Abstract
This work focused on determining the effect of dissolved oxygen concentration (DO) on growth and metabolism of BHK-21 cell line (host cell for recombinant proteins manufacturing and viral vaccines) cultured in two stirred tank bioreactors with different aeration-homogenization systems, as well as pH control mode. BHK-21 cell line adapted to single-cell suspension was cultured in Celligen without aeration cage (rotating gas-sparger) and Bioflo 110, at 10, 30 and 50 % air saturation (impeller for gas dispersion from sparger-ring). The pH was controlled at 7.2 as far as it was possible with gas mixtures. In other runs, at 30 and 50 % (DO) in Bioflo 110, the cells grew at pH controlled with CO2 and NaHCO3 solution. Glucose, lactate, glutamine, and ammonium were quantified by enzymatic methods. Cell concentration, size and specific oxygen consumption were also determined. When NaHCO3 solution was not used, the optimal DOs were 10 and 50 % air saturation for Celligen and Bioflo 110, respectively. In this condition maximum cell concentrations were higher than 4 × 106 cell/mL. An increase in maximum cell concentration of 36 % was observed in batch carried out at 30 % air saturation in a classical stirred tank bioreactor (Bioflo 110) with base solution addition. The optimal parameters defined in this work allow for bioprocess developing of viral vaccines, transient protein expression and viral vector for gene therapy based on BHK-21 cell line in two stirred tank bioreactors with different agitation–aeration systems.
Keywords: BHK-21, Stirred tank bioreactor, Dissolved oxygen, Mammalian cell culture, BHK-21 cell metabolism
Introduction
Mammalian cells culture has been widely used for recombinant proteins and viral vaccines production, as well as gene therapy (Park et al. 2010; Vester et al. 2010; Merten 2006). Among cell lines with extensive utilization for these purposes are Chinese hamster ovary (CHO), NS0 mouse myeloma, Baby hamster kidney (BHK), Human embryonic kidney 293 (HEK-293) and few human-derived cell lines (Krampe and Al-Rubeai 2010). Specifically, BHK cells have found applications primarily in veterinarian viral vaccines (foot-and-mouth disease and rabies viruses), and recently in human recombinant therapeutic proteins such as Factor VIII (Bödeker et al. 1994; Kallel et al. 2003; Auniņš 2010). Microcarrier support (pseudosuspension) and suspension cell culture systems have been explored for this cell line when the scale-up of bioprocess is desired, because of monolayer culture limitations on large-scale (Kallel et al. 2003; Perrin et al. 1995; Butler 2005; Astley et al. 2007). Nevertheless, microcarriers increase process cost and require extra optimization steps during development like microcarrier type and concentration. To overcome these drawbacks, cell lines, including BHK, have been adapted to grow as single cells in suspension. Thus, higher cell densities and concomitant volumetric productivities could be achieved as well as the scalability of the manufacturing process becomes easier (Silva et al. 2008).
Several bioreactors configurations have been assessed for mammalian cell culture. For suspension culture, homogeneous bioreactors similar to those for bacterial and yeast cultures can be used (Kelley et al. 2008). Stirred tank, air-lift and bubble column bioreactors have already been operated successfully with suspension cultures of mammalian cells (Sambanis and Hu 2008; Doelle et al. 2009; Handa-Corrigan et al. 1989). The selection of bioreactors to produce a particular biopharmaceutical from this type of animal cells has to meet special demands such as gentle aeration and agitation in order to avoid cell damage, in parallel some process parameters like pH, temperature and dissolved oxygen must be well controlled (Doelle et al. 2009). Concentrations of toxic metabolites (lactate and ammonia) need to be at low levels (Doelle et al. 2009; Cruz et al. 2000a). The main goal in this task is to obtain high cell and/or product concentration.
The aims of this work was to determine the influence of aeration-homogenization system in stirred tank bioreactors at different dissolved oxygen concentrations as well as pH control mode on BHK-21 cell growth and metabolism. The results could be useful for optimizing viral vaccines production and transient heterologous protein expression.
Materials and methods
Cell line and culture medium
BHK-21 (C-13) cells (Sigma-Aldrich ECACC Cell Lines, Lyon, France) adapted to single cell suspension culture were kindly supplied by Dr. Renaud Wagner from Ecole Superieure Biotechnologie de Strasbourg (France). The culture medium used had the following composition by volume: Iscove’s Modified Dulbecco Medium with glutamine and Phenol red (IMDM, catalog number 12200-036, Gibco, Grand Island, NY, USA) 45.5 %, High glucose Dulbecco′s Modified Eagle Medium (DMEM, catalog number 12100-046, Gibco, NY, USA) 45.5 %, heat inactivated fetal bovine serum (Catalog number 10082-147, Gibco) 5 %, 10 % m/v Pluronic F-68 (Sigma-Aldrich, St. Louis, MO, USA) aqueous solution 2 %, and 4 mM glutamine (Sigma-Aldrich) aqueous solution 2 %.
Inoculum preparation
One milliliter of BHK-21 cells (2 × 106 cell/mL) was thawed and placed in a 75 cm2 tissue culture flask (vertical position) with 30 mL of culture medium for growing. Four days later, the cell suspension was used to inoculate (0.12 × 106 cell/mL) consecutively other tissue culture flasks with 25 and 75 cm2, enough culture medium was added to reach final cellular suspensions of 10 and 30 mL, respectively. Inoculum for bioreactors was generated from 100 to 250 mL spinner flasks (Bellco Glass Inc., Vineland, NJ, USA), with operation volumes of 50 and 100 mL, respectively; the stirring speed was maintained at 30 rpm (Sci-Era quad drive stirrer system with a stirrer (Bellco Biotechnology, Vineland, NJ, USA). Spinners were previously inoculated (0.25 × 106 cell/mL) from 75 cm2 tissue culture flask in the exponential phase. After 72 h, the inoculums for bioreactors were ready, the cell concentration and viability in spinners were 4.27 × 106 ± 0.85 × 106 cell/mL and 100 %, respectively. All the procedures described in this section were performed in an incubator (Thermoforma 3110, Marietta, OH, USA) at 37 °C and 5 % of carbon dioxide atmosphere.
Bioreactor cultures
Batch experiments in bioreactors were carried out in a 5 L Celligen (New Brunswick Scientific, Edison, NJ) without aeration cage and 2 L Bioflo 110 (New Brunswick Scientific, Edison, NJ) at 37 °C, 80 rpm, with 2 and 1 L working volumes, respectively. The initial cell concentration was 0.25 × 106 cell/mL. For each bioreactor, dissolved oxygen was studied at 10, 30 and 50 % air saturation throughout the batch and the pH was controlled at 7.2 until possible using a gas mixture (carbon dioxide, air, oxygen and nitrogen). Two additional experiments were performed in Bioflo 110 at 30 and 50 % air saturation with controlling pH at 7.2 during the whole batch time, adding NaHCO3 solution (8 % m/v) when necessary. Temperature, pH and dissolved oxygen over the course of batch cultures were online acquired by an in house LabVIEW program (National Instruments, Austin, TX ,USA). Other bioreactor operation parameters and aeration-homogenization details are shown in Table 1.
Table 1.
