Abstract
Objective
The neurobiological underpinnings of anorexia nervosa (AN) are poorly understood. In this study we tested whether brain gray matter (GM) and white matter (WM) in adolescents with AN would show alterations comparable to adults.
Method
We used magnetic resonance imaging to study GM and WM volume, and diffusion tensor imaging to assess fractional anisotropy for WM integrity in 19 adolescents with AN and 22 controls.
Results
Individuals with AN showed greater left orbitofrontal, right insular, and bilateral temporal cortex GM, as well as temporal lobe WM volumes compared to controls. WM integrity in adolescents with AN was lower (lower fractional anisotropy) in fornix, posterior frontal, and parietal areas, but higher in anterior frontal, orbitofrontal, and temporal lobes. In individuals with AN, orbitofrontal GM volume correlated negatively with sweet taste pleasantness. An additional comparison of this study cohort with adult individuals with AN and healthy controls supported greater orbitofrontal cortex and insula volumes in AN across age groups.
Conclusions
This study indicates larger orbitofrontal and insular GM volumes, as well as lower fornix WM integrity in adolescents with AN, similar to adults. The pattern of larger anteroventral GM and WM volume as well as WM integrity, but lower WM integrity in posterior frontal and parietal regions may indicate that developmental factors such as GM pruning and WM growth could contribute to brain alterations in AN. The negative correlation between taste pleasantness and orbitofrontal cortex volume in individuals with AN could contribute to food avoidance in this disorder.
Keywords: adolescent, anorexia nervosa, brain, gray matter, white matter
Introduction
Anorexia nervosa (AN) is an eating disorder (ED) associated with intense fear of weight gain, and perception of being overweight despite severe emaciation from self-driven food refusal.1 It is the third most common chronic illness among adolescents2 with a mortality rate 12 times higher than the death rate associated with all causes of death for females 15–24 years old.3 AN shows a difficult to disentangle interplay between neurobiological, psychological and environmental factors4 and little is known about brain biomarkers in children and adolescents with AN.
In the past, functional brain imaging studies implicated striatum, insula, anterior cingulate, amygdala, and orbitofrontal cortex (OFC) in AN, brain regions that contribute to taste and reward processing.5,6 The mechanisms for those alterations remain unclear, but brain gray (GM) and white matter (WM) might underlie altered brain function and behavior.7
Most structural brain imaging studies in EDs come from adult samples and a recent meta-analysis found the available data “inconclusive.”8 Early studies in youth and adults suggested lower total GM and WM volume, 9–12 while studies after recovery in adults found lower13,14 or normal15,16 total brain tissue volumes.8 Studies in adult AN assessing regional volume differences indicated lower GM volumes in insula, frontal operculum, occipital, medial temporal, and cingulate cortex, while one recent study found larger dorsolateral prefrontal GM volume.17–21 After long-term recovery regional brain-tissue volumes in adults with ED history were normal.15 Very few brain structure studies in adolescent AN have been conducted8: One study in mostly adolescents found lower GM in frontal, temporal, parietal, occipital, and cerebellar areas.22 A study in adolescent AN16 that found greater total GM compared to controls, but lower temporal, parietal, frontal, and cingulate cortex volumes, indicated that the rate of localized GM development could be different between groups.
Only some studies corrected for age or total intracranial volume (TIV) and effects of comorbid diagnoses or medication were often not taken into account. Not taking TIV into account could miss group differences pertaining to the more static body size related cranial vault, and comorbid anxiety and depression have been associated with GM alterations independent from an AN diagnosis.23 Furthermore, nutritional status is associated with quickly occurring GM and WM changes.24 After only 2–3 days of dehydration GM and WM volumes are significantly lower, while hyperhydration is associated with higher GM and WM volumes.24 All those factors may contribute to inconsistent results across studies. Recently25 we found in adult AN in a nutritionally highly controlled environment and AN after long term recovery, correcting for TIV, medication use and comorbidity, larger left orbitofrontal cortex gyrus rectus GM volume that correlated with perceived taste pleasantness, as well as larger right insula volume. Those results suggested that altered orbitofrontal and insula cortex volumes could be trait markers for AN related to altered reward function.26–28
Another brain imaging method, diffusion tensor imaging (DTI),29 maps water diffusivity along WM axons, expressed as fractional anisotropy (FA)30 and is considered a measure of axon integrity related to myelination, and density. A second measure, the apparent diffusion coefficient (ADC), measures water diffusivity at the voxel level and higher ADC indicates dispersed water diffusion reflecting cell damage.30 Commonly, high ADC reflecting cell disruption is associated with low axon integrity and FA. One study showed lower fimbria-fornix WM integrity that was related to trait anxiety31 in adult AN compared to controls, while a study in mixed ill and recovered adult AN found lower FA in the posterior thalamic radiation.32
In this study we tested the following hypotheses: 1. Larger left orbitofrontal gyrus rectus as well as right insula volumes, associated with adult AN, are present in adolescents with AN; 2. Orbitofrontal cortex volume predicts taste pleasantness perception in both individuals with AN and control adolescents, and 3. Adolescents with AN have lower WM integrity in the fimbria fornix similar to our previous study in adults, which could point to altered reward processing pathways.33
Method
Subjects
Nineteen individuals with AN (17 restricting-type and 2 binge/purge-type) and 22 healthy control adolescent girls who were similar in age participated in the study. Individuals with AN were recruited from the Children’s Hospital Colorado Eating Disorders Program. The study was approved by the Colorado Multiple Institutional Review Board. Individuals with AN were within 1–2 weeks of inpatient hospital treatment, were closely supervised and followed the program meal plan to avoid acute effects of starvation and dehydration. Control adolescents were recruited through local advertisements. Participants were administered the Computerized Diagnostic Interview Schedule for Children (C-DISC) for DSM-IV diagnoses.34 All participants were right-handed, without history of head trauma, neurological disease, or major medical illness.
