Abstract
Chitosan (CS), a polysaccharide derived from chitin, the second most abundant polysaccharide, is widely used in the medical world because of its natural and nontoxic properties and its innate ability for antibacterial and hemostasis effects. In this study, the novel composites containing CS and cellulose (CEL) (i.e., [CEL + CS]), which we have previously synthesized using a green and totally recyclable method, were investigated for their antimicrobial activity, absorption of anticoagulated whole blood, anti-inflammatory activity through the reduction of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and the biocompatibility with human fibroblasts. The [CEL + CS] composites were found to inhibit the growth of both Gram positive and negative microorganisms. For examples, the regenerated 100% lyophilized chitosan material was found to reduce growth of Escherichia coli (ATCC 8739 and vancomycin resistant Enterococcus faecalis (ATCC 51299) by 78, 36, and 64%, respectively. The composites are nontoxic to fibroblasts; that is, fibroblasts, which are critical to the formation of connective tissue matrix were found to grow and proliferate in the presence of the composites. They effectively absorb blood, and at the same rate and volume as commercially available wound dressings. The composites, in both air-dried and lyophilized forms, significantly inhibit the production of TNF-α and IL-6 by stimulated macrophages. These results clearly indicate that the biodegradable, biocompatible and nontoxic [CEL + CS] composites, particularly those dried by lyophilizing, can be effectively used as a material in wound dressings.
Keywords: chitosan, cellulose, composite material, wound healing, antimicrobial, biocompatible
INTRODUCTION
Chitosan (CS), a linear polysaccharide derived from the N-deacetylation of chitin, which is the second most abundant polysaccharide, is known to possess unique properties including wound healing, hemostasis, antibacterial activity, and is an effective drug carrier.1–9 These unique properties of CS work in concert to synergistically influence three of the four phases of wound healing. In the coagulation phase, the hemostatic effect of CS stops hemorrhage, which is generally more of a problem in acute than chronic wounds. For wound healing to occur, all cells involved in the coagulation, inflammatory, and proliferative phases survive in a moist environment. In a dehydrated or desiccated milieu cells die if they dry. CS maintains cellular and tissue viability through its ability to keep the wound moist. Chronic wounds such as ulcerations caused by venous and arterial insufficiency of the lower extremities, pressure ulcers, and wounds that commonly occur from neuropathy and/or compromised blood perfusion in the diabetic foot, generally are inhibited from healing owing to persistent bacterial bio-burdens during the inflammatory and proliferative phases. Since CS has excellent antimicrobial properties, healing of these wounds will occur over more predictable time frames.
Chitosan is known to have the ability to deliver drugs. CS has the capability to encapsulate, stabilize, and deliver drugs that enhance connective tissue matrix growth (i.e., granulation tissue formation) during the proliferative phase of healing. Unfortunately, in spite of its clear advantages, there are drawbacks, which limit applications of current chitosan-based dressing products. For example, it is not possible to dissolve CS in organic solvents because it has a rigid crystalline structure due to intra- and inter-molecular hydrogen bonds.10–12 As a consequence, an acid such as acetic acid is required to break hydrogen bonds to facilitate dissolution. Subsequent neutralization with a base solution is then needed. Such a procedure is not only costly and time consuming, but also may lead to acid induced changes in the structure of CS.13,14 Furthermore, CS has rather poor rheological properties and will undergo extensive swelling in water. This makes it structurally too weak to be used by itself in any application. To increase the structural strength of CS products, attempts have been made to cross-link chitosan chains with a cross-linking agent or convert its functional group through a chemical reaction. In fact, all current CS-based biochemical and medical devices are based on these methods.15–19 The rather complicated, costly, and multi-step process currently used, that is, it involves the use of environmentally harmful chemicals and solvents and man-made polymers to strengthen its structure, is not desirable as it may inadvertently alter or remove its unique properties, making the CS-based composite materials less biocompatible and toxic. A new method, which can effectively dissolve CS not at high temperature by strong acid/base but rather by recyclable “green” solvent and to improve the structural strength of CS products not by chemical modification with synthetic chemicals and/or polymers but rather by use of naturally occurring biopolymers such as cellulose (CEL), is particularly needed.
Recently, we have developed a new method, which can offer a solution for this problem.20 In this method, we exploited advantages of a simple ionic liquid, butyl methylimmidazolium chloride (BMIm+ Cl−), a green solvent,20 to develop an innovative, simple, pollution-free method to dissolve not only CS but also other polysaccharides including CEL without using any acid or base, thereby avoiding any possible chemical or physical changes. Secondly, we used only naturally occurring biopolymers, such as CEL, as support materials to strengthen structure and expand utilities while keeping the biodegradable, biocompatible and anti-infective, and drug carrier properties of CS-based materials intact. Using this method, we have successfully synthesized composite materials containing CEL and CS with different compositions.20 Preliminary results show that the composite materials obtained have combined advantages of their components, namely superior chemical stability and mechanical stability (from CEL) and excellent antimicrobial properties (from CS). The [CEL + CS] composite materials inhibit growth of a wider range of bacteria than other CS-based materials prepared by conventional methods. Specifically, we found that over a 24 h period, the [CEL + CS] composite materials substantially inhibited growth of bacteria such as methicillin resistant Staphylococcus aureus (MRSA), vancomycin resistant Enterococcus (VRE), Staphylococcus aureus, and Escherichia coli.20
To evaluate the suitability of these novel [CEL + CS] composites for use as wound dressing material, we systematically investigated their antimicrobial activity, ability to absorb anticoagulated whole blood, anti-inflammatory activity through the reduction of tumor necrosis factor-α (TNF-α) and interlukin-6 (IL-6), and their biocompatibility with human fibroblasts. The results of our investigation are reported herein.
