Summary
Many ovarian cancer cells express stress-related molecule MICA/B on their surface that is recognized by Vγ2Vδ2 T cells through their NKG2D receptor, which is transmitted to downstream stress-signaling pathway. However, it is yet to be established how Vγ2Vδ2 T cells-mediated recognition of MICA/B signal is transmitted to downstream stress-related molecules. Identifying targeted molecules would be critical to develop a better therapy for ovarian cancer cells. It is well established that ATM/ATR signal transduction pathways, which is modulated by DNA damage, replication stress, and oxidative stress play central role in stress signaling pathway regulating cell cycle checkpoint and apoptosis. We investigated whether ATM/ATR and its down stream molecules affect Vγ2Vδ2 T cells-mediated cytotoxicity. Herein, we show that ATM/ATR pathway is modulated in ovarian cancer cells in presence of Vγ2Vδ2 T cells. Furthermore, downregulation of ATM pathway resulted downregulation of MICA, and reduced Vγ2Vδ2 T cells-mediated cytotoxicity. Alternately, stimulating ATM pathway enhanced expression of MICA, and sensitized ovarian cancer cells for cytotoxic lysis by Vγ2Vδ2 T cells. We further show that combining currently approved chemotherapeutic drugs, which induced ATM signal transduction, along with Vγ2Vδ2 T cells enhanced cytotoxicity of resistant ovarian cancer cells. These findings indicate that ATM/ATR pathway plays an important role in tumor recognition, and drugs promoting ATM signaling pathway might be considered as a combination therapy together with Vγ2Vδ2 T cells for effectively treating resistant ovarian cancer cells.
Keywords: Vγ2Vδ2 T cells, ovarian cancer, cytotoxicity, ATM/ATR pathway, MICA, combination therapy
Introduction
Human innate immune system provides first line of defense against multiple viral or bacterial attacks, and provides critical surveillance against oncogenic development. Evidences show that individuals with primary immunodeficiency or induced immunosuppression during organ or cell transplantation showed higher risk for tumor development [1]. Alternately, impaired function of innate immune cells such as natural killer (NK) cells, subset of αβ T cells, and γδ T cells lead to increased susceptibility of the host for tumor growth [2, 3]. Among these innate immune cells, γδ T cells (Vγ2Vδ2 subset) are of particular interest due to their dual role in the immune system for bridging the gap between the innate and adaptive immunity, and have been demonstrated critical anti-tumor activities [4–6]. Due to the robust metabolic activities in tumor cells, specific antigens such as MHC class-I chain-related molecules MICA/B were typically found to be highly expressed in most tumor cells compared to normal healthy cells [7]. These antigens were specifically recognized by the Vγ2Vδ2 T cells and were proposed to enhance the cytotoxic activity of Vγ2Vδ2 T cells towards variety of tumor cells [8–11]. The activating receptors, NKG2D, serves as one of the most important receptors present in Vγ2Vδ2 T cells and mediate the recognition process to eliminate the tumor cells. This NKG2D molecule not only interacts to the receptor MICA/B, but also interacts with molecules such as ULBPs [12].
Emerging T cell-based adaptive immunotherapy is under consideration for various cancers as a therapeutic regimen [13]. In the adoptive therapy, T cells are activated, expanded ex vivo and reinjected into the patients with tumors [14, 15]. Adoptive T-cell therapy in renal cancer patients showed no adverse events, and 3 of 5 patients showed slower tumor progression. Patients documented positive response showed an increased number of Vγ2Vδ2 T cells in the peripheral blood and a strong in vitro response to phosphoantigen stimulation [14]. Various trials show promise for development of autologous Vγ2Vδ2 T cell therapies in eligible patients. However, for ovarian cancer, there is currently no effective immunotherapy. Interestingly, chemotherapeutic agents were shown to induce immunogenic tumor cell death, which is crucial for tumor eradication and long-term protection against relapse. Moreover, Vγ2Vδ2 T cells were recruited to the tumor bed after immunogenic chemotherapy and appear to be contributors to the efficacy of chemotherapy [16]. So, developing a combination therapy using chemotherapeutic reagent and Vγ2Vδ2 T cells will be a valuable option to be tested.
The Vγ2Vδ2 T cells induce cytotoxicity in many ovarian tumor cells via induction of apoptosis [17]. However, some of the ovarian tumor cells evade the apoptosis process and became resistant towards Vγ2Vδ2 T cells-mediated cytotoxicity. These resistant cell lines (such as A2780) showed slower proliferation compared to the sensitive cell line (such as OV4); interestingly, we found that the resistant cell line has reduced expression of MICA [17]. We proposed that the tumor cells may evade the Vγ2Vδ2 T cells cytotoxicity by down-regulating their MICA expression and at the same time enter into a dormancy stage, in which their proliferation were slowed down. In the current study, we further investigated the molecular mechanisms involved in the immune escape process. It has been shown that genotoxic stress or inhibitors of DNA-replication could up-regulate the expression of NKG2D ligand through activation of ATM (ataxia telangiectasia mutated) and ATR (ATM- and Rad3-related) protein kinase pathway in human fibroblast and in mouse tumor cell lines, which led to enhance cytotoxic lysis by NK cells [18, 19]. ATM and ATR are activated in response to DNA damage, oxidative stress, and replication stress resulting in apoptosis or cell cycle arrest. After activation ATM phosphorylates Chk2, and ATR phosphorylates Chk1 to start a cascade of downstream signaling events [20]. Activated Chk1 and Chk2 phosphorylate Cdc25 phosphatases, to inhibit their function, and the cells delay progression though the cell cycle [20]. After activation ATR and ATM also phosphorylates H2A variant H2AX at Ser-139 (γH2AX) at the damage sites, or where chromosomes are fragmented by oxidative stress [21]. The γH2AX has been used as a marker for DNA damage, oxidative stress, and replication stress. It was also shown that inhibition of ATM pathway by using synthetic inhibitor such as KU-55933 suppressed cell proliferation and induced apoptosis [22]. In this study, we examined whether the ATM and ATR protein kinases play a role in Vγ2Vδ2 T cells-mediated recognition of ovarian cancer cells. We found that treatment of ovarian cancer cells with Vγ2Vδ2 T cells results in down regulation of ATR and ATM signal transduction in resistant cells, but remain unchanged in sensitive cells. When we treated the cells with Vγ2Vδ2 T cells along with drugs activating ATM pathway, it resulted a significant increase in cytotoxicity of tumor cells. Thus, ATM-Chk2 signal transduction plays a critical role in regulating tumor survival in ovarian cancer upon Vγ2Vδ2 T cell treatment.
