Abstract
In this study, the cytotoxic, genotoxic/antigenotoxic and antioxidant/oxidant activity of copaene (COP), a plant-derived tricyclic sesquiterpene, on human lymphocyte cultures (n = 5) was investigated. COP was added into culture tubes at various concentrations (0, 10, 25, 50, 100, 200 and 400 mg/L). While the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase (LDH) assays were used for viability and cytotoxic evaluations, the micronucleus (MN) and sister chromatid exchange (SCE) assays were used for genetic evaluations. Moreover, total antioxidant capacity (TAC) and total oxidative status analysis were used for biochemical evaluations. According to LDH and MTT assays COP significantly reduced cell proliferation at high concentrations (200 and 400 mg/L). In addition, there was no significant increase (P < 0.05) in both SCE and MN frequencies of cultures treated with COP as compared to controls. We have also concluded that concentrations of COP of 50 and 100 mg/L increased TAC level when compared to the controls. In conclusion, in this study it has been reported for the first time that copaene is not genotoxic and it increases the antioxidant capacity in human lymphocyte cultures.
Keywords: Copaene, Micronucleus, Sister chromatid exchange, Total antioxidant capacity, Total oxidative status
Introduction
Reactive oxygen species (ROS) such as (O·−2), hydrogen peroxide (H2O2), nitric oxide radical (NO·), hydroxyl radical (OH·) and singlet oxygen (1O2) come together with other factors are responsible for several pathologies like cancer, cardiovascular diseases, neural disorders, inflammation, and arteriosclerosis (Barlak et al. 2011; Mitjavila and Moreno 2012; López-Alarcón and Denicola 2013). Enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase, and glutathione-S-transferase, and nonenzymatic antioxidants such as reduced glutathione, vitamin E, and vitamin C and naturally occurring components such as fatty acids, lichen, plant extract and antibody prevent oxidative stress (Turkez and Geyikoglu 2010; Turkez et al. 2012a, b, c, d, e; Dirican et al. 2012; Ramesh et al. 2012).
Plant extracts (flavonoids, terpenoids, lignans, carotenoids, coumarins and saponins) from traditional herbs are a significant source of potential therapeutic compounds for many diseases (Parkin et al. 2001; Perestelo et al. 2010; Pehlivan Karakaş et al. 2012). They can act as direct antioxidants through free radical scavenging mechanisms or as indirect antioxidants possibly by enhancing the enzymatic and nonenzymatic antioxidants status (Ben Sghaier et al. 2011).
Terpenoids are compounds found in the essential oils of many plants that are known to have positive effects on human and animal health (Hamułka et al. 2012; Styrczewska et al. 2013). Sesquiterpenes, which are one of the most common terpenes, are a class of natural products with a diverse range of attractive industrial properties (Wang et al. 2011; Scalcinati et al. 2012). Several biological activities are attributed to sesquiterpenes, such as antimicrobial (Wu et al. 2012), antibacterial (Stojanović-Radić et al. 2012) antioxidant, antifungal (Al-maskri et al. 2011; Conforti et al. 2008) and antigenotoxic (Anter et al. 2011) activities. Copaene (COP) is a tricyclic sesquiterpenes derived from different plants; Cedrelopsis grevei leaves (Afoulous et al. 2013), Xylopia Laevigata (Quintans Jde et al. 2013), Annona reticulate (Chavan et al. 2011) and Ceratitis capitata (Nishida et al. 2000). To our best knowledge, its cytotoxic, genotoxic and antioxidant/oxidant effects on cultured human lymphocytes cells have not yet been explored.
This study is focus on the cytological and biochemical properties of COP. We aimed to assess the cytotoxic (by MTT and LDH assay), oxidative (by TAC and TOS levels) and genotoxic/antigenotoxic (by SCE and MN assay) effects of COP in cultured human lymphocytes cells for the first time.
