Abstract
The purpose of this study was to determine the relationship between VEGF and mini-TyrRS/mini-TrpRS in angiogenesis in hypoxic culture and to begin to comprehend their mechanism in angiogenesis. We designed a VEGF gene silencing assay by using lentivirus vectors, and then western blotting was used to determine the protein expression of VEGF, VEGFR2 and pVEGFR2 in three groups in hypoxic culture at 3, 6, 12, or 24 h: (1) untransfected human umbilical vein endothelial cells (HUVECs) (Control); (2) pGCSIL-GFP lentivirus vector-transduced HUVECs (Mock); and (3) pGCSIL-shVEGF lentivirus vector-transduced HUVECs (Experimental). We also detected the effects of mini-TyrRS/mini-TrpRS peptides on HUVEC proliferation, migration and tube formation after lentivirus vector transfection and VEGFR2 antibody injection. The results indicated that expression of the mini-TyrRS protein was increased, whereas that of mini-TrpRS was specifically decreased in hypoxic culture both in control and mock groups. However, this trend in protein levels of mini-TyrRS and mini-TrpRS was lost in the experimental group after transduction with the pGCSIL-shVEGF lentivirus vector. The protein expression of VEGF was increased in hypoxic culture both in control and mock groups. After transduction with the pGCSIL-shVEGF lentivirus vector, the protein level of VEGF was noticeably decreased in the experimental group; however, for VEGFR2, the results showed no significant difference in VEGFR2 protein expression in any of the groups. For pVEGFR2, we found a distinct trend from that seen with VEGF. The protein expression of pVEGFR2 was sharply increased in hypoxic culture in the three groups. The addition of mini-TyrRS significantly promoted proliferation, migration and tube formation of HUVECs, while mini-TrpRS inhibited these processes in both control and mock groups in hypoxic culture. However, these effects disappeared after transduction with the pGCSIL-shVEGF lentivirus vector in the experimental group, but no significant difference was observed after VEGFR2 antibody injection. The protein expression of VEGF is similar to that of mini-TyrRS in hypoxic culture and plays an important role in the mini-TyrRS/mini-TrpRS-stimulated proliferation, migration and tube formation of HUVECs in hypoxia. These results also suggest that the change in mini-TyrRS and mini-TrpRS expression in hypoxic culture is not related to VEGFR2 and that some other possible mechanisms, are involved in the phosphorylation of VEGFR2.
Keywords: Mini-TyrRS, Mini-TrpRS, Angiogenesis, VEGF, VEGFR2, Hypoxia, HUVEC
Introduction
Aminoacyl-tRNA synthetases are thought to have been central to the origin of life, participating in the transition from the RNA world to the realm of proteins (Brown and Doolittle 1995; Choudhury and Banerjee 2013; Nagel and Doolittle1995; Ribas de Pouplana and Schimmel 2001; Woese et al. 2000). Increasingly, these conserved, universal enzymes have been identified as participants in more recently evolved and divergent cellular processes (Martinis et al. 1999; Francklyn et al. 2002). For example, two human aminoacyl-tRNA synthetases have been found to regulate angiogenesis in addition to their essential roles in protein synthesis (Fu et al. 2012; Sajish et al. 2012; Zeng et al. 2011, 2012, Wakasugi and Schimmel 1999a; Wakasugi and Schimmel 1999b; Wakasugi et al. 2002a; b; Otani et al. 2002a, b; Tzima et al. 2003). One of these molecules is a stimulatory factor, and the other is an inhibitor. Human tyrosyl-tRNA synthetase (TyrRS) and tryptophanyl-tRNA synthetase (TrpRS) are close structural homologues that are grouped together as subclass C within the class I synthetases (Banik and Nandi 2012; Brown et al. 1997). Although the enzymes function independently of each other, they share similar tertiary and quaternary structures and an active site design that was originally seen in bacterial TyrRS and TrpRS (Doublié et al. 1995), see Fig. 1.
Fig. 1.
Schematic diagram of the conversion of human tyrosyl-tRNA synthetase (TyrRS) and tryptophanyl-tRNA synthetase (TrpRS) into cell-signalling factors. a Human TyrRS contains a Rossmann fold domain (yellow), an anticodon recognition domain (green), and a C-terminal domain (purple). Leukocyte elastase cleaves TyrRS into two cell-signalling fragments: mini-TyrRS and C-TyrRS. Mini-TyrRS is angiogenic and induces polymorphonuclear (PMN) cell and human umbilical vein endothelial cell (HUVEC) migration. The cell-signalling activity of mini-TyrRS depends on a three-residue ELR motif, which is highlighted in red. C-TyrRS is 50 % identical in sequence to endothelial and monocyte-activating polypeptide II (EMAP-II) and has EMAP-II-like cell-signalling activities. A seven-residue peptide on C-TyrRS (RVGKIIT) is crucial for the chemotaxis activity of C-TyrRS. b Human TrpRS contains a Rossmann fold domain (yellow), an anticodon recognition domain (green), and an N-terminal domain (blue). The N-terminal domain is removed by either alternative splicing or leukocyte elastase cleavage to generate mini-TrpRS or T2-TrpRS, respectively. The truncated forms of TrpRS inhibit HUVEC cell migration, shear-stress activation, and angiogenesis. (Color figure online)
In our previous study, TyrRS/mini-TrpRS proteins affected proliferation and migration of rat coronary venular endothelial cells (RCVECs) in ischemic tissues (Zeng et al. 2011). It was found that mini-TrpRS does not inhibit the mini-TyrRS-induced proliferation and migration of VE-cadherin knockout endothelial cells (ECs) (Zeng et al. 2012). Because mini-TyrRS induced EC tube formation similar to VEGF (Zeng et al. 2011; Ran et al. 2010), we investigated the relationship between VEGF and mini-TyrRS/mini-TrpRS in this process. The effects of VEGF on angiogenesis are mediated by VEGFR2; thus, we examined whether VEGFR2 functions downstream of mini-TyrRS or mini-TrpRS. To confirm activation of the VEGFR2 receptor, we investigated whether mini-TyrRS induces phosphorylation of VEGFR2 (pVEGFR2). Here, there were two possibilities for mini-TyrRS/mini-TrpRS-induced VEGFR2 activation: (1) mini-TyrRS/mini-TrpRS stimulates the production of VEGF, which then activates VEGFR2, or (2) mini-TyrRS/mini-TrpRS stimulates the activation of VEGFR2 by transactivation mechanisms. To test these possibilities, we designed a VEGF gene silencing assay by using lentivirus vectors, and then western blotting was used to determine the protein expression of VEGF, VEGFR2 and pVEGFR2. However, we also detected the effects of mini-TyrRS/mini-TrpRS peptides on HUVECs proliferation, migration and tube formation after lentivirus vector transduction.