Materials and methods
Derivation of Vγ2Vδ2 T cells
Human peripheral blood was collected (30 ml) from adult healthy donors after obtaining the IRB approval from the Ohio State University Medical Center and obtaining written consents from donors. The ethic committee has also approved the procedure and records are saved in the laboratory. Freshly collected blood was processed to isolate peripheral blood mononuclear cells (PBMC) following the similar protocol published earlier [10, 11]. In brief, the peripheral blood was diluted twice with phosphate buffer saline (PBS, pH 7.4) and carefully layered over 10 ml of Ficoll-Paque Plus solution (GE Healthcare, Uppsala, Sweden). After 30 min of centrifugation in a swinging bucket rotor at 1400 rpm at room temp (24°C);the upper layer was aspirated out and the mononuclear cell layer (buffy coat) was collected. Buffy coat was washed three times with PBS to remove platelets. One million of PBMC in each well was stimulated with 10 µM risedronate in a 24-well plate using 1 ml RPMI 1640 supplemented with 10% fetal bovine serum (FBS, HyClone Lab Inc, Logan, UT), 2 mM glutamine, 1 nM β-mercapto ethanol, 1 nM HEPES, and 100 IU of penicillin and streptomycin at 37°C incubator. Recombinant IL-2, 0.5 nM (PeproTech Inc. Rocky Hill, NJ) was added to the culture on days 3 and 7. Cells were split after day 10 using the complete RPMI 1640 media supplemented with 0.5 nM rIL-2. Flowcytometric (FACS) analysis was performed (using a FACS Calibur machine, BD Biosciences, CA) at day 14, to evaluate phenotype of the expanded cell. FACS analysis data revealed that 99.8% of the expanded cells were CD3+, and 89.5% of them were Vδ2+. Cells were used between 15–19 days of initial culture for further experiments discussed below.
Ovarian cancer cells and chemicals
The ovarian cancer cells (A2780 and OV4; purchased from ATCC, VA, and used within six months of receipt) were cultured in 10 cm or 6 cm culture dishes in DMEM media supplemented with 10% FBS and antibiotics. Chemicals/drugs were used in this study includes H4073 (5 µM, kind gift from Prof. Periannan Kuppusamy, Columbus, OH), Etoposide (500 µM, MP Biomedical LLC, Santa Ana, CA), KU55933 (10 µM, Selleck Chemicals, Houston, TX), Caffeine (5 mM, kind gift from Prof. Jeff Parvin, Columbus, OH), Neocarzinostatin, NCS (500 ng/ml, Cat# N9162, Sigma, St. Louis, MO, USA).
Protein analyses
Total protein analysis was performed using standard western blot (WB) technology. Half a million of tumor cells (A2780 or OV4) were pre-seeded in 6 cm Petri dish for 10 h before adding Vγ2Vδ2 T cells. Control plates were also plated at the same time to maintain equal cell numbers. We did not observe any significant change on cell numbers after 10 h of pre-seeding. Fresh medium (RPMI for A2780 and DMEM for OV4) containing 10% FBS were replaced to the culture plates. Two and half million Vγ2Vδ2 T cells were added to the tumor cells to make a ratio of C:T = 1:5 (where ever applicable). Protein was isolated after 4 and 24 h of addition of Vγ2Vδ2 T cells. Before isolation of total protein, Vγ2Vδ2 T cells were gently removed by washing with 1× PBS. Forty to sixty microgram of total protein from each sample was used for a single WB analysis. Various antibodies have been used for WB, such as ATM, phosphorylated (p) ATM, ATR, pATR, pCHK1, pCHK2, H2AX, γH2AX to detect their level of expressions on tumor cells at various time points of co-culture with Vγ2Vδ2 T cells.
ATM silencing
ATM shRNA was obtained from Sigma Aldrich Inc (ATM shRNA plasmid from bacterial glycerol stock TRCN0000039949 (defined as #1) and TRCN0000039950 (defined as #2) and a irrelevant negative control shRNA plasmid). Transfection with various RNAs in A2780 cells were conducted using Lipofectamine™ 2000 transfection reagents (Invitrogen) according to the manufacturer’s instruction and earliser published methods [23].
Cytotoxicity assays
The cytotoxicity assay was performed using the LIVE/DEAD® Cell-Mediated Cytotoxicity Kit (Invitrogen Inc) and repeated by 3–6 times for each condition. Protocol was followed as suggested with little modification. Five hundred thousand of ovarian cancer cells (A2780) were pre-seeded on a 6 cm dish for 8 h. Cells were then stained with DiOC18 (7.5 µM) for 3 h. After staining, DiOC18 was washed by three times with 1xPBS, then, 1:10 ratio Vγ2Vδ2 T cells were added in combination of various treatment molecules including H4073, Etoposide, KU55933, Caffeine, and NCS. After 24 h, floating cells were collected and adhesion cells were collected by adding non-enzymatic cell dissociation buffer and mixed with corresponding floating cells. Collected cells were then stained with Propidium Iodide (PI 37.5 µM). After adding PI, cells were centrifuged for 5 min at 1500 r/min. Then cells were incubated at 37°C for 30 min. After staining with PI, the cells were evaluated immediately by flowcytometry for the cytotoxicity effect of Vγ2Vδ2 T cells. Upper-gated cells are PI stained dead cancer cells and lower gated cells are PI stain negative live cancer cells that are DiOC18 positive.
MitoSox™Red staining
MitoSOX™ Red mitochondrial superoxide indicator was purchased from Invitrogen. The concentrations were maintained as 0.19 mg/ml to stain 500K cells. In brief, 500K A2780 tumor cells were pre-seeded in 6 cm petri-dish for 8 h in complete RPMI medium. After pre-seeding, medium was replaced with fresh complete RPMI. Tumor cells were co-cultured with Vγ2Vδ2 T cells at 1:10 ratio under different treatment conditions as indicated before including H4073, Etoposide, KU55933, Caffeine, and NCS. After 24 h cells were collected after washing with medium. Very few adherent cancer cells were washed away when we removed non-adherent Vγ2Vδ2 T cells from co-culture. After all cells were collected, staining reagent was added using concentration indicated before. After 10 min of staining, cells were washed one time with 1XPBS and processed immediately for flowcytometric analysis. Adherent cells were collected after adding non-enzymatic cell dissociation buffer (Sigma) and collected after spin down with PBS.
Flowcytometry
A2780 tumor cells were pre-seeded in 6 cm Petri dish for 8 h in complete RPMI medium. After pre-seeding, medium was replaced with fresh complete medium. Tumor cells were co-cultured with Vγ2Vδ2 T cells at 1:10 ratio under various treatment conditions as indicated before including H4073, Etoposide, KU55933, Caffeine, and NCS. After 24 h, all cells were collected after spinning down with existing medium. Adherent cells were collected after adding non-enzymatic cell dissociation buffer (Sigma) and collected after spin down with PBS. PE-conjugated MICA/B and PE-conjugated IgG antibodies were added to the different samples. After 45 min incubation in ice, cells were washed once with PBS and processed for flowcytometry.
Immunofluorescence staining
Immunofluorescence staining was performed to analyze protein expressions after the treatment with various relevant chemicals and drugs in presence or absence of Vγ2Vδ2 T cells. In brief, half a million of A2780 cancer cells (C) were pre-seeded in 6 cm Petri dish 10 h before adding Vγ2Vδ2 T (T) cells. Fresh RPMI medium containing 10% FBS were replaced to the culture plates. Vγ2Vδ2 T cells were added to the tumor cells to make a ratio of C:T = 1:10. Optimum concentration of chemical/drugs was added such as H4073, Etoposide, KU55933, Caffeine or NCS before adding Vγ2Vδ2 T cells. Cells were stained after 24 h of co-culture. Briefly, cells were washed with 1XPBS for three times gently. Cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% triton-X-100 for 30 min. After blocking, cells were stained by various antibodies such as ATM, pATM, ATR, pATR, CHK1, pCHK1, CHK2, pCHK2, H2AX or γH2AX to detect their level of expression on tumor cells.