Materials and methods
Human blood cell cultures
Human peripheral blood cultures were set up according to the slight modification of the protocol described by Evans and O’Riordan (1975). The heparinized blood samples was collected from five healthy women aged from 20 to 25 years old, respectively, non-smoking, non-alcoholic, not under drug therapy and with no recent history of exposure to mutagens. The heparinized blood (0.5 mL) was cultured in 6 mL of culture medium (Chromosome Medium B, Biochrom-Leonorenstr-2-6.D-12247-Berlin, Germany) with 5 mg/mL of phytohemagglutinin (Biochrom). Copaene (Cas: 3856-25-5, C15H24, Sigma-Aldrich®, Steinheim, Germany). COP was dissolved in ethanol (final concentration in culture, 0.1 %). COP at concentrations of 10, 25, 50, 100, 200 and 400 mg/L was added to the cultures just before incubation. Each individual lymphocyte culture without COP was studied as a control group. Ascorbic acid (10 μM, Sigma-Aldrich® Chem. Co. St. Louis, MO, USA) and hydrogen peroxide (25 μM, Sigma-Aldrich®) were also used as the positive controls in TAC and TOS analysis, respectively. Mitomycin C (10−7 M, Sigma-Aldrich®) was used as the positive control in SCE and MN assays.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The whole blood samples were seeded in 96-well plates. Cells were incubated at 37 °C in a humidified 5 % CO2/95 % air mixture and treated with COP at different concentrations for 24 h. Viability of cells was assessed by measuring the formation of a formazan from MTT spectrophotometrically (MTT cell proliferation kit; Cayman Chemical Company, Ann Arbor, MI, USA). Briefly, MTT was added to the cell cultures for 3 h. Formed formazan crystals were dissolved in dimethyl sulfoxide (Sigma-Aldrich®), and the plates were analyzed using Elisa reader (Sigma-Aldrich) at a wavelength of 570 nm.
Lactate dehydrogenase (LDH) assay
LDH assay was carried out by using the LDH-cytotoxicity assay kit (Cayman Chemical), according to the manufacturer’s protocol. In brief, 104–105 cells/well were seeded in 96-well plates and exposed to different concentrations of COP (0–400 mg/L) for 24 h. At the end of exposure, 96-well plate was centrifuged at 400g for 5 min to settle down the COP present in the solution. Then, 100 μL supernatant was transferred to a fresh well of 96-well plate that already contained 100 μL of reaction mixture from Bio-Vision kit and incubated for 30 min at room temperature. After incubation, the absorbance of solution was measured at 490 nm using a microplate reader (Synergy-HT, BioTek, Winooski, VT, USA). LDH levels in the media versus the cells were quantified and compared with the control values according to the instructions of the kit.
Sister chromatid exchange (SCE) assay
With the aim of providing successive visualization of SCEs, 5-bromo-20-deoxyuridine (Sigma, final concentration 20 mM) was added after culture initation. Lymphocyte cultures were incubated in complete darkness for 72 h at 37 °C. Exactly 70 h and 30 min after beginning the incubations, colcemid (Sigma-Aldrich®) was added to the cultures to achieve a final concentration of 0.5 μg/mL−1. After hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation and resuspension, the cell suspension was dropped onto chilled, grease-free microscopic slides, air-dried, aged, and then differentially stained for inspection of the SCE rate according to the fluorescence plus Giemsa (FPG) procedure (Perry and Wolff 1974). For each treatment condition, 25 well-spreaded second division metaphases were scored, and the obtained values were calculated as SCEs per cell. All slides were coded prior to scoring. SCE scoring was carried out by only one person (Togar B) using a light microscope (Prior Scientific, Rockland, MA, USA) at 100× magnification under oil immersion.
Micronuclei (MN) assay
Human lymphocytes were stimulated by COP and cultured for about 72 h; after 44 h of COP stimulation, cytochalasin B (Sigma-Aldrich®, final concentration of 6 μg/mL−1) was added. Cells were harvested by centrifugation and treated with a hypotonic solution (0.075 M KCl/37.4 °C). For the second time, the cells were then centrifuged and a solution (methanol + acetic acid) was added three times, and the resulting cells were resuspended and dropped onto clean slides. To prepare slides, 3–5 drops of the fixed cell suspension were dropped on a clean slide and air-dried. The slides were stained with Giemsa in phosphate buffer (pH 6.8) and scored. MN was scored in 1,000 binucleated cells and the frequency of cells with micronuclei was determined (Fenech 1993).
