Abstract
Recent studies have shown that block wnt/β-catenin signaling pathway is integrant for cardiomyocytes differentiation from bone marrow mesenchymal stem cells (MSCs). By transducing the MSCs with lentivirus which contain β-catenin interference RNA, we screened out the non β-catenin expression clone. In the establishment of knockdown β-catenin in MSCs, we investigated the role of 5-azacytidine (5-aza), salvianolic acid B (salB), and cardiomyocytes lysis medium (CLM) in inducing MSCs to differentiate into cardiomyocyte-like cells. A method for culturing MSCs and cardiomyocytes was established. Purified MSCs were investigated by flow cytometry. The MSCs were positive for CD90 and CD29, but negative for CD34 and CD45. Meanwhile, the cardiomyocytes contracted spontaneously after 24 h of seeding into the plates. The fourth-passage non-β-catenin expression MSCs were divided into eight groups: control group, 5-aza, salB, CLM, 5-aza + salB, 5-aza + CLM, salB + CLM, and 5-aza + salB + CLM. The gene and protein expression of cTnT, α-actin, β-myosin, β-catenin, and GSK-3β were detected by quantitative real-time PCR and Western blotting. Our results showed that cTnT expression in 5-aza + salB + CLM group was ninefold higher than in the control group in the non-β-catenin MSCs model, implying that cardiomyocytes differentiation from MSCs is an extremely complicated process and it is necessary to consider the internal and external environmental conditions, such as suitable pharmaceutical inducers, cardiomyocytes microenvironments, inhibition of the negative signaling pathway and so on.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-013-9605-z) contains supplementary material, which is available to authorized users.
Keywords: Mesenchymal stem cells, Cardiomyocytes, Differentiation, Wnt/β-catenin signaling
Introduction
Myocardial ischemia due to myocardial infarction (MI) leading to the irreversible loss of cardiomyocytes, which are inevitably supplanted by non-contractile fibrosis scars, is one of the cause of death in the world. Stem cell biology recently provided the therapeutic approaches for replacing the non-functional cardiac tissue by cell transplantation. Uniquely, pluripotent cells such as mesenchymal stem cells (MSCs) are a significant source in regenerative medicine. Bone marrow-derived MSCs hold the potential to differentiate into the bona fide cardiomyocytes, osteoblasts, or adipocyte lineages. However, despite this breakthrough, most clinical cell transplantation did not get satisfying results. Transplantation of MSCs directly into the MI heart may result in arrhythmia and the therapeutic effect was not evident.
5-Azacytidine is a demethylation pharmaceutical that is widely used to induce MSCs to differentiate into cardiomyocytes, but it is clinically toxic and used as anti-cancer drug. (Xing et al. 2012). 5-aza is a chemical analogue of cytidine and effective in inducing MSCs to differentiate into cardiomyocytes, but it hardly can be used in the clinics, so it is extremely urgent to find another pharmaceutical to substitute it or to alleviate its toxicity. Recent studies showed that salvianolic acid B (salB), a monomer of salviae miltiorrhizae, has a benign effect on the nervous and cardiovascular systems. salB can reduce brain damage and inflammation after traumatic brain injury, and improve cognitive performance; it also has anti-hyperalgesic function in the treatment of neuropathy pain (Kim et al. 2011; Isacchi et al. 2011; Chen et al. 2011).
Besides, it can protect cardiomyocytes from apoptosis and guarantee the integrity of mitochondria and nuclei of cardiac tissue during acute MI (Xu et al. 2011). Cardiomyocytes lysis medium (CLM), which contains cytokines, growth factors and metabolism-related proteins, can provide a paracrine microenvironment for triggering cardiomyocyte differentiation from bone marrow MSCs (Schittini et al. 2010).
Here, we established the non-β-catenin expression MSCs model, investigated the role of 5-aza, salB, and CLM in triggering cardiomyocytes differentiation from non-β-catenin expression MSCs. The results suggested that the non-β-catenin expression MSCs can get a rather higher differentiation rate into cardiomyocytes after treated with the 5-aza combined with salB and CLM.