Some operation parameters and aeration–homogenization devices for each stirred tank bioreactor
| Bioreactor | Volumetric gas flow (mL/min) | VVM (min−1)* | Aeration–homogenization device | KLa (h−1)** |
|---|---|---|---|---|
| Celligen | 400 | 0.2 | Rotating gas-sparger | 2.37 |
| Bioflo 110 | 200 | Classical (impeller for gas dispersion) | 4.54 |
* VVM Volume of gas per liquid volume per minute
** KLa Volumetric oxygen transfer coefficient was determined experimentally by means of dynamic oxygen-electrode method (Scargiali et al. 2010)
Cell counting and sizing
The cells were counted using improved Neubauer counting chamber (Precicolor, HBG, Giessen-Lützellinden, Germany) with proper sample dilution with Phosphate Buffer Saline. Viable cells were quantified in parallel using the trypan blue exclusion method (Augusto et al. 2010).
In some samples cell concentration and size were determined by Scepter handheld automated cell counter (Millipore, Billerica, MA, USA).
Nutrient and metabolite analysis
Five milliliter samples at different times during batches were taken. They were centrifuged at 750g for 4 min (Sorvall Biofuge Primo R Centrifuge, Thermo Electron Corp., Langenselbold, Germany). Subsequently, supernants were filtered through 0.22 μm filter (Millex-GV filter unit, Sao Paulo, Brazil) and frozen at −20 °C until analysis of nutrients and metabolites.
Glucose, lactate, glutamine and glutamate concentrations from supernant samples were measured using enzyme-coupled reaction and electrochemical detection, in YSI 2700 Select Bioanalyzer (YSI Life Sciences, Yellow Springs, OH, USA).
Ammonium quantification was performed by means of enzymatic–colorimetric method at 340 nm (EnzyChrom™ Ammonia/Ammonium Assay Kit (ENH3-100), BioAssay Systems, Hayward, CA, USA). Briefly, NADH was converted to NAD+ in the presence of NH3, ketoglutarate and glutamate dehydrogenase. The decrease in optical density is directly proportionate to ammonium and ammonia concentration in the samples.
Estimation of maximum specific growth rate, metabolite synthesis and nutrient consumption factors
Maximum specific growth rate, μmax, was calculated by plotting natural logarithm of viable cell concentration versus time during exponential phase (Augusto et al. 2010).
Metabolite (lactate, glutamate and ammonium) to cell yield 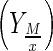 for a given period was determined as Eq. 1:
for a given period was determined as Eq. 1:
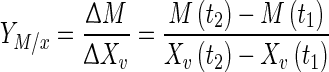 |
1 |
where M(tx) and Xv (tx) are metabolite and cell concentration for a specific moment of the cell culture.
Nutrient (glucose and glutamine) consumption to cell yield 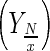 for a given period was calculated as Eq. 2:
for a given period was calculated as Eq. 2:
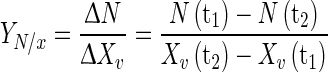 |
2 |
where N(tx) is nutrient concentration for a specific moment of the cell culture.
Nutrient to metabolite conversion yield coefficients 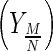 (glutamine–ammonium and glucose–lactate pairs), for a given time interval, were calculated as Eq. 3:
(glutamine–ammonium and glucose–lactate pairs), for a given time interval, were calculated as Eq. 3:
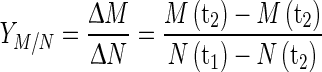 |
3 |
Cell specific oxygen consumption rate
The cell specific oxygen uptake rate (qO2, nmol O2 cell−1 min−1) was determined by dynamic method without modifications. It was quantified from the depletion in the dissolved oxygen concentration after stopping the gas flow. In this condition the mass balance for the dissolved oxygen can be simplified as Eq. 4. The qO2 is obtained as the rate of oxygen uptake ratio (OURd) and cell concentration (Xv). OURd is calculated from the slope of the plot of dissolved oxygen concentration versus time after stopping gas flow (Eq. 4) (Garcia-Ochoa et al. 2010). The procedure was repeated at several moments during the batch experiment. Specific oxygen consumption rate were calculated assuming air saturation concentration of 0.21 mM for oxygen at 37 °C (Cruz et al. 2000a).
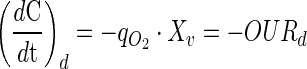 |
4 |
where 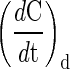 is the accumulation of oxygen in the liquid phase.
is the accumulation of oxygen in the liquid phase.
Results
Influence of aeration–homogenization system in stirred tank bioreactors, dissolved oxygen concentration (batches performed without base addition to control pH)
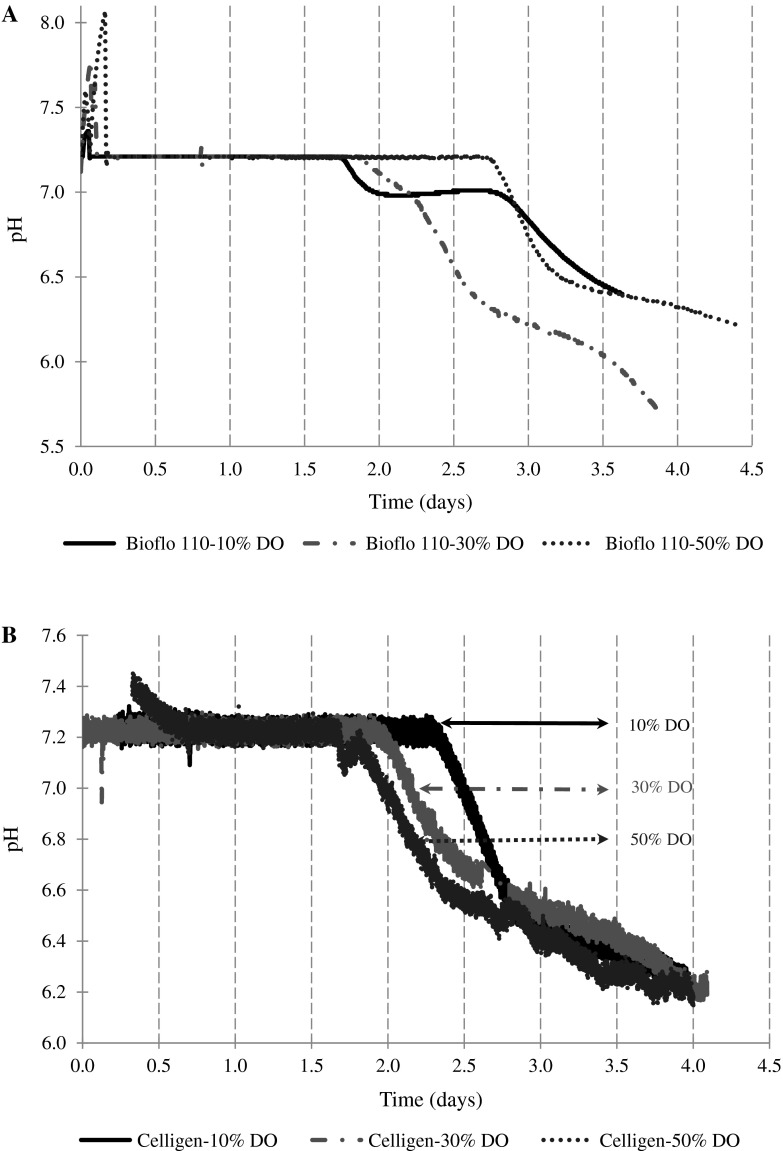
The optimal dissolved oxygen concentration for batch experiments without NaHCO3 solution (8 % m/v) addition was different for each bioreactor under study. The highest maximum cell concentrations were 4.34 × 106 cell/mL and 4.59 × 106 cell/mL for Bioflo 110 (50 % DO) and Celligen (10 % DO), respectively (Table 2). As a rule the best batch cultures (maximum cell concentration as a criterion) with this kind of partial pH control were associated to longer times of culture with pH values oscillating around 7.2. The pH decrease for Bioflo 110 (50 % DO) and Celligen (10 % DO) appeared at 2.75 and 2.32 days, in that order (Fig. 1). Simultaneously, the ammonium and lactate synthesis factors were lower than those related to other batches performed at different DO in this pH control condition (Table 2). The glutamine and glucose metabolism at optimal DO conditions were more efficient. Specifically, for glucose, values of lactate-glucose conversion factors were the lowest and distant of 2 (theoretical value for total conversion to lactate) (Table 2).