Total antioxidant capacity (TAC) and total oxidative status (TOS) analysis
The major advantage of this test is to measure the antioxidant capacity of all antioxidants in a biological sample and not just the antioxidant capacity of a single compound (Kusano and Ferrari 2008). Plasma samples, obtained from the lymphocyte cultures 2 h after incubation with COP, were analyzed using commercial kits (Rel Assay Diagnostics®, Gaziantep, Turkey) for automated Trolox-equivalent total antioxidant capacity (TAC) assay and the total oxidant status (TOS) assay. The major advantage of the TAC assay is that it measures the antioxidant capacity of all antioxidants in a biological sample and not just of a single compound (Kusano and Ferrari 2008). In this test, antioxidants in the sample reduce dark blue-green colored 2,2’-azinobis(3- ethylbenzothiazoline-6-sulfonate) (ABTS) radical to its colorless form. The change in absorbance at 660 nm corresponds to the total antioxidant level in a sample. The assay is calibrated with a stable antioxidant standard solution of vitamin E analog (Trolox-equivalent) (Erel 2005). The TOS assay used here is based on the oxidation of the ferrous ion-chelator complex to ferric ion (Fe3+), which is mediated by oxidants contained in the tested sample. The reaction is further enhanced by other molecules from the reaction medium. The reaction of Fe3+ with chromogen in an acidic medium produces a colored complex. Its intensity corresponds to the total amount of oxidants in the sample and can be measured spectrophotometrically. The TOS assay is calibrated with hydrogen peroxide and the results are expressed in terms of μM hydrogen peroxide equivalent per litre (Erel 2004).
Statistical analysis
Statistical analysis was performed using SPSS software (version 13.0, SPSS, Chicago, IL, USA). The statistical analysis of experimental values in the MTT, SCE, MN, TAC and TOS analysis was performed by Duncan’s test. Statistical decisions were made with a significance level of 0.05.
Results
We investigated the effect of COP at various concentrations on the proliferation of human lymphocytes cells using MTT assay in vitro (Table 1). The results of MTT analysis showed that COP significantly suppressed the proliferation of human lymphocytes cells, at higher concentrations than 100 mg/mL (200 and 400 mg/mL) compared to the control value. We also evaluated the cytotoxicity of COP on lymphocytes by measuring the amount of intracellular LDH release (Table 1). According to the LDH analyses, 200 and 400 mg/mL) concentrations of COP led to cell death via membrane damage (P < 0.05). In addition, MTT analyses demonstrated that COP significantly suppressed the cell proliferation rates in human lymphocytes when exposed to the compound at concentrations of 200 and 400 mg/mL compared to untreated culture (P < 0.05).
Table 1.
Cytotoxicity and cell viability in human lymphocytes cultures maintained 24 h in the presence of COP
| Concentrations (mg/L) | LDH assay (% cytotoxicity) | MTT assay (% cell viability) |
|---|---|---|
| Control | 12.8 ± 4.4a | 99.5 ± 8.3a |
| 10 | 14.5 ± 5.1b | 88.4 ± 8.1b |
| 25 | 14.1 ± 5.9b | 88.9 ± 8.6b |
| 50 | 17.6 ± 5.4c | 89.3 ± 7.1b |
| 100 | 17.3 ± 4.8c | 81.4 ± 7.7c |
| 200 | 36.4 ± 5.6d | 47.3 ± 5.3d |
| 400 | 41.8 ± 5.4d | 46.4 ± 4.8d |
Values are means ± SD; means in the same column followed by the different superscript letters present significant statistical differences at the level of 0.05
Figure 1 shows the SCEs/cell frequencies obtained from the cultures treated with different COP concentrations in comparison to the control group. The treatments with COP did not significantly (P > 0.05) alter the SCE rate results when compared with the untreated culture. On the contrary, Mitomycin C (10–7 M) caused considerable increases of SCE frequencies as compared to the control group. COP was not effective in increasing the SCE frequency compared with the control groups (P > 0.05). It was reported that COP does not affect the genetic material in lymphocytes at a wide range of concentrations in the SCE assay.
