Abstract
Iron overload is a major health problem for patients who have to have continuous blood transfusions. It brings some metabolic problems together. Various iron chelating agents are being used for treatment of hemochromatosis which arises from excess iron accumulation. This study was conducted with the aim of determining whether deferasirox used as an iron chelator in patients with hemochromatosis has genotoxic effects. Commercial form of deferasirox, Exjade was used as test material. Test material showed a general mutagen character in mutant strains of Salmonella typhimurium. Deferasirox has also led to an increase in mutagenity-related polymorphic band count in random amplification of polymorphic DNA test done with bone marrow cells of rats. Similarly, test material has increased micronucleus formation in cultured in vitro human peripheral lymphocytes particularly in 48 h period. Consistently with the abovementioned findings, deferasirox reduced nuclear division index (NDI) compared to controls and some part of these reductions are statistically significant. NDI reductions were found at positive control levels at high concentrations.
Keywords: Iron chelator, Deferasirox, Genotoxicity, Reversion test, RAPD test, Micronucleus test
Introduction
Hemochromatosis is a disease which arises from excess iron accumulation in the body and has severe outcomes that may lead to death unless treated. One of the most important causes of the disease is blood transfusions applied to anemia patients. Because anemia requires recurrent blood transfusions due to insufficient hemoglobin, iron overload occurs in liver, heart and other organs. Excess iron deposition is observed in vital organs due to erythrocyte transfusions and degradation of impaired erythrocytes. Therefore all organs, mainly heart and liver may be damaged. So special drugs called chelators (inducing metal excretion) are used to remove the excess iron from the body and to control this condition. Deferasirox (DFX), a new chelator, is effectively used to control iron load. DFX has a high specific affinity to iron. All patients who have iron overload may use DFX comfortably beginning from small ages like 2 years (Karakas 2009). However severe suspects exist about toxic and mutagenic potentials of chelators. In a study done with deferoxamine (DFO), this agent was found to be highly toxic and mutagenic (Whittaker et al. 2001). DFO together with gamma rays was not only found to be clastogen but it increased genotoxic parameters like frequency of acentric fragment and ring chromosome formation (Juckett et al. 1998). In addition, deferiprone, another chelator used to control iron overload in the body, was detected to form chromosom break less frequently than DFO (Marshall et al. 2003). On the contrary to the abovementioned findings, deferiprone was detected to significantly reduce DNA damage developing as the result of iron deposition (Anderson et al. 2000). Although DFX which was used as test material in our study has many advantages, sufficient data do not exist about its genotoxicity. Therefore we tried to investigate whether the agent has genotoxic and/or cytotoxic effects using salmonella reversion, RAPD (random amplification of polymorphic DNA) and micronucleus test.
Materials and methods
In this study, deferasirox (Exjade, obtained from a pharmacy in Adana / Turkey) (CAS No: 201530-41-8) was used as test material. Gene mutation test in oxotroph strains of Salmonella typhimurium (TA 98 and TA 100), polymorphic band determination with RAPD test in rat bone marrow cells and human peripheral lymphocytes in vitro micronucleus (MN) test were used as short-term genotoxicity tests. Dimethyl sulfoxide (DMSO) was used as solvent for test material that does not completely dissolve in water. Cells used for quantitative analysis were treated with test material at various concentrations and results were compared with their own controls. LD50 of the drug and their concentrations used for treatment were taken into consideration for selection of the test material concentration (Scheme 1).
Scheme 1.
Skeletal formula of deferasirox
Salmonella reversion test
The standard plate-incorporation assay was performed with S. typhimurium strains TA98 and TA100 in the presence and absence of S9 mix, in accordance with Maron and Ames (1983). A volume of 0.5 ml S9 mix containing 50 μL of S9 factor per Petri dish was used for the assay. For the test, DFX was dissolved in DMSO and amounts of 108, 216, 432 and 864 μg/petri dish were used. In parallel, 4-nitrophenylene diamine (4-NPD) (cat. no 10,889-8; Sigma-Aldrich, St. Louis; MO, USA), was used as a positive mutagen (100 μg/petri dish) for TA98 and sodium azide (SA) (cat. no S-2002; Sigma) (1 μg/petri dish) for TA100. In addition, 2-aminofluorene (2-AF) (cat. no A-9031; Sigma) was used as a positive mutagen (20 μg/petri) in the presence of S9 mix on both TA98 and TA100 test strains. Each sample was evaluated with five replicate plates.