Ischemic diseases (such as coronary atherosclerotic heart disease, CAHD) are pathophysiologically caused by hypoxia. Many organs lose their function due to lack of oxygen. Thus, we designed this research simulating the conditions of ischemia to prove the relationship between VEGF and mini-TyrRS/mini-TrpRS in angiogenesis in hypoxic culture and to begin to comprehend their mechanism in angiogenesis. This research may be helpful for healing ischemic diseases such as CAHD.
Materials and methods
Materials
Human umbilical vein endothelial cells (HUVECs, ATCC_Number: CRL-1730TM) were obtained from the Laboratory of Peptides Related with Human Diseases, West China Hospital, Sichuan University (Sichuan, China). The pcDNA3.1 plasmid encoding human VEGF (pcDNA3.1-hVEGF), a third generation self-inactivating lentivirus vector containing a CMV-driven GFP reporter (pGCSIL-GFP), pGC-LV-GFP vector, pHelper 1.0 vector and pHelper 2.0 vector were purchased from Shanghai Genechem Co., Ltd (Shanghai, China). Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA). Low-glucose Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Invitrogen. The ECL chemiluminescence kit and BCA protein quantitative kit were obtained from Pierce (Rockford, IL, USA). The plasmid extraction kit was purchased from Promega (Madison, WI, USA). Goat monoclonal anti-human mini-TyrRS or mini-TrpRS IgG antibodies and horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated GAPDH was purchased from Kang Chen Biotechnology Company (Shanghai, China). The Revert Aid™ First Strand cDNA Synthesis Kit and Protein Marker were purchased from MBI Company (Vilnius, Lithuania). The cytoplasmic protein extraction kit was obtained from Keygen Biotechnology (Nanjing, China).
Plasmid constructs
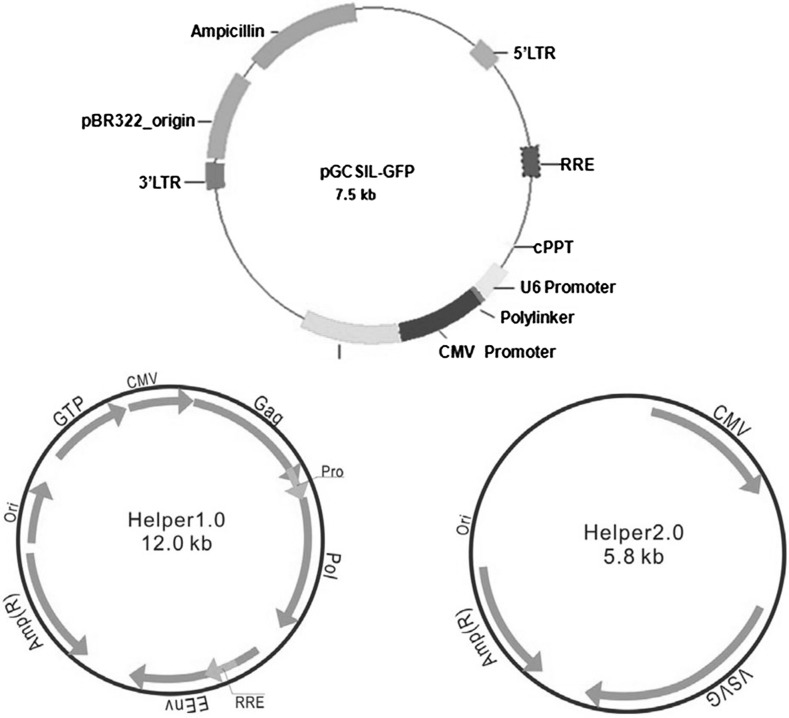
The lentivirus vector system consisted of three parts before packaging, including the pGCSIL-GFP vector, the pHelper 1.0 (gag/pol element) vector and the pHelper 2.0 (VSVG element) vector. Human VEGF siRNA was made with the following sequences: 5′-CCGGAAGATAGAGCAAGACAAGAAATTCAAGAGATTTCTTGTCTTGCTCTATCTTTTTTTG-3′and 3′-AATTCAAAAAAAGATAGAGCAAGACAAGAAATCTCTTGAATTTCTTGTCTTGCTCTA TCTT-5′. Primer pairs for amplifying hVEGF from pcDNA3.1-hVEGF and cloning into the AgeI site of the pGCSIL-GFP vector were as follows: forward: CCTATTTCACCGGTATGATTCCTCATA and reverse: GTAATACTACCGGTGGTTATCCACGCG; the underlined nucleotides indicate AgeI sequences. The full-length cDNA of hVEGF was cloned into pGCSIL-GFP by digesting it with AgeI and ligating the resultant fragments into the AgeI site of the pGCSIL-GFP vector (pGCSIL-shVEGF). PCR and DNA sequencing confirmed accurate insertion of hVEGF cDNA. Plasmid and vectors structure can be seen in Fig. 2.
Fig. 2.
pGCSIL-GFP plasmid and vectors, including the pGC-LV-GFP vector, the pHelper 1.0 vector and the pHelper 2.0 vector, used in these experiments
Production and titration of recombinant lentivirus vectors
For the preparation of lentivirus vectors, pHelper 1.0 plasmid DNA (15 μg), pHelper 2.0 plasmid DNA (10 μg), pGCSIL-GFP plasmid DNA (20 μg) were co-transfected into subconfluent 293T cells using Lipofectamine 2000. Infectious lentiviruses were harvested at 48 h post-transfection. The recombinant pGCSIL-shVEGF and pGCSIL-GFP viral vector titers were determined by fluorescence-activated cell sorting analysis of GFP+ 293T cells. Virus titers were 108 transducing units/ml medium.