In addition, we compared the adolescents of this study with the adult sample (AN: n=19, age=23.1±5.8 years; control individuals: n=24, age=27.4±6.3 years) from our previous study.25
Behavioral Measures
Study participants completed as described previously35: 1. Eating Disorder Inventory–3 (EDI-3) for Drive for Thinness (DT), Bulimia (B), and Body Dissatisfaction (BD). 2. Temperament and Character Inventory (TCI) for Novelty Seeking (NS) and Harm Avoidance (HA). 3. Spielberger State and Trait Anxiety Inventory (STAI). 4. Beck Depression Inventory (BDI). 5. revised Sensitivity to Reward (SR) and Punishment (SP) Questionnaire (SPSRQ). 6. Rating of 1 molar sucrose and a control solution (slightly salty resembling saliva) for sweetness and pleasantness on 9-point Likert scales (0, not sweet/pleasant at all to 9, extremely sweet/pleasant).
MRI Acquisition for GM, WM
Structural brain images were acquired on a GE Signa 3T scanner, axial 3-dimensional T-1 weighted magnetization-prepared rapid acquisition gradient echo (spoiled gradient recall, SPGR, field of view 22 cm, flip angle 10°, slice thickness 1.2 mm, scan matrix 256×256, repetition time [TR] 10 ms, time to echo [TE] 3 ms, voxel size 1.2 mm3).
MRI Acquisition for DTI
For each participant, 26 diffusion-weighted images (DWIs) were acquired for DTI mapping (25 DWI diffusion gradient images and one b0 baseline image). Each DWI included 29 slices acquired in axial anterior-posterior commissure orientation and in a 128×128 matrix, TR=8500 ms, field of view=28 cm, and slice thickness 3.5 mm with 0.5 mm gap.
GM, WM Analysis
Images were manually aligned to the anterior-posterior commissure line. T1-weighted images were preprocessed using SPM8 voxel-based morphometry (VBM) toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) in Matlab R2009b, 7.9.0 (MathWorks, Natick, MA, USA). Images were normalized to MNI space using high-dimensional diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) segmented into GM, WM, and cerebrospinal fluid (CSF). A custom age-specific tissue probability map (TPM) and T1 reference template was created using the Template-O-Matic Toolbox (http://dbm.neuro.uni-jena.de/software/tom/). This toolbox uses data from a large sample of children and adolescents to create age-specific TPMs and T1 images based on the average age of the sample.36 Segmentation procedures in VBM8 automatically removed non-brain tissues including scalp, skull, and dural venous sinus (http://www.fil.ion.ucl.ac.uk/spm/doc/biblio/) and were based on maximum a posteriori probability (MAP) estimation techniques that do not require a priori information about tissue probabilities (variability of head shape and size) of control subjects that may not accurately represent the analyzed sample. After initial segmentation of TIV into GM, WM, and CSF, 2 mixed tissue classes (GM-WM and GM-CSF) were estimated using partial volume estimation.37 The results are an estimation of pure tissue type present in every voxel, and superior to previous SPM methods.38,39 In addition, optimized block-wise nonlocal means (NLM) and classical Markov Random Field (MRF) denoising methods were applied. Nonlinear modulated data (corrected for TIV) were used in the analyses. Images were smoothed to an 8-mm full-width at half maximum Gaussian kernel.
DTI Image Analysis
DTI datasets were processed using NordicICE (http://www.nordicneurolab.com) for 3-dimensionalfiber tracking of axonal projections using “Fiber Assignment by Continuous Tacking” (FACT).40 Fibers are tracked continuously based on water diffusion from a voxel center proceeding according to the vector direction. Where the tract leaves the voxel and enters the next, the direction is changed to that of the neighboring voxel. An exhaustive search tracking method was implemented and a principal eigenvector angle stopping threshold of 41° was used, minimum fiber length was 5 mm an d only fractional anisotropy values greater than 0.2 were included.40,41
Whole brain FA and ADC maps were further analyzed using SPM8. FA and ADC images for each participant were normalized to the average age-specific T1 template, and carefully visually inspected for correct normalization. FA and ADC images were smoothed with a 6-mm FWHM filter and masked with a WM mask. The location of WM FA identified as significantly different across groups was identified by visual inspection and ‘Dissecting the White Matter Tracts: Interactive Diffusion Tensor Imaging Teaching Atlas’ by Hutchins et al.(http://www.asnr2.org/neurographics).
Statistical Analysis
GM/WM analysis
A general linear model (GLM) whole-brain analysis was used (SPM8), a factorial design modeled with diagnosis as 2-level factor (control and AN adolescents) and age and total intracranial volume (TIV) as covariates, as well as use of antipsychotic medication, use of selective serotonin reuptake inhibitor (SSRI) medication and comorbid depression or anxiety, with each assigned a 0 or 1 coding presence or absence. TIV for correction of differences in head size (“global normalization”) was obtained by adding GM, WM, and cerebral spinal fluid (CSF) volumes from tissue-class images in native space using VBM8 (GM+WM+CSF=TIV).
Initially, a voxel-wise F-test was performed, p < 0.001 uncorrected, extent threshold > 50 voxels (suggested for study of orbitofrontal cortex42 and insula43). Results were corrected using SPM8 anatomical automatic labeling (AAL) atlas derived a priori defined anatomical regions (orbitofrontal cortex, insula, caudate, putamen, amygdala), family-wise error (FWE) corrected at p < 0.05, and regional volumes that reached significance were extracted.