Experimental animals
Four healthy Sprague-Dawley rats (2 male and 2 female, 12–16 weeks old) were used for each treatment group and the control group. The test animals were maintained under a 14:10-h light:dark photoperiod without twilight at room temperature (25 ± 2 °C) and fed by laboratory rodent diet.
In vivo RAPD test
Deferasirox (Exjade) in three different concentrations (250, 500 and 1,000 mg/kg) was administered to the rats via oral gavage (oral LD50 ≥ 1,000 mg/kg in rats). Each test had one control, one solvent control (DMSO) and one positive control (urethane). 12 or 24 h after deferasirox administration, rats were killed by cervical dislocation under anasthetic conditions. Then bone marrow was aspirated in warm (37 °C) isotonic solution (0.9 % NaCI) and then genomic DNA of cells was obtained by using precipitation method with phenol/chloroform and ethanol from cells in each concentration obtained from bone marrow samples. Concentrations of the isolated DNA samples were measured using Qubit 2.0 fluorometer device as ng/μl (Schweitzer and Scaiano 2003; McKnight et al. 2006).
The RAPD protocol was applied by modifying the protocols by Noel and Rath (2006). Selected primers consisting of 10 nucleotides were used for RAPD-PCR (polymerase chain reaction). Reactions were done at thermocycler (Techne TC 4000). Reaction products were stored at 4 °C before electrophoresis. Amplified DNA fragments were separated by electrophoresis procedure (at 85 V for 2 h and 30 min in 1.5 % agarose gel). At the end of this procedure, amplified RAPD profiles obtained with below primers were photographed with Vilber Lournat gel imaging system. Polymorphic band profiles were evaluated in order to explain the differences between RAPD profiles of groups treated with various doses of deferasirox. Differences between the band profiles of treatment groups compared with the band profile formed on the control were determined with the scoring method (decrease or increase in band number) and statistical analysis was done.
Data obtained with the scoring were plotted and evaluated with the t test in the Minitab® 15.1.1.0. statistical program. Statistical analysis is based on dividing total polymorphic band counts (N = 590 in this study) of each treatment group with bands obtained with all primers.
Selected primers for RAPD test
| Primer | Primer sequence (5′ → 3′) | G + C percent (%) | Bound temperature (°C) |
|---|---|---|---|
| 1. | Primer GGTGACGCAG | 70 | 34 |
| 2. | Primer GGGTAACGCC | 70 | 34 |
| 3. | Primer CCCGTCAGCA | 70 | 34 |
| 4. | Primer TCCGATGCTG | 60 | 32 |
| 5. | Primer CTGCGCTGGA | 70 | 34 |
| 6. | Primer GTTTCGCTCC | 60 | 32 |
| 7. | Primer GTAGACCCGT | 60 | 32 |
| 8. | Primer AAGAGCCCGT | 60 | 32 |
| 9. | Primer AACGCGCAAC | 60 | 32 |
| 10. | Primer CTCACCGTCC | 70 | 34 |
In vitro micronucleus test
Whether iron chelator deferasirox (Exjade) is genotoxic or not was investigated with the micronucleus test in human peripheral lymphocytes in vitro. For this purpose, human peripheral lymphocytes were treated with deferasirox at three different concentrations (10, 20 and 40 μg/ml) for 24 or 48 h. In vitro micronucleus test was conducted using peripheral blood of two volunteer men and two volunteer women (a total of four subjects) who were healthy, non-smokers, whose BMIs were within normal ranges (18.5–24.9) (Eknoyan 2008) and whose ages were close to each other (age of 23–24 years).
