Abstract
Cisplatin is one of the most effective chemotherapeutic agents but injury may occur at higher doses. The aim of this study was to investigate the effect of bilberry on cisplatin induced toxic effects in rat ovary. Twenty-one female Wistar–Albino rats were utilized to form three groups: In group 1 (control group), each rat received intraperitoneal injection of 1 mL of 0.9 % NaCl saline solution during 10-days. In group 2 (cisplatin group), a single dose of 7.5 mg/kg b.w. cisplatin was given. In group 3 (cisplatin + bilberry group), a single dose of 7.5 mg/kg cisplatin and bilberry at 200 mg/kg b.w. were given for 10 days. Ovaries were surgically removed in all groups and prepared for biochemical and light microscopic investigations at the examination times. Malondialdehyde (MDA) levels and activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) of tissue samples were measured. Histopathological damages in cisplatin administrated rats were seen such as severe edema, vascular congestion, hemorrhage and follicular degeneration in the ovary tissue. Moderate pathological alterations were observed in rats treated with bilberry plus cisplatin. Cisplatin administration significantly increased MDA production and decreased SOD, CAT, GPx and GST activities in the ovarian tissue when compared to the control group (p < 0.05). Cisplatin + bilberry administration increased antioxidant enzymes activities and reduced MDA levels. Bilberry administration seems to reduce the cisplatin induced ovarian toxicity thus it alleviates free radical damage. But it dose not protect completely rat ovary tissues.
Keywords: Bilberry, Cisplatin, Ovarian damage, Rat, Protective effect
Introduction
Cisplatin [CDDP, cis-diamminedichloroplatinum(II)] is a potent chemotherapeutic agent used in the treatment of malignancies (Carter 1984). However, the usage of CDDP is limited because of toxic effects on the tissue and organs such as ovary, kidney, liver etc. at high dose (Dillioglugil et al. 2005; Naziroglu et al. 2004; Zhang et al. 2011; Ueki et al. 2013). Numerous mechanisms contribute to the harmful effect. The cytotoxicity of this drug is believed to result from the platinum binding to DNA. This DNA-platinum complex prevents efficient DNA replication and transcription. This reaction is responsible for the cytotoxic effect of CDDP (Kim et al. 2003). Also, CDDP behaves as a direct cellular toxin, especially when the chloride level is low. The chloride molecules of CDDP exchange water inside of the cell. This reaction results in an increase at the production of reactive oxygen species (ROS). These free radicals and elevated malondialdehyde (MDA) levels alter the cellular structure and damage the tissue (Boulikas and Vougiouka 2003; Satoh et al. 2003).
Oxidative stress is one of the most important mechanisms involved in CDDP-induced toxicity. The mitochondrion is the primary target for CDDP-induced oxidative stress, resulting in loss of mitochondrial protein-SH, inhibition of calcium uptake and a reduction in the mitochondrial membrane potential (Saad et al. 2004). Cells develop various protective mechanisms to protect from oxidative stress. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities are increased in cellular membranes due to CDDP induced nephrotoxicity (Noori and Mahboob 2010; Naqshbandi et al. 2012; Ueki et al. 2013), hepatoxicity (Mansour et al. 2006; Kart et al. 2010; Longo et al. 2011), ovarian toxicity (Gupta et al. 2006; Zhang et al. 2011). These antioxidant enzymes play an important role to avoid the oxidative injury (Gupta et al. 2006). It is shown that antioxidant therapy reverses these raised enzyme activities (Caffrey and Frenkel 2000; Durak et al. 2009).
Gingko biloba extracts significantly prevented hair cell damage induced by cisplatin (Choi et al. 2013). Riboflavin, folate, and the multiple-vitamin supplementation proved to be more efficacious in attenuating the cisplatin-induced intestinal damage and associated changes in apoptosis (Bodiga et al. 2012). Dietary fish oil supplementation ameliorated cisplatin induced specific metabolic alterations and oxidative damage due to its intrinsic biochemical antioxidant properties (Naqshbandi et al. 2012). Several agents/strategies were evaluated to prevent CDDP nephrotoxicity but were not found suitable for clinical practice. Bilberry is a natural antioxidant fruit containing chemical substances known as anthocyanosides (Ichiyanagi et al. 2004). It constitutes a family of flavonoids that are widely distributed in colored fruits and vegetables (Goiffon et al. 1991; Matsumoto et al. 2001). It is an excellent free-radical scavenger which has been utilized in the treatment of cancer and heart diseases. There are a lot of studies demonstrating protective effect of bilberry in different tissues and organs such as vascular tissues, intestine, heart, and retina (Bell and Gochenaur 2006).
Therefore, we thought that bilberry could be efficient against CDDP induced ovarian damage. There is no study indicating preventive effects of bilberry on oxidative stress and histopathologic events in ovaries. In this study, we aimed to show the effects of bilberry on ovarian toxicity due to CDDP administration.