Infection of HUVECs with lentivirus vectors
On the day of transduction, cells were plated at 4 × 103 cells/well in 96-well plates along with lenti-hVEGF at different MOIs in serum-free growth medium containing 5 μg/ml polybrene. After 4 h, serum containing growth medium was added and then replaced after 48 h. Five days post-infection, reporter gene expression was examined using fluorescent microscopy. HUVECs were plated in T-75 flasks and transduced at an MOI of 200 for transplantation; then, HUVECpGCSIL-GFP and HUVECpGCSIL-shVEGF cells were passaged and prepared for cell transplantation. The proportion of GFP-expressing cells upon transplantation was measured by flow cytometry.
Cell culture and experimental groups
HUVECs were cultivated in DMEM low-glucose medium supplemented with 10 % inactivated calf serum and 100 U/mL penicillin and 100 μg/mL streptomycin in an atmosphere of 5 % CO2 in air at 37°C according to the incubator instructions before being used in experiments. In our study, cells were cultivated in hypoxic culture for 3, 6, 12, or 24 h. The hypoxic atmosphere consisted of 1 % O2, 5 % CO2 and 94 % N2 at 37°C according to the instructions of the hypoxia incubator. Three groups were used: (1) untransduced HUVECs (Control), (2) pGCSIL-GFP lentivirus vector-transduced HUVECs (Mock), and (3) pGCSIL-shVEGF lentivirus vector-transduced HUVECs (Experimental). The purity of EC cultures was verified by immunostaining with an antibody against CD31. For all data shown, each individual experiment was performed with an independent preparation of HUVECs.
Gene-silencing effect of VEGF in hypoxia after lentivirus vectors transduction by Real time fluorescent quantitation PCR (RT-PCR)
After hypoxic cultivation for 3, 6, 12, or 24 h, HUVECs (1 × 106 cells) were harvested, washed twice with ice-cold phosphate-buffered saline (PBS) and collected by centrifugation. Total RNA was isolated from HUVECs using Trizol reagent (MRC, Cincinnati, OH, USA) according to the manufacturer’s instructions. Total RNA (5 μL) was converted to complementary DNA (cDNA) using the Revert Aid™ First Strand cDNA Synthesis Kit. A 5 μL aliquot of the resulting cDNA was used as a template for PCR amplification with the following primers: human VEGF, P1 (forward, 5′-CAGATGTGACAAGCCGAGG-3′), P2 (reverse, 5′-CGATGGTGATGGTGTGGTG-3′); GAPDH, P3 (forward, 5′-TGACTTCAACAGCGACACCCA-3′), P4 (reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′). The amplifications were performed by an initial denaturation (94 °C for 2 min), followed by 45 cycles of denaturation, annealing and extension (94 °C for 20 s, 54 °C for 20 s, 72 °C for 30 s), and a final extension (72 °C for 5 min). The GAPDH transcript was also amplified by RT-PCR from the same cDNA template and was used as an internal control. All the primers were designed and synthesised by Genepharma (Shanghai, China). The identity of each PCR product was confirmed by DNA sequencing. The pictures were scanned and analysed by the Gel Doc 1000 gel imaging system.
Effects of mini-TyrRS/mini-TrpRS peptides on HUVEC proliferation after lentivirus vector transduction
The effects of mini-TyrRS/mini-TrpRS peptides on HUVEC proliferation after lentivirus vector transfection were evaluated using the MTT colorimetric assay. HUVECs were seeded in 24-well plates (1 × 104 cells/well) in triplicate and stimulated with mini-TyrRS/mini-TrpRS peptides (100 μg/ml) and VEGFR2 antibody Cediranib (10 μg/ml, AZD2171, NSC-732208, Selleckchem Company, Houston, TX, USA). The cells were washed with PBS, trypsinised, and counted at 3, 6, 12, and 24 h. For the MTT assays, HUVECs were seeded in 96-well plates (1 × 104 cells/well) in triplicate and treated in an identical manner. After treatment with mini-TyrRS/mini-TrpRS peptides, viable cell numbers were estimated by the MTT assay as described in a previous study (Sladowski et al. 1993). Briefly, the medium was removed and replaced with medium containing 5 mg/ml MTT and incubated for 4 h. The medium was then aspirated, and the product was solubilised with dimethyl sulfoxide. Absorbance was measured at 570 nm for each well using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA) according to the manufacturer’s protocol.
Effects of mini-TyrRS/mini-TrpRS peptides on HUVECs migration after lentivirus vector transfection
Cell migration after lentivirus vector transfection was assayed using a modified Boyden chamber technique (Transwell analysis). Porous filters (8 μm pores) were coated with type IV collagen on both sides via passive absorption by incubating with 10 μg/ml collagen in coating buffer for 24 h. Serum-free medium containing mini-TyrRS/mini-TrpRS peptides (100 μg/ml) and VEGFR2 antibody Cediranib (10 μg/ml) was added into the lower chamber as a chemoattractant, and cells (1 × 104) were plated in the upper chamber and allowed to migrate for 3, 6, 12, and 24 h. Non-migrating cells were removed from the upper chamber with a cotton swab, filters were stained with Diff-Quik stain, (IMEB Inc., San Marcos, CA, USA), and migrating cells adherent to the underside of the filter were enumerated using an ocular micrometer and by counting a minimum of ten high-powered fields (HPF). Data are presented as relative migration (number of cells/HPF) and represent the mean ± SD of quadruplicate experiments.
Effects of mini-TyrRS/mini-TrpRS peptides on HUVECs matrigel-induced capillary tube formation after lentivirus vectors transfected
This assay was performed by the method as described (Yeh et al. 1998). Matrigel, (Becton Dickinson, San José, CA, USA), a basement membrane, was diluted to 4 mg/ml with cold D-Hank’s (Becton Dickinson) and added to 24-well plates to a total volume of 400 μL in each well. Plates were stored at 37 °C for 30 min to form a gel layer. After gel formation, 1 ml HUVECs (5 × 105 cells) in a medium containing 20 % foetal bovine serum was applied onto each well after stimulation with mini-TyrRS/mini-TrpRS peptides (100 μg/ml) and VEGFR2 antibody Cediranib (10 μg/ml). They were incubated in the hypoxic atmosphere for 3, 6, 12, or 24 h. After incubation, the cells were observed with an inverted phase-contrast microscope (Olympus, Tokio, Japan) and photographed. The total tube area was analysed in 3 different fields at 40× magnification using Image-proplus 5.1 software.