Results
Differential expression of ATM/ATR signaling pathway molecules in ovarian cancer cells upon Vγ2Vδ2 T cells induction
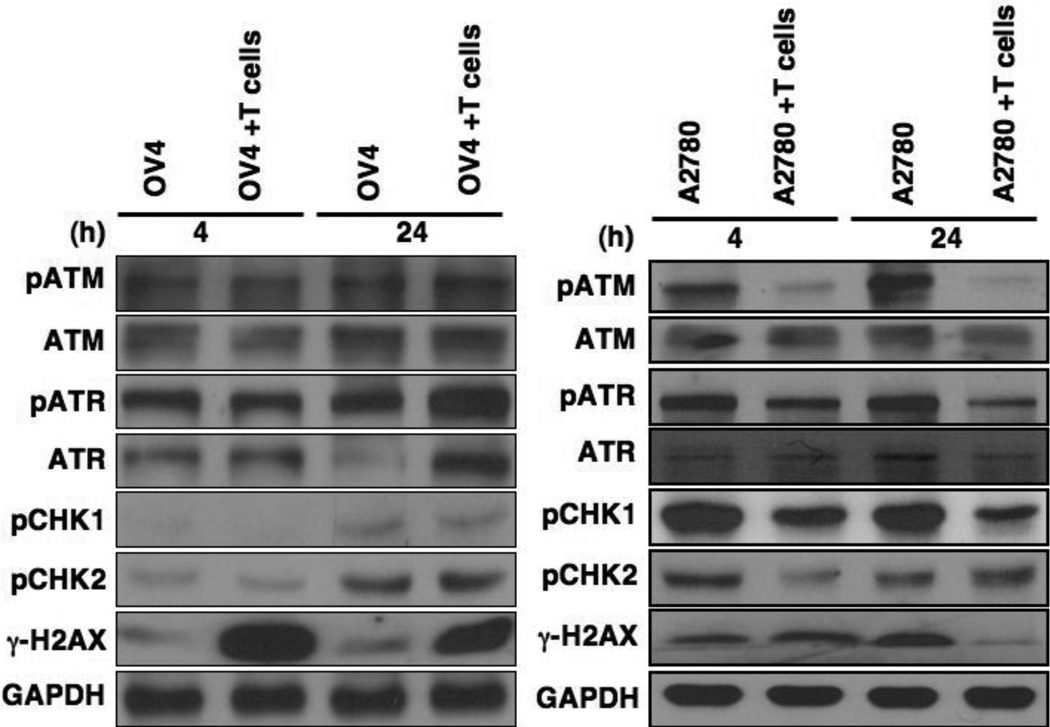
Many epithelial cancer cells express stress-related molecules such as MICA/B on their surface and Vγ2Vδ2 T cells recognize those molecules through NKG2D receptor molecules expressed on them. However, it is yet to be investigated how Vγ2Vδ2 T cells-mediated recognition of MICA/B signal is transmitted to downstream stress-related molecules, and the molecular mechanism of this signal transduction pathway. Exploring this pathway would be critical to develop better therapies for ovarian cancer cells. Upon DNA damage, replication stress, and oxidative stress, ATM/ATR signal transduction pathway plays a central role in regulating cell cycle checkpoint and apoptosis [20]. To identify the stress-related downstream signaling pathways in response to Vγ2Vδ2 T cells, we have used OV4 (sensitive), and A2780 (resistant) ovarian cancer cell lines [17]. Downstream factors of ATM/ATR pathways and their activation by phosphorylation were analyzed using Western blot (Fig. 1). Western blot analysis revealed that there was no significant change in the total protein levels of ATM and ATR at both 4 h and 24 h time points, however pATM and pATR levels were enhanced at 24 h when co-cultured with Vγ2Vδ2 T cells in OV4 cells (Fig. 1, left panel). In contrast, H2AX is significantly phosphorylated in both 4 h and 24 h when co-cultured with Vγ2Vδ2 T cells, suggesting a general stress- and DNA damage-mediated phosphorylation of H2AX. Besides ATR and ATM, H2AX is also phosphorylated by DNA-pK. Therefore, there might be some contribution of DNA-pK in H2AX phosphorylation upon Vγ2Vδ2 T cell induction. A2780 resistant cells did not show any significant difference in total ATM and ATR levels when co-cultured with Vγ2Vδ2 T cells. However, the levels of pATM and pATR were downregulated when compared to total ATM and ATR, upon co-culture with Vγ2Vδ2 T cells, in both 4 h and 24 h time points (Fig. 1, right panel). Accordingly, ATM substrate Chk2 phosphorylation (pChk2) and ATR substrate Chk1 (pChk1) were also downregulated at 4 h and 24 h upon Vγ2Vδ2 T cell induction. This data showed that ATM-Chk2 and ATR-Chk1 signaling pathways are downregulated upon Vγ2Vδ2 T cells induction. We noticed that γH2AX is substantially downregulated at 24 h, but this is not evident at 4 h, further indicating that H2AX phosphorylation is influenced by different kinases besides ATR and ATM. Based on these preliminary observations, we hypothesize that downregulation of ATR-Chk1 and ATM-Chk2 pathways might play a critical role in promoting the resistance of A2780 towards Vγ2Vδ2 T cells-mediated cytotoxicity.
Figure 1. Levels of ATR and ATM signaling pathway molecules in ovarian cancer cells.
A sensitive (OV4) and a resistant (A2780) ovarian cancer cells were co-cultured with ex-vivo-expanded Vγ2Vδ2 T cells at a ratio of Cancer: Vγ2Vδ2 T cells = 1:5, and the cancer cells were harvested at 4 h and 24 h time points to isolate total protein after gentle removal of Vγ2Vδ2 T cells. Total proteins were subjected to the Western blot analysis using relevant Abs as stated.