Table 2.
Nutrient consumption, metabolite synthesis, conversion nutrient to metabolite factors and other characteristic parameters for each batch experiment
| Bioreactor | DO (%) | NaHCO3 addition | Xmax–H (106cell/mL) | Xmax–S (106cell/mL) | μmax (day−1) | Reference time (day) |
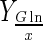 (pg/cell) (pg/cell) |
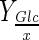 (pg/cell) (pg/cell) |
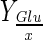 (pg/cell) (pg/cell) |
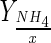 (pg/cell) (pg/cell) |
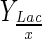 (pg/cell) (pg/cell) |
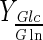 (mol/mol) (mol/mol) |
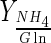 (mol/mol) (mol/mol) |
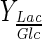 (mol/mol) (mol/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioflo 110 | 10 | Without | 1.85 | 1.73 | 1.30 | 1.78EEP | 197 | 1317 | 10 | 14 | 1094 | 0.05 | 0.59 | 1.68 |
| 2.23MCC | 238 | 1287 | 14 | 34 | 1049 | 0.06 | 1.17 | 1.65 | ||||||
| 30 | Without | 3.63 | 2.95 | 1.93 | 2.23EEP | 139 | 655 | 12 | 14 | 536 | 0.09 | 0.85 | 1.65 | |
| 2.82MCC | 130 | 696 | 11 | 13 | 565 | 0.09 | 0.84 | 1.64 | ||||||
| With | 4.94 | 3.94 | 1.52 | 2.24EEP | 102 | 533 | 3 | 8 | 378 | 0.03 | 0.60 | 1.44 | ||
| 2.78MCC | 93 | 681 | 3 | 9 | 440 | 0.03 | 0.78 | 1.31 | ||||||
| 50 | Without | 4.34 | 3.73 | 1.55 | 2.16EEP | 147 | 539 | 7 | 8 | 372 | 0.05 | 0.42 | 1.40 | |
| 2.83MCC | 115 | 581 | 7 | 8 | 368 | 0.06 | 0.58 | 1.28 | ||||||
| With | 4.24 | 3.68 | 1.68 | 2.30EEP | 141 | 654 | 6 | 4 | 559 | 0.05 | 0.25 | 1.73 | ||
| 2.88MCC | 116 | 988 | 7 | 6 | 752 | 0.06 | 0.44 | 1.54 | ||||||
| Celligen | 10 | Without | 4.59 | 3.51 | 1.81 | 1.98EEP | 111 | 692 | 7 | 0.2 | 492 | 0.06 | 0.01 | 1.44 |