Materials and methods
Statement of ethics
This study involving the use of Sprague–Dawley rats was conducted in strict accordance with Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation and was approved by the Committee on Ethics of Animal Experiments of the Tianjin University of Traditional Chinese Medicine (Permit No. SCXK 2005-0001). All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Materials
Low-glucose Dulbecco’s Modified Eagle’s Medium (L-DMEM), Dulbecco’s Modified Eagle’s Medium/F12 (DF12), pancreatic enzyme solution, collagenase II, BrdU and fetal bovine serum (FBS) were purchased from Gibco (Invitrogen, Applied Biosystems, Carlsbad, CA, USA). Amphotericin, 5-azacytidine, and salB were purchased from Sigma-Aldrich (St. Louis, MO, USA). 4′6-diamidine-2′-phenylindole (DAPI), D-Hank’s balanced salt mixture, High Capacity cDNA, Reverse Transcription kits and QPCR Master Mix were purchased from Cwbio (Beijing, China). PE-conjugated mouse monoclonal antibodies against rat CD45 and CD34, FITC-conjugated mouse monoclonal antibodies against rat CD29 and CD90 were purchased from BD Biosciences (San Jose, CA, USA). Rabbit anti-rat myosin and vimentin polyclonal antibody, goat anti-rabbit FITC-IgG, goat anti-rabbit rhodamine red-X-conjugated IgG and rabbit anti-rat monoclonal antibody to cTnT were purchased from Abcam (Cambridge, UK); rabbit anti-rat monoclonal antibody to GAPDH, was purchased from Epitomocs (Burlingame, CA, USA). Sh-β-catenin lentivirus was purchased from GeneCopoeia (Guangzhou, China). The target sequence was GTGGTATAGAGGCTC (Cho et al. 2009). The interference sequences were as follows: Sense: 5′-CCGGGTGGGTGGTATAGAGGCTCGGATCCGAGCCTCTATACCACCACTTTTTG-3′, Antisense: 5′-AATTCAAAAAGTGGGTGGTATAGAGGCTCGGATTCGGATCCGAGCCTCTATACCACCAC-3′
Methods
MSCs culturing and lentivirus transduction
The lentivirus we used contained the β-catenin interference sequence as well as the puromycin resistance gene. So it was possible to screen the successfully transduced cells by adding puromycin to the culture medium. MSCs were isolated from the bone marrow of 180–200 g male rats (Wakitani et al. 1995). The cells were cultured with complete medium (L-DMEM, 10 % FBS, 100 U/mL penicillin, 100 µg/mL streptomycin) at 37 °C in a 5 % CO2 atmosphere. Non-adherent cells (hematopoietic cells) were removed by replacing the medium after 2 days. The MSCs were spindle-shaped. The medium was changed every other day. Transfections were performed when the cells reached 70–80 % confluence. Briefly, the cells were seeded at 24-hole plate and the medium was changed before transfection, 24 h later the medium was replaced with lentivirus-containing medium (the concentration of the lentivirus was 10 µg/mL; 1 µL lentivirus and 8 µg/mL polybrene within complete medium). On the next day, the medium was changed with fresh complete medium. Then the cells were cultured with selection medium which containing 10 µg/mL puromycin until drug resistant clones appeared. The non-β-catenin expression MSCs from the fourth passage were used for all experiments.
Isolate newborn rat cardiomyocytes and prepare CLM
The cardiomyocytes were isolated from neonatal rat heart tissue (Dell’Ovo et al. 2008). The 0–2 days neonatal rats were euthanized with CO2 and sterilized in 75 % alcohol for 3 min. The cardiac tissue was obtained through chest dissection; pericardium/epicardium and endocardium were discarded, and the myocardia (auricle and ventricle) were processed. Cardiac tissue was cut into 1-mm3 pieces, they were transferred to a 50-ml sterile tube, and washed three times with D-Hank’s solution to remove excess blood. Then 5 mL of 0.0625 % pancreatic enzyme solution was added to the tube, and the mixture was placed in a thermostated oscillating incubator at 37 °C for 5 min at 190 rpm, and the supernatant was discarded. Then 3 mL of 0.0625 % pancreatic enzyme solution and 3 mL 0.1 % collagenase II solution were added to the pellets. The mixture was placed in the incubator at 37 °C for 20 min at 190 rpm, then the supernatant was collected into a new tube, and digestion was terminated via the addition of FBS. This step was repeated three times, until almost no tissue clump remained. The collected supernatant was centrifuged for 8 min at 1,000×g, and the cells were cultured with complete medium (DF12, 20 % FBS, 1 % Brdu) at 37 °C in a humid 5 % CO2 atmosphere. After 24 h, more than 90 % of the cells showed spontaneous contraction under an inverted-phase contrast microscope (DMI3000B, Leica, Wetzlar, Germany). The medium was changed every other day until the cells reached 80 % confluence.
The cardiomyocytes conditioned in the wells were synchronized in the absence of FBS at 37 °C for 12 h under a 5 % CO2 atmosphere. Then the cells were digested with pancreatic enzymes and diluted with L-DMEM at 105 cells/ml. The cells were thawed at −80 °C for 4 h and then thawed at room temperature. This step was repeated three times to ensure that the cells were fractured. Then the tube was centrifuged at 2,000×g for 10 min. The supernatant was collected and filtered with 0.45-μm filters, then stored at −20 °C for later use.