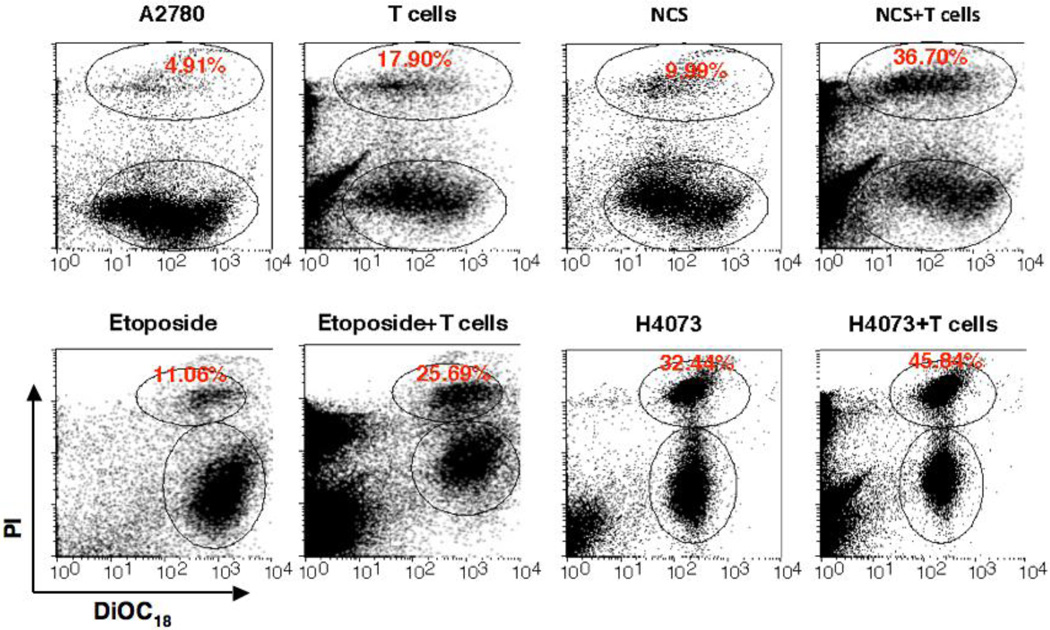
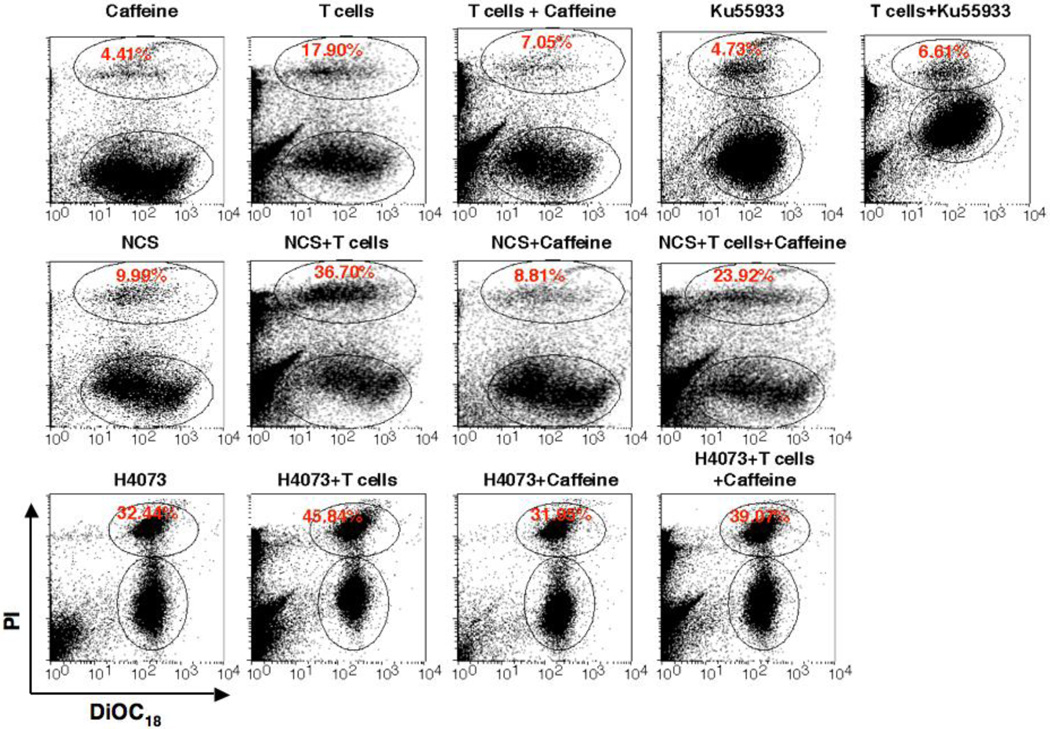
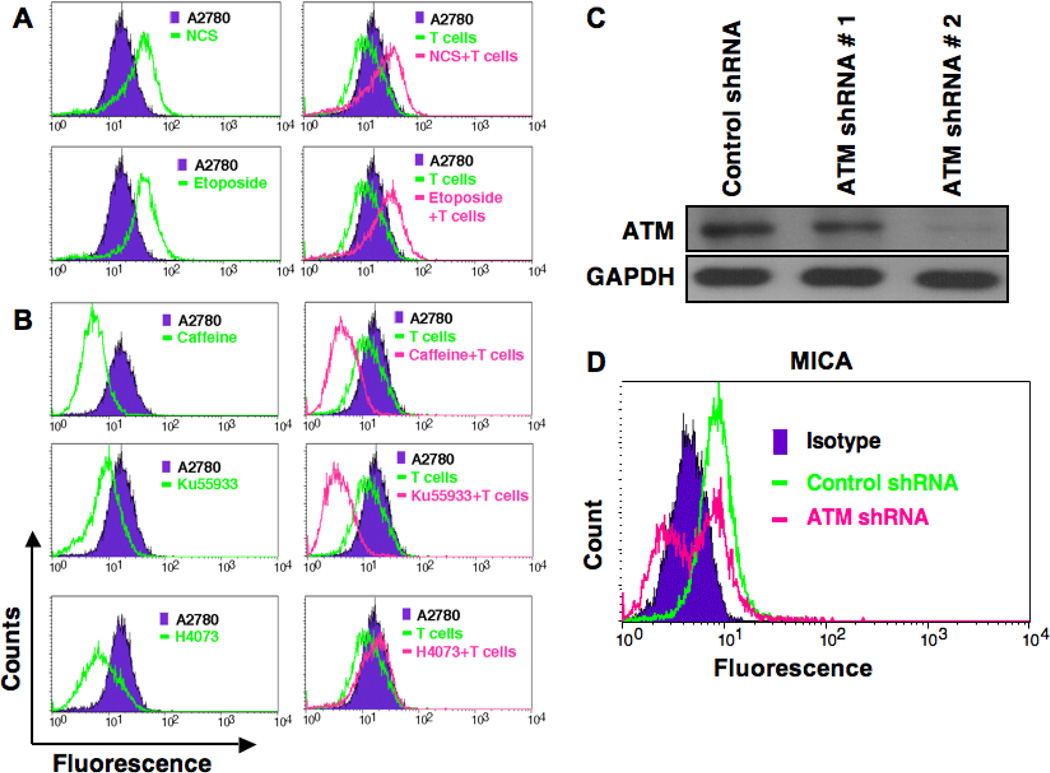
Induction of ATM enhances Vγ2Vδ2 T cells-mediated cytotoxicity and specific inhibitors of ATM reduce cytotoxicity
It has been shown that genotoxic stress or inhibitors of DNA-replication could up-regulate the expression of NKG2D ligand through activation of ATM and ATR protein kinase pathways in human fibroblast and in mouse tumor cell lines, which led enhanced cytotoxic lysis by NK cells [18, 19]. The reduction of ATM activation upon Vγ2Vδ2 T cells induction in resistant ovarian cancer cells (A2780), suggest that somehow regulatory pathways involved in ATM activation are affected in this resistant cell line. Considering this possibility, we hypothesize that upregulation or induction of ATM would enhance Vγ2Vδ2 T cells-mediated cytotoxicity of these resistant cells. We next tested this hypothesis by doing following experiments. We selected three different chemicals or drugs (NCS, Etoposide, and H4073), known to influence ATM signaling pathway, and combined them with Vγ2Vδ2 T cells to determine the cytoxicity in combination treatment. NCS is a macromolecular chromoprotein enediyne antibiotic, which leads to double-strand DNA cleavage [24]. Etoposide results in immediate ATM activation by inhibiting topoisomerase and thus DNA synthesis [25]. Studies implicated that etoposide could be used as a second-line of therapy both for platinum-resistant and platinum-sensitive ovarian cancer patients [26–28]. The H4073, a curcumin analog and food additive has demonstrated anti-tumor activity, but does not induce ATM [29]. We have optimized both the dose of molecules and Vγ2Vδ2 T cells concentration to demonstrate the specific effects on ovarian cancer cells. By using live/dead flowcytometric analysis study, we show that all three molecules have cytotoxic effect to the tumor cells and that the effect could be significantly enhanced by using application of Vγ2Vδ2 T cells (Fig. 2). The values (±SEM) for the level of cytotoxicity are as follows: A2780 = 3.45±0.74; A2780+T = 11.75±3.07; NCS = 9.63±0.74; NCS+T = 24.60±4.29; Etoposide = 7.50±0.18; Etoposide+T = 39.77±1.45; H4073 = 25.69±3.78; H4073+T = 36.72±8.12; Caffeine = 4.80±0.47; Caffeine+T = 7.64±0.34; KU3393 = 5.17±0.44; KU3393+T = 6.04±0.56. In the current experimental setting, the optimum dose of NCS and Etoposide were not able to induce remarkable lysis of tumor cells; however, when Vγ2Vδ2 T cells were added, the cytotoxicity effect was significantly boosted indicating synergistic effect of NCS and Etoposide with Vγ2Vδ2 T cells treatment. In contrast, H4073 was shown to be able to induce strong apoptosis to the A2780 cells; however, in combination with Vγ2Vδ2 T cells, no synergistic effect of apoptosis was observed. Our data support a possibility of the involvement of the ATM pathway in enhanced cytotoxicity of resistant ovarian cells. To further assess this possibility, we used two ATM inhibitors such as Caffeine (general inhibitor) and KU55933 (specific inhibitor) to abrogate or reduce ATM signaling in these ovarian cancer cells. Tumor cells were subjected to various treatments to optimize the synergistic effect of ATM on Vγ2Vδ2 T cell-mediated cytotoxicity in a live/dead flowcytometric assays (Fig. 3). Both caffeine and KU55933 were able to abolish the cytotoxic effect of Vγ2Vδ2 T cells. There is no significant difference in the inhibitory effect between caffeine and KU55933, indicating a possible high dependency of Vγ2Vδ2 T cells on the ATM pathway. In contrast, when ATM stimulator NCS was added, the cytototixic effect of Vγ2Vδ2 T cells was boosted by two-fold. Interestingly, Caffeine or KU55933 (data not shown) alone was able to reduce the boost, indicating that ATM indeed play an important role in regulating the apoptotic response induced by Vγ2Vδ2 T cells in A2780 cells. However, there was no significant inhibitory effect of Vγ2Vδ2 T cells-mediated cytotoxicity on tumor cells from caffeine in the presence of H4073, which does not induce ATM, confirming the caffeine and KU55933 effect was ATM specific.