| 2.95MCC | 100 | 864 | 6 | 5.0 | 604 | 0.06 | 0.04 | 1.42 | ||||||
| 30 | Without | 3.07 | 2.97 | 1.69 | 2.10EEP | 181 | 1045 | 27 | 5.5 | 919 | 0.15 | 0.25 | 1.78 | |
| 2.80MCC | 146 | 1059 | 19 | 4.4 | 914 | 0.13 | 0.25 | 1.75 | ||||||
| 50 | Without | 3.16 | 3.35 | 1.49 | 2.01EEP | 204 | 1090 | 22 | 22 | 902 | 0.11 | 0.88 | 1.67 | |
| 2.67MCC | 223 | 1619 | 25 | 26 | 1335 | 0.11 | 0.94 | 1.67 |
All factors were calculated using as initial moment, the time when the first sample of batch experiments was taken. Reference times, end of exponential phase (EEP) and maximum cell concentration (MCM), were used to define the end of time interval to calculate factors. Gln, Glc, Glu, NH4 and Lac represent glutamine, glucose, glutamate, ammonium and lactate, respectively. Xmax–H and Xmax–S represent maximum viable cell concentration determined by hemocytometer and total cell concentration by Millipore® Scepter handheld automated cell counter, respectively
Fig. 1.
pH profiles for the experiments performed in Celligen and Bioflo 110 bioreactors without addition of base solution to control pH. a Batches in Bioflo 110. b Batches in Celligen
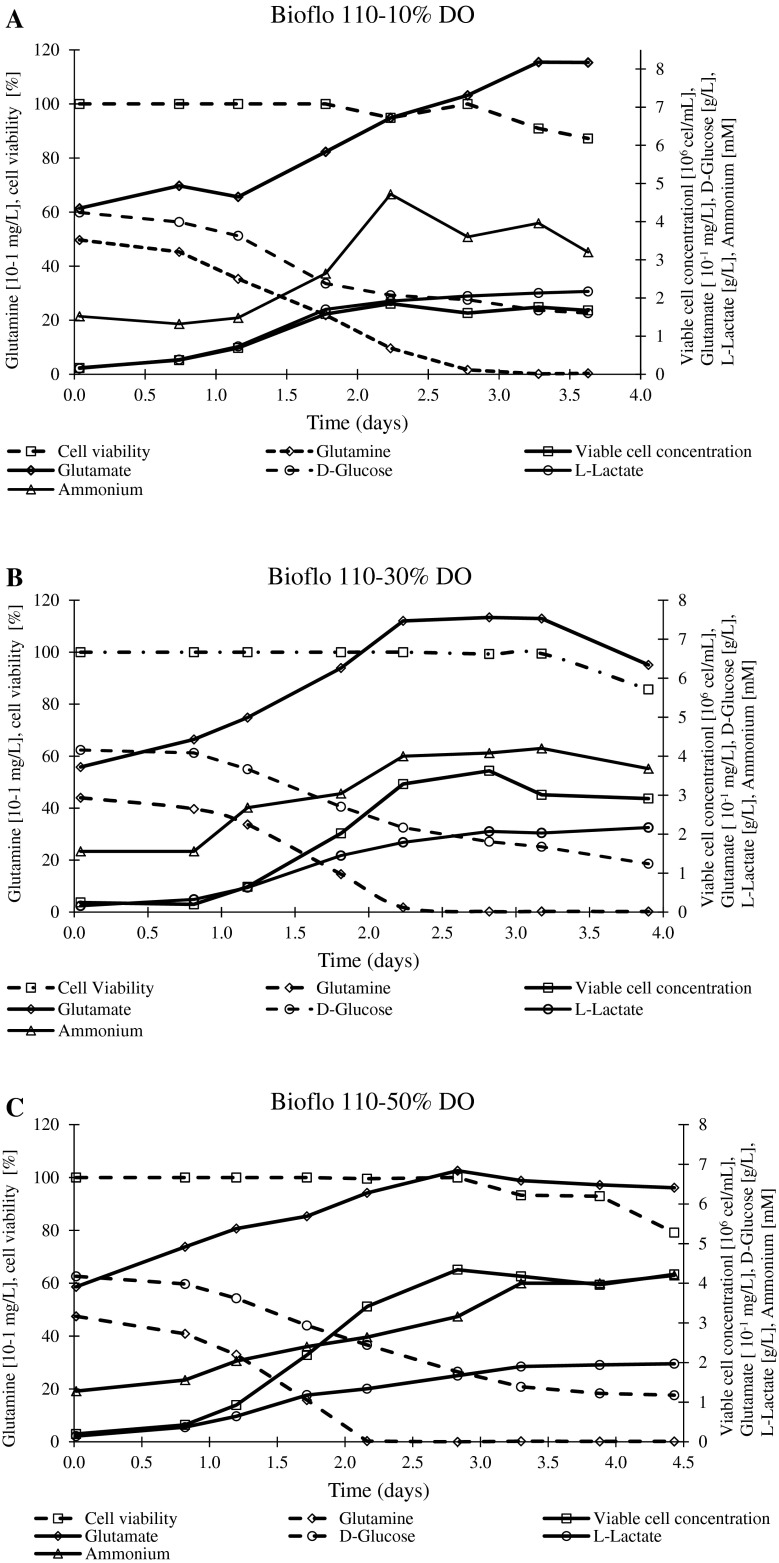
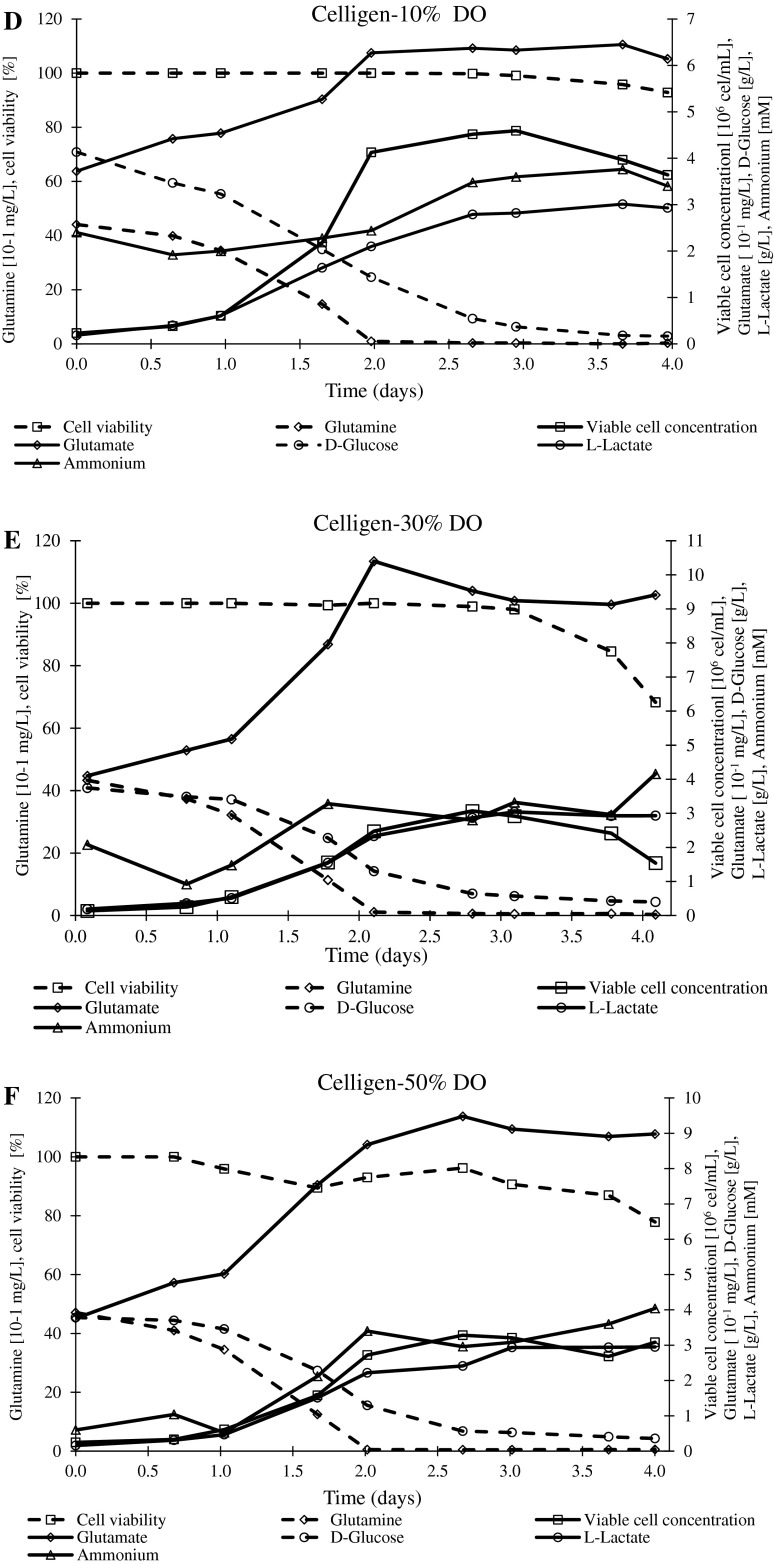
The μmax values were in the 1.30–1.93 day−1 and 1.49–1.81 day−1 ranges for Bioflo 110 and Celligen, respectively (Table 2). The end of exponential phase in all experiments was around 2 days after bioreactor inoculation (Fig. 2). In almost all experiments, the viability was inferior to 90 % after 4 days from the inoculum, the exception was the batch performed in Celligen at 10 % DO (cell viability = 92.9 %) (Fig. 2d). This batch matched with the lowest ammonium synthesis (Table 2).
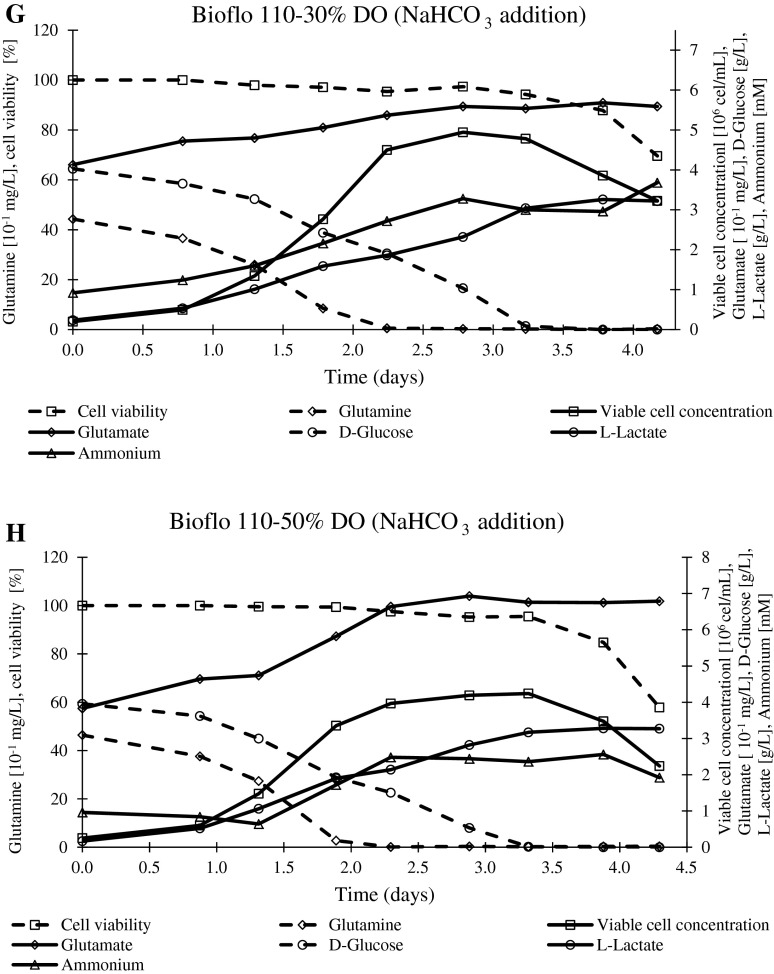
Fig. 2.