Identification of MSCs
Flow cytometric analysis showed that MSCs expressed CD29, CD90, but not CD45 and CD34 (Wei et al. 2011). MSCs cultured in wells were harvested by treatment with 0.25 % tyrpsin, and then incubated for 1 h at 4 °C with PE-conjugated mouse monoclonal antibodies against rat CD45 and CD34, and with FITC-conjugated mouse monoclonal antibodies against rat CD29 and CD90. Control tubes were incubated with FITC-, PE-conjugated antibodies against rat IgG. The cells were washed with phosphate buffer solution (PBS) three times. Then the cells were analyzed by flow cytometry (BD, San Jose, CA, USA).
Induction method
After the fourth passage, the non-expressing MSCs were divided into eight groups based on different treatment conditions: (1) control group, (2) 5-aza group, (3) salB group, (4) 5-aza + salB group, (5) CLM group, (6) 5-aza + CLM group, (7) salB + CLM group, and (8) 5-aza + salB + CLM group. Each group was cultured for 2 weeks. Each group was synchronized (i.e., the medium was changed to L-DMEM) for 24 h, induced by the above mentioned pharmaceuticals for 24 h, then substituted by complete medium. The medium was changed every other day. The 5-aza concentration was 10 µmol/L, the salB concentration was 22 µmol/L, and the ratio of CLM to normal medium was 1:1.
Immunofluorescence assay detection of myosin and vimentin
Induced non-β-expression MSCs were washed three times with PBS and fixed by incubation for 5 min in 4 % paraformaldehyde. The cells were then permeabilized by incubation for 30 min in 0.5 % Triton ×-100 in PBS and blocked by incubation for 60 min with 5 % normal rabbit serum (Bioss, Beijing, China). Blocked cells were incubated for 1 h at 37 °C with rabbit anti-rat myosin and vimentin polyclonal antibody (dilution 1:100) at 4 °C overnight. The secondary antibody, goat anti-rabbit FITC-IgG (dilution 1:100) and Goat anti-rabbit rhodamine red-X-conjugated IgG (dilution 1:100), were added to the cells, which were then incubated for 30 min at room temperature. Nuclei were stained by incubation with 4′6-diamidine-2′-phenylindole (DAPI) for 10 min at room temperature. The cells were washed with PBS for three times after each step, blocked with glycerol, and examined under a fluorescence microscope.
Quantitative real-time PCR detection of the mRNA level
Total RNA was extracted from cultured MSCs, which had been induced for 2 weeks, using an RNA kit (Sigma-Aldrich, E.N.Z.A. DNA/RNA/protein isolation kit) according to the manufacturer’s instructions. The RNA concentration was determined using a micro-spectrophotometer device. The primer sequences are shown in Table 1. We synthesized cDNA from 2 μg of total RNA, according to the manufacturer’s instructions. The quantitative reaction was conducted according to the QPCR kit (Cwbio, Beijing, China). The reaction condition was as follows: 95 °C 10 min; 95 °C 1 s, 60 °C 1 min, 40 cycles.
Table 1.
Primers for real-time quantitative PCR
| Gene | Upstream | Downstream |
|---|---|---|
| GAPDH | GTCTTCTGAGTGGCAGTGAT | TGCTGAGTATGTCGTGGAG |
| cTnT | GCAGGCTCTTCATGCCCAACT | CGCTCTGCCCGACGCTTTT |
| β-Catenin | TCCACCAGTTGCCTTTATCA | CTGAGAAACTTGTCCGATGC |
| Gsk-3β | GTACCAGAACAGCCAGGGCTAC | CGGTCGGTTACTAGCAGAGCTT |
| α-Actin | TCTATGAGGGCTACGCTTTG | GCCAATAGTGATGACTTGGC |
| β-Myosin | GAAAGGTGAAGCTCAGTTTCAA | TTCGGTGTCTCCTTGATTGG |
Western blotting detection of the protein expression
Proteins were obtained from adherent cells. Cell lysates were prepared by homogenizing the cells in lysis buffer. Quantification of the protein was conducted using a modified bicinchoninic acid (BCA) assay (Cwbio). Protein samples were prepared by boiling them for 3 min after the addition of the loading buffer (Cwbio). Proteins were then separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 8 or 10 % gel and transferred onto polyvinylidene fluoride (PVDF) membrane by electroblotting. After being blocked in non-fat milk for 1 h, the membranes were incubated at 4 °C overnight with the primary antibody (rabbit anti-rat cTnT and GAPDH). The next day, the membranes were washed and incubated with the goat anti-rabbit horseradish peroxidase-conjugated secondary antibody [purchased from Abcam (Cambridge, UK)] at room temperature for 1 h. Membranes were washed, and protein bands were visualized using an enhanced chemiluminescence kit (Cwbio) under a ChemiDoc imaging system (Bio Rad Laboratories, Berkeley, CA, USA). Membranes were incubated in stripping buffer at room temperature for 30 min, and washed. Then they were re-probed with a second primary antibody (goat anti-rabbit IgG, purchased from Abcam) for additional protein detection.