Western-blot analysis
Cytoplasmic protein extracts were prepared according to the instructions of the cytoplasmic protein extraction kit. The extracted proteins (35 μg) were subjected to 10 % SDS-PAGE. After electrophoresis, proteins were transferred onto PVDF membranes (Millipore, Billerica, MA, USA), and the membranes were then placed into blocking buffer containing non-fat dry milk. Goat monoclonal anti-human mini-TyrRS/mini-TrpRS IgG antibodies (final dilution 1:500) were used as the primary antibody, and HRP-conjugated rabbit anti-goat IgG (final dilution 1:10,000) was used as the secondary reagent. An internal control, β-actin, and a protein marker were also used. Detection was performed using an ECL chemiluminescence kit. The Western blot membranes were scanned and analysed by the Gel Doc 1000 gel imaging system (Bio-Rad Company, Hercules, CA, USA).
Statistical analysis
Results are expressed as the mean ± standard deviation. Comparison of means was performed using the analysis of variance procedure (Student–Newman–Keuls test, SPSS 16.0 for Windows). A p value < 0.05 was considered a statistically significant difference.
Results
Gene-silencing effect of VEGF in hypoxia after lentivirus vector transduction
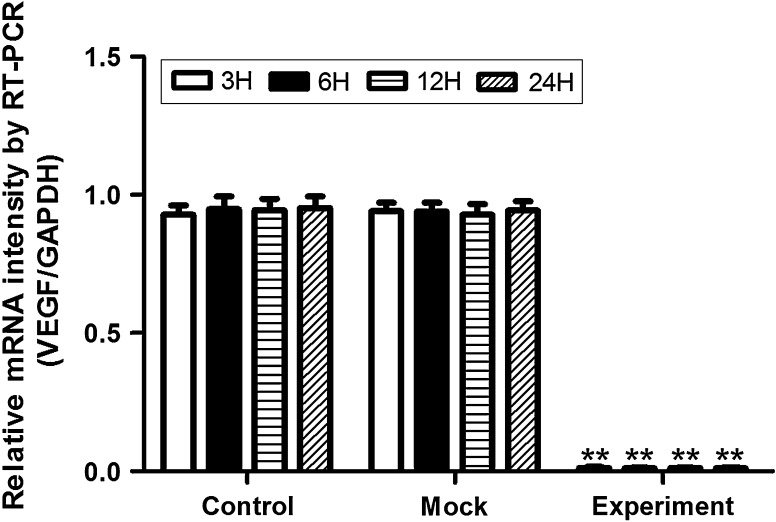
RT-PCR was used to detect the gene silencing effect of VEGF after lentivirus vector transduction (Fig. 3). The results indicated that the relative mRNA intensity of VEGF was sharply decreased in hypoxic culture after lentivirus vector transduction in the experimental group and showed a significant difference compared with the control and mock groups. The silencing effect was more than 99 % at 3, 6, 12, and 24 h. However, there was no statistically significant difference between the control and mock groups.
Fig. 3.
Gene silencing effect of VEGF in hypoxia after lentivirus vector transduction, by RT-PCR. The results indicated that the relative mRNA intensity of VEGF was sharply decreased in hypoxic culture after lentivirus vector transduction in the experimental group, while no statistically significant difference was seen in the control or mock groups. **p < 0.01 versus control or mock groups at 3, 6, 12, and 24 h
Mini-TyrRS and mini-TrpRS protein expression induced by VEGF gene silencing
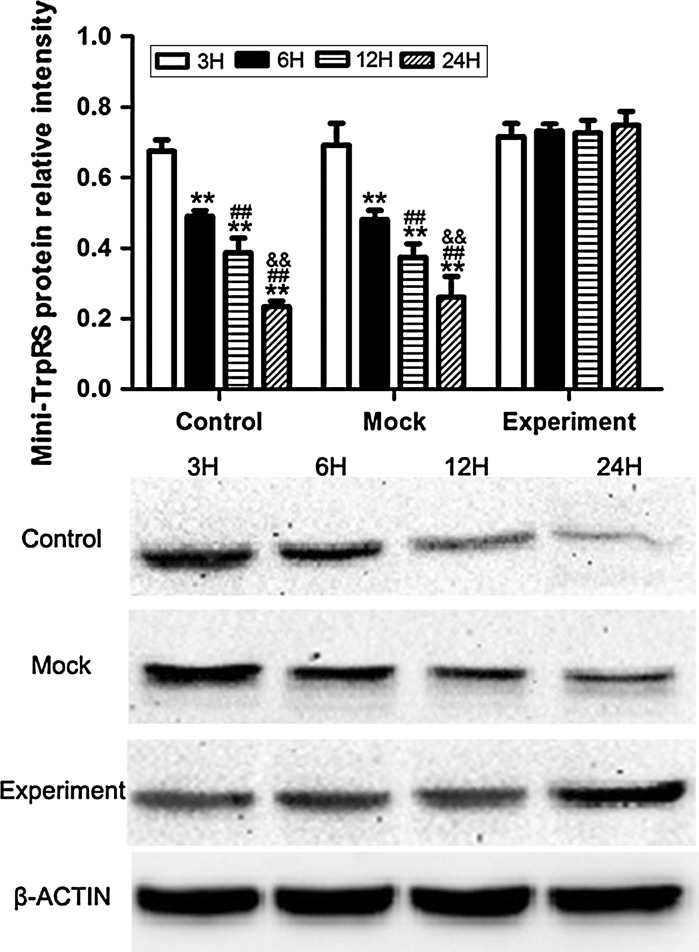
Western blotting (Figs. 4, 5) was used to compare the levels of mini-TyrRS and mini-TrpRS protein induced by VEGF gene silencing among the three groups of HUVECs: (1) untransduced HUVECs (Control); (2) pGCSIL-GFP lentivirus vector-transduced HUVECs (Mock); and (3) pGCSIL-shVEGF lentivirus vector-transduced HUVECs (Experimental). The results showed that expression of the mini-TyrRS protein was increased in hypoxic culture at 3, 6, 12, and 24 h in both the control and mock groups, while this trend in the protein level of mini-TyrRS was lost in the experimental group after transduction with the pGCSIL-shVEGF lentivirus vector, where there was no statistically significant difference at 3, 6, 12, and 24 h (Fig. 4). For mini-TrpRS, protein expression was specifically decreased in hypoxic culture at 3, 6, 12, and 24 h in both the control and mock groups, while this effect was also lost after transduction with the pGCSIL-shVEGF lentivirus vector, and no statistically significant difference in expression was seen at 3, 6, 12, and 24 h (Fig. 5).