MATERIALS AND METHODS
Materials
The preparation and complete characterization of the [CEL + CS] composite materials used in this study was described in our previous publication.20 Minimal essential medium (MEM), 10% Fetal Bovine Serum (FBS), and gentamicin were obtained from Atlanta Biologicals, Lawrenceville, GA. CellTiter 96® aqueous non-radioactive cell proliferation assay was obtained from Promega, Madison, WI. One Percent Penicillin–Streptomycin and phorbol 12-myristate 13-acetate (PMA) were obtained from VWR, Radnor, PA. The enzyme-linked immunosorbent assay (ELISA) kit was from R&D Systems, Minneapolis, MN and was used according to the manufacturer’s instructions. Images of the fibroblasts and macrophages were taken with an Olympus microscopic camera using CellSens Imaging Software (Olympus, Center Valley, PA). Kendall – Curity AMD gauze sponges, 8 and 12 ply (Covidien, Mansfield, MA), Promogran matrix wound dressing (Johnson & Johnson, New Brunswick, NJ), Aquacel and DuoDERM CGF (ConvaTec, Princeton, NJ), Allevyn adhesive (Smith + Nephew, Andover, MA) are wound dressing that were purchased commercially.
Bactericidal activity
Bacterial killing assays were performed in the presence and absence of the composites with varying concentrations of CS and CEL. The bacterial strains used in this protocol included Escherichia coli (ATCC 8739), Staphylococcus aureus (ATCC 25923), methicillin resistant S. aureus (ATCC 33591), and vancomycin resistant Enterococcus faecalis (ATCC 51299). The strains were maintained on blood agar at 4°C. According to a modified protocol from Pinto et al.,21 bacterial cells were grown in nutrient broth for 18–20 h at 37°C with agitation. The cells were diluted in fresh medium and incubated for 24 h at 37°C in the presence of the composites. Serial dilutions of the bacteria were plated onto nutrient agar and incubated for 24 h. Bacterial colony forming units (CFUs) were quantified and compared to bacteria grown in the absence of composites.
Blood absorption
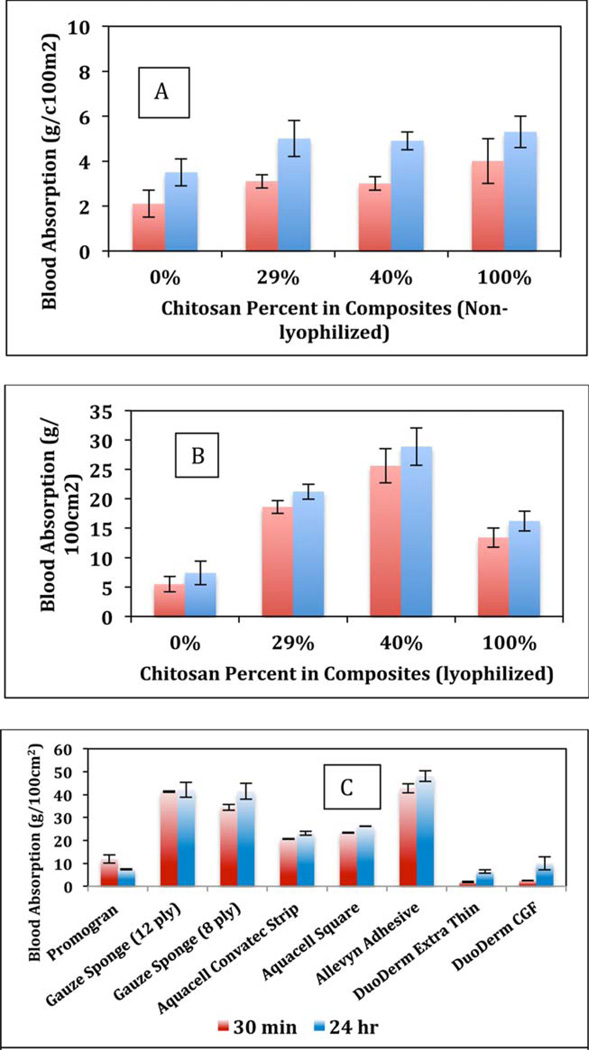
The capability of the commercially available wound dressings [Figure 1(C)] and [CEL + CS] composites to absorb blood and the rate of absorption were examined using the procedure reported by Terrill et al.22 In brief, each type of composite or dressing was premeasured and preweighed before testing. The composites or the dressing materials were then placed in a square petri dish with approximately 30 mL of whole blood donated by the Zablocki VA Medical Center, Milwaukee, WI. The dishes were incubated at 37°C. Before being weighed, the material was suspended above the dish for 30 s to release all unabsorbed blood. The composites and dressings were weighed at 30 min and 24 h. The amount of blood absorbed was then calculated as g/100 cm2.
FIGURE 1.
Comparison of blood absorbed over time by air-dried and lyophilized [CEL + CS] composites (A and B) and commercially available wound dressing materials (C) at 30 min (red) and 24 h (blue). At least three independent experiments were performed. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fibroblast adherence and growth
The adherence and growth of fibroblasts in the presence of the [CEL + CS] composites were assessed with modifications from Kloth et al.23 Essentially, human fibroblasts (ATCC CRL-2522) were grown in minimal essential medium (MEM) supplemented with 10% FBS and 0.25 mg/mL gentamicin according to ATCC guidelines until at least the 2nd passage. Cells were seeded into the wells containing membrane composites at a concentration of 8 × 104 cells/mL per well. Cells were imaged by an Olympus microscope camera using CellSens Imaging Software.
Viability assay
The proliferation of fibroblasts was assessed by the CellTiter 96® aqueous non-radioactive cell proliferation assay by the reduction of MTS [3-(4,5-dimethylthiazol-2-yl)–5-(3-carboxymethoxyphenyl)–2-(4-sulfophenyl)–2H-tetrazolium] into a formazan product. The protocol was modified according to Silva et al. In brief, the MTS reagent was added to each well in a 5:1 ratio with the fresh noncolored culture medium.24 The cells were incubated at standard culture conditions for 4 h and the optical density (490 nm) was measured.
Cell culture
The human monocytic cell line THP-1 (ATCC TIB-202) was cultured in RPMI-1640 medium supplemented with 10% FBS and 1% Penicillin–Streptomycin. Stimulation and differentiation of monocytes to macrophages was performed by adding 0.2 µM phorbol 12-myristate 13-acetate (PMA) to the medium. The cell line was maintained under 5% CO2 humidified atmospheric conditions in a 37°C incubator.