Statistical analysis
Each experiment was conducted three times. Statistical analyses were performed using one-way analysis of variance (ANOVA). All data are expressed as mean ± SD. Significant difference was considered P < 0.05.
Results
Purified MSCs were cultured
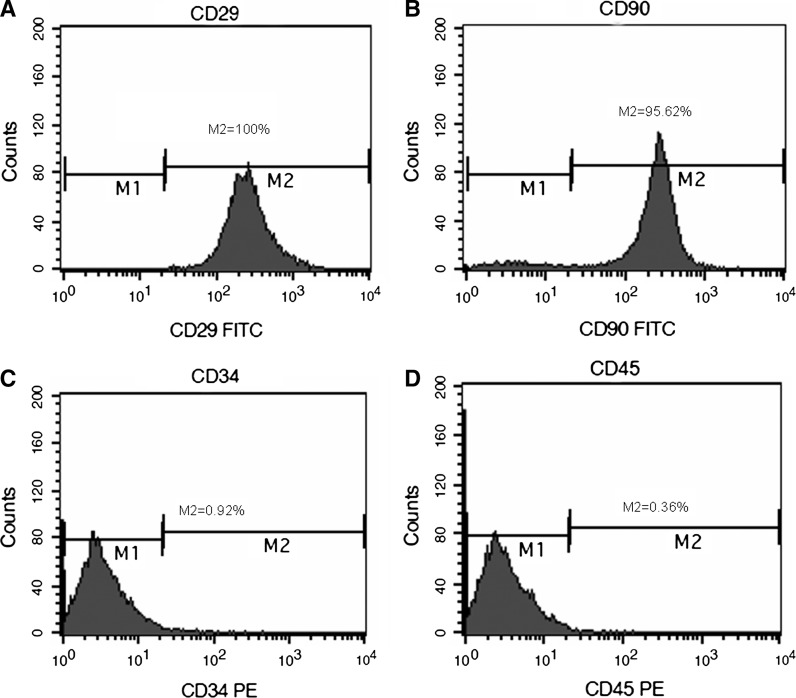
Cultured MSCs and cardiomyocytes were identified by a flow cytometer. MSCs were positive for CD90 (M2 = 95.26 %) and CD 29 (M2 = 100 %), but negative for CD45 (M2 = 0.36 %) and CD34 (M2 = 0.92 %) (Fig. 1a–d).
Fig. 1.
Identification of MSCs. MSCs were identified by flow cytometry. MSCs expressed CD29, CD90, but not CD34, CD45
Knockdown β-catenin in MSCs
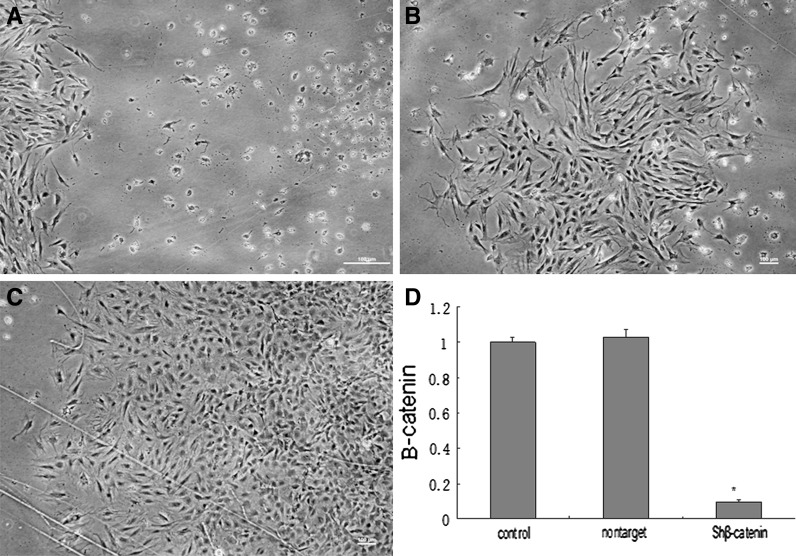
β-Catenin knockdown was performed by transducing MSCs with lentivirus containing interference shRNA plasmids (Genecopoeia). The sh-β-catenin containing lentivirus transduced MSCs efficiently and the transduction rate was more than 90 %. The fluorescence expression of non-β-catenin expression MSCs was analyzed by fluorescence microscope. Since the lentivirus used contained the β-catenin interference sequences as well as the puromycin resistance gene, the selection of the successfully transduced cells was performed by adding puromycin at 10 µg/mL to the culture medium. Puromycin selected was conducted at the third day after MSCs transfection. Two weeks later we obtained a resistant clone, and the β-catenin expression was investigated by QPCR (Fig. 2).