Fig. 4.
Western blotting was used to compare the levels of mini-TyrRS protein induced by VEGF gene silencing among three groups of HUVECs. The results showed that expression of the mini-TyrRS protein was increased in hypoxic culture at 3, 6, 12, and 24 h in both the control and mock groups, while this trend was lost in the experimental group after transduction with pGCSIL-shVEGF lentivirus vectors, and no statistically significant difference was seen at 3, 6, 12, and 24 h. **p < 0.01 versus 3H protein expression, ##p < 0.01 versus 6H protein expression, &&p < 0.01 versus 12H protein expression
Fig. 5.
Western blotting was used to compare the levels of mini-TrpRS protein induced by VEGF gene silencing among three groups of HUVECs. Protein expression was specifically decreased in hypoxic culture at 3, 6, 12, and 24 h in both the control and mock groups. This trend was lost after transduction with the pGCSIL-shVEGF lentivirus vector, and no statistically significant difference was seen at 3, 6, 12, and 24 h. **p < 0.01 versus 3H protein expression, ##p < 0.01 versus 6H protein expression, &&p < 0.01 versus 12H protein expression
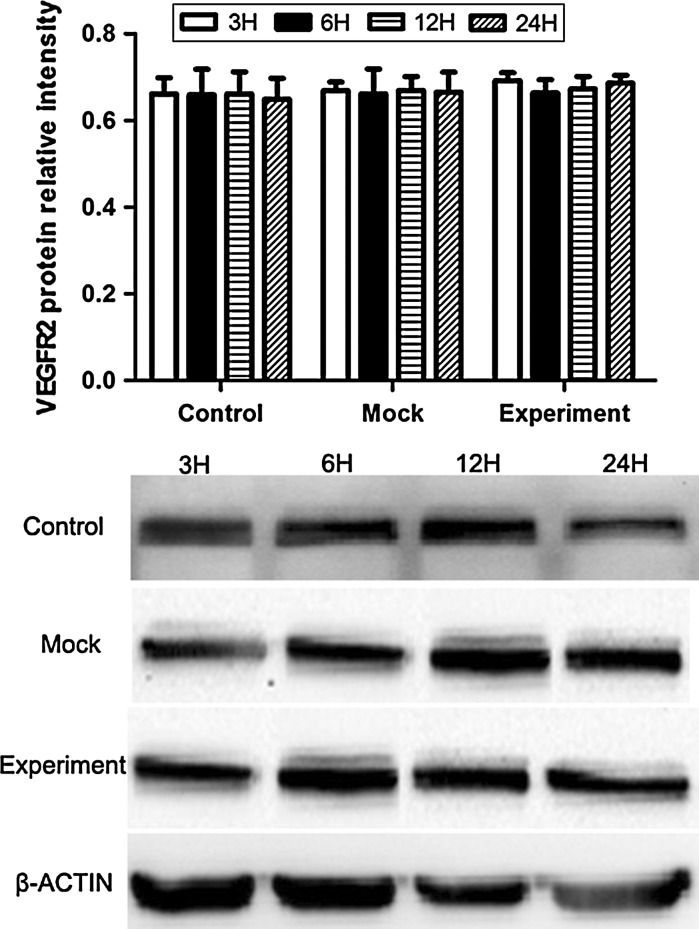
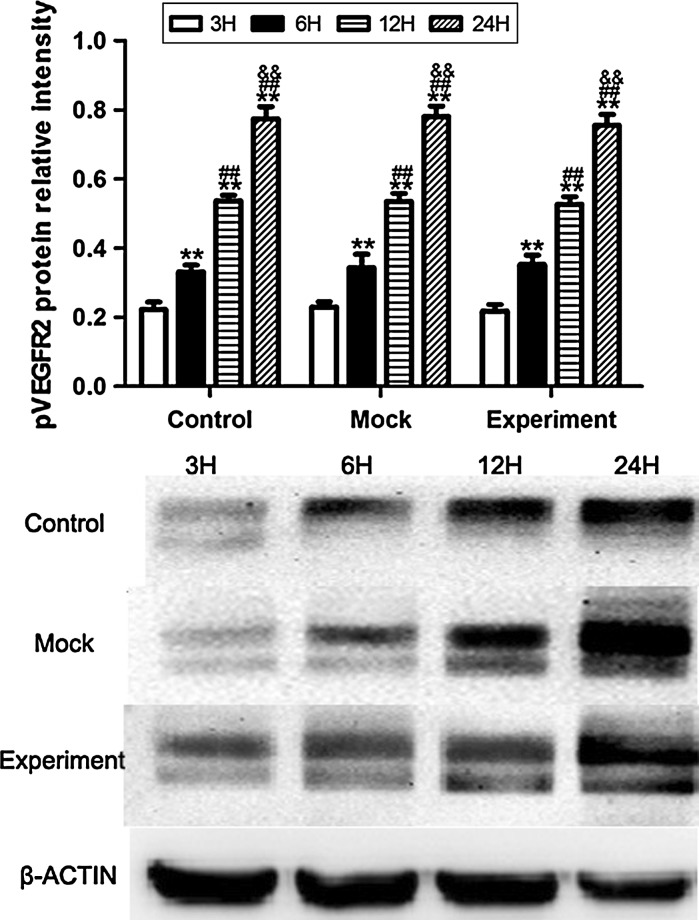
VEGF, VEGFR2 and pVEGFR2 protein expression induced by VEGF gene silencing
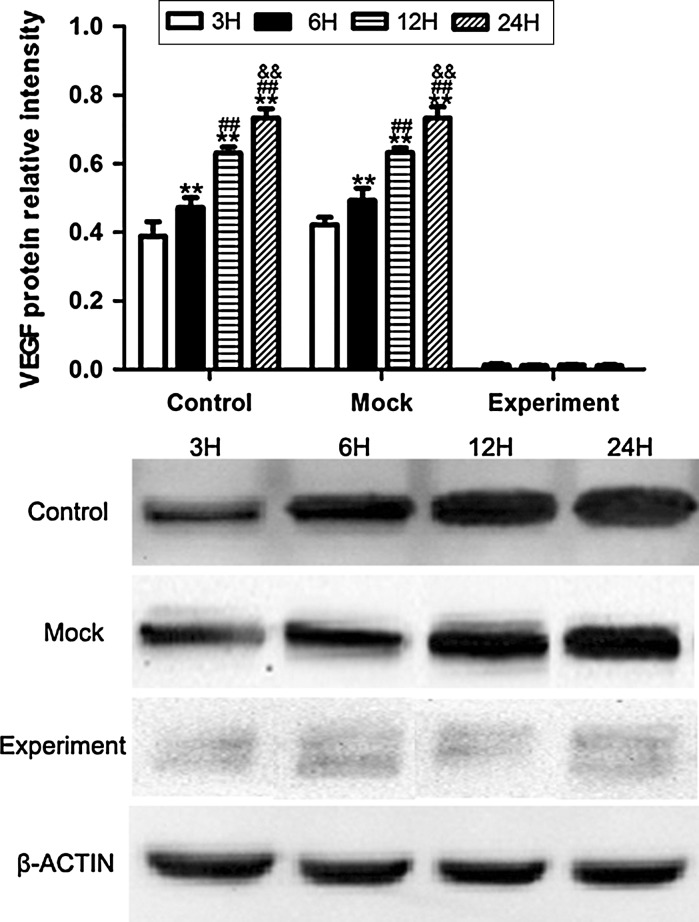
The protein expression of VEGF was increased in hypoxic culture at 3, 6, 12, and 24 h both in the control and mock groups, while after transduction with pGCSIL-shVEGF lentivirus vectors, the protein level of VEGF was noticeably decreased in the experimental group, and no significant difference was detected at 3, 6, 12, and 24 h (Fig. 6). For VEGFR2, the results showed that there was no significant difference in VEGFR2 protein expression in any of the groups (Fig. 7). These results may suggest that the change in mini-TyrRS and mini-TrpRS expression in hypoxic culture is not related to VEGFR2. In contrast, for pVEGFR2, we found a distinct trend from that seen with VEGF. The protein expression of pVEGFR2 was sharply increased in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and experimental groups (Fig. 8). We conclude that some other possible mechanism, but not VEGF, is involved in the phosphorylation of VEGFR2.
Fig. 6.
Protein expression of VEGF was increased in hypoxic culture at 3, 6, 12, and 24 h in both the control and mock groups, while after transduction with pGCSIL-shVEGF lentivirus vector, the protein level of VEGF was noticeably decreased in the experimental group, and no significant difference was detected at 3, 6, 12, and 24 h. **p < 0.01 versus 3H protein expression, ##p < 0.01 versus 6H protein expression, &&p < 0.01 versus 12H protein expression