Materials and methods
Chemicals
CDDP, purity 98 % was obtained from a pharmacy (Yozgat, Turkey). The treatment protocol used in this study was according to a previously reported study (Mansour et al. 2006). Bilberry was supplied by a drugstore (Yozgat, Turkey). The treatment protocol used in this study to treat against liver and kidney toxicity has been previously reported by Bao et al. (2008a, b).
Animals and experimental procedure
The experimental study protocol was approved by the Ethical Committee of the Cukurova University Medical Faculty. Twenty-one female adult Wistar–Albino rats weighing between 150 and 220 g were taken from the Cukurova University’s Experimental Animal Laboratory TIBDAM Institute. Animals were acclimatized to the laboratory conditions 1 week before the start of the experiment. All rats were housed 10 days at 24 ± 2 °C and provided with 12 h sunlight. Ad libitum feeding method was performed with standard laboratory diet.
All animals were allocated randomly into three groups. In group 1 (n = 7, control group), each rat received intraperitoneal (ip) injection of 1 mL of 0.9 % NaCl saline solution during 10-days. In group 2 (n = 7, CDDP group), a single dose ip of 7.5 mg/kg body weight (b.w.) CDDP was administered. In group 3 (n = 7, CDDP + bilberry group), a single dose ip of 7.5 mg/kg b.w. CDDP and bilberry at 200 mg/kg b.w. was given for 10 days. After the completion of the drug doses, all rats were sacrificed. Ovaries were surgically removed, immediately washed with cold saline solution (0.9 % NaCl). Ovarian tissues were fixed 10 % formalin and stored at −80 °C until analysis. Ovarian tissues were prepared for biochemical and light microscopic investigations at the examination times.
Sample collection and biochemical assays
The ovarian tissues were homogenized using a Teflon homogenizer (Heidolph Silent Crusher M, Schwabach, Germany), and then the homogenates were centrifuged at 10,000×g for 15 min at 4 °C. All processes were carried out at 4 °C. 0.5–1.0 g ovarian tissue homogenate was prepared for the analysis by biochemical assays. MDA level and SOD, CAT, GPx and GST activities were determined by measuring the absorbance of the samples in a spectrophotometer (Shimadzu UV 1800, Kyoto, Japan). Protein concentration was determined using bovine serum albumin (BSA) as standard according to Lowry et al. (1951).
To determine the activity of CAT, 10 % homogenates of ovarian tissue, prepared in 0.9 % NaCl, were centrifuged at 8,500×g at 4 °C for 15 min. In the supernatant (after dilution with phosphate buffer, pH 7.0), CAT enzyme activity was measured by assessing the hydrolysis of H2O2. The decrease was detected by absorbance at 240 nm (Aebi 1984). Its activity was expressed as nmol/mg protein.
GPx activity was measured using H2O2 as substrate according to the method described by Paglia and Valentine (1987). The reaction was monitored indirectly as the oxidation rate of NADPH at 240 nm for 3 min. Enzyme activity was expressed as nmol/mg protein.
SOD activity was measured as the inhibition of autoxidation of pyrogallol, according to the method of Marklund and Marklund (1974). Activity was monitored at 440 nm for 180 s (s). Data are expressed as U of SOD/mg hemoglobin.
GST activity was determined on the basis of conjugation of GSH (Glutathione) with 1-chloro-2,4-dinitrobenzene (CDNB) (Habig et al. 1974). Increases in absorbance were recorded at 340 nm for 3 min. The specific activity of GST was expressed as nmol/mg protein.
MDA is generated by lipid peroxidation and was measured after incubation at 95 °C with thiobarbituric acid (TBA) in aerobic conditions (pH 3.4). The MDA content was assayed using the TBA test (Ohkawa et al. 1979). Absorbance was measured at 532 nm to determine the MDA content. Specific activity was expressed as nmol/mg protein.
Histopathological examinations
For histopathological examinations ovary samples were fixed for 24 h in 10 % formalin solution and embedded in paraffin after dehydration. Then 5–6 μm thick slices were cut from ovary blocks with a microtom and stained with hematoxylin and eosin (H&E). The sections were examined and photographed by using an Olympus BX-51 light microscope (Olympus Corp., Tokyo, Japan). At least five randomly selected ovarian sections were examined for the degree of vascular congestion, hemorrhage, follicular degeneration, leukocyte infiltration and interstitial edema. Each slide was examined and the severity of the changes observed were scored by using a scale of grade 0, grade I, grade II and grade III.
Statistical analysis
The Statistical Package Program for the Social Sciences (SPSS 11.0; SPSS Inc., Chicago, IL, USA) version 11.0 was used for statistical analysis. The significance was calculated using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison procedure to calculate the significance. p < 0.05 value was taken as statistically significant.
Results
Effects of CDDP and bilberry treatment on ovary histology
There was no difference between the CDDP + bilberry-treated and the control group with respect to the macroscopic features.