Fig. 2.
Knockdown of β-catenin in MSCs. a The transfected MSCs were screened with puromycin (at 10 µg/mL). Only the cells transduced by lentivirus survived. The dead cells did not contain lentivirus. b, c After a week, the living cells formed clones. d The β-catenin mRNA expression in β-catenin-knockdown MSCs was compared with the control group and the nontarget group by qPCR. Scale bar 100 µm
Morphological changes
Twenty four hours after seeding, MSCs were spindle-shaped (Fig. 3a). The cardiomyocytes were spindle, triangle or irregular shaped (Fig. 3c), they were spontaneously beating at a frequency about 30–50/min (supplementary movie). The MSCs in 5-aza + salB + CLM induced group were bigger than the MSCs of the control group and had a triangle or irregular shape (Fig. 3).
Fig. 3.
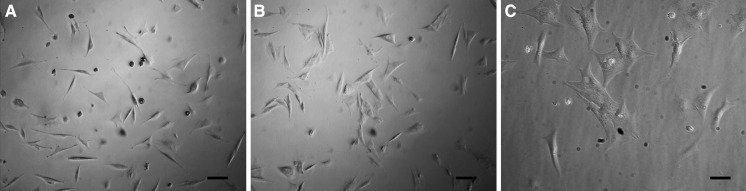
The microscopic picture of the MSCs in each induction group. a The control group. b The 5-aza + salB + CLM group. c Purified cardiomyocytes. The original MSCs are spindle-shaped and only have two cusps; the cardiomyocytes were more like rectangles and showed spontaneous contraction under microscope. The spindle-shaped MSCs gradually possessed three or four cusps during the differentiation process. Scale bar 50 µm
Myosin and vimentin expression in MSCs and cardiomyocytes
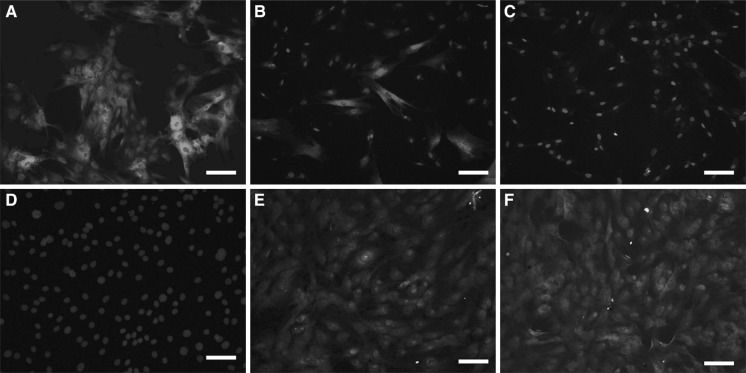
Myosin is involved in the contraction of muscle cells. Vimentin is one of the markers in MSCs. As shown in Fig. 4a–c, vimentin expression was negative in cardiomyocytes and almost 100 % positive in MSCs, while only 25 % positive in 5-aza + salB + CLM group. On the contrary, myosin expression was negative in MSCs, and almost 100 % positive in cardiomyocytes, while more than 70 % positive in the 5-aza + salB + CLM group (Fig. 4d–f).
Fig. 4.