Profiles of viable cell concentration, cell viability, nutrients (glutamine and D-glucose) and metabolites (glutamate, ammonium and L-Lactate). a Bioflo 110–10 % DO. b Bioflo 110–30 % DO. c Bioflo 110–50 % DO. d Celligen-10 % DO. e Celligen-30 % DO. f Celligen-50 % DO. g Bioflo 110–30 % DO (with NaHCO3 addition). h Bioflo 110–50 % DO (with NaHCO3 addition)
Influence of pH control mode
When pH was controlled during the whole batch, similar (50 % DO) and improved (30 % DO) values of maximum cell concentrations with respect to those for batches without base solution addition were observed (Table 2; Fig. 2). Concurrently, the glucose was totally exhausted (Fig. 2).
Glutamine consumption to cell yields for the batch performed at 30 % DO with base addition was lower than the same batch without NaHCO3 solution (8 % m/v) addition at the end of the exponential phase (36 %) and maximum cell concentration (39 %), meanwhile the same values for this parameter were observed at 50 % DO with both pH control modes (Table 2). Besides, the yield of lactate and ammonium per cell were also inferior at 30 % DO with base addition with respect to similar batches without base addition. Opposite performance was observed at 50 % DO (Table 2).
General findings
Discrepancies between cell counting by hemocytometer and scepter handheld automated cell counter were detected. This was most evident for cell concentration higher than 3.5 × 106 cell/mL (Table 2). Probably, the changes of culture medium at high cell concentration interfere in impedance-based particle detection used by the scepter counter.
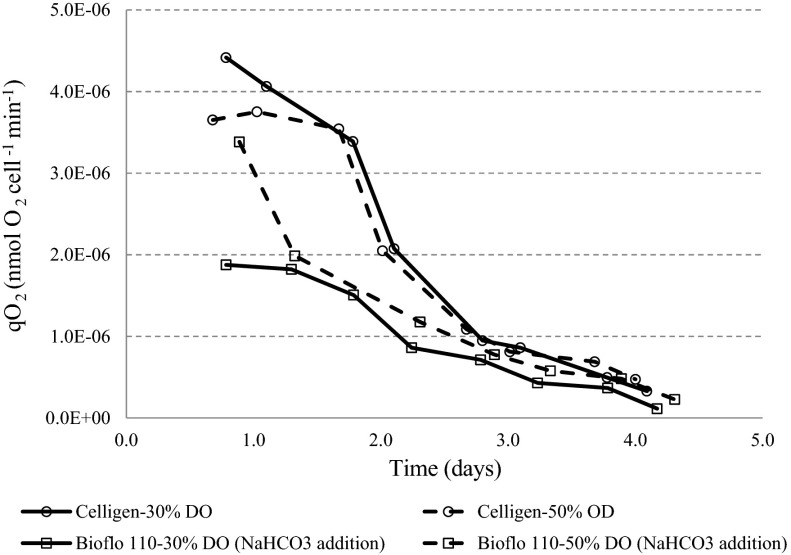
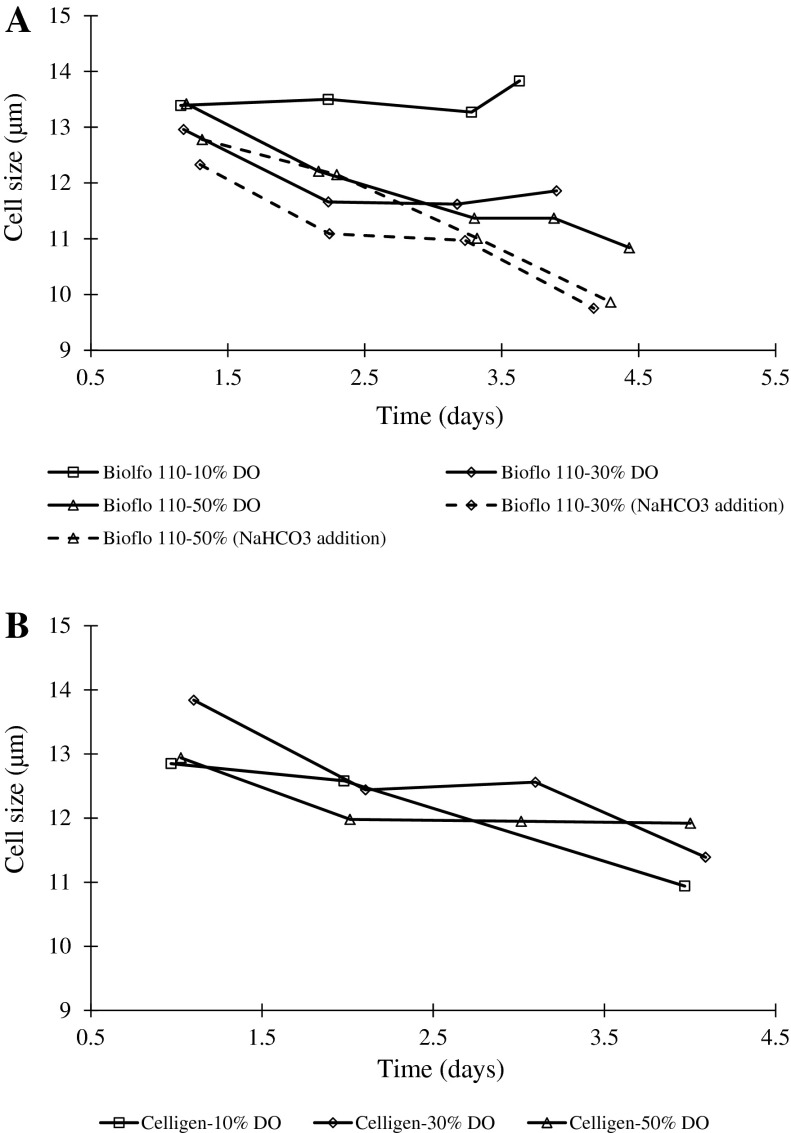
The oxygen cell consumption was higher in batches performed without base addition in Celligen. However, in both experimental conditions, a decrease in the cell specific oxygen consumption rate throughout the experiment was confirmed (Fig. 3). At the end of the batches, the qO2 values were similar (1.14–4.75 10−7 nmol O2 cell−1 min−1). A similar inverse relationship between cell size and culture time was also observed. As a conclusion, for this parameter, highest maximum cell concentrations were associated to smallest cell size at the end of batch culture. When maximum cell concentrations were higher than 4 × 106 cell/mL, the cell sizes were smaller than 11 μm (Fig. 4).
Fig. 3.
Cell specific oxygen consumption rate profile for batches performed in different bioreactors with or without NaHCO3 addition
Fig. 4.
Cell size profiles defined by Millipore® Scepter handheld automated cell counter. a Bioflo. b Celligen
Discussion
Despite the high number of publications about BHK-21 culture in bioreactors and metabolism (Kallel et al. 2003; Perrin et al. 1995; Teixeira et al. 2005; Cruz et al. 2000b), combined studies about different bioreactors assessment at different dissolved oxygen concentrations are not often addressed. Similarly, the impact of pH control mode (with or without base addition) is not usually set.