All imaging procedures were similar in adolescents and adults, including MRI scanner, sequence, preprocessing, and statistical analyses including covariates. Adolescents and adults with AN were similar on clinical variables as in this study (see Supplement 1).
FA/ADC analysis
GLM was used for group comparison similar to GM/WM. Thresholds based on previous studies of p < 0.005 uncorrected, 50 voxel contiguity, were used to create the result maps.31 For the resulting clusters, mean FA/ADC values were extracted using the SPM marsbar toolbox.
Demographic and extracted regional brain data were analyzed using SPSS (IBM-SPSS, Chicago, IL) and independent samples t-test. We applied linear regression analyses to test behavior-brain relationships and results were false discovery rate corrected.
Results
Demographic and Behavioral Data
Adolescents with AN and controls (Table 1) were similar in age. Individuals with AN showed the expected lower body mass index (BMI), lower novelty seeking, but higher harm avoidance, depression, drive for thinness, body dissatisfaction, as well as state and trait anxiety, compared to controls. Sensitivity to punishment was significantly higher in those with AN vs. controls, together with a trend to higher sensitivity to reward. Sucrose sweetness-perception was similar between groups, but pleasantness rating was lower in the AN group.
Table 1.
Demographic Variables
| CW, n=22 | AN, n = 19 | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p-value | |
| Age (years) | 14.8 | 1.8 | 15.4 | 1.4 | −1.11 | 0.292 |
| BMI (kg/m2) | 21.3 | 1.9 | 16.2 | 1.1 | 10.19 | <0.001 |
| Novelty Seeking | 22.5 | 5.0 | 15.1 | 5.3 | 4.60 | <0.001 |
| Harm Avoidance | 10.9 | 5.9 | 21.2 | 7.6 | −4.88 | <0.001 |
| Depression | 2.5 | 2.6 | 19.9 | 9.8 | −8.02 | <0.001 |
| Drive for Thinness | 1.0 | 1.8 | 13.7 | 6.8 | −8.38 | <0.001 |
| Bulimia | 0.6 | 1.0 | 1.1 | 1.5 | −1.05 | 0.298 |
| Body Dissatisfaction | 1.5 | 2.2 | 15.6 | 9.1 | −7.05 | <0.001 |
| Punishment Sensitivity | 4.8 | 3.6 | 11.0 | 4.7 | −4.68 | <0.001 |
| Reward Sensitivity | 6.1 | 3.8 | 8.4 | 3.8 | −1.97 | 0.056 |
| State Anxiety | 40.4 | 10.7 | 53.7 | 11.9 | −3.75 | 0.001 |
| Trait Anxiety | 39.9 | 9.5 | 51.8 | 15.5 | −3.01 | 0.005 |
| Sweetness, 1M Sucrose | 8.45 | 0.74 | 8.53 | 0.73 | 2.35 | 0.763 |
| Pleasantness, 1M Sucrose | 5.05 | 2.38 | 3.26 | 2.47 | −0.30 | 0.024 |
| Medication Use | n | % | n | % | ||
| SSRI | 0 | 0.0 | 7 | 36.8 | ||
| Atypical Antipsychotic | 0 | 0.0 | 3 | 15.8 | ||
| SSRI + Atypical Antipsychotic | 0 | 0.0 | 1 | 5.3 | ||
| Comorbid Diagnoses | ||||||
| Major Depression | 0 | 0.0 | 1 | 5.3 | ||
| Anxiety Disorder | 0 | 0.0 | 2 | 10.5 | ||
| Major Depression + Anxiety Disorder | 0 | 0.0 | 2 | 10.5 | ||
Note: AN=anorexia nervosa; BMI=body mass index; CW=control adolescents; SSRI=selective serotonin reuptake inhibitor;
Brain Volume Results
Brain total GM, WM, CSF, and total intracranial volumes were similar between groups (Table 2).
Table 2.