Fig. 1.
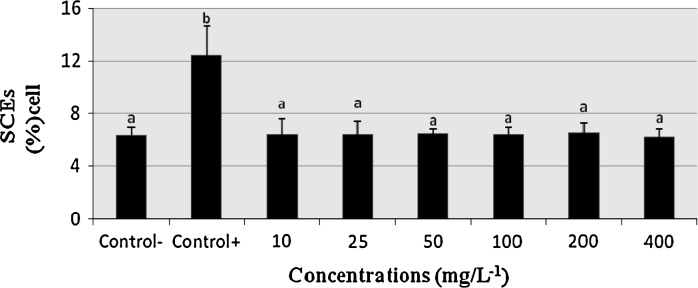
The frequencies of sister chromatit exchange (SCE) values in human lymphocyte treated with copaene (COP) in cultures for 72 h. SCE sister chromatid exchanges; Control− negative control; Control+ positive control; mitomycin C (10−7 M). The bars marked by different letter are different from each other at a level of 0.05
The effects of COP on the MN number in human lymphocyte cultures are shown in Fig. 2. Compared to the negative-control group, mitomycin C (10−7 M) caused considerable increases of MN frequencies, COP at all applied concentrations (10–400 mg/L) did not alter MN/1000 cell frequencies in human lymphocytes in vitro (P > 0.05).
Fig. 2.
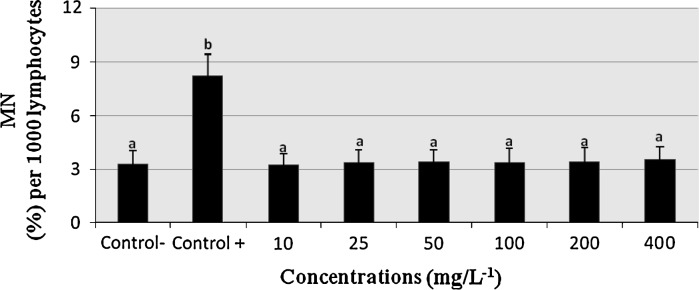
The percentages of micronucleus (%) in cultured human lymphocytes exposed to various concentrations of COP. MN Micronucleus; Control− negative control; Control+ positive control; (mitomycin C: 10−7 M). The bars marked by different letter are different from each other at a level of 0.05
As shown in Table 2, two concentrations of COP (50 and 100 mg/L−1) caused a significant increase of TAC levels in human lymphocytes compared with the controls. On the other hand, COP did not change the TOS levels in cultured lymphocytes at all concentrations.
Table 2.