Fig. 7.
Western blotting was used to compare the levels of VEGFR2 protein induced by VEGF gene silencing among the three groups of HUVECs. The results showed that there was no significant difference in VEGFR2 protein expression in any of the groups
Fig. 8.
Western blotting was used to compare the levels of VEGFR2 protein induced by VEGF gene silencing among the three groups of HUVECs. The protein expression of pVEGFR2 was sharply increased in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and experimental groups. **p < 0.01 versus 3H protein expression, ##p < 0.01 versus 6H protein expression, &&p < 0.01 versus 12H protein expression
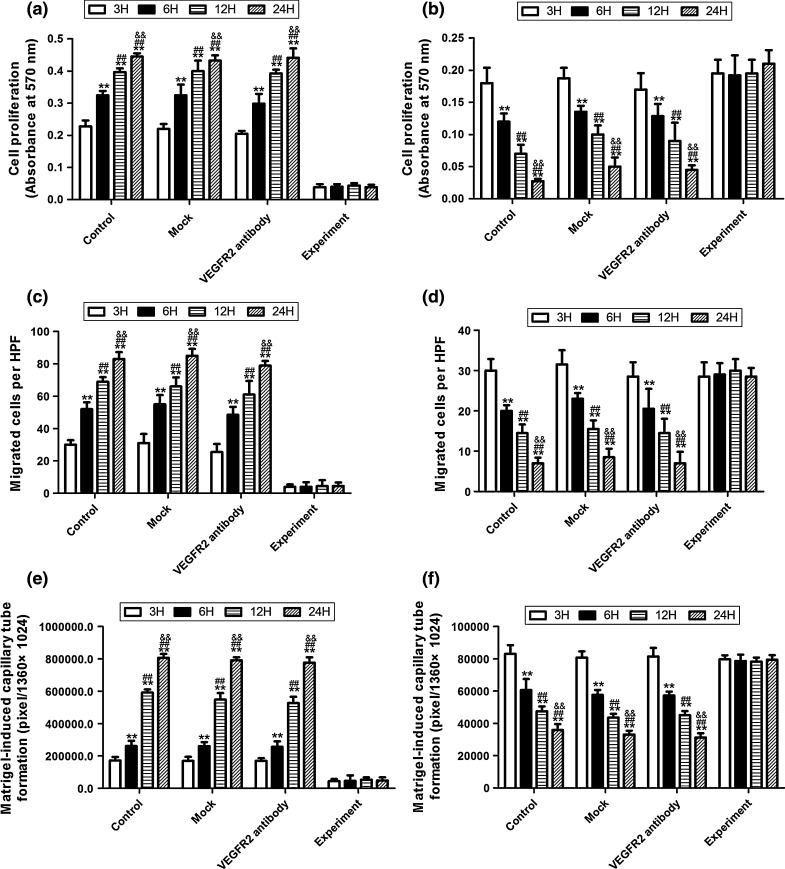
Effects of mini-TyrRS/mini-TrpR-mediated proliferation, migration and tube formation on HUVECs in hypoxic culture after transfection with the pGCSIL-shVEGF lentivirus vector
To determine the effects of mini-TyrRS/mini-TrpRS peptides on HUVEC proliferation, a necessary process for angiogenesis, we conducted experiments involving the direct addition of mini-TyrRS/mini-TrpRS peptides and then determined the proliferative effects using MTT viability assays. The addition of mini-TyrRS alone significantly promoted proliferation of HUVECs, while the addition of mini-TrpRS inhibited proliferation in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and VEGFR2 antibody groups (Figs. 8a, 9a). However, these effects disappeared after transduction with the pGCSIL-shVEGF lentivirus vector at 3, 6, 12, and 24 h in the experimental group (Fig. 9a, b). In Transwell Boyden chamber assays, mini-TyrRS also increased cell migration, while mini-TrpRS inhibited migration in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and VEGFR2 antibody groups (Fig. 9c, d), similar to their effects on mini-TyrRS/mini-TrpRS-induced HUVEC proliferation. Additionally, VEGF gene silencing by lentivirus vector transduction markedly attenuated the mini-TyrRS and mini-TrpRS-induced cell migration (Fig. 9c, d). Matrigel-induced capillary tube formation was also used in this experiment. Mini-TyrRS also increased tube formation, while mini-TrpRS inhibited tube formation in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and VEGFR2 antibody groups (Fig. 9e, f). These data suggest that VEGF may play an important role in the mini-TyrRS/mini-TrpRS-stimulated proliferation, migration and tube formation of HUVECs in hypoxic culture.