In the present work a classical stirred tank bioreactor (Bioflo 110) was compared to a modified cell lift impeller bioreactor (Celligen) (Mirro and Voll 2009). The modifications consisted of removing the aeration cage (useful for cell culture with microcarriers) and the working volume level was lower in relation to the discharge ports. Thus, the agitation device was a rotating gas-sparger. To avoid cell damage caused by shear forces resulting from mechanical agitation and aeration, it was included in culture medium composition, Pluronic F-68 (0.2 % m/v) was included in culture medium composition, its protecting effect was reinforced with fetal bovine serum addition (Molina-Grima et al. 1997).
The maximum cell concentrations (1.85−4.94 × 106 cell/mL, Table 2) for both bioreactors under study, operating in different conditions, were higher or similar to other reported values for this parameter in BHK-21 or recombinant BHK-21 cells suspension cultures using fetal bovine serum or serum-free or protein-free media in batch mode (1−3.2 × 106 cell/mL) (Handa-Corrigan et al. 1989; Cruz et al. 2002; Moreira et al. 1995; Ishaque et al. 2007). In general, the studies related with increase of cell concentration in BHK-21 cell lines are focused on the optimization of the glutamine and glucose metabolism (Cruz et al. 2000a; Cruz et al. 2000b; Butler and Jenkins 1989). Rarely, the influence of cell physical environment and the concentration of other important molecule for both metabolic pathways, oxygen, are explored for this purpose. In the present work, low glucose and glutamine consumption to cell yield, as well as low lactate-glucose and ammonium-glutamine yield coefficients were observed in both bioreactors when highest maximum cell concentrations were reached (Table 2). This suggests that 10 and 50 % DO for Celligen and Bioflo 110 (without base addition) are proper conditions for more efficient glucose and glutamine metabolism of BHK-21 cell with a culture medium with 4 g/L of glucose and 3.72 mM of glutamine. The earlier drop of initial pH (7.2) (Fig. 1) in those experiments with low values of maximum cell concentration could be explained by the high production of lactate and ammonium, metabolites responsible for pH decrease and growth inhibition (Cruz et al. 2000a). However, carbon dioxide (CO2) accumulation effect (inducing pH decrease) in culture media should not be neglected. The differences between optimal DO for each bioreactor could be justified by different efficient conditions for CO2 stripping out. It has been reported that aeration systems designed for effective oxygen transfer will not necessarily be efficient for CO2 removal (Birch 2010). Probably, gas mixtures to keep constant low DO (10 %) are suitable to CO2 stripping from culture broth and favoring BHK-21 metabolism and respiration in a stirred tank bioreactor with a rotating gas-sparger (Celligen with modifications).
The effective pH control throughout the whole batch prevents the intracellular acidification and as a consequence, normal cell physiology is guaranteed. Thus the negative effects of lactate and ammonium ions is reduced, because of the reduction of their intrinsic toxicity and osmolarity effects. Therefore, the improvement of maximum cell concentration and glutamine consumption to cell yield at 30 % DO in the Bioflo 110 as well as the total glucose total consumption (30 and 50 % DO) when base solution was added is justified by this pH control mode in bioreactor (Table 2; Fig. 2b, e). However, an increase in the maximum cell concentration was not observed at 50 % DO. Probably, the bioconversion yield (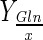 ,
, 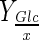 ) without or with base addition was similar because gas mixture compositions at this DO are able to maintain pH constant until the minimum limit value for glutamine consumption to cell yield for BHK-21 cell line at 50 % DO. Thus, it was demonstrated that base addition to control pH at 7.2 was not necessary to improved maximum cell concentration at 50 % DO in the Bioflo 110 bioreactor (Figs. 1a, 2c, h).
) without or with base addition was similar because gas mixture compositions at this DO are able to maintain pH constant until the minimum limit value for glutamine consumption to cell yield for BHK-21 cell line at 50 % DO. Thus, it was demonstrated that base addition to control pH at 7.2 was not necessary to improved maximum cell concentration at 50 % DO in the Bioflo 110 bioreactor (Figs. 1a, 2c, h).
Based on the direct relationship between the end of the exponential phase and glutamine depletion, glutamine could be considered the limiting substrate. However, the increase of this amino acid concentration in culture medium is not a suitable alternative in order to increase maximum cell concentration because high ammonium synthesis could be induced at glutamine concentration above 4 mM (Butler and Jenkins 1989). Ammonium levels for this culture medium (3.72 mM of glutamine) were already high enough to promote apoptosis in BHK cells (>2 mM) (Teixeira et al. 2005). In addition, ammonium is released by cells due to the amino acids (Schneider et al. 1996), and not only due to glutamine metabolism; the values of 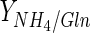 are much lower than 2 (theoretical value for a complete oxidation of glutamine through the tricarboxylic acid cycle) (Table 2). This result suggests that glucose is the principal energetic source for BHK cells in both bioreactors in the 10–50 % DO range, in particular for Celligen 10 % DO (Table 2). On the other hand, batch with low maximum cell concentrations in Celligen (30 and 50 % DO) were associated to high
are much lower than 2 (theoretical value for a complete oxidation of glutamine through the tricarboxylic acid cycle) (Table 2). This result suggests that glucose is the principal energetic source for BHK cells in both bioreactors in the 10–50 % DO range, in particular for Celligen 10 % DO (Table 2). On the other hand, batch with low maximum cell concentrations in Celligen (30 and 50 % DO) were associated to high 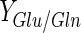 values. This could be justified by the enhanced action of glutaminase (responsible for enzymatic glutamine degradation into glutamate and ammonium in bioreaction broth) contained in fetal bovine serum (Ozturk and Palsson 1990), at 30 and 50 % DO in the rotating gas-sparger bioreactor (Table 2).
values. This could be justified by the enhanced action of glutaminase (responsible for enzymatic glutamine degradation into glutamate and ammonium in bioreaction broth) contained in fetal bovine serum (Ozturk and Palsson 1990), at 30 and 50 % DO in the rotating gas-sparger bioreactor (Table 2).
Considering that lactate was only produced by aerobic glycolysis (lactate formation is also possible from glutamine (Schneider et al. 1996)), more than 64 % 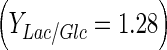 of glucose was metabolized following this metabolic route for all experiments (Table 2); the rest was metabolized through tricarboxylic acid cycle. Under normal condition, mammalian cells in bioreactors consume large amounts of glucose; nearly 90 % of this monosaccharide is converted to lactate (Mulukutla et al. 2010). For DO concentration which ensures highest cell concentration in Celligen without aeration cage (10 % air saturation), upto 71 % of glucose was converted to lactate. With the same criterion, for the best conditions in stirred tank bioreactor assessed in this work (DO at 30 % air saturation and pH control with base solution addition), the lactate–glucose conversion percentage was at most of 66 %. Thus, the use of glucose in optimal conditions for both bioreactors was similar to other reported values for BHK-21 culture at high glucose concentrations (
of glucose was metabolized following this metabolic route for all experiments (Table 2); the rest was metabolized through tricarboxylic acid cycle. Under normal condition, mammalian cells in bioreactors consume large amounts of glucose; nearly 90 % of this monosaccharide is converted to lactate (Mulukutla et al. 2010). For DO concentration which ensures highest cell concentration in Celligen without aeration cage (10 % air saturation), upto 71 % of glucose was converted to lactate. With the same criterion, for the best conditions in stirred tank bioreactor assessed in this work (DO at 30 % air saturation and pH control with base solution addition), the lactate–glucose conversion percentage was at most of 66 %. Thus, the use of glucose in optimal conditions for both bioreactors was similar to other reported values for BHK-21 culture at high glucose concentrations (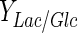 , in 1.5–1.6 mol/mol range) (Cruz et al. 1999) and suitable for mammalian cell culture (much less than 90 %) (Mulukutla et al. 2010).