Total antioxidant capacity (TAC) and total oxidative status (TOS) levels in cultured human lymphocytes incubated with COP and in the control cultures
| Concentrations(mg/L) | TAC (Trolox equiv./mmol L−1) | TOS (H2O2 equiv./μmol L−1) |
|---|---|---|
| Control− | 6.4 ± 0.3a | 11.2 ± 2.2a |
| Control+ | 12.5 ± 0.8d | 38.2 ± 4.2b |
| 10 | 7.6 ± 0.5b | 11.6 ± 2.8a |
| 25 | 7.8 ± 0.5b | 11.7 ± 2.5a |
| 50 | 9.4 ± 0.6c | 11.3 ± 2.4a |
| 100 | 9.1 ± 0.5c | 11.2 ± 2.6a |
| 200 | 7.4 ± 0.4b | 11.4 ± 2.5a |
| 400 | 6.1 ± 0.5a | 11.8 ± 3.1a |
Values are means ± SD; means in the same column followed by the different superscript letters present significant statistical differences at the level of 0.05
Discussion
In this study the cytotoxic, genotoxic/antigenotoxic and antioxidant/oxidant activity of COP in vitro was investigated using human lymphocyte cultures. According to the literature, in vitro cytological, genetical and biochemical effects of COP have still not been evaluated. For this reason, we have studied the cytological, genetical and biochemical effects of other sesquiterpenes. LDH and MTT assays were used to measure the effects of COP applications on cytotoxicity and cell viability in cultured human lymphocytes. Analysis of MTT results revealed that COP caused decreases of cell viability of human cells at concentrations higher than 100 mg/L. Likewise, LDH assay demonstrated that COP exhibited cytotoxic effects on human blood cells at 200 and 400 mg/L. Our results are in accordance with recent studies which have revealed that several sesquiterpenes such as britannin (in HepG-2, MCF-7, MDBK and A-549 cells), artesunate (in rat hepatic stellate cells), gossypol (in human retinoblastoma cells), dihydroartemisinin (in HepG2 human hepatoma cells), and deoxynivalenol (in human lymphocyte cells) exhibited antiproliferative effects in a concentration-dependent manner (Meky et al. 2001; Moghadam et al. 2012; Hsiao et al. 2012; Wang et al. 2012). In addition, Fiori et al. (2011) reported that guaiazulene inhibited cell growth in human gingival fibroblasts (under UV irradiation) in a concentration-dependent manner. Besides, Königs et al. (2008) studied the effect of deoxynivalenol in human primary hepatocytes and compared these data to the effects in the HepG2 cell line. They found that deoxynivalenol had a distinct cytotoxic effect on human primary hepatocytes. Our results reported that COP, as other sesquiterpenes, showed a concentration-dependent cytotoxic effect.
In this study, we have evaluated the genetic effect of COP on human lymphocyte cultures using SCE and MN assay. Our findings indicate that COP is neither genotoxic nor mutagenic on human lymphocytes since the observed mean values of the frequency of SCE and MN per cell was not found significantly different from the control values on both cells. In parallel to this finding beta-caryophyllene (a natural bicyclic sesquiterpene) at concentrations of up to 100 mg/L did not produce any cytotoxicity and genotoxicity, as shown by the frequency of micronuclei (MN) in cultured human lymphocytes (Di Sotto et al. 2010). Likewise, it was reported that zerumbone (a phytochemical sesquiterpene) from a type of edible ginger did not induce genotoxicity in cultured human peripheral blood lymphocytes (Al-Zubairi et al. 2010). Moreover, baccharin (a kind of sesquiterpene that was isolated from the aerial parts of Baccharis dracunculifolia) treatment led to significant increases of DNA damages (by comet test) in Chinese hamster lung V79 fibroblast cells, but this difference was not found in the MN assay (de Oliveira et al. 2012). On the other hand, recent studies reported that gossypol and ptaquiloside (a norsesquiterpene) were found to be genotoxic in human lymphocyte cells by using SCE assay (Best and McKenzie 1988; Gil da Costa et al. 2012). Likewise, nivalenol (a sesquiterpene mycotoxin) showed organ specific genotoxicity in cultured Chinese hamster ovary (CHO) cells and in several mouse organs and tissues like liver, kidney, thymus, bone marrow and mucosa of stomach, jejunum, and colon using single cell gel electrophoresis (SCGE) assay (Tsuda et al.1998). In addition, ptaquiloside increased DNA damage in human gastric epithelial cells as well as in a mouse model (Gomes et al. 2012). Furthermore, Aquino et al. (2011) reported that artesunate showed weak genotoxic effects at low doses and clastogenic effects at high doses in bone marrow cells from male Swiss mice.