Cytokine measurement
The cultured monocytes were grown and diluted to a concentration of 5 × 105 cells/mL. A 24-well tissue culture plate was seeded with 1.6 mL of cells and [CEL + CS] composites were added to appropriate wells. TNF-α and interleukin-6 (IL-6) levels were measured and data analyzed using an ELISA kit according to manufacturer’s instructions. Images of the macrophages were taken with an Olympus microscopic camera using CellSens Imaging Software.
Statistical analysis
Statistics on data were performed with Microsoft Excel and StatPlus® software. Data are presented ± standard error of the mean. A p value of ≤ 0.05 was considered significant.
RESULTS AND DISCUSSION
Chitosan has proven to have efficient antimicrobial activity. 24–26 It has been widely used in the food and agricultural industries and in wound dressings because of its characteristic antibacterial activity toward both Gram positive and Gram negative organisms.27 To assess the antimicrobial abilities of the [CEL + CS] composites, bacteria were grown in the presence of the composites and then plated out onto growth agar and measured by the amount of colonies formed compared to a standard growth control. The results for the percent growth reduction of the different composites are shown in Table I. It is evident that there is bactericidal activity with the lyophilized 100% chitosan sample (Chitosan L) for all the bacteria except for S. aureus, with the greatest effect on E. coli and VRE. The 50% composite had the greatest effect on S. aureus. Even though there was a bactericidal effect on MRSA with lyophilized 100% CS, 50%, and 29% [CS + CEL] composites; the standard deviations associated with each growth reduction value was relatively high. It seems, therefore, that statistically, there was no significant growth reduction among all of the composites for MRSA. Since errors associated with results obtained for effect on P. aeruginosa are relatively large the results are inconclusive. This may be due to the fact that it was found that the inner surface of the test tube used in this assay was found to be coated with biofilm. This is hardly surprising because this microbe is known to be a strong producer of biofilms, and as described below, the chitosan composites were found to have no effect on bacterial biofilms.
TABLE I.
Percent Growth Reduction of Bacteria Growth in the Presence of Chitosan Composites
| 100% Chitosan |
100% Chitosan (L) |
50% Chitosan |
29% Chitosan |
|
|---|---|---|---|---|
| S. aureus | 35 (±30) | −5.3 (±76) | 70 (±11) | 54 (±38) |
| MRSA | −0.4 (±1) | 36 (±42) | 2 (±21) | 14 (±14) |
| VRE | −21 (±65) | 64 (±35) | 30 (±26) | 38 (±19) |
| E. coli | 15 (±34) | 78 (±17) | 9 (±23) | 39 (±31) |
| P. aeruginosa | −41 (±6.3) | 42.5 (±71) | −218 (±22) | 80 (±52) |
The bacteria used in this study were selected because they were found to have the highest morbidity and mortality associated with wound infections. Similar effect was also observed by Burkatovskaya and co-workers who reported that Chitosan acetate has greater effect against microorganisms both in vitro and in a mouse wound model than other types of nonchitosan wound treatments.27–29 Ignatova et al.30,31 demonstrated that electrospun mats of quarternized chitosan killed bacteria, especially S. aureus and E. coli within 2 h. Modified chitosan material, such as quarternized or those supplemented with silver nanoparticles were reported to have more of an effect with microorganisms than chitosan alone.21,30,31 Cai et al.32 showed an inhibition of E. coli growth, but not with S. aureus with a chitosan/silk fibroin composite. Also, chitosan–dextran hydrogel composite has shown to exhibit antimicrobial activity against Staphylococcus, Streptococcus, Clostridium, and E. coli,33 and Wu et al.34 showed a chitosan dose dependent bacteriostatic effect on S. aureus and E. coli with their chitosan/cellulose blend of composites.
In this study, we used chitosan composites in their natural state or in a composite with cellulose. Modifications to chitosan material can be expensive, potentially toxic, and complicated to produce. To be able to use chitosan in its natural state in a wound dressing, the benefits with respect to biodegradability, toxicity, and production would be advantageous. Results obtained in this study clearly indicate that the novel [CEL + CS] composite previously synthesized by us can effectively kill both Gram positive (S. aureus and VRE) and Gram negative (E. coli and P. aeruginosa) bacteria over a period of 24 h. Clearly, their antimicrobial activities are superior to those reported for composites made from chitosan and man-made polymers. Bacteriostatic and bactericidal properties are known to be important for wound healing applications in preventing infection and even possible sepsis. These effects of the chitosan composites on the wound pathogens illustrate their great potential as components in wound dressings.
Subsequently, bacterial biofilms were assessed for a change in metabolic activity in the presence of the chitosan material. It was found that after 24 h, the chitosan composites did not decrease the metabolic activity compared to the (no-chitosan) control biofilm (data not shown). It is hardly surprising to observe lack of effect of the chitosan composites on biofilms because biofilms are known to envelop bacteria with a polymeric matrix that prevents topical antibiotics and antiseptic agents from reaching the infecting microbes.35 In fact, currently, all available broad spectrum antimicrobial agents that are typically used on chronic wounds (e.g., sodium hypochlorite, cadexomer iodine, chlorhexidine, and hydrogen peroxide) are not able to eradicate biofilms. Of interest is the recent report to indicate that low intensity electric fields can completely override the inherent resistance of biofilm bacteria to biocides and antibiotics. Experiments are now in progress to investigate this possibility.36
An ideal wound dressing material should maintain a delicate balance of absorption of fluids with the ability to maintain a moist healing environment for proper tissue repair. The results obtained for absorption of whole blood by air-dried [CEL + CS] composites, lyophilized [CEL + CS] composites and commercial dressings including Allevyn, Aquacel, Curity Gauze, Promogram, and DuoDerm over 30 min and 24 h are shown in Figure 1(A–C), respectively. Compared to the other fiber-based commercial dressings, the present lyophilized [CEL + CS] composites have comparable levels of whole blood absorption. In this study, whole blood was used instead of saline or prepared artificial transudate or exudate material because it was previously found that the types of dressings, including foam, alginates, gauze, hydrocolloids, and collagen absorb whole blood different from the other two artificial media.37 To be more consistent with the physiological environment of a wound, we chose to examine the absorptivity with whole blood. Terrill et al. also stated that a dressing that absorbs over 35 g/100 cm2 would be good for bleeding wounds.22
It is evident from Figure 1(A,B) that for all compositions, [CEL + CS] composites dried by lyophilizing absorb relatively higher amount of blood compared to those air-dried. Depending on composition, lyophilized composites absorb at least 2–6× more blood compared to those by air-dried composites.