, in 1.5–1.6 mol/mol range) (Cruz et al. 1999) and suitable for mammalian cell culture (much less than 90 %) (Mulukutla et al. 2010).
The maximum lactate concentration at the end of the exponential phase among performed batches was 2.33 g/L (26 mM), for Celligen at 30 % air saturation (Fig. 2). Therefore, the BHK-21 cell culture in conditions considered in this work should not be inhibited by lactate, because it has been reported that inhibitory lactate concentrations for BHK-21 cells were around 28 mM in suspension cultures (Cruz et al. 2000a).
The proximity of conversion factors for two critical moments of the batch (Table 2), the end of the exponential phase and the maximum cell concentration, was confirmed. In general, the time difference is around 12 h. This also suggests a substrate (glutamine) inhibition of growth as was previously mentioned (Radlett et al. 1971).
The maximum specific growth rate for mammalian cells varies in the range of 0.55–1.39 day−1 (Dietmair et al. 2012). In both Celligen and Bioflo BHK-21 cultures, the maximum specific growth rates were higher or next to the superior limit of the usual range for this variable (Table 2). This is an important parameter for maximizing production of viruses when they are more active in cycling cells. It has been demonstrated by several authors that enhanced cell growth rates should lead to an increased viral production rate (Merten 2004). Thus, BHK-21 cell line, culture medium and bioreactor configurations used in this study are suitable for viral vaccine manufacturing, transient protein expression and gene therapy vector production.
Regarding, cell specific oxygen uptake rates, their profiles and values for evaluated conditions in both bioreactors were similar to others as previously reported (Cruz et al. 2000a; Cruz et al. 1999) for the BHK-21 cell line at high initial glucose concentration (4 g/L). The decrease of cell specific oxygen uptake rates over the course of bioconversion could be justified by changes in medium composition and cell growth phase (Garcia-Ochoa et al. 2010; Jorjani and Ozturk 1999). The lactate concentration increase has a direct influence on cell specific oxygen uptake rate decrease (Cruz et al. 2000a). Higher initial cell specific oxygen uptake rates in Celligen is an evidence of more efficient glucose metabolism in this bioreactor compared to the Bioflo 110 bioreactor at the beginning of batches (Fig. 3).
Low cell size was an indicative of the high cell concentration in both bioreactor (Figs. 2, 4). One of the causes of this relationship could be the osmolarity increase. Higher osmolarity results in a decrease of cell volume due to the enhanced efflux of water (Seewöster and Lehmann 1997). It has been reported that accumulation of lactate leads to high osmolarity (Li et al. 2010). As high lactate concentrations were directly correlated with high cell concentrations (Fig. 2), this metabolite could be the main component for the increase in osmolarity.
Conclusion
Taking into consideration the highest maximum BHK-21 cell concentration as choice criterion, best dissolved oxygen concentrations for assessed stirred tank bioreactors were 10 (rotating gas-sparger) and 50 % (with impeller for gas dispersion) air saturation. As a consequence, the impact of aeration–homogenization system on BHK-21 cell growth and metabolism was quantified. These findings are valid when no NaHCO3 solution (8 % m/v) is added to control pH. Addition of base solution in conjunction with CO2 in aeration mixtures could be significant, but it depends on dissolved oxygen concentration. In this work, maximum BHK-21 cell concentration at 30 % air saturation in classical stirred tank was improved in 36 % when the pH was controlled during the whole bioreaction time with respect to a similar experiment perfomed without base addition. Nevertheless, no differences were detected for 50 % air saturation for the same bioreactor. The optimal parameters defined in this work allow for bioprocess development for manufacturing of viral vaccines, transient protein expression and viral vector production for gene therapy purposes based on the use BHK-21 cells grown in suspension in two types of bioreactors operating in batch mode.
Acknowledgments
The authors would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for post-doctoral fellowship (2010/52521-6), Fundação para o Desenvolvimento Tecnológico da Engenharia (FDTE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 483009/2010-5) for scientific grants. First author gratefully acknowledges his wife and daughter, Relma and Giovanna for the inspiration to write this paper.
Contributor Information
Eutimio Gustavo Fernández Núñez, Phone: +55-11-3091-2282, Email: eutimiocu@yahoo.com.
Alexandre Gonçalves de Rezende, Phone: +55-11-3726-7222.
Daniela Cristina Ventini Monteiro, Phone: +55-11-3726-7222.
Vera Lucia Lopes Boldorini, Phone: +55-11-3726-7222.
Soraia Attie Calil Jorge, Phone: +55-11-3726-7222.
Renato Mancini Astray, Phone: +55-11-3726-7222.
Carlos Augusto Pereira, Phone: +55-11-3726-7222.
References
- Astley K, Naciri M, Racher A, Al-Rubeai M. The role of p21cip1 in adaptation of CHO cells to suspension and protein-free culture. J Biotechnol. 2007;130:282–290. doi: 10.1016/j.jbiotec.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Augusto EFP, Moraes AM, Piccoli RAM, Barral MF, Suazo CAT, Tonso A, Pereira CA. Nomenclature and guideline to express the amount of a membrane protein synthesized in animal cells in view of bioprocess optimization and production monitoring. Biologicals. 2010;38:105–112. doi: 10.1016/j.biologicals.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Auniņš JG. Viral vaccine production in cell culture. In: Flickinger MC, editor. Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. New York: Wiley; 2010. pp. 1–35. [Google Scholar]
- Birch JR. Suspension culture, animal cells. In: Flickinger MC, editor. Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Hoboken: Wiley; 2010. pp. 1–28. [Google Scholar]
- Bödeker BGD, Newcomb R, Yuan P, Braufman A, Kelsey W (1994) Production of recombinant factor VIII from perfusion cultures: I. Large-scale fermentation. In: Spier RE, Griffiths JB, Berthold W (eds) Animal cell technology: Products of today, prospects for tomorrow. Butterworth-Heinemann, Oxford, UK, pp. 580–583
- Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68:283–291. doi: 10.1007/s00253-005-1980-8. [DOI] [PubMed] [Google Scholar]
- Butler M, Jenkins H. Nutritional aspects of the growth of animal cells in culture. J Biotechnol. 1989;12:97–110. doi: 10.1016/0168-1656(89)90009-6. [DOI] [Google Scholar]
- Cruz HJ, Ferreira AS, Freitas CM, Moreira JL, Carrondo MJT. Metabolic responses to different glucose and glutamine levels in baby hamster kidney cell culture. Appl Microbiol Biotechnol. 1999;51:579–585. doi: 10.1007/s002530051435. [DOI] [PubMed] [Google Scholar]