Figure 2. ATM-inducing molecules enhance Vγ2Vδ2 T cells-mediated cytotoxic lysis of ovarian cancer cells.
Cytotoxicity of Vγ2Vδ2 T cells towards ovarian cancer cells was performed in presence of NCS, Etoposide, and H4073 molecules using a LIVE/DEAD cell-mediated viability/cytotoxicity kit and flowcytometric analysis following protocol mentioned in the Materials and Methods section. Upper-gated cells are PI stained dead cancer cells and lower gated cells are PI-stain negative live cancer cells that are DiOC18 positive.
Figure 3. ATM inhibition reduces cytotoxic lysis of ovarian cancer cells by Vγ2Vδ2 T cells.
The Vγ2Vδ2 T cells-mediated cytotoxicity of ovarian cancer cells was evaluated in presence of NCS, Etoposide, and H4073 molecules along with ATM inhibitors. Flowcytometric analysis was performed to evaluate cytotoxicity using a LIVE/DEAD cell-mediated viability/cytotoxicity kit. Upper-gated cells are PI stained dead cancer cells and lower gated cells are PI-stain negative live cancer cells that are DiOC18 positive.
Oxidative stress and ROS production in mitochondria of cancer cells upon induction of Vγ2Vδ2 T cells
It has been shown that ATM signaling pathway is activated upon certain levels of reactive oxygen species (ROS) induction [30]. Therefore, we next investigated whether ROS was the major contributor in the tumor cell lysis. ROS production was evaluated in tumor cells using MitoRed staining technique and flowcytometric evaluation in various treatment conditions. ATM inducers or ATM inhibitors alone could generate ROS production in the mitochondria of tumor cells. Even though Vγ2Vδ2 T cells were able to increase the ROS production compared with control tumor cells, addition of NCS or Etoposide didn't show any significant enhancement of ROS production (data not shown). Treating the cells with caffeine or KU55933 has showed slight decrease in ROS production compared to controls. However, H4073 significantly increased the ROS production in the presence of Vγ2Vδ2 T cells indicating that ROS production might play an important role in H4073 induced apoptosis (data not shown). However, we predict that in our experimental condition, overall ROS production doesn't seems to play an important role in the synergistic effect of Vγ2Vδ2 T cells and ATM inducers-induced cytolysis.
Downstream signaling molecules of ATM/ATR pathway involved in lysis of tumor cells in combination therapy
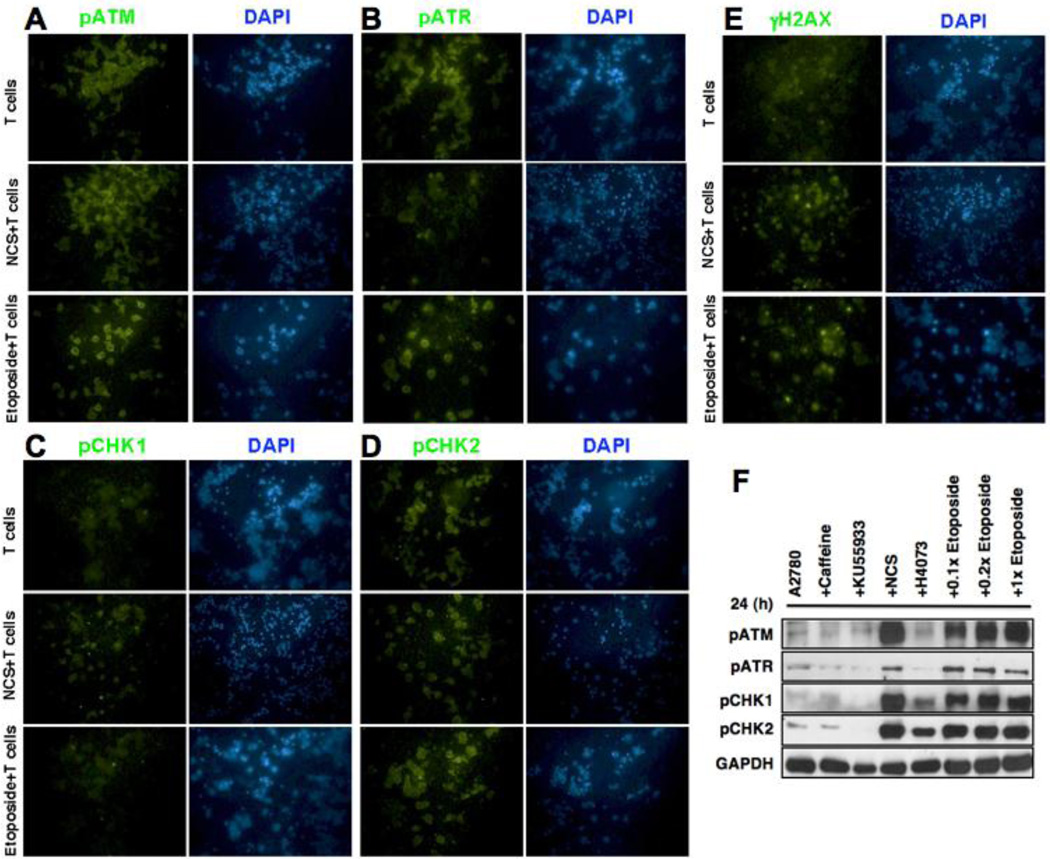
Imunocytochemical analysis was performed to better understand the modulation of the stress-related signaling molecules in tumor cells in the presence of ATM stimulatory chemicals (NCS and Etoposide) and Vγ2Vδ2 T cells after 24 h of co-culture (Fig. 4). As expected, NCS and Etoposite increased the expression of pATM and pATR, which leads to increased susceptibility towards tumor cells (Fig. 4AB). Additonally, the pChk2 and γH2AX levels also showed increase in their levels upon combination treatment (Fig. 4DE). No significant change was observed on pCHK1 level, suggesting that ATR-Chk1 pathway might not be the predominant pathway activated when treated with Etoposide and NCS (Fig. 4C). We further confirmed our results by Western blot analysis. Our results also show that NCS, and Etoposide induced activation (phosphorylation) of ATM/ATR and their substrate Chk2/Chk1 respectively (Fig. 4F). Conversely, ATM inhibitors (caffeine, and KU55933) reduce expressions of the ATM/ATR and their substrate Chk2/Chk1 molecules (Fig. 4F).