The method of Rotfuss et al. (1986) was modified for the in vitro micronucleus test. For this purpose, 0.2 ml of peripheral blood obtained from healthy subjects was inoculated onto 2.5 ml of chromosome medium (Pb-Max, Gibco, Invitrogen, Carlsbad, CA, USA) and incubated at 37 °C for 68 h. Dissolver control (DMSO) 10 μl/2.7 ml medium, positive control (mitomycin-C) 0.25 μg/ml and test material were added to culture medium 20 and 44 h after the beginning of the culture. 10 μg/ml cytochalasin was added to the culture 44 h after the beginning of the incubation.
At the end of incubation, culture tubes were centrifuged (2,000 rpm 5 min), supernatant was thrown away and hypotonic solution (0.4 % KCI) at 37 °C was added drop by drop to the cells remaining at the bottom of the tube and the cells were let to swell with incubation at 37 °C for 5 min. Cell culture was centrifuged after treatment with hypotonic solution (1,200 rpm 10 min), supernatant was thrown away and cold first fixative (1/5/6 = glacial acetic acid/methanol/ % 0.9 NaCI) was added and the cells were fixed at room temperature for 20 min. After the incubation, the culture tube was centrifuged, supernatant was thrown away and cells were treated with cold second fixative (1/5 = glacial acetic acid/methanol) at room temperature for 20 min. Treatment with the second fixative was repeated once more and preparations were prepared after fixation. Preparations were stained with 5 % Giemsa prepared in Sorensen buffer for 10 min and closed with Entellan®.
1,000 binuclear cells were examined to detect cells with micronucleus in preparations prepared from blood cultures of each group (belonging to each control and treatments). A total of 1000 intact cells were scored to determine the frequency of cells, with 1, 2, 3, or 4 nuclei, and to calculate the NDI (nuclear division index) for cytotoxicity of agents using the formula: NDI = ((1 x M1) + (2 x M2) + (3 x M3) + (4 x M4))/N; where M1–M4 represent the number of cells with one to four nuclei and N is the total number of intact cells scored (Fenech 2000).
Results
Salmonella reversion test
The potential of deferasirox to cause gene mutation was tested at four non-toxic concentration (108, 216, 432 and 864 μg/petri) in absence or presence of metabolic activator (S9 mix). In absence of metabolic activator, all DFX doses except the lowest dose significantly increased reversion mutations compared to dissolver control in the TA 98 strain. In presence of metabolic activator, both untreated control and solvent control led to an increase in the revertant colony number except for the lowest concentration. In the tests done in absence of metabolic activator in TA 100 strain, two low concentrations (108 and 216 μg/petri dish) have led to increase in the number of revertant colonies compared to control and solvent control. The number of colonies decreased compared to controls probably due to toxicity at two high concentrations. In the studies done in presence of metabolic activator, revertant colony number increased compared to control and dissolver control at all concentrations. However, increase of revertant colony number was found statistically significant at two concentrations (216 and 864 μg/petri) (Table 1).
Table 1.