The morphology of many different stages of developing follicles were detected normal in the control group (Fig. 1). After 10 days of CDDP exposure, edema, hemorrhage and vascular congestion were detected in ovary tissues. In addition to these, leukocyte infiltration and follicular degeneration were observed in these tissues (Fig. 2a, b). Treatment with bilberry effectively reduced the ovarian tissue damage. 10 days after bilberry + CDDP was given to rats, edema and vascular congestion were detected (Fig. 3a, b). Hemorrhage, leukocyte infiltration and follicular degeneration were not observed in the bilberry + CDDP-treatment group.
Fig. 1.
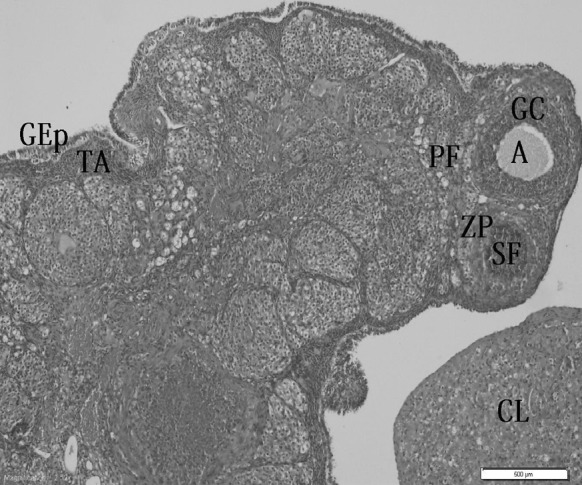
Ovary section of control rats showing normal morphology of many different stages of developing follicles. A antrum, GC granular cell, PF primary follicle, ZP zona pellucida, GEp germinal epithelial, TA tunica albuginea, CL corpus luteum, SF secondary follicle, ×200
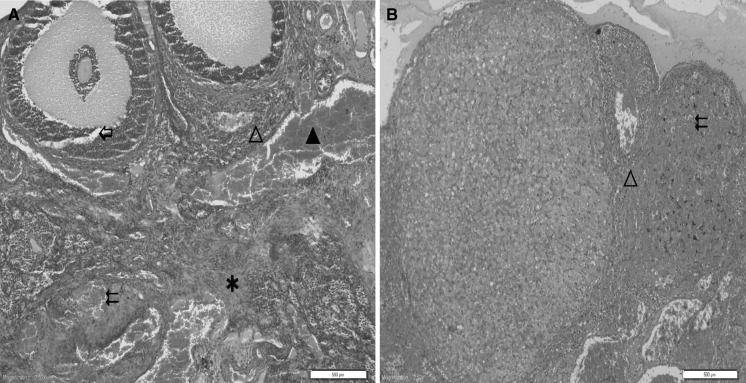
Fig. 2.
a, b Ovary sections of cisplatin-treated rats showing severe asterisk edema, double left wing oriented arrow hemorrhage, filled triangle vascular congestion, open triangle leukocyte infiltration and left wing oriented white arrow follicular degeneration, ×200
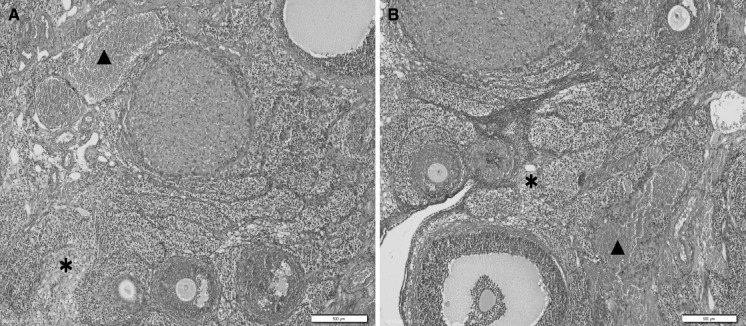
Fig. 3.
a, b Ovary section of cisplatin + bilberry treated rats showing mild asterisk edema and filled triangle vascular congestion, ×200
Total tissue damage scores were shown in Table 1. The pathological changes were scored as follows: grade 0, normal; grade I, mild edema, mild vascular congestion, no hemorrhage and no follicular degeneration, no leukocyte infiltration; grade II, moderate edema, moderate vascular congestion, no hemorrhage and no leukocyte infiltration; grade III, severe edema, severe vascular congestion, hemorrhage, follicular degeneration and leukocyte infiltration.
Table 1.
Histopathological grading of the ovary lesions in the treatment groups with respect to the number of animals
| Groups | Histopathological scores | |||
|---|---|---|---|---|
| 0 | I | II | III | |
| Control | 5 | – | – | – |
| Cisplatin | – | – | 1 | 4 |
| Cisplatin + bilberry | 1 | 3 | 1 | – |
Grade 0: normal; grade I: mild level (mild edema, mild vascular congestion, no hemorrhage and no follicular degeneration, no leukocyte infiltration); grade II: moderate level (moderate edema, moderate vascular congestion, no hemorrhage and no leukocyte infiltration); grade III: severe level (severe edema, severe vascular congestion, hemorrhage, follicular degeneration and leukocyte infiltration) of histopathological changes
Evaluation of biochemical results
No death was observed in any of the experimental groups. The course of the enzymatic activities were presented in Figs. 4, 5, 6, 7 and 8 (mean ± SD). There were differences in the MDA and enzyme activities among the different treatment groups.