Whole Brain Volumes Represent Raw Values
| CW, n = 22 | AN, n = 19 | |||||
|---|---|---|---|---|---|---|
| Whole Brain Volumes |
Mean | SD | Mean | SD | t | p-value |
| Gray Matter Volume (cm3) | 667.38 | 68.33 | 662.62 | 44.88 | 0.243 | 0.809 |
| White Matter Volume (cm3) | 472.28 | 45.53 | 459.79 | 44.91 | 0.881 | 0.383 |
| Cerebral Spinal Fluid Volume (cm3) | 252.35 | 49.23 | 230.74 | 24.16 | 1.739 | 0.09 |
| Total Intracranial Volume (cm3) | 1392.02 | 86.5 | 1353.16 | 91.81 | 1.388 | 0.173 |
|
Regional Gray Matter Volume Contrasts AN Adolescents > Controls |
MNI Coordinates | Cluster Size | T/Z |
Cluster Level pFWE-corr |
Peak Level pFWE-corr |
|
| L Middle Orbitofrontal Cortex | −33, 60, −9 | 189 | 4.21/3.75 | 0.011 | 0.009 | |
| R Fusiform Gyms | 20, −34, −15 | 137 | 5.43/4.57 | 0.001 | 0.032 | |
| R Fusiform Gyms | 27, −10, −36 | 107 | 4.88/4.22 | 0.005 | 0.042 | |
| L Fusiform Gyms | −32, −7, −41 | 100 | 4.26/3.79 | 0.024 | 0.047 | |
| R Hippocampus | 29, −27, −8 | 113 | 4.74/4.12 | 0.003 | 0.018 | |
| L Hippocampus | −33, −19, −11 | 74 | 4.01/3.61 | 0.018 | 0.026 | |
| R Insula | 41,5,7 | 69 | 4.06/3.64 | 0.029 | 0.047 | |
| R Parahippocampal Gyrus | 18, −10, −29 | 579 | 6.41/5.15 | <0.001 | 0.001 | |
| L Parahippocampal Gyrus | −21, −13, −27 | 116 | 4.42/3.90 | 0.008 | 0.021 | |
| L Gyrus Rectus | −5, 30, −26 | 19 | 3.99/3.59 | 0.014 | 0.038 | |
| L Gyrus Rectus | −9, 30, −26 | 25 | 3.95/3.56 | 0.016 | 0.035 | |
| L Gyrus Rectus | −6, 53, −24 | 41 | 4.19/3.73 | 0.009 | 0.028 | |
|
Regional White Matter Volume Contrasts AN Adolescents > Controls |
MNI Coordinates | Cluster Size | T/Z |
Cluster Level pFWE-corr |
Peak Level pFWE-corr |
|
| R Hippocampus | 29, −21, −20 | 208 | 5.60/4.68 | <0.001 | 0.007 | |
| R Parahippocampal Gyrus | 29, −25, −20 | 237 | 5.74/4.76 | <0.001 | 0.006 | |
| R Middle Temporal Gyrus | 50, −31, −6 | 82 | 5.98/4.91 | 0.001 | 0.078 | |
| L Superior Temporal Gyrus | −48, −33, 22 | 98 | 3.86/3.49 | 0.071 | 0.045 | |
Note: Regional brain volume contrasts based on group comparison corrected for total intracranial volume, age, comorbid diagnoses and medication use. AN=anorexia nervosa; CW=control adolescents; FWE-corr=family-wise error corrected; L=left; MNI=Montreal Neurological Institute; R=right.
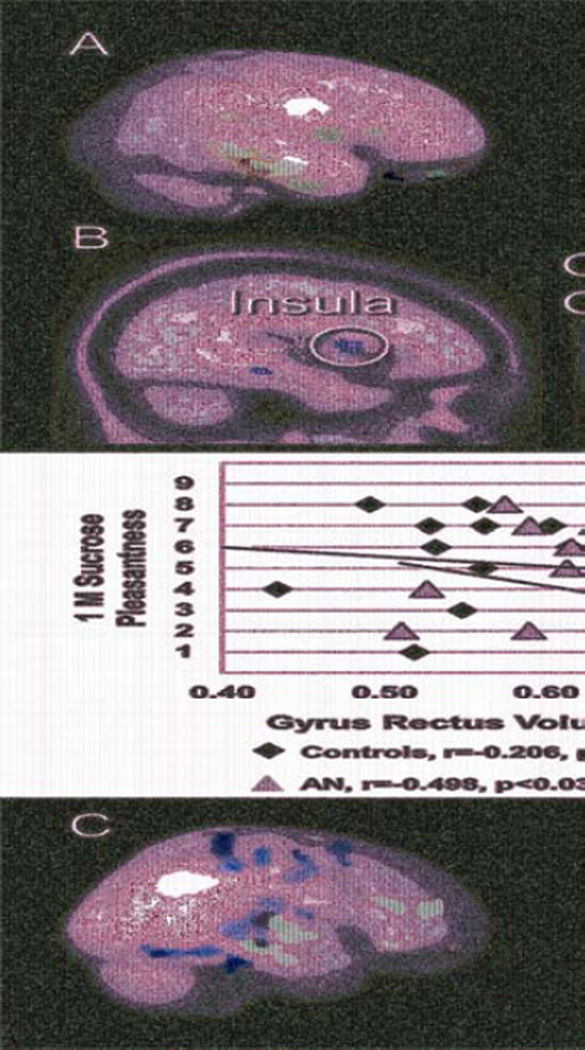
GM volume was greater in individuals with AN compared to control adolescents in left orbitofrontal gyrus rectus, bilateral fusiform gyrus, bilateral hippocampus, right insula, and bilateral parahippocampal gyrus and WM volume was greater in individuals with AN compared to control adolescents in right hippocampus and parahippocampal gyrus, right middle temporal gyrus, and left superior temporal gyrus (Figure 1).
Figure 1.
Group differences in brain volumes and white matter integrity. Note: A. Red indicates anorexia nervosa (AN) > Controls (CW) for gray matter; blue indicates AN > Controls for white matter. B. Greater left orbitofrontal gyrus rectus volume in adolescent AN is negatively correlated with 1M sucrose taste pleasantness. C. White matter integrity (fractional anisotropy). Green indicates Controls > AN in superior frontal, parietal and temporal lobes; blue indicates AN > CW in frontal, orbitofrontal, and temporal lobes.