Figure 4. Expression of ATM/ATR pathway molecules in presence Vγ2Vδ2 T cells and ATM inducing factor.
Ovarian cancer cells were co-cultured with Vγ2Vδ2 T cells for 24 h at 1:10 ratios along with ATM inducing factor (NCS), and various ATM/ATR pathway molecules were assessed using immunocytochemical techniques (A–E). F. Level of activation (phosphorylated form) in ATM/ATR and their substrate (ChK2/Chk1) molecules in A2780 cells with NCS, H4073, and Etoposide stimulus or with ATM-inhibitors (caffeine or Ku55933).
ATM stimulator induces surface expression of MICA
To understand the contributory role of ATM signaling pathway, and its cross-talk with already known molecules activated by Vγ2Vδ2 T cells, next we examined the levels of MICA expression on ovarian cancer cells. We investigated whether the synergistic cytotoxicity by ATM inducer and Vγ2Vδ2 T cell treatment, is mediated by modulation of MICA expression. Therefore, we tested whether ATM inducer has any effect on MICA expression on the tumor cells. Flowcytometric analysis revealed that NCS and Etoposide, inducers of ATM, also enhanced surface expression of MICA in A2780 cells. As such these cells reduced surface expression of MICA in the presence of Vγ2Vδ2 T cells and become resistant to the cytotoxic lysis [17]. However, in the presence of ATM inducers these cells could not reduce surface expression of MICA when Vγ2Vδ2 T cells were added; instead they increased the MICA expression (Fig. 5A). These data showed that ATM inducers directly promote MICA expression, which then enhance the cytotoxic effect in resistant tumor cells when ATM inducers and Vγ2Vδ2 T cells were combined. If this is true, then the ATM inhibitors should show an effect on MICA expression. In the absence of Vγ2Vδ2 T cells, inhibiting ATM by caffeine or KU55933 leads to down-regulation in surface expression of MICA on A2780 cells (Fig. 5B). However, stimulating ATM using NCS lead to an up-regulation of surface expression of MICA. These results indicated that activated ATM in A2780 cells leads to positive expression of MICA, which was able to boost the recognition of tumor cells by Vγ2Vδ2 T cells. This is consistent with finding in our previous published paper, where we showed that the MICA down-regulation played a critical role in A2780 cell’s resistance towards Vγ2Vδ2 T cells cytotoxicity compared to OV4 cell line [17]. Molecules such as Etoposide were also able to promote MICA expression, which might follow similar mechanisms to NCS. When co-cultured with Vγ2Vδ2 T cells similar pattern was also observed in NCS and Etoposide (Fig. 5A). H4073 was able to slightly increase the expression of MICA compared to no drug treatment in the context of Vγ2Vδ2 T cells; even though the effect is much smaller compared to NCS and Etoposide, which may explain the little synergistic effect observed in H4073+ Vγ2Vδ2 T cells. This result emphasize that MICA regulation by ATM signaling pathway may play a critical role in the resistance of A2780 towards Vγ2Vδ2 T cells treatment, and stimulating ATM might be important in sensitizing A2780 cell line by up-regulating MICA expression.
Figure 5. ATM-inducing molecule enhances surface expression of MICA in ovarian cancer cells and ATM-inhibitor reduces it.
A. Ovarian cancer cells were co-cultured with Vγ2Vδ2 T cells for 24 h at 1:10 ratios in presence or absence of ATM-inducing factors (NCS or etoposide) and assessed for surface expression of MICA level using flowcytometry. Appropriate isotype Ab was used as a control. B. The surface expression of MICA molecule on ovarian cancer cells was also assessed by flowcytometry in presence or absence of Vγ2Vδ2 T cells and ATM-inhibiting factors (caffeine or Ku55933). C. ATM knock down in A2780 cells was evaluated by WB methods. Upper panel shows level of ATM and lower panel shows level of internal control GAPDH. D. Flowcytometric analysis for MICA. ATM knocked down cells was subjected to flowcytometric analysis for surface expression of MICA molecule.
To further confirm the direct involvement of ATM pathway in regulation of MICA molecule in ovarian cancer cells, we have knocked down ATM in A2780 cells using shRNA-mediated knock down approach. ATM knock down was evaluated by WB methods and found that one of the ATM shRNA (#2) is more effective in knocking down of ATM than the other (#1) (Fig. 5C). To investigate the effect of ATM knockdown on surface expression of MICA on A2780 cells, flowcytometric analysis was performed for MICA. We found that knock down of ATM by using ATM shRNA (#2) in A2780 cells also downregulated MICA expression in ovarian cancer cells (Fig. 5D).
Discussion
The cytotoxic T cells, particularly Vγ2Vδ2 T cells, are important for the consideration of immunotherapy due to the fact that they: 1) recognize transformed cells independent of antigen processing or presentation by classical MHC molecules, and 2) possess the strong anti-tumor effector functions. It has been shown that aminobisphosphonates, a class of drugs used as adjuvant cancer therapy for the treatment of malignant osteolytic bone disease, have effects of potently activating the anti-tumor effector functions of human peripheral Vγ2Vδ2 T cells.