Fig. 9.
Effect of mini-TyrRS/mini-TrpRS peptides on HUVECs proliferation, migration and tube formation after pGCSIL-shVEGF lentivirus vector transduction. The addition of mini-TyrRS alone significantly promoted proliferation, migration and tube formation of HUVECs in hypoxic culture at 3, 6, 12, and 24 h in the control, mock, and VEGFR2 antibody groups. However, these effects disappeared after transduction with pGCSIL-shVEGF lentivirus vectors in the experimental group (A,C,E). While the addition of mini-TrpRS alone significantly inhibited proliferation, migration and tube formation of HUVECs in hypoxic culture at 3, 6, 12, and 24 h in the control, mock and VEGFR2 antibody groups, these effects disappeared after transduction with pGCSIL-shVEGF lentivirus vectors in the experimental group (B,D,F). **p < 0.01 versus 3H protein expression, ##p < 0.01 versus 6H protein expression, &&p < 0.01 versus 12H protein expression
Discussion
It has recently been reported in endothelial cells that mini-TyrRS/mini-TrpRS release is induced by TNF-a, undergoes binding, and induces phosphorylation of Src, Akt, Erk, and VEGF-receptor 2, which is required for tube formation (Greenberg et al. 2008; Biselli et al. 2008). Thus, in the present study, we aimed to test the relationship between VEGF and mini-TyrRS/mini-TrpRS in angiogenesis in hypoxic culture and to begin to comprehend their role in the mechanism of angiogenesis. The results of this research indicated that the expression of mini-TyrRS protein was increased, whereas the expression of mini-TrpRS was specifically decreased, in hypoxic culture in both the control and mock groups, but these trends in mini-TyrRS and mini-TrpRS expression were lost in the experimental group after transduction with pGCSIL-shVEGF lentivirus vectors. The protein expression of VEGF was increased in hypoxic culture both in the control and mock groups, whereas after transduction with the pGCSIL-shVEGF lentivirus vector, the protein level of VEGF was noticeably decreased in the experimental group. For VEGFR2, the results showed that there was no significant difference in VEGFR2 protein expression in any of the groups, while for pVEGFR2, we found a distinct trend compared with that seen in VEGF. The protein expression of pVEGFR2 was sharply increased in hypoxic culture in all three groups. The addition of mini-TyrRS alone significantly promoted proliferation, migration and tube formation of HUVECs, while mini-TrpRS inhibited the processes in hypoxic culture in the control, mock and VEGFR2 antibody groups. However, all of these effects disappeared after transduction with the pGCSIL-shVEGF lentivirus vector in the experimental group.
Researchers have found a group of angiogenesis regulatory factors: the CXC chemotactic factors. The functions of these factors are dependent on whether they have the ELR motif (Clark-Lewis et al. 1993). CXC chemotactic factors that have the ELR motif can promote angiogenesis, while CXC chemotactic factors that do not have the ELR motif are angiogenesis antagonists. This situation is analogous to that for human mini-TyrRS and mini-TrpRS. Mini-TyrRS has the ELR motif and promotes angiogenesis, but mini-TrpRS does not have the ELR motif; thus, they have totally opposite functions (Jeong et al. 2000). To confirm activation of the VEGFR2 receptor, there were two possibilities for mini-TyrRS/mini-TrpRS-induced VEGFR2 activation: (1) they stimulate the production of VEGF, which then activates VEGFR2, or (2) they stimulate the activation of VEGFR2 by transactivation mechanisms. Our results show that mini-TyrRS/mini-TrpRS-induced angiogenesis is dependent on VEGF in hypoxia, while it is independent of VEGFR-2. VEGF transactivation occurs downstream of the mini-TyrRS/mini-TrpRS receptor CXCR. This VEGF transactivation is associated with receptor complex formation between VEGF and CXCR. In contrast, for pVEGFR2, we found a similar trend to that seen with VEGF. The protein expression of pVEGFR2 was sharply increased in hypoxic culture in the control, mock and experimental groups, meaning that the level of pVEGFR2 expression also relied on hypoxia. This result also indicated that although mini-TyrRS/mini-TrpRS-induced angiogenesis is independent of VEGFR-2 in hypoxia, pVEGFR-2 may be involved in angiogenesis, similar to VEGF. Further experiments will be performed to prove this relationship.
From the results of our research, we conclude that the protein expression of VEGF was similar to that of mini-TyrRS in hypoxic culture and plays an important role in the mini-TyrRS/mini-TrpRS-stimulated proliferation, migration and tube formation of HUVECs in hypoxia. Our results also suggest that the change in mini-TyrRS and mini-TrpRS expression in hypoxic culture is not related to VEGFR2 but rather to some other possible mechanism that is involved in the phosphorylation of VEGFR2, which may be involved in angiogenesis similar to the way that VEGF is.