Absorption amount by the air-dried [CEL + CS] composites are comparable to the commercial nonlyophilized dressings (Promogram and DuoDerm), all between 2 and 7 g/100 cm2. The lyophilized [CEL + CS] composites showed comparable results for absorption over time to the commonly used lyophilized dressings such as gauze, Aquacel, and Allevyn. Moreover, we found that the absorption values of the composites approached the absorption amounts of the standard gauze and the Allevyn dressing, which may be used for moderate to heavily bleeding wounds. In particular, 29, 40, and 50% chitosan lyophilized materials are more absorptive than the Aquacel dressing materials. It is known that the fibrous make-up of gauze can lead to an increase of inflammation and on removal can cause trauma to the wound viable tissues with associated pain.
Of particular importance is the observation that all of the lyophilized [CEL:CS] composites did have rapid absorption rates, such that the amount of blood absorbed at 30 min and 24 h did not differ greatly. In contrast, the air-dried [CEL:CS] composites did absorb at a slower rate. This finding is not insignificant and may be descriptive to what type of wound would benefit by a specific composite.
The results presented clearly indicate that the blood absorption capability of the [CEL + CS] composites is comparable to those of commercial dressings, and that the composites have ability to maintain moisture balance for wound healing. Such ability is one of the most important factors in the development of a biodegradable and biocompatible novel wound dressing material.
The [CEL + CS] composites developed for wound dressing material must help to promote wound healing by creating a moist microenvironment for proper tissue regeneration. The presence of fibroblasts is critical for healing and the interaction of the fibroblasts with the composites must promote cellular and collagen proliferation. Previous reports have shown that chitosan materials are nontoxic to fibroblasts.38–40 It is expected that chitosan composites can play a role in physiological regeneration of tissue during the wound healing process.
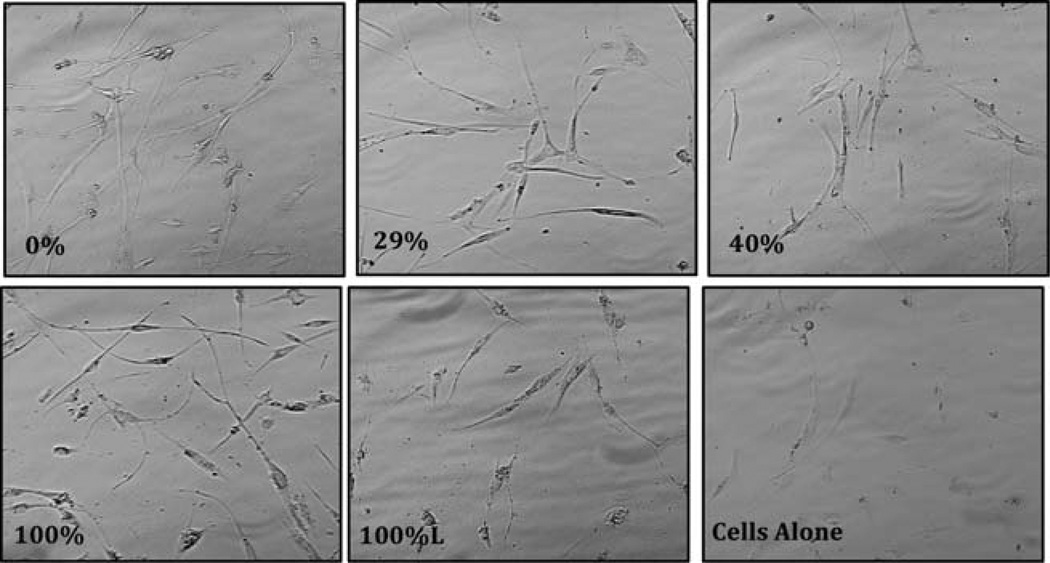
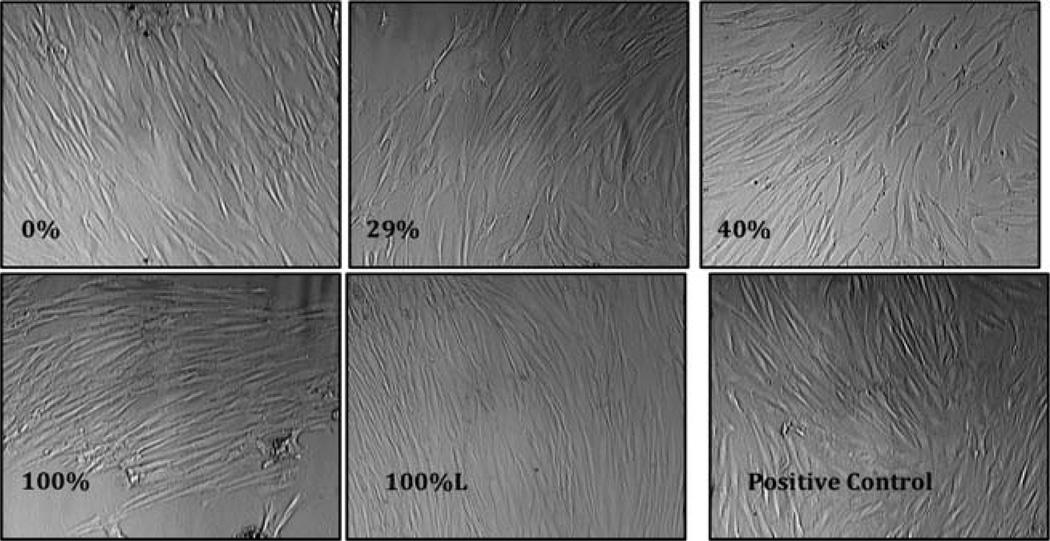
In this study we analyzed the morphology and the proliferation capabilities of adherent fibroblasts by examining morphology microscopically. Results of the morphological analysis in the short term (3 days) and long term (7 days) are shown in Figures 2 and 3, respectively. Adherent fibroblasts were seen in wells after 3 days compared to the control well. The 7 day images show healthy fibroblasts in wells containing all chitosan composites. The adherent cells in 0, 29, 40, 100, and 100% lyophilized (i.e., 100%L) showed a typical morphology of an elongated body and more cells than the other wells. As shown, compared to the control well, the chitosan composites did exhibit proliferation. The data in this study supports the use of the modified chitosan composites and the continued development of the material for proper scaffolding during wound healing.