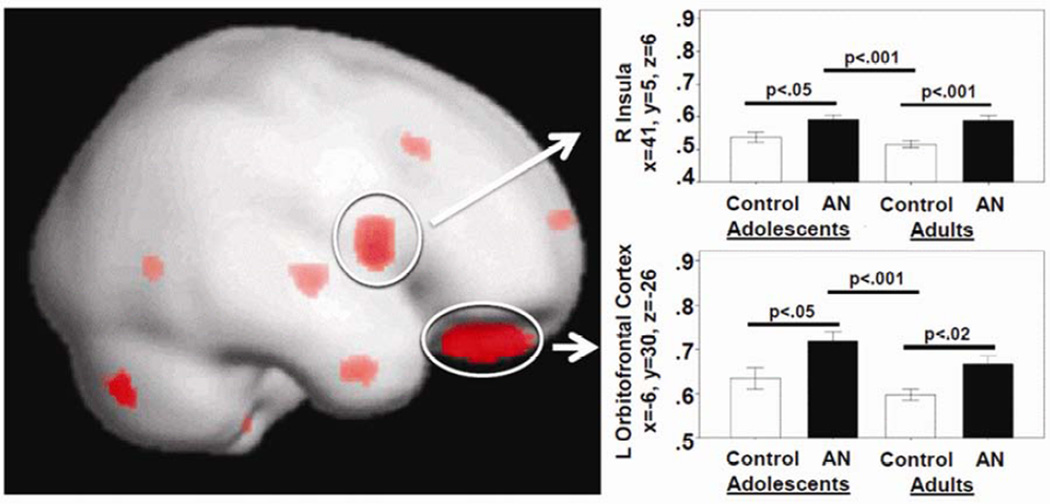
The 4-group GM GLM between adolescent and adult groups indicated significant differences (FWE p < 0.05) (Figure 2): right prefrontal cortex (x=11, y=32, z=26, adolescents and adults with AN > adult controls), right Insula (x=41, y=5, z=6, adolescents with AN > adolescent and adult controls, adults with AN > adult controls), right putamen (x=29, y=6, z=10, adolescents, adults with AN > adult controls), left orbitofrontal gyrus rectus (x=−6, y=30, z=−26, adolescents with AN > adolescent, adult controls, adults with AN > adult controls; x=−8, y=47, z=−26, adolescents with AN > adolescent and adult controls), right gyrus rectus (x=6, y=33, z=−26, adolescents and adults with AN > adult controls).
Figure 2.
Results from the 4-group analysis, contrasting adolescent and adults with anorexia nervosa (AN) and control individuals (CW). Note: Red indicates areas of group difference, which are rendered on a semitransparent standard brain with displayed results both on the outside as well as within deeper brain structures including the insula. L=left; R=right.
We carefully considered whether to include the 2 binge eating/purging type individuals with AN in the study. Detailed analyses indicated that both ED behavior scores as well as brain imaging results (see Supplement 1) were comparable to the restricting type AN individuals and did not confound the results and were therefore included.
DTI Results
DTI FA (Figure 1, Table 3) was greater in controls compared to AN in left fornix, bilateral cingulum, right forceps major, right superior and left posterior corona radiata. DTI FA was greater in AN compared to controls in left superior longitudinal fasciculus, bilateral anterior corona radiata, and bilateral inferior fronto-occipital fasciculus.
Table 3.
Diffusion Tensor Imaging (DTI) Results
| MNI Coordinates | |||||
|---|---|---|---|---|---|
| Pathway/Region | Cluster Size | x | y | z | T/Z |
|
FA Controls > AN Adolescents |
|||||
| Cingulum, R | 182 | 12 | −14 | 42 | 4.06/3.64 |
| Forceps Major, R | 51 | 20 | −62 | 34 | 3.59/3.28 |
| Superior Corona Radiata, R | 77 | 30 | −28 | 26 | 3.37/3.11 |
| Cingulum, R | 61 | 4 | 10 | 60 | 3.77/3.42 |
| Cingulum, L | 52 | −12 | −4 | 46 | 3.66/3.33 |
| Fornix, L | 235 | −18 | −20 | 22 | 4.29/3.81 |
| Posterior Corona Radiata, L | 156 | −22 | −40 | 62 | 4.41/3.89 |
| Fornix, L | 65 | −2 | −22 | 12 | 3.60/3.29 |
|
FA AN Adolescents > Controls |
|||||
| Inferior Fronto-Occipital Fasciculus, R | 340 | 20 | 54 | −8 | 4.09/3.67 |
| Anterior Corona Radiata, R | 84 | 22 | 46 | 14 | 3.75/3.41 |
| Anterior Corona Radiata, L | 79 | −20 | 50 | 18 | 4.16/3.71 |
| Inferior Fronto-Occipital Fasciculus, L | 91 | −48 | −26 | −10 | 4.60/4.03 |
| Superior Longitudinal Fasciculus, L | 79 | −60 | −12 | 4 | 4.21/3.75 |
|
ADC AN Adolescents > Controls |
|||||
| Corpus Callosum, Splenium, R | 106 | 22 | −36 | 0 | 3.73/3.39 |
| Corticospinal Tract, R | 132 | 28 | −24 | 64 | 4.04/3.62 |
| Posterior Corona Radiata, Corticopontine Tract, R | 97 | 22 | −38 | 70 | 4.03/3.62 |
| Superior Longitudinal Fasciculus, R | 106 | 52 | −54 | 10 | 4.35/3.85 |
| Corticopontaine Tract, L | 632 | −26 | −38 | 66 | 5.74/4.77 |
| Superior Longitudinal Fasciculus, L | 66 | −40 | −52 | 48 | 3.46/3.18 |
| Fornix, L | 211 | −8 | −32 | 2 | 4.41/3.89 |
Note: ADC=apparent diffusion coefficient; AN=anorexia nervosa; FA= fractional anisotropy; L=left; MNI=Montreal Neurological Institute; R=right.
DTI ADC was higher in AN compared to controls in left fornix, right corpus callosum, right corticospinal tract, right posterior corona radiata, bilateral corticopontine tract, and bilateral superior longitudinal fasciculus.
Correlation Results
Control adolescents
GM volume significantly negatively correlated with age in the left gyrus rectus (x=−6 y=53 z=−24, r=−0.652, p < 0.009).
Adolescents with AN
Orbitofrontal cortex volume negatively correlated with sweet taste-pleasantness (Figure 2).