In our study, the in vitro antioxidant/oxidant capacity of COP was determined by measuring TAC and TOS levels. Two concentrations of COP (50 and 100 mg/L) caused an increase in antioxidant capacity in cell cultures. The Copaiba oil (containing COP sesquiterpene) from Copaifera multijuga Hayne was the most potent, inhibiting both NO production and the pleurisy induced by zymosan (Veiga Junior et al. 2007). In addition, it was found that farnesol effectively suppress 1,2-dimethylhydrazine (DMH) induced colonic mucosal damage by ameliorating oxidative stress, inflammatory and apoptotic responses (Khan and Sultana 2011). Besides, atractylon showed antioxidant activity in 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) radical scavenging assay (Hwang et al. 1996). In another study, Haraguchi et al. (1997) demonstrated that 7-hydroxy-3,4-dihydrocadalin and 7-hydroxycadalin, and flavonoids, quercetin, kaempferol sesquiterpenes protected mitochondrial enzyme activity against oxidative stress. Our findings are in accordance with previous reports on antioxidant features of other sesquiterpenes, since our TAC analysis indicated that treatments with COP also supported the antioxidant capacity of the cells in vitro. Also, all concentrations of COP did not cause an increase in oxidative stress in human blood cell cultures.
As a result this study revealed that COP in the culture of human is not genotoxic and also increased the capacity of the antioxidant for the first time.
Acknowledgments
The authors are grateful to all volunteers for the blood samples.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem Toxicol. 2013;56:352–362. doi: 10.1016/j.fct.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Al-maskri AY, Hanif MA, Al-Maskari MY, Abraham AS, Al-sabahi JN, Al-Mantheri O. Essential oil from Ocimum basilicum (Omani Basil): a desert Crop. Nat Prod Commun. 2011;6:1487–1490. [PubMed] [Google Scholar]
- Al-Zubairi AS, Abdul AB, Syam MM. Evaluation of the genotoxicity of zerumbone in cultured human peripheral blood lymphocytes. Toxicol In Vitro. 2010;24:707–712. doi: 10.1016/j.tiv.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Anter J, Romero-Jiménez M, Fernández-Bedmar Z, Villatoro-Pulido M, Analla M, Alonso-Moraga A, Muñoz-Serrano A. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J Med Food. 2011;14:276–283. doi: 10.1089/jmf.2010.0139. [DOI] [PubMed] [Google Scholar]
- Aquino I, Perazzo FF, Maistro EL. Genotoxicity assessment of the antimalarial compound artesunate in somatic cells of mice. Food Chem Toxicol. 2011;49:1335–1339. doi: 10.1016/j.fct.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Barlak Y, Değer O, Colak M, Karatayli SC, Bozdayi AM, Yücesan F. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Sci. 2011;9:74. doi: 10.1186/1477-5956-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sghaier M, Skandrani I, Nasr N, Franca MG, Chekir-Ghedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: a structure-activity relationship study. Environ Toxicol Pharmacol. 2011;32:336–348. doi: 10.1016/j.etap.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Best RG, McKenzie WH. Variable Sister-chromatid exchange response in human lymphocytes exposed in vitro to gossypol acetic acid. Mutat Res. 1988;206:227–233. doi: 10.1016/0165-1218(88)90165-6. [DOI] [PubMed] [Google Scholar]
- Chavan MJ, Wakte PS, Shinde DB (2012) Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat Prod Res 26:1515–1518 [DOI] [PubMed]
- Conforti F, Menichini F, Loizzo MR, Statti AG, Rapisarda A, Menichini F, Houghton PJ. Antioxidant, alpha-amylase inhibitory and brine-shrimp toxicity studies on Centaurea centaurium L. methanolic root extract. Nat Prod Res. 2008;22:1457–1466. doi: 10.1080/14786410802098071. [DOI] [PubMed] [Google Scholar]