Vimentin and myosin expression in MSCs and cardiomyocytes. a Vimentin expression in MSCs. b Vimentin expression in the 5-aza + salB + CLM group. c Vimentin expression in cardiomyocytes. d Myosin expression in MSCs. e Myosin expression in the 5-aza + salB + CLM group. f Myosin expression in cardiomyocytes. Scale bar 50 µm
The cardiomyocytes differentiation rate from non-β-catenin expressing MSCs
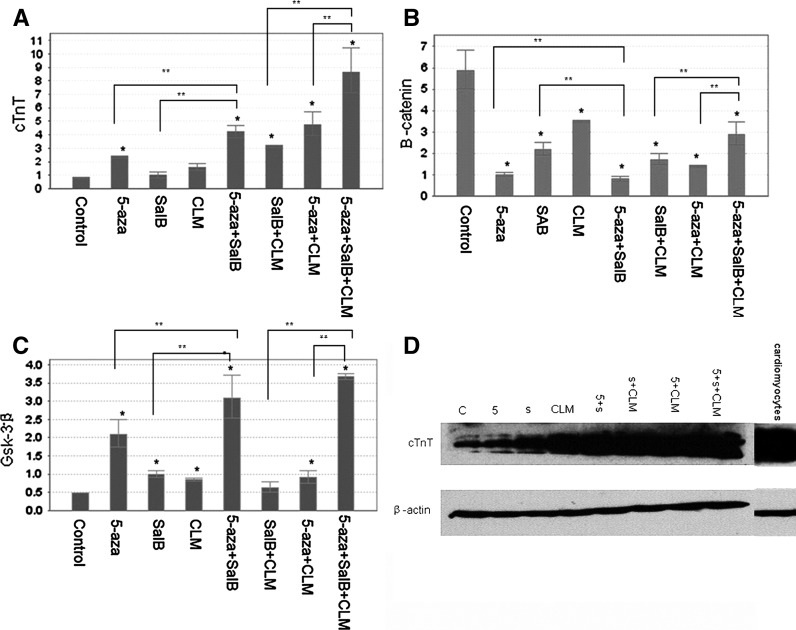
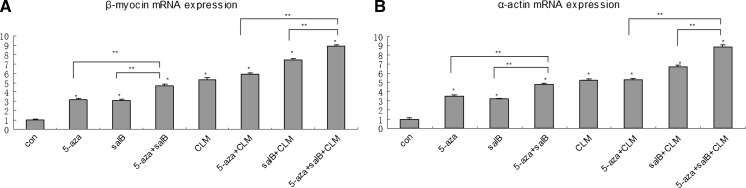
The non-β-catenin expressing MSCs were induced by 5-aza, salB, 5-aza + salB, CLM, 5-aza + salB + CLM and 5-aza + salB + CLM. After 2 weeks, QPCR was conducted to detect mRNA expression of the cTnT, α-actin, β-catenin, β-myosin, and GSK-3β genes. The mRNA expression of cTnT, α-actin and β-myosin in the 5-aza + salB + CLM group was almost ninefold higher than in the control group (Figs. 5, 6). The expression was almost 2.2-fold, fourfold, 1.5-fold, threefold, and 4.5-fold, respectively, higher for the 5-aza group, for the 5-aza + salB group, for the CLM group, for the salB + CLM group, and for the 5-aza + CLM group than for the control group. The salB group did not show a significant difference compared with the control group (Fig. 5a). The expression of β-catenin in the induced groups was much lower than that of the control group (Fig. 5b). However, the expression of Gsk-3β in the 5-aza + salB + CLM group was seven times higher than in the control group. Gsk-3β expression in other induced groups was also higher than in the control group (Fig. 5c). The protein expression of cTnT exceeded that of the control group in any of the induction groups, and the 5-aza + salB + CLM group showed the most significant effect (Figs. 5d, 7).
Fig. 5.
mRNA and protein expressions after knockdown of β-catenin. a cTnT mRNA expression; b β-catenin mRNA expression; c Gsk-3β mRNA expression; d cTnT protein expression. The group sequence from left to right is control group, 5-aza, salB, CLM, 5-aza + salB, salB + CLM, 5-aza + CLM, 5-aza + salB + CLM groups and the purified cardiomyocytes. Results are shown as the mean ± SD values. Experiments were repeated three times. *P < 0.05 compared to the control group. **P < 0.05 compared between the two groups as shown in the diagrammes
Fig. 6.
α-Actin and β-myosin mRNA expression. a β-myosin mRNA expression. b α-actin mRNA expression. Results are shown as the mean ± SD values. Experiments were repeated three times. *P < 0.05 compared to the control group. **P < 0.05 compared between the two groups as shown in the diagrammes
Fig. 7.
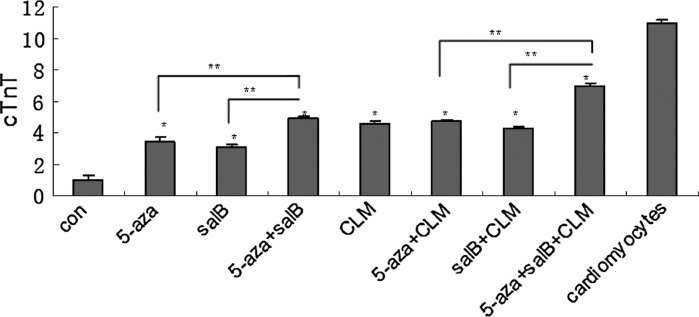
Relative protein expression. Expressions of cTnT after knockdown of β-catenin were quantitated by densitometric analysis of the immunoblots. *P < 0.05 compared to the control group. **P < 0.05 compared between the two groups as shown in the picture
Discussion
Mesenchymal stem cells are one kind of pluripotent stem cells which can differentiate into cardiomyocytes, adipocyte, and osteoblast. But the mechanism of MSCs differentiation is variable. Extra cellular inhibitors of BMPs, Wnts, and Nodal are the principal inducers that commit MSCs to a cardiac fate (Mercola et al. 2011), while MSCs differentiate into osteoblast by activating Wnt/β-catenin signaling (Hamidouche et al. 2008; Qiang et al. 2009). Song et al. (2011) reported that activate PKC signaling pathway by phorbol myristate acetate can promote cardiomyocytes differentiation rate from MSCs. Kilian et al. (2010) demonstrated that cell shape has a strong effect on the differentiation of MSCs from bone marrow. Cells in cell culture flask promoting increased contractility led to preferential osteogenesis when MSCs were exposed to a mixture of lineage cues, whereas MSCs cultured in culture flask that promote low contractility preferred to follow an adipocyte lineage.