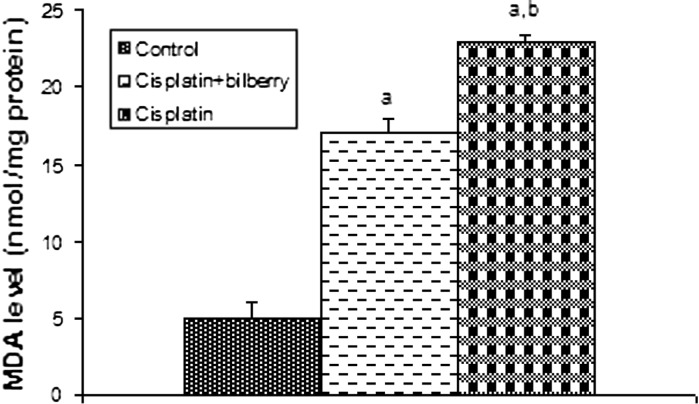
Fig. 4.
Effects of a single high dose of treatment of cisplatin with or without bilberry treatment in parallel on MDA content in the ovary tissues of rats. a Comparison of the control and experimental groups. b Comparison of the cisplatin + bilberry and the cisplatin groups. Data represent the mean ± SD of seven rats in each group. Significance at p < 0.05
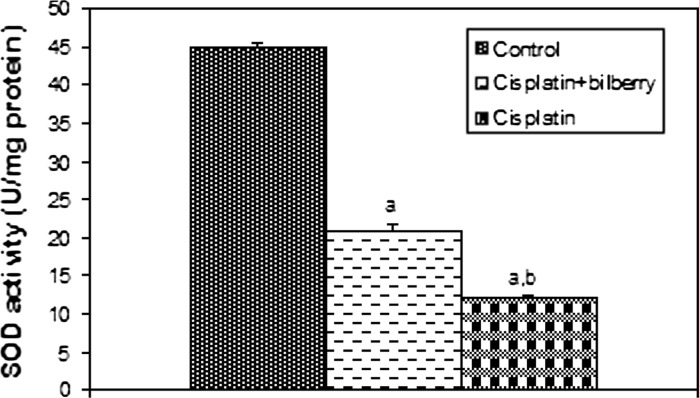
Fig. 5.
Effects of a single high dose of treatment of cisplatin with or without bilberry treatment in parallel on SOD activity in the ovary tissues of rats. a Comparison of the control and experimental groups. b Comparison of the cisplatin + bilberry and the cisplatin groups. Data represent the mean ± SD of seven rats in each group. Significance at p < 0.05
Fig. 6.
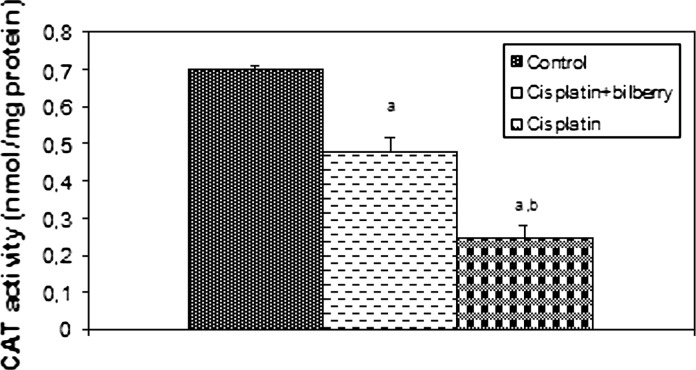
Effects of a single high dose of treatment of cisplatin with or without bilberry treatment in parallel on CAT activity in the ovary tissues of rats. a Comparison of the control and experimental groups. b Comparison of the cisplatin + bilberry and the cisplatin groups. Data represent the mean ± SD of seven rats in each group. Significance at p < 0.05
Fig. 7.
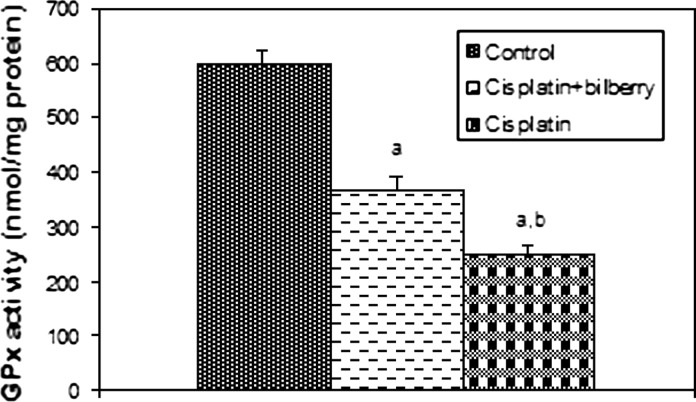
Effects of a single high dose of treatment of cisplatin with or without bilberry treatment in parallel on GSH-Px activity in the ovary tissues of rats. a Comparison of the control and experimental groups. b Comparison of the cisplatin + bilberry and the cisplatin groups. Data represent the mean ± SD of seven rats in each group. Significance at p < 0.05
Fig. 8.