FIGURE 2.
Images (40×) of human fibroblast in the presence and absence of [CEL + CS] composites for 3 days. Only one composite is lyophilized (100%L); the rest are all air-dried. The experiment was performed in duplicate.
FIGURE 3.
Images (40×) of human fibroblast in the presence and absence of [CEL + CS] composites for 7 days. Only one composite is lyophilized (100%L), the rest are all air-dried. The experiment was performed in duplicate.
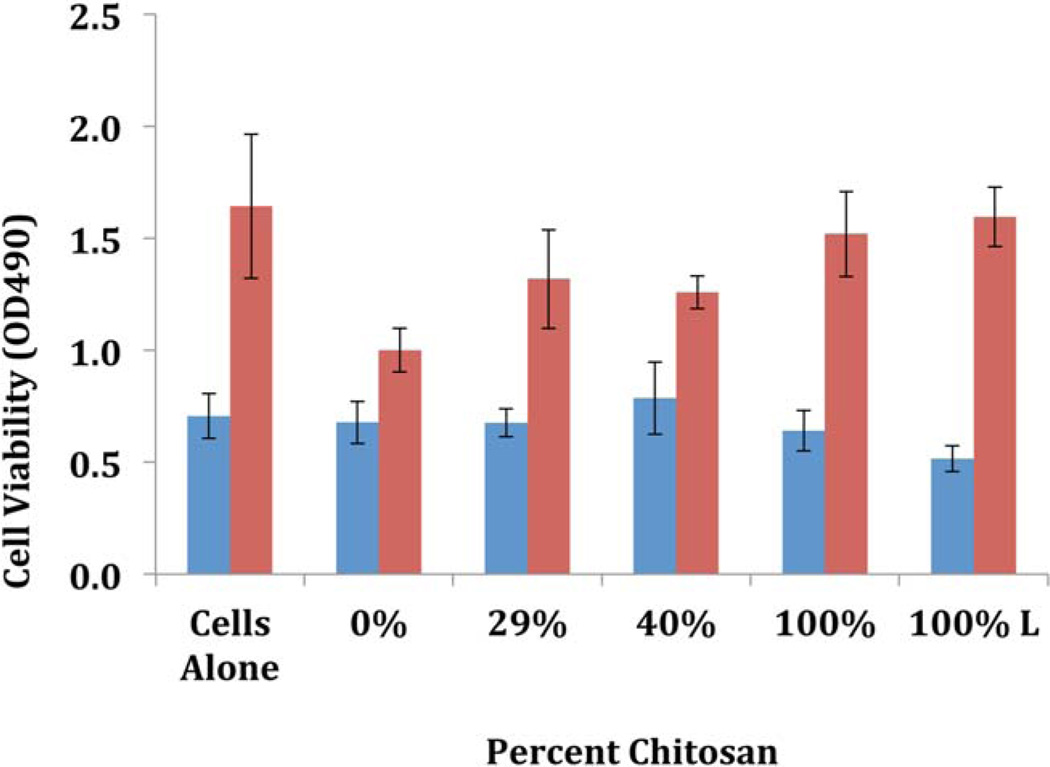
Proliferation and viability of fibroblasts (Figure 4) in the presence and absence of chitosan composites were measured by the MTS assay (see Materials). Similar to Fakhry et al.,41 our study shows the proliferation of fibroblasts over time (from 3 to 7 days) and in the presence of chitosan there is a slowing of growth and proliferation although the cells still maintain the proper morphology. These results are in contrast to previous studies with modified chitosan material showing a direct correlation between time of incubation and amount of proliferation.32,40 Mori et al.42 theorized that materials with higher amounts of chitosan can inhibit growth of fibroblasts indirectly. Chitosan that can bind proteins and the fetal bovine serum used in the culture medium may be causing the caustic effect on the fibroblasts. Silva et al.40 more recently showed the ability to increase the host response of proliferation by fibroblasts with argon and nitrogen gases in plasma surface modification to their chitosan composites.
FIGURE 4.