A growing body of evidence proved that wnt/β-catenin signaling pathway plays a negative role in cardiomyocytes differentiation from MSCs. The wnt/β-catenin signaling promotes cardiogenesis before gastrulation and inhibits heart specification later (Ueno et al. 2007). Wnt/β-catenin signaling is activated by wnts interacting with its receptors such as Frizzled, then the disheveled protein negatively regulates glycogen synthase kinase-3β (Gsk-3β), leading to the accumulation of β-catenin in the cytoplasma. The β-catenin is then translocated into nucleus, where it activates the expression of wnt/β-catenin responsive genes. Without wnt signal, β-catenin is phosphorylated by GSK-3β and then undergoes degradation mediated by ubiquitin (Gwak et al. 2006). Cho et al. (2009) found that glycogen synthase kinase (Gsk)-3β rather than Gsk-3α played the vital role in mediating cardiomyocyte differentiation in bone-marrow derived MSCs. Injection with Gsk-3β-overexpressing bone marrow-derived MSCs attenuates cardiac dysfunction after MI, which means inhibiting the wnt/β-catenin is necessary in the cardiomyocyte differentiation process from MSCs. Blocking the wnt/β-catenin signaling can enhance MSCs engraftment, granulation tissue formation and myocardial repair (Alfaro et al. 2008; Zelarayán et al. 2008). It is pretty sure that inhibiting wnt/β-catenin signaling pathway is necessary to induce MSCs to differentiate into cardiomyocytes.
We built the non-β-catenin expression MSCs model so as to further study the conditions that effectively induce cardiomyocytes differentiation from MSCs. Significant efforts have been directed to studying the factors that influence the cardiomyocyte differentiation from MSCs. Several studies reported that MSCs treated with 5-aza expressed cardiomyocyte specific markers such as α-atin, sarcomeric β-myosin and troponin-T (Wakitani et al. 1995; Liu et al. 2003). MSCs can be directly injected into a chronic MI scar, improve ventricular function (Schuleri et al. 2009), but it has side effects, such as arrhythmia. While the side effects can be alleviated by pretreated MSCs with neuropeptide Y (NPY) before injecting MSCs into MI heart (Schuleri et al. 2010).
In our present study, we showed that 5-aza combined with salB and CLM can trigger MSCs into cardiomyocytes at a high rate. After induction by 5-aza + salB + CLM, the morphology of MSCs evolved toward that of cardiomyocytes (Fig. 3). The MSCs became much bigger and irregular. Also vimentin protein expression was reduced while myosin protein expression was enhanced in the 5-aza + salB + CLM group when detected by immunofluorescence (Fig. 4). This means that the induced MSCs gradually acquired the morphological characteristics of cardiomyocytes. The cTnT protein expression in the 5-aza + salB group was significantly higher than that in the 5-aza and salB groups; cTnT expression was significantly higher in the 5-aza + salB + CLM group than in the 5-aza + CLM and salB + CLM groups. We can conclude that the induction effect was stronger when 5-aza was combined with salB. 5-aza can induce differentiation while salB can protect the MSCs from poisonous and injurious factors of 5-aza when they were combined together. Although the cTnT expression in the 5-aza + salB + CLM group was almost ninefold higher than in the control group, the MSCs did not beat spontaneously. There is much more work to do on inducing MSCs to differentiate into functional beating cardiomyocytes which can be used to replace non-functional cardiomyocytes.
Electronic supplementary material
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81173413, www.nsfc.gov.cn), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (Nos. 20101210110004 and 20101210120008, www.cutech.edu.cn), and Tianjin Research program of Application Foundation and Advanced Technology (No. 09JCYBJC12200, www.tsyc.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Qing Gao, Email: 616264342@qq.com.
Yingchang Fan, Phone: +86-15-22078055, FAX: +86-22-59596279, Email: fych@tjutcm.edu.cn.