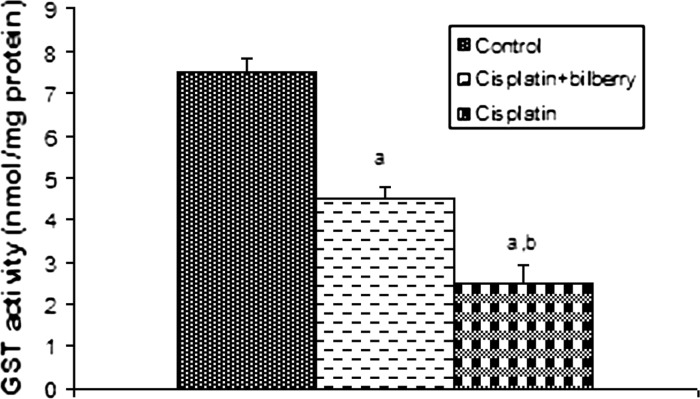
Effects of a single high dose of treatment of cisplatin with or without bilberry treatment in parallel on the GST content in the ovary tissues of rats. a Comparison of the control and experimental groups. b Comparison of the cisplatin + bilberry and the cisplatin groups. Data represent the mean ± SD of seven rats in each group. Significance at p < 0.05
Malondialdehyde levels (MDA)
The MDA level significantly increased in the CDDP-treated group compared to the control group (p < 0.01). A significant decrease of MDA level was observed in the bilberry + CDDP-treated group compared to the CDDP-treated group (p < 0.05), (Fig. 4).
Superoxide dismutase activity (SOD)
SOD activity decreased in the CDDP-treated group compared to the control group (p < 0.01). SOD activity increased in the bilberry + CDDP-treated group compared to the CDDP-treated group (p < 0.05), (Fig. 5).
Catalase activity (CAT)
CAT activity significantly decreased in the CDDP and bilberry + CDDP-treated groups compared to the control group. CAT activity increased in the bilberry + CDDP-treated group compared to the CDDP-treated group (p < 0.05), (Fig. 6).
Glutathione peroxidase activity (GPx)
A significant decreased of GPx activity was observed in the CDDP-treated group compared to the control groups (p < 0.01). GPx activity increased in the bilberry + CDDP-treated group compared to the CDDP-treated group (p < 0.05), (Fig. 7).
Glutathione-S-transferase activity (GST)
GST activity was statistically significantly decreased in the CDDP-treated groups compared to the control group. A significant increase in GST activity was observed in the bilberry + CDDP-treated groups compared with the CDDP-treated group (p < 0.05) (Fig. 8).
Discussion
Antineoplastic drugs are used to treat malignancies of the testis, metastatic ovarian tumors, lung cancer and many other solid tumors (Sweetman 2002). However, most of these agents are gonadotoxic and ovarian failure occurs in up to 40 % of patients (Dixit et al. 1997). The improvement of antineoplastic therapy has resulted in an increase in survival rates. Therefore, the prevention of chemotheraphy related, especially CDDP induced gonadotoxicity has become more of an issue. In humans, CDDP has been demonstrated to cause increased incidences of premature ovarian failure (Meirow 2000), ovarian dysfunctions such as menstrual disorders, premature menopause, infertility, etc., which resulted in a profound impact on patients’ self-esteem and their life quality and also increased medical costs (Wallace et al. 1989; Nasir et al. 1997; Stroud et al. 2009). It was reported that chemotherapeutic medicines leading to either temporary or permanent infertility severely affected the ovaries and hormonal balance (DeVita et al. 2005). In rats, CDDP has been shown to cause ovarian damage, at varying degrees ranging from minimal effects to ovarian failure, increased follicular apoptosis, decreased expression anti-Müllerian hormone (AMH), alterations in the estrous cycle, and a decrease in the number of AMH-producing follicles (Borovskaya et al. 2004; Yucebilgin et al. 2004; Yeh et al. 2006, 2008). Our study has demonstrated that CDDP caused significant toxicity in the ovarian tissues of rats.
Matsuo et al. (2007) reported that GnRH agonists are useful against chemotheraphy induced ovarian toxicity. They claimed that addition of Leuprorelin acetate protected primordial follicles from harmful effect of CDDP. Yeh et al. (2006) utilized the antioxidant sodium-2-mercaptoethanesulfonate (mesna) to preserve ovaries from CDDP induced toxic effect in rat model. It was found that the administration of mesna during low-dose CDDP treatment protected the ovaries by decreasing the loss of growing follicles in the ovaries (Yeh et al. 2008). Oxidative stress induced by CDDP in the rat ovary tissue causes infertility in female rats. Mirtazapine reverses this in a dose-dependent manner (Altuner et al. 2013). Sharma and Goyal (2012) demonstrated the protective effects of Heliotropium eichwaldi against CDDP-induced nephrotoxicity in mice. CDDP damages the granulosa cells by oxidative stress to diminish the ovarian reserve but mesna (2-mercaptoethane sulfonate), a Food and Drug Administration approved antioxidant, could attenuate the cisplatin-induced ovarian damages (Li et al. 2013). So, we hypothesized that utilization of a protective agent such as bilberry would be useful to preserve ovary against the CDDP induced toxicity.