Discussion
This study replicates now in adolescents previous findings in adults that AN is associated with larger orbitofrontal and insula cortex, and adolescents with AN show lower WM integrity in the fornix similarly to adults with the disorder.
Greater GM, WM Volume and FA in Individuals With AN
Most previous studies reported lower brain volumes in AN, while we found higher localized GM and WM in adolescents and adults with AN. The use of analysis software that is more accurate in GM/WM separation and CSF calculation could have contributed these results,38,39 as well as the fact that AN individuals’ food intake was highly supervised, reducing acute effects of malnutrition. Little research on this topic exists in eating disorders, but one study did find increase in GM and WM volume with short-term weight restoration, supporting the potential benefit of our approach.44 The inclusion of age, depression and anxiety diagnoses, and medication use as covariates, may also have contributed to these novel findings.
The location of peak orbitofrontal cortex gyrus rectus group differences in the current study (x=−5, y=30, z=−26) was almost similar to findings in our adult study (x=−6, y=29, z=−26), and this overlap was supported by the 4-group analysis with data in adults that were described previously.25 The medial orbitofrontal cortex is an important higher order brain region for processing of reward expectation and value,27,28 aids in controlling how much we eat of a certain food (sensory specific satiety),45 and has been associated with food avoidance.46 The factors contributing to higher orbitofrontal gyrus rectus volume is unclear. One potential explanation is that the trajectory of orbitofrontal GM development in AN may be delayed, reaching peak volumes later than in controls and thus resulting in greater cortical thickness and volume.47 Another possibility could be effects of repeated food restriction in AN, but this will need to be tested longitudinally. As in previous studies, sweet pleasantness was lower in individuals with AN compared to controls,48 and gyrus rectus volume in adolescents with AN was negatively related to sweet pleasantness rating, suggesting that enlarged orbitofrontal cortex could directly be involved in food avoidance in adolescent AN. Interestingly, WM FA was also greater in the orbitofrontal gyrus (corona radiata) and superiorly in the adjacent prefrontal WM (superior longitudinal fasciculus) and this could point further to a larger developmental alteration in this region.
Also similarly to adults,25 adolescents with AN had higher right insula GM volume, with peak coordinates (x=41, y=5, z=7) very close to the adult group (x=42, y=9, z=4) in the middle insula, and also supported by the 4-group contrast. The middle insula has been associated with gustation, but even more so with interoception.49 The fixed perception of being fat while severely underweight in anorexia nervosa50 could thus be related to larger right sided insula volume.
Differing from adult AN were larger GM and WM volumes in the temporal lobe fusiform gyrus, hippocampus and parahippocampal gyrus, as well as greater FA in the connecting middle and superior temporal lobe structures (fronto-occipital fasciculus in our adolescent AN sample. The fusiform gyrus is an important structure in external body recognition51 and body size perception.52 Importantly, previous research found GM and WM in this area related to body weight changes,53 and this region is consistently activated when viewing food images.54 Furthermore, hippocampus and parahippocampus show decreased activation during satiety states.55,56 Thus higher volume and FA in those areas could contribute to altered body perception and thus core symptoms of AN.
Lower FA in Individuals With AN
Lower fornix FA in adolescents with AN is consistent with studies in adults, as is lower cingulum FA, although the adult sample had more posteriorly located lower cingulum FA.31 The fornix projects from the hippocampus as fimbria-fornix57 superior-anteriorly toward the midline, forming the body of the fornix. It winds around and between the lateral ventricles and projects inferiorly to the anterior commissure, and from there to hypothalamus and mammillary bodies, thalamus and cingulate cortex, and bilateral nucleus accumbens.58 Fornix lesions in rodents result in altered feeding and drinking patterns,59 reward processing,33 and resistance to behavior extinction60 but the fornix is also an important limbic structure, supporting emotion regulation by frontal cortical brain regions.61 Thus, abnormal fornix integrity could lead to altered feedback between limbic and higher order brain structures including hippocampus, amygdala, ventral striatum, cingulate, and orbitofrontal cortex.62 In contrast to our study in adult AN 31 we did not find correlations between harm avoidance or state or trait anxiety with fornix FA. Whether this is a relationship that develops later during development will need further exploration.
In addition, FA in the corona radiata and forceps major, the occipital part of the corpus callosum, was lower in adolescents with AN. The corona radiata fiber bundles connect the wide spread cerebral cortex63 with the basal ganglia and spinal cord, and corona radiata lesions have been found in central taste disorders.64 The corpus callosum facilitates communication between left and right-sided brain structures, and an increasing number of studies now implicate the corpus callosum in taste processing.65–67 The functional significance of the corona radiata and corpus callosum FA alterations require further study but could be related to altered taste and reward processing in AN. As expected, ADC was higher in areas of lower FA and we therefore do not separately discuss regional ADC alterations.