References
- Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Liu WB, Chao XD, Zhang L, Qu Y, Huo JL, Zhou F. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull. 2011;84:163–168. doi: 10.1016/j.brainresbull.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Cho J, Rameshwar P, Sadoshima J. Distinct roles of glycogen synthase kinase (GSK)-3α and GSK-3β in mediating cardiomyocyte differentiation in murine bone marrow-derived mesenchymal stem cells. J Biol Chem. 2009;284:36647–36658. doi: 10.1074/jbc.M109.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3β—overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Ovo V, Bandi E, Coslovich T, Florio C, Sciancalepore M, Decorti G, Sosa S, Lorenzon P, Yasumoto T, Tubaro A. In vitro effects of yessotoxin or a primary culture of rat cardiomyocytes. Toxicol Sci. 2008;106:392–399. doi: 10.1093/toxsci/kfn187. [DOI] [PubMed] [Google Scholar]
- Gwak J, Cho M, Gong SJ, Won J, Kim DE, Kim EY, Lee SS, Kim M, Kim TK, Shin JG, Oh S. Protein-kinase-C-mediated β-catenin phosphorylation negatively regulates the wnt/β-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- Hamidouche Z, Haÿ E, Vaudin P, Charbord P, Schüle R, Marie PJ, Fromigué O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/beta-catenin signaling-dependent Runx2 expression. FASEB J. 2008;22:3813–3822. doi: 10.1096/fj.08-106302. [DOI] [PubMed] [Google Scholar]
- Isacchi B, Fabbri V, Galeotti N, Bergonzi MC, Karioti A, Ghelardini C, Vannucchi MG, Bilia AR. Salvianolic acid B and its liposomal formulations: anti-hyperalgesic activity in the treatment of neuropathic pain. Eur J Pharm Sci. 2011;44:552–558. doi: 10.1016/j.ejps.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Park SJ, Kim JM, Jeon SJ, Kim DH, Cho YW, Son KH, Lee HJ, Moon JH, Cheong JH, Ko KH, Ryu JH. Cognitive dysfunctions induced by a cholinergic blockade and Aβ 25–35 peptide are attenuated by salvianolic acid B. Neuropharmacology. 2011;61:1432–1440. doi: 10.1016/j.neuropharm.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Liu Y, Song J, Liu W, Wan Y, Chen X, Hu C. Growth and differentiation of rat bone marrow stromal cells: Does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res. 2003;58:460–468. doi: 10.1016/S0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD., Jr Bortezomib induces osteoblast differentiation via Wnt-independent activation of β-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittini AV, Celedon PF, Stimamiglio MA, Krieger M, Hansen P, da Costa FD, Goldenberg S, Dallagiovanna B, Correa A. Human cardiac explant-conditioned medium: soluble factors and cardiomyogenic effect on mesenchymal stem cells. Exp Biol Med. 2010;235:1015–1024. doi: 10.1258/ebm.2010.010003. [DOI] [PubMed] [Google Scholar]
- Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuleri KH, Feigenbaum GS, Centola M, Weiss ES. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:275–286. doi: 10.1152/ajpheart.00765.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hwang HJ, Chang W, Song BW, Cha MJ, Kim IK, Lim S, Choi EJ, Ham O, Lee CY, Park JH, Lee SY, Choi E, Lee C, Lee M, Lee MH, Kim SH, Jang Y, Hwang KC. Cardiomyocytes from phorbol myristate acetate activated mesenchymal stem cells restore electromechanical function in infarcted rat hearts. Proc Natl Acad Sci USA. 2011;108:296–301. doi: 10.1073/pnas.1015873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Wei F, Wang T, Liu J, Du Y, Ma A. The subpopulation of mesenchymal stem cells that differentiate toward cardiomyocytes is cardiac progenitor cells. Exp Cell Res. 2011;317:2661–2670. doi: 10.1016/j.yexcr.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Xing Y, Lv A, Wang L, Yan X. The combination of angiotensin II and 5-azacytidine promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Mol Cell Biochem. 2012;360:279–287. doi: 10.1007/s11010-011-1067-z. [DOI] [PubMed] [Google Scholar]
- Xu L, Deng Y, Feng L, Li D, Chen X, Ma C, Liu X, Yin J, Yang M, Teng F, Wu W, Guan S, Jiang B, Guo D. Cardio-protection of salvianolic acid B through inhibition of apoptosis network. PLoS One. 2011;6:e24036. doi: 10.1371/journal.pone.0024036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelarayán LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, Zafiriou MP, van der Nagel R, Dietz R, de Windt LJ, Balligand JL, Bergmann MW. β-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci USA. 2008;105:19762–19767. doi: 10.1073/pnas.0808393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.