Mutagenicity of deferasirox in Salmonella typhimurium TA98 and TA100 strains in absence or presence of S9
| Test subst. | Conc. (μg/petri dish) | TA98 | TA100 | ||
|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | ||
| Control | – | 33.50 ± 3.43 | 24.50 ± 2.01 | 217.0 ± 12.6 | 167.5 ± 9.27 |
| DMSO | 80 μl | 29.17 ± 2.73 | 34.17 ± 3.88 | 190.2 ± 16.0 | 177.17 ± 6.18 |
| 4-NPDa | 100 | 7,213 ± 725 | – | – | – |
| SAb | 1 | – | – | 1,143 ± 123 | – |
| 2-AFc | 20 | – | 808 ± 206 | – | 617.5 ± 78.4 |
| DFXd | 108 | 33.67 ± 1.87 | 36.17 ± 5.34 | 244.2 ± 11.6b2 | 190.0 ± 33.4 |
| DFX | 216 | 39.17 ± 2.8b1 | 54.50 ± 3.41a3b2 | 231.7 ± 11.3b1 | 241.0 ± 15.7a2b2 |
| DFX | 432 | 35.83 ± 2.18b1 | 46.67 ± 5.13a2 | 195.3 ± 26.8 | 183.2 ± 13.7 |
| DFX | 864 | 35.00 ± 0.89b2 | 55.33 ± 4.51a3b2 | 147.67 ± 7.68a3b2 | 230.7 ± 20.2a1b1 |
A total of six petri dishes were evaluated for the detection of revertant colonies
Significant difference with control (a), solvent control (b). a1b1: P < 0.05; a2b2: P < 0.01; a3b3: P < 0.001
a4-Nitrophenylene diamine
bSodium azide
c2-Aminoifluorene
dDeferasirox
RAPD test
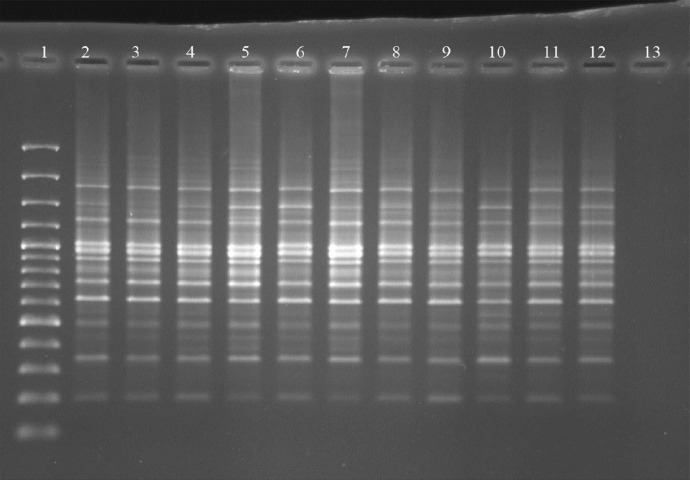
According to the obtained results, all untreated control values were accepted as zero in band scoring, all of the comparisons with this control were found significant. When band differences in these groups were evaluated, a very high band polymorphism was found compared to both dissolver control and positive control in rat bone marrow cells in all DFX doses applied for 12 h and the difference was statistically very significant (P < 0.001) (Fig. 1). However a significantly higher band polymorphism was detected compared to dissolver control and positive control only for the highest dose (1,000 mg/kg) in 24 h applications (Table 2).
Fig. 1.
RAPD-PCR gel image: The DNA of the female rats, RAPD-PCR was performed with Primer 8 (PM8). 1 DNA Marker (from top to bottom: 3,000, 2,000, 1,500, 1,200, 1,000, 900, 800, 700, 600, 500, 400, 300, 200 and 100 bp sized), 2 Control, 3 Solvent Control (DMSO) (12 h), 4 Positive Control (Urethane) (12 h), 5 1000 mg/kg Deferasirox (12 h), 6 500 mg/kg Deferasirox (12 h), 7 250 mg/kg Deferasirox (12), 8 Solvent Control (DMSO) (24 h), 9 Positive Control (Urethane) (24 h), 10 1000 mg/kg Deferasirox (24 h), 11 500 mg/kg Deferasirox (24 h), 12 250 mg/kg Deferasirox (24 h), 13 No DNA
Table 2.