Developmental Aspects of Brain Structure
Childhood and adolescence are times of intense morphologic brain development. The medial orbitofrontal cortex peaks around age 9, while insula cortical thickness peaks at age 18 years, and the middle and inferior temporal cortex around age 11.47 More GM volume in adolescents with AN in those areas may suggest either higher growth or delayed pruning and cortical thinning. This does not solve the questions though, what may cause such alterations, whether they contribute to illness onset or whether illness food restriction itself affects this process of brain development. FA usually increases with greater myelination and age during childhood and adolescence,68 however the relationship to cognitive-emotional functioning is still obscure, and higher FA does not necessarily mean better function.69
The results are in contrast to various studies that found lower brain volumes in AN. The brain analysis method we used shows improved accuracy from other analysis methods 38,39 and we do not believe that there is a methodological systematic error. Older VBM versions used Bayesian statistics tissue priors of control subjects that may not accurately represent the analyzed sample, which may be particularly important for AN brains that may not conform to standard templates. This approach improves tissue segmentation accuracy because it does not depend on standard template assumptions and normalizes images to a custom template created from the specific study population. Furthermore, similar orbitofrontal and insula results in adolescents with anorexia as well as adults25 point toward consistent brain alterations. While we made every effort to reduce effects of acute malnutrition, past or more recent effects of underfeeding may also have contributed to the differences across groups. Comorbidity and use of medication are a concern and potential confound in brain imaging studies. We accounted for those factors by using them as covariates in the brain imaging analysis. Two of the youth with AN were of the binge eating/purging subtype. The behavioral and brain imaging measures in the 2 binge eating/purging individuals fell well within the range of the restricting type individuals, and we therefore did not exclude those individuals from the study. However, it could be possible that the neurologic underpinnings or developmental trajectories may differ between restricting and binge eating/purging subtypes, which could be a confound. The 4-group GM analysis showed also group differences other than our a priori hypotheses for orbitofrontal cortex and insula. However, a detailed and sufficient discussion of those results would go beyond the space limitations of this manuscript.
The neurobiology of AN is poorly understood. The results from this study provide new insights into brain structure alterations in youth and how this could be related to pathophysiology and behavior. The results further identify AN as a brain disorder, and may help patients and their families understand better this complex disorder. The similarity in results compared to adults further strengthens this argument. Furthermore, future interventions may be able to stimulate or reduce activity in altered brain regions, which may facilitate the treatment process.
In summary, this novel study comparing adolescents with AN and healthy controls, replicates and supports findings in adult AN of greater left orbitofrontal cortex gyrus rectus and right insula volumes and that these regions are associated with the pathophysiology of AN. Those alterations could be trait markers that may contribute to illness onset, or an effect of AN that may hinder recovery. The additional findings in adolescents with AN of negative relationship between gyrus rectus GM volume and taste pleasantness could indicate that structural alterations in those areas are directly involved in core symptoms of adolescent AN.
Supplementary Material
Acknowledgments
A Davis Foundation Award of the Klarman Family Foundation Grants Program in Eating Disorders, National Institute of Mental Health (NIMH) grant K23 MH080135, and NIMH grant R01 MH096777 provided funding for all aspects of the study to Dr. Frank.
The authors would like to thank all the individuals who participated in this study, as well as the staff at the Eating Disorders Program at the Children’s Hospital Colorado and the Eating Disorder Center Denver.
Disclosure: Dr. Frank has received grant or research support from the National Institutes of Health and has served as a consultant to the Eating Disorder Center of Denver.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Drs. Hagman and Yang, and Ms. Shott report no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association. Handbook of Psychiatric Measures. 4. Washington DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders - Text Revision (DSM-IVTR) [Google Scholar]
- 2.USDHHS. Eating Disorders Information Sheet. Health. PHSsOiWs. ed2000. [Google Scholar]
- 3.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 4.Bulik CM. Exploring the gene-environment nexus in eating disorders. J Psychiatry Neurosci. 2005;30(5):335–339. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience. 2009;10(8):573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 6.Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Current Topics in Behavioral Neurosciences. 2011;6:37–57. doi: 10.1007/7854_2010_85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao X, Xu D, Bansal R, et al. Multimodal magnetic resonance imaging: The coordinated use of multiple, mutually informative probes to understand brain structure and function. Human Brain Mapping. 2013;34(2):253–271. doi: 10.1002/hbm.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Eynde F, Suda M, Broadbent H, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. European Eating Disorders Review. 2012;20(2):94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 9.Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J Pediatr. 1996;129:794–803. doi: 10.1016/s0022-3476(96)70021-5. [DOI] [PubMed] [Google Scholar]
- 10.Kornreich L, Shapira A, Horev G, Danziger Y, Tyano S, Mimouni M. CT and MR evaluation of the brain in patients with anorexia nervosa. AJNR Am J Neuroradiol. 1991;12:1213–1216. [PMC free article] [PubMed] [Google Scholar]
- 11.Laessle RG, Krieg JC, Fichter MM, Pirke KM. Cerebral atrophy and vigilance performance in patients with anorexia and bulimia nervosa. Neuropsychobiology. 1989;21(4):187–191. doi: 10.1159/000118575. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GW, Ellinwood EH, Jr, Rockwell WJ, Herfkens RJ, Nishita JK, Guthrie LF. Cerebral atrophy in bulimia. Biol Psychiatry. 1989;25(7):894–902. doi: 10.1016/0006-3223(89)90269-2. [DOI] [PubMed] [Google Scholar]
- 13.Lambe EK, Katzman DK, Mikulis DJ, Kennedy SH, Zipursky RB. Cerebral gray matter volume deficits after weight recovery from anorexia nervosa. Arch Gen Psychiatry. 1997;54(6):537–542. doi: 10.1001/archpsyc.1997.01830180055006. [DOI] [PubMed] [Google Scholar]
- 14.Muhlau M, Gaser C, Ilg R, et al. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry. 2007;164(12):1850–1857. doi: 10.1176/appi.ajp.2007.06111861. [DOI] [PubMed] [Google Scholar]