Antioxidants were often used to antagonize the adverse effects caused by cisplatin by virtue of blocking oxidative stress (Li et al. 2013). Overproduction of the oxygen free radicals induced oxidative stress, which was responsible for the CDDP-related tissue toxicity (Cepeda et al. 2007). Oxygen free radicals affected the cell components such as lipid, protein, DNA and carbohydrates, in which lipids were the most sensitive. Oxygen free radicals enhanced lipid peroxidation which not only changed the fluidity and permeability of biomembranes, but also generated MDA, the stable end product, which caused the cross-linking and polymerization of macromolecules, such as proteins, nucleic acid, etc. Therefore, MDA level is a measure of lipid peroxidation and oxidative injury of the cell (Li et al. 2013). It is increased in oxidative stress (Celik and Suzek 2009). SOD, CAT, GPx and GST activities are increased to prevent ovarian tissue injury. These enzymatic systems localized in cellular membranes are the most important means to protect the tissue against injuries (Gupta et al. 2006; Sasaki and Joh 2007; Kara et al. 2012). But, when the cells are exposed to highly elevated oxidative stress the antioxidant mechanism could be damaged leading to decreased antioxidant enzyme activities and their gene expressions (Rodriguez et al. 2004). Similarly, in the previous studies, it has been reported that SOD, CAT, GPx and GST activities decreased in rat tissues by CDDP exposure (Li et al. 2013). The decreased antioxidant enzymes activities might be associated with toxicity of CDDP on rat ovarian tissues.
Bilberry is a natural product and contains unique subtances preventing cellular damage. The pigments of the plant serve as a scavenger against the toxic effects of free radicals. It has been utilized for the treatment of oxidative stress on various tissues and organs (Jang et al. 2005; Jakesevic et al. 2011). Ichiyanagi et al. (2004) identified the reactivities of twelve major anthocyanins in bilberry extracts towards reactive nitrogen species. They demonstrated that bilberry acts as a scavenger for superoxide anions and hydroxyl radicals. Milbury et al. (2007) reported that bilberry upregulates the oxidative stress related enzymes such as heme oxygenase-1 and glutathione-S-transferase. There are no data about the effect of bilberry against CDDP induced toxic effect in rat ovary. To the best our knowledge, this is the first study evaluating the effect of bilberry to protect ovarian tissue from damage by CDDP. In this prospective randomized trial, bilberry decreased the MDA levels and increased the protective enzymatic activities due to CDDP induced toxicity in rat ovary.
Histopathological changes were seen in the rat tissue after even a single dose of the CDDP. Attia (2012) demontrated that resveratrol has a protective role in CDDP-induced genotoxicity and apoptosis in somatic and germ cells of mice. Also, Ozer et al. (2011) have shown that misoprostol treatment after CDDP application protected the renal tissues from CDDP’s side effect. Atessahin et al. (2006) have investigated histological examinations in testes and further indicated significant damage to Sertoli, Leydig and germ cell populations induced by CDDP. In another study, apoptosis in ovaries was studied after CDDP administration (Yeh et al. 2008). In this study, administration of CDDP to rats increased severe edema, vascular congestion, hemorrhage, leukocyte infiltration and follicular degeneration in the ovary tissue. However, moderate pathological alterations were observed in rats treated with bilberry + CDDP. Only edema and vascular congestion were detected in this group. Thus, it appears that bilberry ameliorates CDDP induced toxicity but it dose not protect completely in rat ovary tissues.
In conclusion, bilberry has protective effects on histopathological changes induced by CDDP in rat ovarian tissue in our study. Ovarian tissue SOD, CAT, GPx and GST activities were significantly higher in the CDDP + bilberry group than the CDDP group (p < 0.05). Ovarian MDA levels were significantly lower in the CDDP + bilberry group than in the CDDP-treatment groups (p < 0.05). In the present experimental study, we observed that supplementation of bilberry prevented the ovarian tissue from the toxic influence of CDDP. Although our results suggested that bilberry can reduce CDDP ovarian toxicity, the degree of protection it provides is limited.
Acknowledgments
We would like to thank Esra Guven for her help in our study.