Band polymorphism arising in rat bone marrow cells treated with different doses of deferasirox
| Treatment | Conc. | Time (h) | Average number of polymorphic bands ± SE |
|---|---|---|---|
| – | – | 0.000 ± 0.0000 | |
| DMSO | 80 μl | 12 | 0.0678 ± 0.0104 |
| Urethane | 400 mg/kg | 12 | 0.0661 ± 0.0102 |
| DFX | 250 mg/kg | 12 | 0.1390 ± 0.0143 a3b3 |
| DFX | 500 mg/kg | 12 | 0.1831 ± 0.0159 a3b3 |
| DFX | 1,000 mg/kg | 12 | 0.1559 ± 0.0149 a3b3 |
| DMSO | 80 μl | 24 | 0.1356 ± 0.0141 |
| Urethane | 400 mg/kg | 24 | 0.1102 ± 0.0129 |
| DFX | 250 mg/kg | 24 | 0.1288 ± 0.0138 |
| DFX | 500 mg/kg | 24 | 0.1356 ± 0.0141 |
| DFX | 1,000 mg/kg | 24 | 0.1881 ± 0.0161 a3b3 |
Significant difference with solvent control (a), positive control (b). a1b1: P < 0.05; a2b2: P < 0.01; a3b3: P < 0.001
Micronucleus test
MN fromation was found significantly high compared to controls in the culture treated with the highest dose (40 μg/ml) of DFX in the 48 h period (Table 3). In this test, the positive control MMC increased micronucleus formation very much in all applications as expected.
Table 3.
Micronuclear binuclear cell ‰ (MNBN) detected in human lymphocyte cultures treated with different concentrations of DFX for 24 and 48 h and controls
| Treatment | Conc. (μg/ml) | Treatment time (h) | ‰ MNBN cell frequency ± SE | NDI ± SE |
|---|---|---|---|---|
| Control | – | – | 2.50 ± 0.50 | 1.4483 ± 0.032 |
| DMSO | 10 μl/2.7 ml | 24 | 5.00 ± 0.91 | 1.4155 ± 0.038 |
| MMC | 0.25 | 24 | 23.50 ± 2.10 | 1.2575 ± 0.008 |
| DFX | 10 | 24 | 4.50 ± 0.86c3 | 1.4155 ± 0.045c1 |
| DFX | 20 | 24 | 3.75 ± 0.85c3 | 1.3918 ± 0.038c1 |
| DFX | 40 | 24 | 6.25 ± 1.65c2 | 1.069 ± 0.207 |
| DMSO | 10 μl/2.7 ml | 48 | 2.75 ± 0.75 | 1.4055 ± 0.043 |
| MMC | 0.25 | 48 | 71.00 ± 9.70 | 1.1249 ± 0.086 |
| DFX | 10 | 48 | 4.00 ± 0.70c3 | 1.3857 ± 0.073c1 |
| DFX | 20 | 48 | 4.00 ± 0.40a1c3 | 1.3098 ± 0.094a2b1c2 |
| DFX | 40 | 48 | 14.00 ± 2.12a1b1c3 | 1.0845 ± 0.062a2b1 |
Significant difference with control (a), solvent control (b) and positive control (c). a1b1c1: P < 0.05; a2b2c2: P < 0.01; a3b3c3: P < 0.001
The nuclear divison index decreased in the cultures treated with DFX. However this decline was found to be significant compared to control and dissolver control at the two highest concentrations (20 and 40 μg/ml) of the 48 h treatment. Both 24 h and 48 h DFX applications reduced the NDI value at the highest concentration (40 μg/ml) to the level of the positive control (Table 3).
Discussion
In vast majority of the cytogenetic studies done with some chelators, removal of cellular iron was stated to significantly contribute to genome integrity. Of them, deferoxamine (DFO) was reported to show antigenotoxic effect against hydroxyl radical-originated DNA breaks developing as the result of the Fenton/Haber–Weiss reaction though the removal of iron from the cell (Anderson et al. 2000; Coogan et al. 1986; Stinson et al. 1992; Beall et al. 1996; Zhang et al. 1996; Witte et al. 2000; Chakrabarti et al. 2001).