- 15.Wagner A, Greer P, Bailer U, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59(3):291–293. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Fornieles J, Bargallo N, Lazaro L, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. 2009;43(3):331–340. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Boghi A, Sterpone S, Sales S, et al. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res. 2011;192(3):154–159. doi: 10.1016/j.pscychresns.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Brooks SJ, Barker GJ, O'Daly OG, et al. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joos A, Kloppel S, Hartmann A, et al. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182(2):146–151. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Suchan B, Busch M, Schulte D, Gronemeyer D, Herpertz S, Vocks S. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res. 2010;206(1):63–67. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Friederich HC, Walther S, Bendszus M, et al. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59(2):1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Gaudio S, Nocchi F, Franchin T, et al. Gray matter decrease distribution in the early stages of Anorexia Nervosa restrictive type in adolescents. Psychiatry Res. 2011;191(1):24–30. doi: 10.1016/j.pscychresns.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 23.van Tol MJ, van der Wee NJ, van den Heuvel OA, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67(10):1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 24.Streitburger DP, Moller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7(8):e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013 May 17; doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolls E, Critchley H, Verhagen J, Kadohisa M. The representation of information about taste and odor in the orbitofrontal cortex. Chemosensory Perception. 2010;3:16–33. [Google Scholar]
- 27.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 28.Noonan MP, Kolling N, Walton ME, Rushworth MF. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur J Neurosci. 2012;35(7):997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmahmann JD, Pandya DN. Cerebral white matter--historical evolution of facts and notions concerning the organization of the fiber pathways of the brain. Journal of the History of Neuroscience. 2007;16(3):237–267. doi: 10.1080/09647040500495896. [DOI] [PubMed] [Google Scholar]
- 30.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 31.Kazlouski D, Rollin MD, Tregellas J, et al. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192(2):109–116. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frieling H, Fischer J, Wilhelm J, et al. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa--a voxel based diffusion tensor imaging (DTI) study. J Psychiatr Res. 2012;46(9):1237–1242. doi: 10.1016/j.jpsychires.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Salinas JA, White NM. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behav Neurosci. 1998;112(4):812–826. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Frank GK, Reynolds JR, Shott ME, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41(3):903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One. 2012;7(9):e45081. doi: 10.1371/journal.pone.0045081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Olivi A, Hertig SJ, van Zijl P, Mori S. Automated fiber tracking of human brain white matter using diffusion tensor imaging. Neuroimage. 2008;42(2):771–777. doi: 10.1016/j.neuroimage.2008.04.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cacioppo JT, Berntson GG. Social neuroscience: key readings. New York: Psychology Press; 2005. [Google Scholar]
- 43.Paxinos G, Mai JK. The human nervous system. 2nd. Amsterdam; Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 44.Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord. 2011;44(5):406–411. doi: 10.1002/eat.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolls B, Rolls E, Rowe E, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- 46.Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eiber R, Berlin I, de Brettes B, Foulon C, Guelfi JD. Hedonic response to sucrose solutions and the fear of weight gain in patients with eating disorders. Psychiatry Res. 2002;113:173–180. doi: 10.1016/s0165-1781(02)00232-9. [DOI] [PubMed] [Google Scholar]
- 49.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214(5 – 6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konstantakopoulos G, Varsou E, Dikeos D, et al. Delusionality of body image beliefs in eating disorders. Psychiatry Res. 2012;200(2 – 3):482–488. doi: 10.1016/j.psychres.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Peelen MV, Downing PE. Selectivity for the human body in the fusiform gyrus. J Neurophysiol. 2005;93(1):603–608. doi: 10.1152/jn.00513.2004. [DOI] [PubMed] [Google Scholar]
- 52.Hummel D, Rudolf AK, Brandi ML, et al. Neural adaptation to thin and fat bodies in the fusiform body area and middle occipital gyrus: An fMRI adaptation study. Human Brain Mapping. 2012 Jul 17; doi: 10.1002/hbm.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. International Journal of Obesity. 2012;36(5):656–664. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55(1):296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 55.Leidy HJ, Lepping RJ, Savage CR, Harris CT. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity. 2011;19(10):2019–2025. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44(3):1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17(5):396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- 58.Sudheimer K, Winn B, Kerndt G, et al. The Human Brain Atlas. Radiology Department, Communications Technology Laboratory, and College of Human Medicine, Michigan State University [Google Scholar]
- 59.Osborne B, Dodek AB. Disrupted patterns of consummatory behavior in rats with fornix transections. Behavioral and Neural Biology. 1986;45(2):212–222. doi: 10.1016/s0163-1047(86)90783-1. [DOI] [PubMed] [Google Scholar]
- 60.Osborne B, Silverhart T, Markgraf C, Seggie J. Effects of fornix transection and pituitary-adrenal modulation on extinction behavior. Behav Neurosci. 1987;101(4):504–512. doi: 10.1037//0735-7044.101.4.504. [DOI] [PubMed] [Google Scholar]
- 61.Dalgleish T. The emotional brain. Nature Reviews Neuroscience. 2004;5(7):583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 62.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4 – 5):727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 63.Parent A, Carpenter MB. Carpenter's human neuroanatomy. 9th. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 64.Onoda K, Ikeda M, Sekine H, Ogawa H. Clinical study of central taste disorders and discussion of the central gustatory pathway. J Neurol. 2012;259(2):261–266. doi: 10.1007/s00415-011-6165-z. [DOI] [PubMed] [Google Scholar]
- 65.Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T. Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res. 2011;1370:99–111. doi: 10.1016/j.brainres.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 66.Hayama T, Ogawa H. Callosal connections of the cortical taste area in rats. Brain Res. 2001;918(1 – 2):171–175. doi: 10.1016/s0006-8993(01)02941-9. [DOI] [PubMed] [Google Scholar]
- 67.Salvolini U, Polonara G, Mascioli G, Fabri M, Manzoni T. Functional topography of the human corpus callosum. Bull Acad Natl Med. 2010;194(3):617–631. discussion 631-612. [PubMed] [Google Scholar]
- 68.Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41(2):223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 69.Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci U S A. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.