Fibroblast viability was measured at 30 min (blue bars) and 24 h (red bars) by the conversion of MTS to a formazan product in the presence of active metabolic enzymes (see text for detailed information). The fibroblast metabolic activity was tested in the presence and absence of [CEL + CS] chitosan composites. Only one composite is lyophilized (100%L), the rest are all air-dried. The experiments were performed in duplicate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
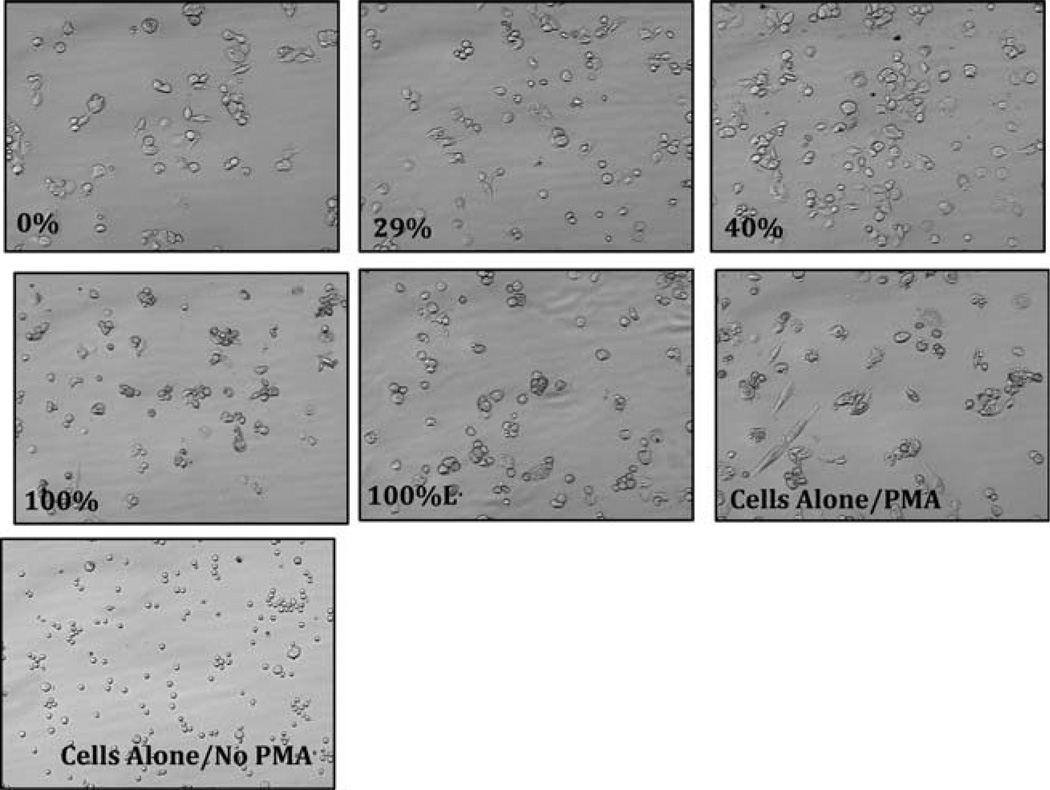
The wound environment requires a proper balance between a multitude of factors including blood clotting, cellular survival, and tissue repair. Another benefit to proper wound healing is for the dressing material to possess anti-inflammatory activity, since proinflammatory cytokines (TNF-α and IL-6) are primary contributors to the inflammation associated with chronic wounds, which stalls and prevents them from proceeding into the proliferative phase of tissue regeneration. Consequently it is important to assess the biocompatibility of potential dressing materials. Accordingly, the [CEL + CS] composites were tested for anti-inflammatory effects on macrophage derived TNF-α and IL-6. Results obtained, shown as images for TNF-α (Figure 5) and quantitative for both TNF-α and IL-6 [Figure 6(A,B)] indicate that, similar to fibroblasts, monocytes were viable and able to differentiate into macrophages in the presence of the chitosan composites especially the 100% and 100%L material.
FIGURE 5.
Images of macrophages (40×) in the presence and absence of [CEL + CS] composites. Only one composite is lyophilized (100%L), the rest are all air-dried. Monocytes were stimulated to become macrophages with PMA (see text for detailed information). The macrophages were cultured in the presence and absence of the [CEL + CS] composites. The experiments were performed in duplicate.
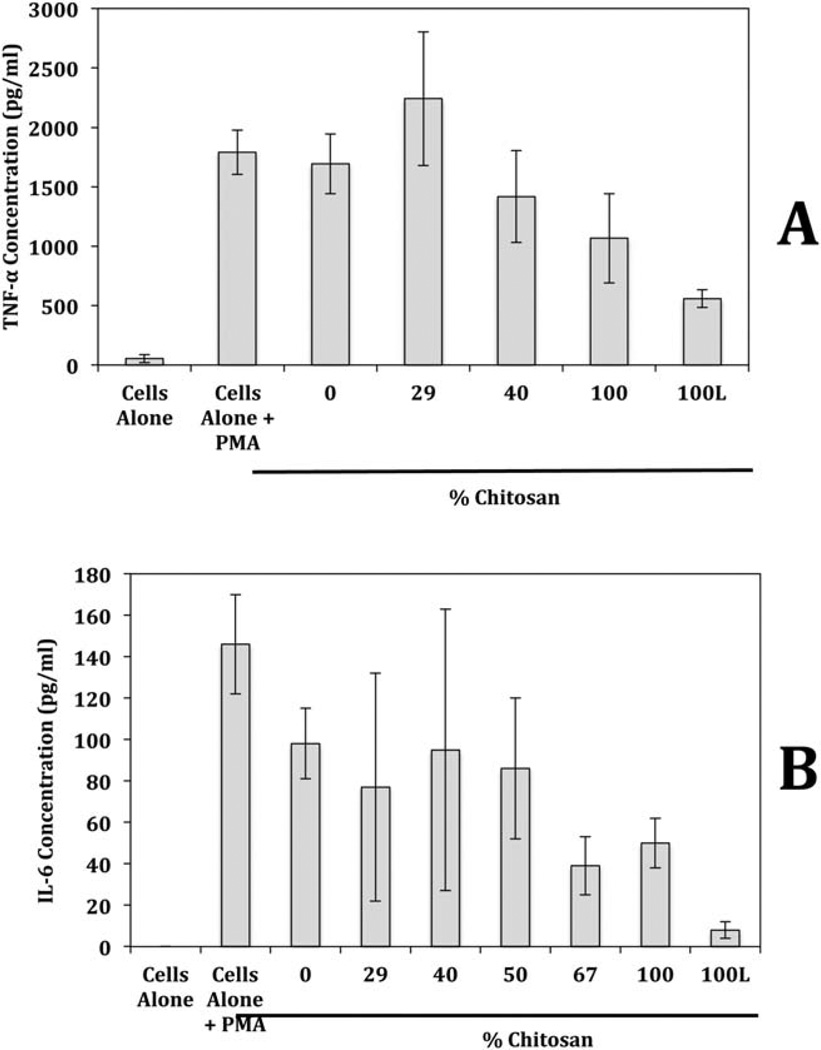
FIGURE 6.