Despite the relative complexity of immunotherapy, such treatment may have an important role, in conjunction with other therapies in the treatment of cancers refractory to conventional treatments alone. Clinical studies have recently shown that adding immunotherapy to chemotherapy has survival benefits in comparison to chemotherapy alone [31]. Moreover, chemotherapeutic agents can sensitize tumors to immune cell-mediated killing [32–34]. For instance, some can increase sensitivity of tumor cells via up-regulation of death receptors DR5 and Fas, ligands to TRAIL and FasL respectively [6]. Similarly, Vγ2Vδ2 T cells could be selectively activated by naturally occurring phosphoantigens, which are accumulated in remarkably higher amount in stressed cells, or synthetic drugs offers new avenues for the development of effective T cell-based immunotherapies. Currently, several protocols based on the in vivo activation of Vγ2Vδ2 T cells with phosphoantigens or aminobisphosphonates, or the adoptive transfer of in vitro expanded Vγ2Vδ2 T cells are in development for the treatment of several tumors [6].
Several mechanisms were identified in tumor cells by which they defend against Vγ2Vδ2 T cells, including inhibiting recruitment of tumor infiltrating lymphocytes, secreting immunosuppressive molecules, promoting immunosuppressive activities such as blocking dendritic cells maturation and promote regulatory T cells [35]. All these studies do not address, the molecular signaling pathways activated in tumor cells upon Vγ2Vδ2 T cells induction. We anticipate that identifying Vγ2Vδ2 T cells-mediated signaling pathways, and establishing the molecular mechanism of their function would help develop a more effective Vγ2Vδ2 T cell-mediated therapy. The Vγ2Vδ2 T cells induce oxidative stress, which upregulates NKG2D ligand MICA [36–38]. Furthermore, MICA upregulation transmits its signal through the phosphoinositide 3-kinase related kinases (PI3K) pathway [39]. The PI3K/AKT/mTOR pathway can become abnormally activated in many human tumors and thereby contributes to cell growth, cell proliferation, and angiogenesis [40–42]. We previously shown that ovarian tumor cell lysis due to Vγ2Vδ2 T cell induction were mediated via AKT and ERK pathway [17]. Thus, our results showed an oxidative stress-mediated regulation of PI3K/AKT/mTOR pathway [43]. Importantly, it has been shown that ATM is activated by phosphorylation upon oxidative stress, and results in DNA damage response [21, 44, 45]. Oxidative stress results in DNA single- and double-strand breaks, DNA-DNA and DNA-protein cross links, and base modifications, resulting in activation of ATM kinase and its downstream signaling pathway. This damage response then activates DNA repair, cell cycle checkpoint, changes in gene expression profiles, and apoptosis [21, 46]. Even though a link between NKG2D ligand activation and ATM damage response pathway has been implicated [47], whether ATM signal transduction is directly modulated upon Vγ2Vδ2 T cell induction has not been established.
Our results indicated that in sensitive ovarian tumor cells, ATM and ATR phosphorylation enhanced (Fig. 1, left panel). Interestingly, in resistant cells ATM-Chk2 pathway is downregulated upon the recognition of Vγ2Vδ2 T cells (Fig. 1, right panel), which resulted downregulation of MICA and subsequent reduction of cytotoxicity and cell death. Our observation of downregulation of ATM and its substrates upon Vγ2Vδ2 T cells induction is striking and led us to do the experiments using ATM enhancer and inhibitors. We observed that ATM-inducing drugs were able to stimulate ATM level, which promotes cytotoxicity. On the other hand, when we inhibited ATM signaling pathway by using ATM-specific inhibitors, it reduced the cytotoxic lysis of resistant cells after Vγ2Vδ2 T cells induction. Thus, both ATM enhancers and inhibitors showed that ATM-Chk2 signaling pathway plays a critical role in enhancing the susceptibility of tumor cells against Vγ2Vδ2 T cells. We investigated the mechanism further, and revealed the cross-talk between the MICA and ATM pathway by examining the changes in the MICA expression level upon using ATM enhancers and inhibitors. We revealed that MICA expression is upregulated when ATM signaling pathway is upregulated and MICA expression is downregulated when ATM signaling is downregulated. These data support a direct cross-talk between ATM pathway and MICA regulation. At this point, we do not know how ATM influences MICA regulation, but it shows that ATM signal transductions are directly linked to MICA regulation and cytotoxicity to Vγ2Vδ2 T cells (Figs. 2, 3 & 5). This direct link between ATM and MICA is further confirmed by using ATM knock down experiments (Figs. 5). It is highly likely that ATM upregulation might influence the apoptosis pathway, as it has been shown that ATM can promote apoptosis [48–50]. It will be highly interesting to elucidate further signaling mechanisms involved in the process of sensitization, which will be greatly helpful to develop improved combination therapies for ovarian cancer.
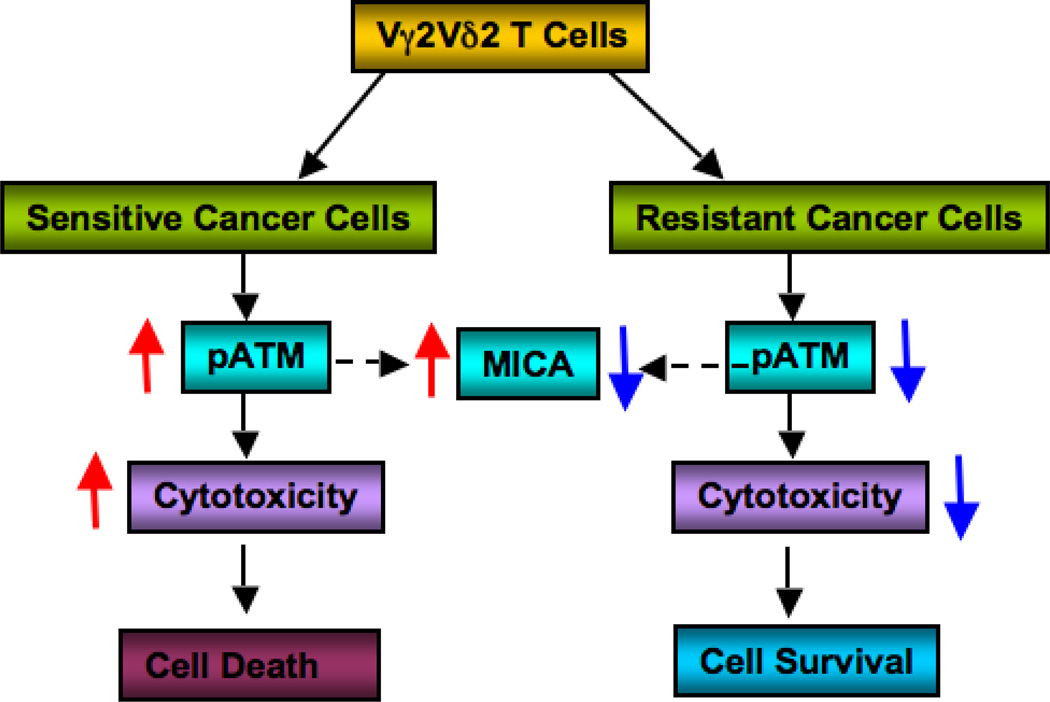
Our results clearly demonstrate that inhibition of ATM pathway activation results in resistance to Vγ2Vδ2 T cell-mediated cell death. Therefore, enhancing ATM activation along with Vγ2Vδ2 T cell treatment would promote the cytotoxicity of resistant ovarian cancer cells. To our knowledge, this is the first report of direct cross-talk of ATM signaling and Vγ2Vδ2 T cell treatment. The molecular mechanism of the inhibition of ATM pathway upon Vγ2Vδ2 T cell-mediated induction is not clear yet, but it is obvious that ATM activation is affected by inhibition of its phosphorylation. Taken together, we propose that ovarian cancer cells inhibit ATM signaling pathway upon Vγ2Vδ2 T cell treatment, which in turn inhibit MICA surface expression, and reduce the cytotoxicity, resulting in resistance (Fig. 6). This also supports the mechanism that Vγ2Vδ2 T cells induce cytotoxicity through ATM signaling pathway. Based on our results, for the first time we tested a new combination therapy where drugs like Etoposide or NCS could be considered as a combination therapeutic regimens in conjunction with Vγ2Vδ2 T cellsmediated immunotherapy for the treatment of ovarian cancers. Moreover, if CD8+ αβ T cells and NK cells function similar to the Vγ2Vδ2 T cells, then this combination therapy would be used to enhance cytotoxicity for these treatments. As resistance to chemotherapy is challenging in ovarian cancer and many other cancer types, we believe that this new therapeutic approach would highly benefit not only ovarian cancers, but also other cancer types.