- de Oliveira PF, Leandro LF, Montanheiro G, Bastos JK, da Silva Filho AA, Tavares DC. Baccharin prevents genotoxic effects induced by methyl methanesulfonate and hydrogen peroxide in V79 cells. J Food Sci. 2012;77:T138–T142. doi: 10.1111/j.1750-3841.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- Di Sotto A, Mazzanti G, Carbone F, Hrelia P, Maffei F. Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat Res. 2010;699:23–28. doi: 10.1016/j.mrgentox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dirican E, Turkez H, Toğar B (2012) Modulatory effects of Thymbra spicata L. different extracts against the mercury induced genotoxicity in human lymphocytes in vitro. Cytotechnology 64:181–186 [DOI] [PMC free article] [PubMed]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–2785. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Fiori J, Teti G, Gotti R, Mazzotti G, Falconi M. Cytotoxic activity of guaiazulene on gingival fibroblasts and the influence of light exposure on guaiazulene-induced cell death. Toxicol In Vitro. 2011;25:64–72. doi: 10.1016/j.tiv.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Gil da Costa RM, Oliveira PA, Bastos MM, Lopes CC, Lopes C. Ptaquiloside-induced early-stage urothelial lesions show increased cell proliferation and intact β-catenin and e-cadherin expression. Environ Toxicol. 2012 doi: 10.1002/tox.21803. [DOI] [PubMed] [Google Scholar]
- Gomes J, Magalhães A, Michel V, Amado IF, Aranha P, Ovesen RG, Hansen HC, Gärtner F, Reis CA, Touati E. Pteridium aquilinum and its ptaquiloside toxin induce DNA damage response in gastric epithelial cells, a link with gastric carcinogenesis. Toxicol Sci. 2012;126:60–71. doi: 10.1093/toxsci/kfr329. [DOI] [PubMed] [Google Scholar]
- Hamułka J, Wawrzyniak A, Sulich A. The assessment of beta-carotene, lycopene and lutein intake selected group of adults. Rocz Panstw Zakl Hig. 2012;63:179–185. [PubMed] [Google Scholar]
- Haraguchi H, Ishikawa H, Sanchez Y, Ogura T, Kubo Y, Kubo I. Antioxidative constituents in Heterotheca inuloides. Bioorg Med Chem. 1997;5:865–871. doi: 10.1016/S0968-0896(97)00029-1. [DOI] [PubMed] [Google Scholar]
- Hsiao WT, Tsai MD, Jow GM, Tien LT, Lee YJ. Involvement of Smac, p53, and caspase pathways in induction of apoptosis by gossypol in human retinoblastoma cells. Mol Vis. 2012;18:2033–2042. [PMC free article] [PubMed] [Google Scholar]
- Hwang JM, Tseng TH, Hsieh YS, Chou FP, Wang CJ, Chu CY. Inhibitory effect of atractylon on tert-butyl hydroperoxide induced DNA damage and hepatic toxicity in rat hepatocytes. Arch Toxicol. 1996;70:640–644. doi: 10.1007/s002040050323. [DOI] [PubMed] [Google Scholar]
- Khan R, Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem Biol Interact. 2011;192:193–200. doi: 10.1016/j.cbi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Königs M, Schwerdt G, Gekle M, Humpf HU. Effects of the mycotoxin deoxynivalenol on human primary hepatocytes. Mol Nutr Food Res. 2008;52:830–839. doi: 10.1002/mnfr.200700439. [DOI] [PubMed] [Google Scholar]
- Kusano C, Ferrari B. Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J Cell Mol Biol. 2008;7:1–15. [Google Scholar]
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal Chim Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Meky FA, Hardie LJ, Evans SW, Wild CP. Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem Toxicol. 2001;39:827–836. doi: 10.1016/S0278-6915(01)00029-1. [DOI] [PubMed] [Google Scholar]
- Mitjavila MT, Moreno JJ. The effects of polyphenols on oxidative stress and the arachidonic acid cascade: implications for the prevention/treatment of high prevalence diseases. Biochem Pharmacol. 2012;84:1113–1122. doi: 10.1016/j.bcp.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Moghadam MH, Hajimehdipoor H, Saeidnia S, Atoofi A, Shahrestani R, Read RW, Mosaddegh M. Anti-proliferative activity and apoptotic potential of britannin, a sesquiterpene lactone from Inula aucheriana. Nat Prod Commun. 2012;7:979. [PubMed] [Google Scholar]
