80107-5. [DOI] [PubMed] [Google Scholar]
- 32.Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. doi:10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 33.Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994;17(10):406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 34.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 35.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272(51):32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 36.Sweatt JD. Toward a molecular explanation for long-term potentiation. Learn Mem. 1999;6(5):399–416. doi: 10.1101/lm.6.5.399. [DOI] [PubMed] [Google Scholar]
- 37.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 38.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 40.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6(7):541–555. doi: 10.1038/nrd2221. doi:10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 41.Stornetta RL, Zhu JJ. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2011;17(1):54–78. doi: 10.1177/1073858410365562. doi:10.1177/1073858410365562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15(21):1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 44.Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem. 2003;278(26):23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20(7):2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267(28):20033–20038. [PubMed] [Google Scholar]
- 47.Baron R, Fourcade E, Lajoie-Mazenc I, Allal C, Couderc B, Barbaras R, Favre G, Faye JC, Pradines A. RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody. Proc Natl Acad Sci U S A. 2000;97(21):11626–11631. doi: 10.1073/pnas.97.21.11626. doi:10.1073/pnas.97.21.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solski PA, Helms W, Keely PJ, Su L, Der CJ. RhoA biological activity is dependent on prenylation but independent of specific isoprenoid modification. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 2002;13(8):363–373. [PMC free article] [PubMed] [Google Scholar]
- 49.Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71(6):528–553. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64(1):58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- 51.Tejada-Simon MV, Villasana LE, Serrano F, Klann E. NMDA receptor activation induces translocation and activation of Rac in mouse hippocampal area CA1. Biochem Biophys Res Commun. 2006;343(2):504–512. doi: 10.1016/j.bbrc.2006.02.183. doi:10.1016/j.bbrc.2006.02.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26(4):1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. doi:10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103(27):10444–10449. doi: 10.1073/pnas.0603914103. doi:10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG. TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci U S A. 2008;105(44):17181–17186. doi: 10.1073/pnas.0800846105. doi:10.1073/pnas.0800846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20(14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klyubin I, Cullen WK, Hu NW, Rowan MJ. Alzheimer’s disease Abeta assemblies mediating rapid disruption of synaptic plasticity and memory. Molecular brain. 2012;5:25. doi: 10.1186/1756-6606-5-25. doi:10.1186/1756-6606-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomura I, Takechi H, Kato N. Intraneuronally injected amyloid beta inhibits long-term potentiation in rat hippocampal slices. J Neurophysiol. 2012;107(9):2526–2531. doi: 10.1152/jn.00589.2011. doi:10.1152/jn.00589.2011. [DOI] [PubMed] [Google Scholar]
- 58.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Megill A, He K, Kirkwood A, Lee HK. Consequences of inhibiting amyloid precursor protein processing enzymes on synaptic function and plasticity. Neural plasticity. 2012;2012:272374. doi: 10.1155/2012/272374. doi:10.1155/2012/272374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005;2(1):e18. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem. 2005;280(19):18755–18770. doi: 10.1074/jbc.M413895200. [DOI] [PubMed] [Google Scholar]
- 62.Ostrowski SM, Wilkinson BL, Golde TE, Landreth G. Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem. 2007;282(37):26832–26844. doi: 10.1074/jbc.M702640200. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K. Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. Faseb J. 2008;22(1):47–54. doi: 10.1096/fj.07-8175com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302(5648):1215–1217. doi: 10.1126/science.1090154. doi:10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- 65.Leuchtenberger S, Kummer MP, Kukar T, Czirr E, Teusch N, Sagi SA, Berdeaux R, Pietrzik CU, Ladd TB, Golde TE, Koo EH, Weggen S. Inhibitors of Rho-kinase modulate amyloid-beta (Abeta) secretion but lack selectivity for Abeta42. J Neurochem. 2006;96(2):355–365. doi: 10.1111/j.1471-4159.2005.03553.x. doi:10.1111/j.1471-4159.2005.03553.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang PL, Niidome T, Akaike A, Kihara T, Sugimoto H. Rac1 inhibition negatively regulates transcriptional activity of the amyloid precursor protein gene. J Neurosci Res. 2009;87(9):2105–2114. doi: 10.1002/jnr.22039. doi:10.1002/jnr.22039. [DOI] [PubMed] [Google Scholar]