In our study, DFX shows a mutagen character in S. typhimurium strain. However, it should not be overlooked that the rat S9 liver fraction was used in Salmonella reversion test in this study. There are differences between rat S9 liver fraction and human S9 liver fraction in terms of mutagenic response to procarcinogens (Hakura et al. 2003). Nevertheless our results are remarkable in order to indicate the genotoxic potential of the test material. In addition, a significantly increased band polymorphism was seen in the in vivo RAPD test done with rat bone marrow cells. However dose–response relationship was not confirmed. Significant increases obtained as the result of the RAPD test support the genotoxic profile of the test material. Band polymorphism significantly increased in bone marrow cell DNA in the 12 h application of test material rather than 24 h application. This result may be related in the in vivo half life (8–16 h) of the agent. Besides, significant increases in the formation of micronuclei were detected in cultured human peripheral lymphocytes. This result is likely related to the clastogenic or aneugenic potential of the test substance. In parallel with these results, an old chelator DFO, showed a highly toxic and also mutagenic effect in L5178Y rat lymphoma cells independently from the presence or absence of S9 (Whittaker et al. 2001). DFO was detected not to be clastogenic alone but increased the frequency of acentric fragments and ring chromosome formations together with gamma rays (Juckett et al. 1998).
We have detected some important findings about deferasirox genotoxicity. This is probably due to a failure of the DNA repair system. The removal of iron from the cell by deferasirox may have caused defects in DNA repair system. Iron is the cofactor of ribonucleotide reductase (RNR) enzyme responsible for deoxyribonucleotide synthesis. This enzyme is activated in DNA damage or replication delay situations (Elledge and Davis 1989; Elledge et al. 1993; O’Donnell et al. 2010; Dyavaiah et al. 2011). Removal of cellular iron protects DNA against reactive oxygen attack. However, genotoxic effects may have as result the suppression of RNR enzymes responsible for DNA repair.
In our study, DFX showed a significant cytotoxic effect. This result is highly similar to that of previous studies. For example, in vitro iron salt application in cell cultures of kaposi sarcoma strongly stimulates the cell growth. Iron chelators like DFO, DFX and ciclopirox olamine are reported to block wnt signal and cell proliferation in colorectal cancer cell line (Song et al. 2011). When antiproliferative effects of iron chelators, DFX and O-trensox were compared in an human hepatocarcinoma cell line and human hepatocyte culture, Deferasirox was detected to be fairly effective in the induction of DNA fragmentation, inhibition of DNA replication and reduction of cell viability. These chelators were reported to block cell cycle in G0-G1 and S phases, respectively. According to these results, DFX has been suggested to have a very effective antitumoral effect in cancer treatment (Chantrel-Groussard et al. 2006). Similarly, advancement of cell cycle was reported to be dependent on intracellular iron level and chelators reduce cell proliferation by removing iron. This antiproliferative effect was reported to be inhibited in the presence of exogenous iron (Pires et al. 2006). As mentioned above, DFX inhibits ribonucleotide reductase enzyme by removing cellular iron and consequently leads to cytotoxicity by impairing DNA synthesis mechanisms (Zhang and Enns 2009). Supporting these data, excess iron load has been reported to cause hyperproliferation in some cell types and iron chelation was reported to show antiproliferative effects (Brown et al. 2006; Steegmann-Olmedillas 2011). Likewise, DFX affects the synthesis of molecules that play key roles in carcinogenesis like metastasis, cell cycle control, and apoptosis (Lui et al. 2013). In another study, DFO and DFX suppressed growth of oesophageal tumour through depleting of iron from the cells (Ford et al. 2013).
According to our results, we may state that the test material has shown an antiproliferative property related to iron chelation. This feature is similar to known effects of chemical agents used for cancer treatment. According to this approach, we consider that DFX or similar ones may lead to new horizons in tumor supression by optimizing their antiproliferative effect besides their main function.
Acknowledgments
This work was supported by the Basic Science Research and Support Group at the TUBITAK (Project no: 111T017).