Figure 6. Interaction of Vγ2Vδ2 T cells and ovarian cancer cells.
Schematics showing involvement of ATM pathway in Vγ2Vδ2 T cells-mediated cytotoxicity.
Highlights.
-
○
Vγ2Vδ2 T cells modulate ATM/ATR pathway in ovarian cancer cells
-
○
Downregulation of ATM/ATR correlates with downregulation of MICA
-
○
Upregulation of ATM/ATR correlates with enhanced MICA expression
-
○
ATM/ATR upregulation mediates enhanced cytotoxicity by Vγ2Vδ2 T cells
-
○
ATM promoting drug combined with Vγ2Vδ2 T cells for effective ovarian cancer lysis
Acknowledgements
This work was supported in part by National Institutes of Health grants, K01 AR054114 (NIAMS), SBIR R44 HL092706-01 (NHLBI), R21 CA143787 (NCI), Pelotonia Idea Award (OSUCCC), and The Ohio State University start-up fund. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Authors have no competing financial interests.
Author’s contribution
Conceived and designed the experiments: JL, HD. Performed the experiments: JL, RA, SK, MD, MJ. Analyzed the data: RA, ML, AR, VP, CS, HD. Contributed reagents/materials/analysis tools: VP, CS. Wrote the paper: JL, AR, HD. All authors read and approved the final manuscript.
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 6.Caccamo N, Dieli F, Meraviglia S, Guggino G, Salerno A. Gammadelta T cell modulation in anticancer treatment. Curr Cancer Drug Targets. 2010;10:27–36. doi: 10.2174/156800910790980188. [DOI] [PubMed] [Google Scholar]
- 7.Bonneville M, Fournie JJ. Sensing cell stress and transformation through Vgamma9Vdelta2 T cell-mediated recognition of the isoprenoid pathway metabolites. Microbes Infect. 2005;7:503–509. doi: 10.1016/j.micinf.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–1618. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 11.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 12.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 13.Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L. Harnessing gammadelta T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Aggarwal R, Kanji S, Das M, Joseph M, Pompili V, Das H. Human ovarian tumor cells escape gammadelta T cell recognition partly by down regulating surface expression of MICA and limiting cell cycle related molecules. PLoS One. 2011;6:e23348. doi: 10.1371/journal.pone.0023348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–410. doi: 10.1016/j.tibs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 21.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Yang DQ. The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther. 2010;9:113–125. doi: 10.1158/1535-7163.MCT-08-1189. [DOI] [PubMed] [Google Scholar]
- 23.Ray A, Milum K, Battu A, Wani G, Wani AA. NER initiation factors, DDB2 and XPC, regulate UV radiation response by recruiting ATR and ATM kinases to DNA damage sites. DNA Repair (Amst) 2013;12:273–283. doi: 10.1016/j.dnarep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Andrea AD, Haseltine WA. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978;75:3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T, Halicka HD, Traganos F, Seiter K, Darzynkiewicz Z. Induction of ATM activation, histone H2AX phosphorylation and apoptosis by etoposide: relation to cell cycle phase. Cell Cycle. 2007;6:371–376. doi: 10.4161/cc.6.3.3835. [DOI] [PubMed] [Google Scholar]
- 26.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 27.Ozols RF. Oral etoposide for the treatment of recurrent ovarian cancer. Drugs. 1999;58 Suppl 3:43–49. doi: 10.2165/00003495-199958003-00007. [DOI] [PubMed] [Google Scholar]
- 28.Hoskins PJ, Swenerton KD. Oral etoposide is active against platinum-resistant epithelial ovarian cancer. J Clin Oncol. 1994;12:60–63. doi: 10.1200/JCO.1994.12.1.60. [DOI] [PubMed] [Google Scholar]
- 29.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, Trigg NJ, Rivera BK, Kalai T, Hideg K, Kuppusamy P. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol Cancer Ther. 2010;9:1169–1179. doi: 10.1158/1535-7163.MCT-09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 32.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'Connor J, Wedel N, Roboz GJ, Miller C, Chopra R, Jurcic JC, Brown R, Ehmann WC, Schulman P, Frankel SR, De Angelo D, Scheinberg D. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 33.Linck D, Lentini G, Tiemann M, Fauser AA, Parwaresch R, Basara N. Sequential application of chemotherapy and monoclonal CD 20 antibody: successful treatment of advanced composite-lymphoma. Leuk Lymphoma. 2005;46:285–288. doi: 10.1080/10428190400015535. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 35.Capietto AH, Martinet L, Fournie JJ. How tumors might withstand gammadelta T-cell attack. Cell Mol Life Sci. 2011;68:2433–2442. doi: 10.1007/s00018-011-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 37.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Fujiyama Y, Andoh A, Bamba T, Okabe H. Oxidative stress increases MICA and MICB gene expression in the human colon carcinoma cell line (CaCo-2) Biochim Biophys Acta. 2001;1526:10–12. doi: 10.1016/s0304-4165(01)00099-x. [DOI] [PubMed] [Google Scholar]
- 39.Dituri F, Mazzocca A, Giannelli G, Antonaci S. PI3K functions in cancer progression, anticancer immunity and immune evasion by tumors. Clin Dev Immunol. 2011;2011:947858. doi: 10.1155/2011/947858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAuliffe PF, Meric-Bernstam F, Mills GB, Gonzalez-Angulo AM. Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin Breast Cancer. 2010;10(Suppl 3):S59–S65. doi: 10.3816/CBC.2010.s.013. [DOI] [PubMed] [Google Scholar]
- 41.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 42.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 43.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 45.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000;28:1387–1404. doi: 10.1016/s0891-5849(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 47.Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–447. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, Yan J, Tang D. ERK kinases modulate the activation of PI3 kinase related kinases (PIKKs) in DNA damage response. Histol Histopathol. 2013 doi: 10.14670/HH-28.1547. [DOI] [PubMed] [Google Scholar]
- 49.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Ambrose M, Gatti RA. Pathogenesis of ataxia-telangiectasia: the next generation of ATM functions. Blood. 2013;121:4036–4045. doi: 10.1182/blood-2012-09-456897. [DOI] [PMC free article] [PubMed] [Google Scholar]