Conflict of interest
The authors declare no conflict of interest.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Altuner D, Gulaboglu M, Yapca OE, Cetin N (2013) The effect of mirtazapine on cisplatin-induced oxidative damage and infertility in rat ovaries. Sci World J 2013:Article ID 3272240, 6 [DOI] [PMC free article] [PubMed]
- Atessahin A, Karahan I, Turk G, Gur S, Yilmaz S, Ceribasi AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics testicular damage and oxidative stress in rats. Reprod Toxicol. 2006;21:42–47. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Attia SM. Influence of resveratrol on oxidative damage in genomic DNA and apoptosis induced by cisplatin. Mutat Res. 2012;741:22–31. doi: 10.1016/j.mrgentox.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Bao L, Yao XS, Yau CC, Tsi D, Chia CS, Nagai H, Kurihara H. Protective effects of Bilberry (Vaccinium myrtillus L.) extract on restraint stress-induced liver damage in mice. J Agric Food Chem. 2008;56:7803–7807. doi: 10.1021/jf800728m. [DOI] [PubMed] [Google Scholar]
- Bao L, Yao XS, Tsi D. Protective effects of Bilberry (Vaccinium myrtillus L.) extract on KBrO3-induced kidney damage in mice. J Agric Food Chem. 2008;56:420–425. doi: 10.1021/jf072640s. [DOI] [PubMed] [Google Scholar]
- Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. Appl Physiol. 2006;100:1164–1170. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- Bodiga VL, Bodiga S, Surampudi S, Boindala S, Putcha U, Nagalla B, Subramaniam K, Manchala R. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition. 2012;28:572–580. doi: 10.1016/j.nut.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Borovskaya TG, Goldberg VE, Fomina TI, Pakhomova AV, Kseneva SI, Poluektova ME, Goldberg ED. Morphological and functional state of rat ovaries in early and late periods after administration of platinum cytostatics. Bull Exp Biol Med. 2004;137:31–335. doi: 10.1023/B:BEBM.0000035121.85533.08. [DOI] [PubMed] [Google Scholar]
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level (review) Oncol Rep. 2003;10:1663–1682. [PubMed] [Google Scholar]
- Caffrey PB, Frenkel GD. Selenium compounds prevent the induction of drug resistance by cisplatin in human ovarian tumor xenografts in vivo. Cancer Chemother Pharmacol. 2000;46:74–78. doi: 10.1007/s002800000127. [DOI] [PubMed] [Google Scholar]
- Carter S. Cisplatin-past present and future. In: Hacker MP, editor. Platinum coordination complexes in cancer chemotherapy. Boston: Martinus Nijhoff Press; 1984. pp. 359–376. [Google Scholar]
- Celik I, Suzek H. Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol Environ Saf. 2009;72:905–958. doi: 10.1016/j.ecoenv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Kim SW, Lee JB, Lim HJ, Kim YJ, Tian C, So HS, Park R, Choung YH. Gingko biloba extracts protect auditory hair cells from cisplatin-induced ototoxicity by inhibiting perturbation of gap junctional intercellular communication. Neuroscience. 2013;244:49–61. doi: 10.1016/j.neuroscience.2013.04.001. [DOI] [PubMed] [Google Scholar]
- DeVita VT, Hellman S, Rosenberg SA. Cancer: principals and practice of oncology. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Dillioglugil MO, Maral KH, Gulkac MD, Ozon KA, Ozdogan HK, Acar O, Dillioglugil O. Protective effects of increasing vitamin E and a doses on cisplatin-induced oxidative damage to kidney tissue in rats. Urol Int. 2005;75:340–344. doi: 10.1159/000089171. [DOI] [PubMed] [Google Scholar]
- Dixit M, Yang JL, Poirier MC, Price JO, Andrews PA, Arteaga CL. Abrogation of cisplatin induced programmed cell death in human breast cancer cells by epidermal growth factor antisense RNA. J Natl Cancer Inst. 1997;89:365–373. doi: 10.1093/jnci/89.5.365. [DOI] [PubMed] [Google Scholar]
- Durak D, Uzun FG, Kalender S, Ogutcu A, Uzunhisarcikli M, Kalender Y. Malathion-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ Toxicol. 2009;24:235–242. doi: 10.1002/tox.20423. [DOI] [PubMed] [Google Scholar]
- Goiffon JP, Brun M, Bourrier MJ (1991) High-performance liquid chromatography of red fruit anthocyanins. J Chromatogr A 537:101–121
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Ichiyanagi T, Yoshihiko H, Matsuo S, Konishi T. Simultaneous comparison of relative reactivities of twelve major anthocyanins in bilberry towards reactive nitrogen species. Chem Pharm Bull. 2004;52:1312–1315. doi: 10.1248/cpb.52.1312. [DOI] [PubMed] [Google Scholar]
- Jakesevic M, Aaby K, Borge GIA, Jeppsson B, Ahrne S, Molin G. Antioxidative protection of dietary bilberry chokeberry and Lactobacillus plantarum HEAL19 in mice subjected to intestinal oxidative stress by ischemia-reperfusion. BMC Complement Altern Med. 2011;1:11–12. doi: 10.1186/1472-6882-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YP, Zhou J, Nakanishi K, Sparrow RJ. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cell. Photochem Photobiol. 2005;8:529–536. doi: 10.1562/2004-12-14-RA-402.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M, Daglioglu YK, Kuyucu Y, Tuli A, Tap A. The effect of edaravone on ischemia–reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2012;162:197–202. doi: 10.1016/j.ejogrb.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol. 2010;62:45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Kim M, Park YJ, Kim OJ, Lee GY, Chung EJ, Sung YK, Kim JC, Han I, Sohn YO. Gene expression profiles related with overcoming cisplatin resistance in human cancer cell lines. Cancer Ther. 2003;1:21–29. [Google Scholar]
- Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, Zhou D. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J Gynecol Oncol. 2013;24:177–185. doi: 10.3802/jgo.2013.24.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V, Gervasi PG, Lubrano V. Cisplatin induced toxicity in rat tissues: the protective effect of Lisosan G. Food Chem Toxicol. 2011;49:233–237. doi: 10.1016/j.fct.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;19:265–275. [PubMed] [Google Scholar]
- Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–661. doi: 10.5483/BMBRep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanamura S, Kawakami T, Sato Y, Hirayama MJ. Preparative-scale isolation of four anthocyanin components of black currant (Ribes nigrum L.) fruits. Agric Food Chem. 2001;49:1541–1545. doi: 10.1021/jf001245y. [DOI] [PubMed] [Google Scholar]
- Matsuo G, Kimio U, Atsuhiko S, Takahashi S, Fujiyoshi N, Takemoto S, Terada A, Fukui A, Kamura T. GnRH agonist acts as ovarian protection in chemotheraphy induced gonadotoxicity: an experiment using a rat model. Kurume Med J. 2007;54:25–29. doi: 10.2739/kurumemedj.54.25. [DOI] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/S0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Graf B, Curran-Celentano JM, Blumberg JB. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. IOVS. 2007;48:2343–2349. doi: 10.1167/iovs.06-0452. [DOI] [PubMed] [Google Scholar]
- Naqshbandi A, Khan W, Rizwan S, Rehman S, Khan F. Studies on the protective effect of dietary fish oil on cisplatin induced nephrotoxicity in rats. Food Chem Toxicol. 2012;50:265–273. doi: 10.1016/j.fct.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Nasir J, Walton C, Lindow SW, Masson EA. Spontaneous recovery of chemotherapy-induced primary ovarian failure: implications for management. Clin Endocrinol (Oxf) 1997;46:217–219. doi: 10.1046/j.1365-2265.1997.771575.x. [DOI] [PubMed] [Google Scholar]
- Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal liver and lens tissues in rats. Toxicology. 2004;15:221–230. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Noori S, Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J Clin Biochem. 2010;25:86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid rection. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ozer MK, Asci H, Oncu M, Calapoglu M, Savran M, Yesilot S, Candan IA, Cicek E. Effects of misoprostol on cisplatin-induced renal damage in rats. Food Chem Toxicol. 2011;49:1556–1559. doi: 10.1016/j.fct.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantative and qualitative characterization of glutathione peroxidase. J Lab Med. 1987;70:158–165. [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079X.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Saad SY, Najjar TA, Alashari M. Role of nonselective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin Exp Pharmacol Physiol. 2004;31:862–867. doi: 10.1111/j.1440-1681.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant protective agents. J Clin Biochem Nutr. 2007;40:1–12. doi: 10.3164/jcbn.40.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, Sasaki T, Makino H. A novel free radical scavenger edarabone protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003;305:1183–1190. doi: 10.1124/jpet.102.047522. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Goyal N. Protective effect of Heliotropium eichwaldi against cisplatin-induced nephrotoxicity in mice. Zhong Xi Yi Jie He Xue Bao. 2012;10:555–560. doi: 10.3736/jcim20120511. [DOI] [PubMed] [Google Scholar]
- Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertil Steril. 2009;92:417–427. doi: 10.1016/j.fertnstert.2008.07.1714. [DOI] [PubMed] [Google Scholar]
- Sweetman SC. The complete drug reference. London: Pharmaceutical Press; 2002. [Google Scholar]
- Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis-platinum. Med Pediatr Oncol. 1989;17:409–413. doi: 10.1002/mpo.2950170510. [DOI] [PubMed] [Google Scholar]
- Yeh J, Kim BS, Liang YJ, Peresie J. Müllerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun. 2006;348:337–344. doi: 10.1016/j.bbrc.2006.06.195. [DOI] [PubMed] [Google Scholar]
- Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol. 2008;198:463–466. doi: 10.1016/j.ajog.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, Erhan Y. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust NZJ Obstet Gynaecol. 2004;44:6–9. doi: 10.1111/j.1479-828X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang C, Wang H, Wang K, Du Y, Zhang J. Combination of tetrandrine with cisplatin enhances cytotoxicity through growth suppression and apoptosis in ovarian cancer in vitro and in vivo. Cancer Lett. 2011;304:21–32. doi: 10.1016/j.canlet.2011.01.022. [DOI] [PubMed] [Google Scholar]