References
- Anderson D, Yardley-Jones A, Vives-Bauza C, Chua-Anusorn W, Cole C, Webb J. Effect of iron salts, haemosiderins, and chelating agents on the lymphocytes of a thalassaemia patient without chelation therapy as measured in the comet assay. Teratog Carcinog Muagen. 2000;20:251–264. doi: 10.1002/1520-6866(2000)20:5<251::AID-TCM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Beall HD, Liu Y, Siegel D, Bolton EM, Gibson NW, Ross D. Role of NAD(P)H:quinone oxidoreductase (DT-diaphorase) in cytotoxicity and induction of DNA damage by streptonigrin. Biochem Pharmacol. 1996;51:645–652. doi: 10.1016/S0006-2952(95)00223-5. [DOI] [PubMed] [Google Scholar]
- Brown KE, Mathahs MM, Broadhurst KA, Weydert J. Chronic iron overload stimulates hepatocyte proliferation and cyclin D1 expression in rodent liver. Transl Res. 2006;148:55–62. doi: 10.1016/j.trsl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Bai C, Subramanian KS. DNA-protein crosslinks induced by nickel compounds in isolated rat lymphocytes: role of reactive oxygen species and specific amino acids. Toxicol Appl Pharmacol. 2001;170:153–165. doi: 10.1006/taap.2000.9097. [DOI] [PubMed] [Google Scholar]
- Chantrel-Groussard K, Gaboriau F, Pasdeloup N, Havouis R, Nick H, Pierre JL, Brissot P, Lescoat G. The new orally active iron chelator ICL670A exhibits a higher antiproliferative effect in human hepatocyte cultures than O-trensox. Eur J Pharmacol. 2006;541:129–137. doi: 10.1016/j.ejphar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Coogan TP, Rosenblum IY, Barsotti DA. Bleomycin-induced DNA-strand damage in isolated male germ cells. Mutat Res. 1986;162:215–218. doi: 10.1016/0027-5107(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Dyavaiah M, Rooney JP, Chittur SV, Lin Q, Begley TJ. Autophagy-dependent regulation of the DNA damage response protein ribonucleotide reductase 1. Mol Cancer Res. 2011;9:462–475. doi: 10.1158/1541-7786.MCR-10-0473. [DOI] [PubMed] [Google Scholar]
- Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- Elledge SJ, Davis RW. DNA damage induction of ribonucleotide reductase. Mol Cell Biol. 1989;9:4932–4940. doi: 10.1128/mcb.9.11.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge SJ, Zhou Z, Allen JB, Navas TA. DNA damage and cell cycle regulation of ribonucleotide reductase. BioEssays. 1993;15:333–339. doi: 10.1002/bies.950150507. [DOI] [PubMed] [Google Scholar]
- Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455:81–95 [DOI] [PubMed]
- Ford SJ, Obeidy P, Lovejoy DB, Bedford M, Nichols L, Chadwick C, Tucker O, Lui GY, Kalinowski DS, Jansson PJ, Iqbal TH, Alderson D, Richardson DR, Tselepis C. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br J Pharmacol. 2013;168:1316–1328. doi: 10.1111/bph.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakura A, Suzuki S, Sawada S, Sugihara T, Hori Y, Uchida K, Kerns WD, Sagami F, Motooka S, Satoh T. Use of human liver S9 in the Ames test: assay of three procarcinogens using human S9 derived from multiple donors. Regul Toxicol Pharmacol. 2003;37:20–27. doi: 10.1016/S0273-2300(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Juckett MB, Shadley JD, Zheng Y, Klein JP. Desferrioxamine enhances the effects of gamma radiation on clonogenic survival and the formation of chromosomal aberrations in endothelial cells. Radiat Res. 1998;149:330–337. doi: 10.2307/3579694. [DOI] [PubMed] [Google Scholar]
- Karakaş Z. Talasemi tedavisinde güncel yaklaşımlar ve yeni şelatörler. Turkiye Klinikleri J Pediatr Sci. 2009;5:15–27. [Google Scholar]