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant No. 81200153) and the Science Foundation of Health Department, Sichuan Province (Grant No. 120213).
References
- Banik SD, Nandi N. Mechanism of the activation step of the aminoacylation reaction: a significant difference between class I and class II synthetases. J Biomol Struct Dyn. 2012;30:701–715. doi: 10.1080/07391102.2012.689701. [DOI] [PubMed] [Google Scholar]
- Biselli PM, Guerzoni AR, de Godoy MF, Pavarino-Bertelli EC, Goloni-Bertollo EM (2008) Vascular endothelial growth factor genetic variability and coronary artery disease in Brazilian population. Heart Vessels 23:371–375 [DOI] [PubMed]
- Brown JR, Doolittle WF (1995) Root of the universal tree of life based on ancient aminoacyl-tRNA synthetase gene duplications. Proc Natl Acad Sci USA 92:2441–2445 [DOI] [PMC free article] [PubMed]
- Brown JR, Robb FT, Weiss R, Doolittle WF (1997) Evidence for the early divergence of tryptophanyl- and tyrosyl-tRNA synthetases. J Mol Evol 45:9–16 [DOI] [PubMed]
- Choudhury A, Banerjee R. The N-terminal fragment of Acanthamoeba polyphaga mimivirus tyrosyl-tRNA synthetase (TyrRSapm) is a monomer in solution. FEBS letter. 2013;587:590–599. doi: 10.1016/j.febslet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M (1993) Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA 90:3574–3577 [DOI] [PMC free article] [PubMed]
- Doublié S, Bricogne G, Gilmore C, Carter CW Jr (1995) Tryptophanyl-tRNA synthetase crystal structure reveals an unexpected homology to tyrosyl-tRNA synthetase. Structure 3:17–31 [DOI] [PubMed]
- Francklyn C, Perona JJ, Puetz J, Hou YM (2002) Aminoacyl-tRNA synthetases: versatile players in the changing theater of translation. RNA 8:1363–1372 [DOI] [PMC free article] [PubMed]
- Fu G, Xu T, Shi Y, Wei N, Yang XL (2012) tRNA-controlled nuclear import of a human tRNA synthetase. J Biol Chem 287:9330–9334 [DOI] [PMC free article] [PubMed]
- Greenberg Y, King M, Kiosses WB, Ewalt K, Yang X, Schimmel P, Reader JS, Tzima E (2008) The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J 25:1597–1605 [DOI] [PubMed]
- Jeong EJ, Hwang GS, Kim KH, Kim MJ, Kim S, Kim KS (2000) Structural analysis of multi-functional peptide motifs present in human bifunctional tRNA synthetase: identification of RNAbinding residues and functional implications for tandem repeats. Biochemistry 39:15775–15782 [DOI] [PubMed]
- Martinis SA, Plateau P, Cavarelli J, Florentz C (1999) Aminoacyl-tRNA synthetases: a family of expanding functions. Mittelwihr, France, October 10–15 [DOI] [PMC free article] [PubMed]
- Nagel GM, Doolittle RF. Phylogenetic analysis of the aminoacyl-tRNA synthetases. J Mol Evol. 1995;40:487–498. doi: 10.1007/BF00166617. [DOI] [PubMed] [Google Scholar]
- Otani A, Slike BM, Dorrell MI, Hood J, Kinder K, Ewalt KL, Cheresh D, Schimmel P, Friedlander M (2002a) A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci USA 99:178–183 [DOI] [PMC free article] [PubMed]
- Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M (2002b) Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8:1004–1010 [DOI] [PubMed]
- Ran X, Wang H, Chen Y, Zeng Z, Zhou Q, Zheng R, Sun J, Wang B, Lv X, Liang Y, Zhang K, Liu W (2010) Aquaporin-1 expression and angiogenesis in rabbit chronic myocardial ischemia is decreased by acetazolamide. Heart Vessels 25:237–247 [DOI] [PubMed]
- Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/S0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- Sajish M, Zhou Q, Kishi S, Valdez DM Jr, Kapoor M, Guo M, Lee S, Kim S, Yang XL, Schimmel P (2012) Trp-tRNA synthetase bridges DNA-PKcs to PARP-1 to link IFN-γ and p53 signaling. Nat Chem Biol 8:547–554 [DOI] [PMC free article] [PubMed]
- Sladowski D, Steer SJ, Clothier RH, Balls M (1993) An improved MTT assay. J Immunol Methods 157:203–207 [DOI] [PubMed]
- Tzima E, Reader JS, Irani-Tehrani M, Ewalt KL, Schwartz MA, Schimmel P (2003) Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress-activated responses of endothelial cells. Proc Natl Acad Sci USA 100:14903–14907 [DOI] [PMC free article] [PubMed]
- Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Schimmel P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J Biol Chem. 1999;274:23155–23159. doi: 10.1074/jbc.274.33.23155. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P (2002a) Introduction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem 277:20124–20126 [DOI] [PubMed]
- Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, Cheresh DA, Schimmel P (2002b) A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci USA 99:173–177 [DOI] [PMC free article] [PubMed]
- Woese CR, Olsen GJ, Ibba M, Söll D (2000) Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol Mol Biol Rev 64:202–236 [DOI] [PMC free article] [PubMed]
- Yeh CH, Peng HC, Huang TF. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta-antagonist and inducing apoptosis. Blood. 1998;92:3268–3276. [PubMed] [Google Scholar]
- Zeng R, Chen YC, Zeng Z, Liu WQ, Jiang XF, Liu R, Qiang O, Li X (2011) Effect of mini-tyrosyl-tRNA synthetase/mini-tryptophanyl-tRNA synthetase on ischemic angiogenesis in rats: proliferation and migration of endothelial cells. Heart Vessels 26:69–80 [DOI] [PubMed]
- Zeng R, Chen YC, Zeng Z, Liu XX, Liu R, Qiang O, Li X (2012) Inhibition of mini-TyrRS-induced angiogenesis response in endothelial cells by VE-cadherin-dependent mini-TrpRS. Heart Vessels 27:193–201 [DOI] [PubMed]