- Cruz HJ, Freitas CM, Alves PM, Moreira JL, Carrondo MJT. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb Tech. 2000;27:43–52. doi: 10.1016/S0141-0229(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Cruz HJ, Moreira JL, Carrondo MJT. Metabolically optimised BHK cell fed-batch cultures. J Biotechnol. 2000;80:109–118. doi: 10.1016/S0168-1656(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Cruz HJ, Conradt HS, Dunker R, Peixoto CM, Cunha AE, Thomaz M, Burger C, Dias EM, Clemente J, Moreira JL, Rieke E, Carrondo MJT. Process development of a recombinant antibody/interleukin-2 fusion protein expressed in protein-free medium by BHK cells. J Biotechnol. 2002;96:169–183. doi: 10.1016/S0168-1656(02)00028-7. [DOI] [PubMed] [Google Scholar]
- Dietmair S, Nielsen LK, Timmins NE. Mammalian cells as biopharmaceutical production hosts in the age of omics. Biotechnol J. 2012;7:75–89. doi: 10.1002/biot.201100369. [DOI] [PubMed] [Google Scholar]
- Doelle HW, Fiechter A, van Griensven M, Kasper C, Pörtner R, Schlegel G, Shimizu S, Stahl F, Suck K, Yamada H, Zorn H. Biotechnology 6. Special applications. Ullmann’s encyclopedia of industrial chemistry. New York: Wiley; 2009. pp. 73–112. [Google Scholar]
- Garcia-Ochoa F, Gomez E, Santos VE, Merchuk JC. Oxygen uptake rate in microbial processes: an overview. Biochem Eng J. 2010;49:289–307. doi: 10.1016/j.bej.2010.01.011. [DOI] [Google Scholar]
- Handa-Corrigan A, Emery AN, Spier RE. Effect of gas–liquid interfaces on the growth of suspended mammalian cells: mechanisms of cell damage by bubbles. Enzyme Microb Tech. 1989;11:230–235. doi: 10.1016/0141-0229(89)90097-5. [DOI] [Google Scholar]
- Ishaque A, Thrift J, Murphy JE, Konstantinov K. Over-expression of Hsp70 in BHK-21 cells engineered to produce recombinant factor VIII promotes resistance to apoptosis and enhances secretion. Biotechnol Bioeng. 2007;97:144–155. doi: 10.1002/bit.21201. [DOI] [PubMed] [Google Scholar]
- Jorjani P, Ozturk SS. Effects of cell density and temperature on oxygen consumption rate for different mammalian cell lines. Biotechnol Bioen. 1999;64:349–356. doi: 10.1002/(SICI)1097-0290(19990805)64:3<349::AID-BIT11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kallel H, Rourou S, Majoul S, Loukil H. A novel process for the production of a veterinary rabies vaccine in BHK-21 cells grown on microcarriers in a 20-l bioreactor. App Microbiol Biotechnol. 2003;61:441–446. doi: 10.1007/s00253-003-1245-3. [DOI] [PubMed] [Google Scholar]
- Kelley BD, Chiou TW, Rosenberg M, Wang DIC. Industrial animal cell culture. In: Rehm HJ, Reed G, editors. Biotechnology set. 2. Weinheim: Wiley; 2008. pp. 23–38. [Google Scholar]
- Krampe B, Al-Rubeai M. Cell death in mammalian cell culture: molecular mechanisms and cell line engineering strategies. Cytotechnology. 2010;62:175–188. doi: 10.1007/s10616-010-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Vijayasankaran N, Shen AY, Kiss R, Amanullah A. Cell culture processes for monoclonal antibody production. MAbs. 2010;2:466–477. doi: 10.4161/mabs.2.5.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten O-W. State-of-the-art of the production of retroviral vectors. J Gene Med. 2004;6:S105–S124. doi: 10.1002/jgm.499. [DOI] [PubMed] [Google Scholar]
- Merten O-W. Introduction to animal cell culture technology—past, present and future. Cytotechnology. 2006;50:1–7. doi: 10.1007/s10616-006-9009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirro R, Voll K. Which impeller is right for your cell line? A guide to impeller selection for stirred-tank bioreactors. BioProcess Int. 2009;7:52–57. [Google Scholar]
- Molina-Grima E, Chisti Y, Moo-Young M. Characterization of shear rates in airlift bioreactors for animal cell culture. J Biotechnol. 1997;54:195–210. doi: 10.1016/S0168-1656(97)00043-6. [DOI] [Google Scholar]
- Moreira JL, Alves PM, Feliciano AS, Aunins JG, Carrondo MJT. Serum-free and serum-containing media for growth of suspended BHK aggregates in stirred vessels. Enzyme Microb Tech. 1995;17:437–444. doi: 10.1016/0141-0229(94)00071-X. [DOI] [Google Scholar]
- Mulukutla BC, Khan S, Lange A, Hu WS. Glucose metabolism in mammalian cell culture: new insights for tweaking vintage pathways. Trends Biotechnol. 2010;28:476–484. doi: 10.1016/j.tibtech.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Ozturk SS, Palsson BO. Chemical decomposition of glutamine in cell culture media: effect of media type, pH, and serum concentration. Biotechnol Prog. 1990;6:121–128. doi: 10.1021/bp00002a005. [DOI] [PubMed] [Google Scholar]
- Park JH, Park HH, Park TH. Cellular engineering for the high-level production of recombinant proteins in mammalian cell systems. Korean J Chem Eng. 2010;27:1042–1048. doi: 10.1007/s11814-010-0278-4. [DOI] [Google Scholar]
- Perrin P, Madhusudana S, Gontier-Jallet C, Petres S, Tordo N, Merten O-W. An experimental rabies vaccine produced with a new BHK-21 suspension cell culture process: use of serum-free medium and perfusion-reactor system. Vaccine. 1995;13:1244–1250. doi: 10.1016/0264-410X(94)00022-F. [DOI] [PubMed] [Google Scholar]
- Radlett PJ, Telling RC, Stone J, Whiteside JP (1971) Improvements in the growth of BHK-21 cells in submerged culture. Appl Microbiol 22:534–537 [DOI] [PMC free article] [PubMed]
- Sambanis A, Hu WS. Cell culture bioreactors. In: Rehm HJ, Reed G, editors. Biotechnology set. 2. Weinheim: Wiley; 2008. pp. 105–125. [Google Scholar]
- Scargiali F, Busciglio A, Grisafi F, Brucato A. Simplified dynamic pressure method for kLa measurement in aerated bioreactors. Biochem Eng J. 2010;49:165–172. doi: 10.1016/j.bej.2009.12.008. [DOI] [Google Scholar]
- Schneider M, Marison IW, von Stockar U. The importance of ammonia in mammalian cell culture. J Biotechnol. 1996;46:161–185. doi: 10.1016/0168-1656(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Seewöster T, Lehmann J. Cell size distribution as a parameter for the predetermination of exponential growth during repeated batch cultivation of CHO cells. Biotechnol Bioeng. 1997;55:793–797. doi: 10.1002/(SICI)1097-0290(19970905)55:5<793::AID-BIT9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Silva AC, Delgado I, Sousa MFQ, Carrondo MJT, Alves PM. Scalable culture systems using different cell lines for the production of Peste des Petits ruminants vaccine. Vaccine. 2008;26:3305–3311. doi: 10.1016/j.vaccine.2008.03.077. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Cunha AE, Clemente JJ, Moreira JL, Cruz HJ, Alves PM, Carrondo MJT, Oliveira R. Modelling and optimization of a recombinant BHK-21 cultivation process using hybrid grey-box systems. J Biotechnol. 2005;118:290–303. doi: 10.1016/j.jbiotec.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Vester D, Rapp E, Kluge S, Genzel Y, Reichl U. Virus–host cell interactions in vaccine production cell lines infected with different human influenza A virus variants: a proteomic approach. J Proteomics. 2010;73:1656–1669. doi: 10.1016/j.jprot.2010.04.006. [DOI] [PubMed] [Google Scholar]