- Lui GY, Obeidy P, Ford SJ, Tselepis C, Sharp DM, Jansson PJ, Kalinowski DS, Kovacevic Z, Lovejoy DB, Richardson DR. The iron chelator, deferasirox, as a novel strategy for cancer treatment: oral activity against human lung tumor xenografts and molecular mechanism of action. Mol Pharmacol. 2013;83:179–190. doi: 10.1124/mol.112.081893. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised method for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Marshall R, Tricta F, Galanello R, Leoni G, Kirkland D, Minto S, Spino M. Chromosomal aberration frequencies in patients with thalassaemia major undergoing therapy with deferiprone and deferoxamine in a comparative crossover study. Mutagenesis. 2003;18:457–463. doi: 10.1093/mutage/geg014. [DOI] [PubMed] [Google Scholar]
- McKnight RE, Gleason AB, Keyes JA, Sahabi S. Binding mode and affinity studies of DNA-binding agents using topoisomerase I DNA unwinding assay. Bioorg Med Chem Lett. 2006;17:1013–1017. doi: 10.1016/j.bmcl.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Noel S, Rath SK. Randomly amplified polymorphic DNA as a tool for genotoxicity: an assessment. Toxicol Ind Health. 2006;22:267–275. doi: 10.1191/0748233706th267oa. [DOI] [PubMed] [Google Scholar]
- O’Donnell JP, Gehman M, Keeney JB (2010) Regulators of ribonucleotide reductase inhibit Ty1 mobility in Saccharomyces cerevisiae. Mob DNA 1:23. doi:10.1186/1759-8753-1-23 [DOI] [PMC free article] [PubMed]
- Pires VS, Gaboriau F, Guillon J, Nascimento S, Dassonville A, Lescoat G, Desplat V, Rochette J, Jarry C, Sonnet P. Modulation of cell proliferation in rat liver cell cultures by new calix[4]arenes. J Enzyme Inhib Med Chem. 2006;21:261–270. doi: 10.1080/14756360600700384. [DOI] [PubMed] [Google Scholar]
- Rotfuss A, Schütz P, Bochum S, Volm T, Eberhardt E, Kreirenberg R, Vogel W, Speit G. Induced micronucleus frequencies in peripheral lymphocytes as a screening test for carriers of a BRCA1 mutation in breast cancer families. Cancer Res. 1986;60:390–394. [PubMed] [Google Scholar]
- Schweitzer C, Scaiano JC. Selective binding and local photophysics of the fluorescent cyanine dye PicoGreen in double-stranded and single-stranded DNA. Phys Chem Chem Phys. 2003;5:4911–4917. doi: 10.1039/b305921a. [DOI] [Google Scholar]
- Song S, Christova T, Perusini S, Alizadeh S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, Joseph B, Subramaniam R, Aman A, Chau A, Hogge DE, Weir SJ, Kasper J, Schimmer AD, Al-awar R, Wrana JL, Attisano L. Wnt inhibitor screen reveals iron dependence of β-catenin signaling in cancers. Cancer Res. 2011;71:7628–7639. doi: 10.1158/0008-5472.CAN-11-2745. [DOI] [PubMed] [Google Scholar]
- Steegmann-Olmedillas JL. The role of iron in tumour cell proliferation. Clin Transl Oncol. 2011;13:71–76. doi: 10.1007/s12094-011-0621-1. [DOI] [PubMed] [Google Scholar]
- Stinson TJ, Jaw S, Jeffery EH, Plewa MJ. The relationship between nickel chloride-induced peroxidation and DNA strand breakage in rat liver. Toxicol Appl Pharmacol. 1992;117:98–103. doi: 10.1016/0041-008X(92)90222-E. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Seifried HE, San HRH, Clarke JJ, Dunkel VC. Genotoxicity of iron chelators in L5178Ymouse lymphoma cells. Environ Mol Mutagen. 2001;38:347–356. doi: 10.1002/em.10033. [DOI] [PubMed] [Google Scholar]
- Witte I, Zhu BZ, Lueken A, Magnani D, Stossberg H, Chevion M. Protection by desferrioxamine and other hydroxamic acids against tetrachlorohydroquinone-induced cyto-and genotoxicity in human fibroblasts. Free Radic Biol Med. 2000;28:693–700. doi: 10.1016/S0891-5849(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–715. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bandy B, Davison AJ. Effects of metals, ligands and antioxidants on the reaction of oxygen with 1,2,4-benzenetriol. Free Radic Biol Med. 1996;20:495–505. doi: 10.1016/0891-5849(95)02089-6. [DOI] [PubMed] [Google Scholar]