Production of (A) TNF-α and (B) IL-6 by macrophages in the presence and absence of [CEL + CS] composites. Macrophages were cultured in the presence and absence of [CEL + CS] composites. Only one composite is lyophilized (100%L), the rest are all air-dried. The amount of TNF-α and IL-6 produced by the macrophages was quantified by ELISA.
Macrophages play an important role in our innate immunity to foreign substances, but can also mediate many responses when there is injury to tissue. These cells can phagocytize or engulf micro-organisms and cellular debris. They respond to tissue damage and infectious microorganisms by phagocytosis and the release of chemical mediators known as cytokines. TNF-α and IL-6, proinflammatory cytokines are released and can help to regulate the immune response, but it can also lead to tissue damage to the host. Proinflammatory cytokines are upregulated during wound healing and regeneration. Mori et al.43 showed in a TNF receptor deficient wound mouse model that the time of healing and tissue regeneration was increased significantly. Their study concluded the need for an anti-inflammatory environment for accelerated wound healing. It has been noted that the over production of IL-6 has been associated with an increase in the production of scarring during wound healing.44
The proinflammatory cytokines can be used as biomarkers for the proper differentiation of monocyte to macrophage and the inflammatory response of the macrophage.45–47 TNF-α and IL-6 were measured quantitatively to assess the inflammatory response in the presence of the chitosan composites and the results are shown in Figure 6(A,B), respectively. As shown, compared to the control macrophages (Cells alone + PMA), the 0, 29, and 40% chitosan composites showed no significant change in the amount of TNF-α secreted within a 3 day period. The TNF-α concentrations of 100% and 100% lyophilized chitosan composites were significantly lower, p = 0.05 and 0.01, respectively, than control showing a potential anti-inflammatory effect. The IL-6 levels were reduced considerable in the presence of 67, 100, and 100% lyophilized chitosan material. This anti-inflammatory effect with chitosan has been shown previously. 47,48 Oliveira et al.,49 showed that when chitosan was exposed to monocytes as the sole stimulant, after 10 days of incubation, the cytokine levels, including TNF-α and IL-6, reached anti-inflammatory levels. Those results correlate with our study clearly indicate the anti-inflammatory effects of [CEL + CS] composites on PMA derived macrophages.
In summary, we have successfully demonstrated that the novel [CEL + CS] composites, which we have previously synthesized using a green and totally recyclable method, possess all properties needed to be used effectively as a wound dressing. Specifically, the composites are antibacterial, hemostatic, biocompatible, good absorbent for anticoagulated whole blood, and are able to maintain moisture balance for wound healing. For example, the composites were found to inhibit the growth of both Gram positive and negative micro-organisms (including Escherichia coli (ATCC 8739), Staphylococcus aureus (ATCC 25923), methicillin resistant S. aureus (ATCC 33591), and vancomycin resistant Enterococcus faecalis (ATCC 51299)). They are nontoxic to fibroblasts, namely fibroblasts were found to grow and proliferate in the presence of the composites. They effectively absorb blood, and at the same rate and volume as commercially available wound dressings. The composites, in both air-dried and lyophilized forms, significantly inhibit the production of TNF-α and IL-6 by stimulated macrophages. Experiments are now in progress to evaluate wound dressing based on the composites.
ACKNOWLEDGMENTS
Contract grant sponsor: The National Institute of General Medical Sciences of the National Institutes of Health; contract grant number: R15GM099033
The authors are grateful to Ms. Anna J Frazier for her competent technical assistance.
REFERENCES
- 1.Dai T, Tegos GP, Barkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordenave N, Grelier S, Coma V. Hydrophobization and antimicrobial activity of chitosan and paper-based packaging materials. Biomacromolecules. 2010;11:88–96. doi: 10.1021/bm9009528. [DOI] [PubMed] [Google Scholar]
- 3.Rabea EI, Badawy M, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 4.Altiok D, Altiok E, Tihminlioglu F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci Mater Med. 2010;21:2227–2236. doi: 10.1007/s10856-010-4065-x. [DOI] [PubMed] [Google Scholar]
- 5.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano AP, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiyozumi T, Kanatani Y, Ishihara M, Saitoh D, Shimizu J, Yura H, Suzuki S, Okada Y, Kikuchi M. Medium (DMEM/F12)-containing chitosan hydrogel as adhesive and dressing in autologous skin grafts and accelerator in the healing process. J Biomed Mater Res B. 2006;79B:129–136. doi: 10.1002/jbm.b.30522. [DOI] [PubMed] [Google Scholar]
- 7.Jain D, Banerjee R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J Biomed Mater Res B. 2008;86:105–112. doi: 10.1002/jbm.b.30994. [DOI] [PubMed] [Google Scholar]
- 8.Varshosaz J, Tabbakhian M, Salmani Z. Designing of a thermo-sensitive chitosan/poloxamer in situ gel for ocular delivery of ciprofloxacin. Open Drug Delivery J. 2008;2:61–70. [Google Scholar]
- 9.Naficy S, Razal JM, Spinks GM, Wallace GG. Modulated release of dexamethasone from chitosan-carbon nanotube films. Sens Actuators A. 2009;155:120–124. [Google Scholar]
- 10.Finkenstadt VL, Millane RP. Crystal structure of Valonia cellulose 1b. Macromolecules. 1998;31:7776–7783. [Google Scholar]
- 11.Augustine AV, Hudson SM, Cuculo JA. Cellulose sources and exploitation. In: Kennedy JF, Philipps GO, Williams PA, editors. Aspects of Cellulose Structure. New York: E. Horwood; 1990. p. 59. [Google Scholar]
- 12.Dawsey TR. Cellulosic polymers, blends and composites. In: Gilbert RD, editor. Water Soluble Cellulose Derivative and Their Commercial Use. New York: Carl Hanser Verlag; 1994. p. 157. [Google Scholar]