- 67.Boo JH, Sohn JH, Kim JE, Song H, Mook-Jung I. Rac1 changes the substrate specificity of gamma-secretase between amyloid precursor protein and Notch1. Biochem Biophys Res Commun. 2008;372(4):913–917. doi: 10.1016/j.bbrc.2008.05.153. doi:10.1016/j.bbrc.2008.05.153. [DOI] [PubMed] [Google Scholar]
- 68.Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11(5):545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 69.Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7(11):1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 70.Grimm MO, Rothhaar TL, Hartmann T. The role of APP proteolytic processing in lipid metabolism. Exp Brain Res. 2012;217(3-4):365–375. doi: 10.1007/s00221-011-2975-6. [DOI] [PubMed] [Google Scholar]
- 71.Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Muller WE, Wood WG. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009;35(2):251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gartner U, Holzer M, Arendt T. Elevated expression of p21ras is an early event in Alzheimer’s disease and precedes neurofibrillary degeneration. Neuroscience. 1999;91(1):1–5. doi: 10.1016/s0306-4522(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 73.Gartner U, Holzer M, Heumann R, Arendt T. Induction of p21ras in Alzheimer pathology. Neuroreport. 1995;6(10):1441–1444. doi: 10.1097/00001756-199507100-00020. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–242. doi: 10.1038/nn1630. doi:10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 75.Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008;283(20):14132–14143. doi: 10.1074/jbc.M708034200. doi:10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH. The beta-amyloid protein of Alzheimer’s disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain. 2008;131(Pt 1):90–108. doi: 10.1093/brain/awm260. [DOI] [PubMed] [Google Scholar]
- 77.Mohamed A, Saavedra L, Di Pardo A, Sipione S, Posse de Chaves E. beta-amyloid inhibits protein prenylation and induces cholesterol sequestration by impairing SREBP-2 cleavage. J Neurosci. 2012;32(19):6490–6500. doi: 10.1523/JNEUROSCI.0630-12.2012. doi:10.1523/JNEUROSCI.0630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordle A, Landreth G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25(2):299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280(40):34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 80.Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, Schubert-Zsilavecz M, Karas M, Muller WE, Wood WG. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther. 2005;312(2):786–793. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 81.Franke C, Noldner M, Abdel-Kader R, Johnson-Anuna LN, Gibson Wood W, Muller WE, Eckert GP. Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis. 2007;25(2):438–445. doi: 10.1016/j.nbd.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Cespedes-Rubio A, Jurado FW, Cardona-Gomez GP. p120 catenin/alphaN-catenin are molecular targets in the neuroprotection and neuronal plasticity mediated by atorvastatin after focal cerebral ischemia. J Neurosci Res. 2010;88(16):3621–3634. doi: 10.1002/jnr.22511. [DOI] [PubMed] [Google Scholar]
- 83.Lee M, You HJ, Cho SH, Woo CH, Yoo MH, Joe EH, Kim JH. Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to beta-amyloid in C6 astroglioma cells. Biochem J. 2002;366(Pt 3):937–943. doi: 10.1042/BJ20020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28(46):12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamano T, Yen SH, Gendron T, Ko LW, Kuriyama M. Pitavastatin decreases tau levels via the inactivation of Rho/ROCK. Neurobiol Aging. 2012;33(10):2306–2320. doi: 10.1016/j.neurobiolaging.2011.10.020. doi:10.1016/j.neurobiolaging.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Mans RA, Chowdhury N, Cao D, McMahon LL, Li L. Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience. 2010;166(2):435–444. doi: 10.1016/j.neuroscience.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mans RA, McMahon LL, Li L. Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience. 2012;202:1–9. doi: 10.1016/j.neuroscience.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60(6):729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- 89.Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L. Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease. J Biol Chem. 2013;288(50):35952–35960. doi: 10.1074/jbc.M113.503904. doi:10.1074/jbc.M113.503904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. review of human trials and recommendations. Arch Neurol. 2010;68(11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2010;68(10):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lansbury PT, Jr., Justman CJ, Grammatopoulos TN, Lynch BA, Liu Z. Treatment of proteinopathies using a farnesyl transferase inhibitor. United States Patent 20100160372. 2010