- Nishida R, Shelly TE, Whittier TS, Kaneshiro KY. α-Copaene, a potential rendezvous cue for the mediterranean fruit fly, Ceratitis capitata? J Chem Ecol. 2000;26:87–100. doi: 10.1023/A:1005489411397. [DOI] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Pehlivan Karakaş F, Yildirim A, Türker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk J Biol. 2012;36:641–665. [Google Scholar]
- Perestelo NR, Jiménez IA, Tokuda H, Hayashi H, Bazzocchi IL. Sesquiterpenes from Maytenus jelskii as potential cancer chemopreventive agents. J Nat Prod. 2010;73:127–132. doi: 10.1021/np900476a. [DOI] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Quintans Jde S, Soares BM, Ferraz RP, Oliveira AC, da Silva TB, Menezes LR, Sampaio MF, Prata AP, Moraes MO, Pessoa C, Antoniolli AR, Costa EV, Bezerra DP. Chemical constituents and anticancer effects of the essential oil from leaves of Xylopia laevigata. Planta Med. 2013;79:123–130. doi: 10.1055/s-0032-1328091. [DOI] [PubMed] [Google Scholar]
- Ramesh T, Kim SW, Hwang SY, Sohn SH, Yoo SK, Kim SK. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Scalcinati G, Partow S, Siewers V, Schalk M, Daviet L, Nielsen J. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb Cell Fact. 2012;11:117. doi: 10.1186/1475-2859-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanović-Radić Z, Lj Comić, Radulović N, Blagojević P, Denić M, Miltojević A, Rajković J, Mihajilov-Krstev T. Antistaphylococcal activity of Inula helenium L. root essential oil: eudesmane sesquiterpene lactones induce cell membrane damage. Eur J Clin Microbiol Infect Dis. 2012;6:1015–1025. doi: 10.1007/s10096-011-1400-1. [DOI] [PubMed] [Google Scholar]
- Styrczewska M, Kulma A, Kostyn K, Hasiewicz-Derkacz K, Szopa J. Flax terpenoid pathway as a source of health promoting compounds. Mini Rev Med Chem. 2013;13:353–364. [PubMed] [Google Scholar]
- Tsuda S, Kosaka Y, Murakami M, Matsuo H, Matsusaka N, Taniguchi K, Sasaki YF. Detection of nivalenol genotoxicity in cultured cells and multiple mouse organs by the alkaline single-cell gel electrophoresis assay. Mutat Res. 1998;415:191–200. doi: 10.1016/S1383-5718(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b(1) toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Aydin E, Aslan A. Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology. 2012;64:679–686. doi: 10.1007/s10616-012-9447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoğlu F, Dirican E, Tatar A. In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology. 2012;64:607–612. doi: 10.1007/s10616-012-9454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO. Ameliorative effect of supplementation with l-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte cultures. Cytotechnology. 2012;64:687–699. doi: 10.1007/s10616-012-9449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Togar B, Polat E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology. 2012;64:459–464. doi: 10.1007/s10616-011-9424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga Junior VF, Rosas EC, Carvalho MV, Henriques MG, Pinto AC. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne–a comparative study. J Ethnopharmacol. 2007;112:248–254. doi: 10.1016/j.jep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Wang C, Yoon SH, Jang HJ, Chung YR, Kim JY, Choi ES, Kim SW. Metabolic engineering of Escherichia coli for α-farnesene production. Metab Eng. 2011;13:648–655. doi: 10.1016/j.ymben.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang BW, Peng LX. Impact of artesunate on the expression and secretion of transforming growth factor-b1 of primary rat hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi. 2012;20:294–299. doi: 10.3760/cma.j.issn.1007-3418.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Wu YX, Chen YJ, Liu CM, Gao K. Four new sesquiterpenoids from Ligularia cymbulifera. J Asian Nat Prod Res. 2012;14:1130–1136. doi: 10.1080/10286020.2012.733002. [DOI] [PubMed] [Google Scholar]