- 13.Cai J, Liu Y, Zhang L. Dilute solution properties of cellulose in LiOH/urea aqueous system. J Polym Sci B Pol Phys. 2006;44:3093–30105. [Google Scholar]
- 14.Fink HP, Weigel P, Purz HJ, Ganster J. Structure formation of regenerated cellulose materials from NMMO- solutions. Prog Polym Sci. 2001;26:1473–1524. [Google Scholar]
- 15.Miao J, Zhang F, Takieddin M, Mousa S, Linhardt RJ. Adsorption of doxorubicin on poly(methyl methacrylate)-chitosan-heparin-coated activated carbon beads. Langmuir. 2012;28:4396–4403. doi: 10.1021/la3000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy SJ, Gregory KW, Morgan JW. Tissue dressing assemblies systems, and methods formed from hydrophilic polymer sponge structures such as chitosan. 062896. WO Patent No. 2005
- 17.El-Mekawy A, Hudson S, El-Baz A, Hamza H, El-Halafawy K. Preparation of chitosan films mixed with superabsorbent polymer and evaluation of its haemostatic and antibacterial activities. J Appl Polym Sci. 2010;116:3489–3496. [Google Scholar]
- 18.Sandoval M, Albornoz C, Munoz S, Fica M, Garcia-Huidobro I, Mertens R, et al. Addition of chitosan may improve the treatment efficacy of triple bandage and compression in the treatment of venous leg ulcers. J Drugs Dermatol. 2010;10:75–80. [PubMed] [Google Scholar]
- 19.Jayakumar R, Prabaharan M, Sudheesh PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotech Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Tran CD, Duri S, Harkins A. Recycle synthesis, characterization and properties of polysaccharide ecocomposite materials. J Biomed Mater Res A. 2013;101:2248–2257. doi: 10.1002/jbm.a.34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto RJB, Fernandes SCM, Freire CSR, Sadocco P, Causio J, eto CP, Trindade T. Antibacterial activity of optically transparent nanocomposite films based on chitosan or its derivatives and silver nanoparticles. Carbohydr Res. 2012;348:77–83. doi: 10.1016/j.carres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Terrill P, Sussman G, Bailey M. Absorption of blood by moist wound healing dressings. Prim Intention. 2003;1:7–10. [Google Scholar]
- 23.Kloth LC, Berman JE, Laatsch LJ, Kirchner PA. Bactericidal and cytotoxic effects of chloramine-T on wound pathogens and human fibroblasts in vitro. Adv Skin Wound Care. 2007;20:331–345. doi: 10.1097/01.ASW.0000276408.53632.0b. [DOI] [PubMed] [Google Scholar]
- 24.Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 25.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Jayakumar R, Prabaharan M, Sudheesh PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotech Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano AP, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignatova M, Starbova K, Markova N, Naolova N, Rashkov I. Electrospun nano-fibre mats with antibacterial properties from quarternized chitosan and poly(vinyl alcohol) Carbohydr Res. 2006;341:2098–2107. doi: 10.1016/j.carres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Ignatova M, Manolova N, Rashkov I. Novel antibacterial fibers of quarternized chitosan and poly(vinyl pyrrolidine) prepared by electrospinning. Eur Polym J. 2007;43:1112–1122. [Google Scholar]
- 32.Cai ZX, Mo XM, Zhang KH, Fan LP, Yin AL, He CL, Wang HS. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int J Mol Sci. 2010;11:3529–3539. doi: 10.3390/ijms11093529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz MA, Cabral JD, Brooks HJ, Moratti SC, Hanton LR. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob Agents Chemother. 2012;56:280–287. doi: 10.1128/AAC.05463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu YB, Yu SH, Mi FL, Wu CW, Shyu SS, Peng CK, Chao AC. Preparation and characterization on mechanical and antibacterial properties of chitosan/cellulose blends. Carbohydr Polym. 2004;57:435–440. [Google Scholar]
- 35.Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costerton JW, Ellis B, Kan L, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulton JA, Blasiole KN, Cottingham T, Tornero M, Graves M, Smith LG, Mirza S, Mostow EN. Wound dressing absorption: A comparative study. Adv Skin Wound Care. 2012;25:315–320. doi: 10.1097/01.ASW.0000416003.32348.e0. [DOI] [PubMed] [Google Scholar]
- 38.Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials. 2001;22:261–268. doi: 10.1016/s0142-9612(00)00183-6. [DOI] [PubMed] [Google Scholar]
- 39.Silva SS, Santos MI, Coutinho OP, Mano JF, Reis RL. Physical properties and biocompatibility of chitosan/soy blended membranes. J Mater Sci Mater Med. 2005;16:575–579. doi: 10.1007/s10856-005-0534-z. [DOI] [PubMed] [Google Scholar]
- 40.Silva SS, Luna SM, Gomes ME, Benesch J, Pashkuleva I, Mano JF, Reis RL. Plasma surface modifications of chitosan membranes: Characterization and preliminary cell response studies. Macromol Biosci. 2008;8:568–576. doi: 10.1002/mabi.200700264. [DOI] [PubMed] [Google Scholar]
- 41.Fakhry A, Schneider GB, Zaharias R, Senel S. Chitosan supports the initial attachment and spreading of osteoblasts preferentially over fibroblasts. Biomaterials. 2004;25:2075–2079. doi: 10.1016/j.biomaterials.2003.08.068. [DOI] [PubMed] [Google Scholar]
- 42.Mori T, Okumura M, Matsuura M, Ueno K, Tokura S, Okamoto Y, Minami S, Fujinaga T. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 1997;18:947–951. doi: 10.1016/s0142-9612(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 43.Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J. 2002;16:963–974. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- 44.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JM. Biological responses to materials. Ann Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 46.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–R9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 48.Lee HS, Stachelek SJ, Tomczyk N, Finley MJ, Composto RJ, Eckmann DM. Correlating macrophage morphology and cytokine production resulting from biomaterial contact. Soc Biomater. 2012 doi: 10.1002/jbm.a.34309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. Chitosan drives anti- inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur Cells Mater. 2012;24:136–153. doi: 10.22203/ecm.v024a10. [DOI] [PubMed] [Google Scholar]